Abstract
The receptor that mediates the increase in glucose transport (GT) in response to β-adrenoceptor (β-AR) agonists was characterized in the rat skeletal muscle cell line L6, using the 2-deoxy-[3H]-D-glucose assay.
The β3-AR agonist BRL37344 (pEC50=6.89±0.21), the β-AR agonist isoprenaline (pEC50=8.99±0.24) and the β2-AR agonist zinterol (pEC50=9.74±0.15) increased GT as did insulin (pEC50=6.93±0.15). The highly selective β3-AR agonist CL316243 only weakly stimulated GT.
The pKB values calculated from the shift of the pEC50 values of the agonists in the presence of the β1-AR selective antagonist CGP 20712A or the β3-AR selective antagonist SR 59230A were not indicative of activation of β1- or β3-ARs. Only (−)-propranolol and the β2-AR selective antagonist ICI 118551 caused marked rightward shifts of CR curves to isoprenaline (pKB=10.2±0.2 and 9.6±0.3), zinterol (pKB=9.0±0.1 and 9.4±0.3) and BRL 37344 (pKB=9.4±0.3 and 8.4±.2), indicating participation of β2-ARs.
The pharmacological analysis was supported by reverse transcription and polymerase chain reaction analysis of L6 mRNA, which showed high levels of expression of β2-AR but not β1- or β3-AR in these cells.
Forskolin and dibutyryl cyclic AMP produced negligible increases in GT while the phosphatidylinositol-3 kinase inhibitor, wortmannin, significantly decreased both insulin- and zinterol-stimulated GT, suggesting a possible interaction between the insulin and β2-AR pathways.
This study demonstrates that β2-ARs mediate the increase in GT in L6 cells to β-AR agonists, including the β3-AR selective agonist BRL 37344. This effect does not appear to be directly related to increases in cyclic AMP but requires P13K.
Keywords: β-adrenoceptor, β1-adrenoceptor, β2-adrenoceptor, β3-adrenoceptor, cyclic AMP, glucose transport, insulin, phosphatidylinositol-3 kinase
Introduction
Insulin-mediated glucose transport (GT) is severely impaired in type 2 diabetes. Since skeletal muscle accounts for ∼85% of the total glucose metabolised (Defronzo et al., 1981), there is great interest in identifying insulin-independent mechanisms that stimulate GT in skeletal muscle. Skeletal muscle expresses both GLUT1 and GLUT4 glucose transporters (Gaster et al., 2000) and although there is no significant change in GLUT4 protein levels in the skeletal muscle of patients with type 2 diabetes, sensitivity to insulin is reduced in this tissue (Schalin-Jantti et al., 1994). In rat tissues in vivo the β3-AR selective agonist BRL 37344 promotes GT (Abe et al., 1993; Liu & Stock, 1995), and another selective agonist, CL 316243, decreases blood glucose in obese diabetic mice (Yoshida et al., 1994). These effects are not indirectly mediated by an increase in plasma insulin levels, as BRL 37344 and CL 316243 also increase GT in rat isolated tissues, including skeletal muscle, by a mechanism independent of insulin (Abe et al., 1993; Liu et al., 1996). In the whole muscle preparation, however, these agonists may act on a variety of different cell populations, including adipocytes, which express β3-AR and respond to β3-AR agonists with increased GT (Nikami et al., 1996). BRL 37344 has also been shown to produce an increase in GT in the absence of insulin in a homogenous population of differentiated L6 rat skeletal muscle cells (Tanishita et al., 1997). However, a definitive pharmacological characterisation of the receptor subtype mediating this response has not been made.
Rat skeletal muscle expresses high levels of β2-AR and moderate levels of β1- but not β3-AR mRNA (Roberts et al., 1999), and in accord with this finding cyclic AMP levels in rat soleus muscle are increased in response to β2, but not β3-AR stimulation (Roberts & Summers, 1998). A study that used monoclonal antibodies against human β3-AR indicated that this receptor was present in human gastrocnemius muscle (Chamberlain et al., 1999), although, as in the rat, functional studies in human skeletal muscle have demonstrated the presence of β2-, but not β1- or β3-ARs (Hagström-Toft et al., 1998).
L6 cells grow as myoblasts in medium containing 10% foetal calf serum (FBS) and differentiate and undergo fusion into myotubes when transferred to medium containing 2% FBS (Ewart et al., 1998). The expression of GLUT1 and GLUT4 glucose transporters in L6 cells is regulated during differentiation (Mitsumoto et al., 1991). Since these cells also show an increase in GT in response to BRL 37344 (Tanishita et al., 1997) they represent a good model for studying the non-insulin dependent GT mechanisms of skeletal muscle.
The present study aimed to characterize the receptor that mediates GT increase in L6 cells in response to β-AR agonists. We used differentiated L6 myotubes to measure GT using the 2-deoxy-[3H]-D-glucose ([3H]-2-DG) method(Tanishita et al., 1997) and a number of selective agonists and antagonists to characterize the β-AR involved.
Methods
Cell culture
Rat L6 myoblasts (ATCC) were grown as a monolayer in Dulbecco's Modified Eagle's Medium (DMEM) containing 4.5 g l−1 glucose, supplemented with 10% FBS, 8 mM L-Glutamine, 10 mM HEPES buffer, 2.5 μg ml−1 fungizone (Amphostat B) and 80 μg ml−1 gentamycin sulphate, at 37°C in air containing 5% CO2. For differentiation, the cells were seeded into 12-well plates at ∼8×105 cells per well and maintained in DMEM supplemented with 2% FBS for 7 days until fusion occurred, with medium renewal every second day. The cells used for [3H]-2-DG assays and reverse transcription/polymerase chain reaction (RT/PCR) experiments were restricted to passages 5 to 10.
2-deoxy-[3H]-D-glucose uptake assay
GT was measured using the [3H]-2-DG glucose method (Tanishita et al., 1997). Briefly, the cells were washed with HEPES Buffered Saline (HBS) and incubated in serum free DMEM for 16–20 h the day before each experiment. [3H]-2-DG transport was measured on day 7. Cytochalasin B (10 μM) was used to determine non-facilitated GT (Birnbaum, 1989). In our experiments cytochalasin B inhibited the basal GT by 70–75%. [3H]-2DG (50 nM) uptake was allowed to proceed for 15 min at 37°C, and then terminated by rapid washing with ice-cold HBS. The uptake of [3H]-2DG during this time was linear. For quantitation of [3H]-2-DG uptake cells were digested with 0.2 M NaOH for 2–3 h at 70°C, mixed with 3.5 ml EcoLite and counted.
RNA extraction
RNA was extracted from L6 cells at day 7, and from heart, cerebellum and white adipose tissue (WAT) of a male Sprague Dawley rat (280 g) using Trizol reagent according to the manufacturer's protocol (Life Technologies, Inc.). The RNA was assessed for purity by 260/280 absorbency ratio and gel electrophoresis. All RNA samples were frozen at −70°C, until they were used for RT/PCR within 2–3 months.
Reverse transcription (RT) and polymerase chain reaction (PCR)
RT was performed on 1 μg of isolated RNA, using RT reaction mix consisting of 10×RT buffer (Promega), 10 mM dNTPs, 0.5 μg μl−1 oligo(dT), 20 mM MgCl2, and 20 U reverse transcriptase and 18 U RNAsin (Promega). The samples were incubated at 42°C for 45 min, then at 95°C for 5 min before being placed on ice. 20 μl of 1 mM EDTA was added to each sample, and the RT products were then stored at −70°C until they were used for PCR within 1 month. PCR amplification was performed on 4 μl of cDNA. Specific oligonucleotide rat primers were used to amplify β1-AR (forward, 5′-CCGCTGCTACAACGACCCCAAG-3′ and reverse, 5′-AGCCAGTTGAAGAAGACGAAGAGGCG-3′), β2-AR (forward, 5′-GGTTATCGTCCTGGCCATCGTGTTTG-3′ and reverse, 5′-TGGTTCGTGAAGAAGTCACAGCAAGTCTC-3′), β3-AR (intron spanning forward, 5′-TAGTCCTGGTGTGGATCGTGTCCGC-3′ and reverse, 5′-CGCTCACCTTCATAGCCATCAAACC-3′), and β-actin (forward, 5′-ATCCTGCGTCTGGACCTGGCTG-3′ and reverse, 5′-CCTGCTTGCTGATCCACATCTGCTG-3′). One of the primers for each PCR reaction was labelled with γ33P-adenosine 5′-triphosphate (Geneworks). PCR reactions contained 1×PCR buffer (Life Technologies), 200 μM dNTP's, 2 mM magnesium acetate, 40 ng of each primer and 1 U Taq polymerase (Life Technologies), and then amplified for 16 (β-actin) and 27 (β2- and β3-ARs) cycles at 64°C, or 30 cycles at 60°C (β1-AR). Following amplification, 4 μl of PCR products were electrophoresed on a 1.3% agarose gel. The gel was then transferred onto Hybond N+ membrane by Southern blotting in 0.4 M NaOH/1 M NaCl overnight. After blotting, the membrane was soaked in 0.5 M Tris-HCl (pH 7.5)/1 M NaCl for 5 min, washed in 2×SSC (0.3 M NaCl/30 mM sodium citrate) for 5 min, and air-dried for 30 min. The membranes were exposed to phosphorimager plates for 6–20 h and the bands quantified using the ImageQuaNT software (Molecular Dynamics). The β-actin was used as an internal control.
Drugs and reagents
The authors would like to thank the following companies and individuals for gifts of: SR 59230A, 3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propranol oxalate (Dr L. Manara, Sanofi-Midi), CL 316243, (R,R)-5-[2-[[2-(3-chlorophenyl) -2-hydroxyethyl] -amino]-propyl]1,3-benzodioxole-2,2-dicarboxylate (Dr T. Nash, Wyeth-Ayerst) and (±)-CGP 20712A (2-hydroxy-5(2-((2-hydroxy-3-(4-((1-methyl-4-trifluoromethyl)1H-imidazole-2-y1)-phenoxy)propyl)amino)ethoxy)-benzamide monomethane sulphonate) (Dr G. Anderson, Ciba-Geigy AG Switzerland). The drugs and reagents used were as follows: zinterol hydrochloride (Bristol-Myers Squibb, Noble Park, Australia); (−)-propranolol, (±)-ICI 118551, (erythro-DL-1(7-methylin-dian-4-yloxy)-3-isopropylaminobutan-2-ol) (Imperial Chemical Industries Wilmslow, Cheshire, U.K.); BRL 37344, 4-[2-[(2-hydroxy-2-(3-chlorophenyl)ethyl)-amino]propyl]-phenoxyacetic acid (Tocris Cookson Ltd., Ballwin MO, U.S.A.); (−)-isoprenaline, wortmannin, forskolin, dibutyryl cyclic AMP (N6,2′-O-dibutyryladenosine 3′.5′-cyclic monophosphate), cytochalasin B, insulin (Sigma Chemical Co., St. Louis MO, U.S.A.); DMEM, L-glutamine, Amphostat B, HEPES buffer, trypsin/EDTA (Trace Biosciences, Sydney, Australia); gentamicin sulphate (David Bull Laboratories, Melbourne, Australia); 2-deoxy-[3H]-D-Glucose (NEN™ Life Science Products, Inc., Boston MA, U.S.A.); Trizol reagent (Life Technologies, Rockville MD, U.S.A.). Stock solutions of forskolin, cytochalasin B and wortmannin were made up in 10% dimethyl sulphoxide; the final concentration of dimethyl sulphoxide in the assay media was 0.01%. The remaining drugs were made up in 2 mM L(+)-ascorbic acid. None of the vehicles had statistically significant effects on the basal GT. The primers were synthesised at: Gibco BRL, Life Technologies, Rockville MD, U.S.A., Horward Florey Institute, Melbourne, Australia or Gene Works Pty Ltd., Australia.
Statistics
Results are presented as mean values±s.e.mean. The statistical significance of differences between the control (untreated cells) and experimental groups was analysed by Student's unpaired t-test, one-way ANOVA or two-way ANOVA followed when appropriate by Student's unpaired t-test at the levels indicated.
Results
The effects of insulin and β-AR agonists on glucose transport in L6 myotubes
Insulin-stimulated glucose transport (GT) was used in most experiments as a positive control for cell viability. Concentration-response (CR) curves for stimulation of GT were constructed using L6 cells differentiated into myotubes (Figure 1a,b). GT was stimulated by insulin, the non-selective β-AR agonist isoprenaline, the β2-AR agonist zinterol, and the β3-AR agonist BRL 37344 (Table 1 and Figure 1c). The magnitude of the BRL 37344-induced increase in GT was consistent with previous findings (Tanishita et al., 1997). BRL 37344 and insulin displayed similar pEC50 values, whereas isoprenaline and zinterol had potencies about three orders of magnitude greater. The β-AR agonists produced similar maximal responses to insulin. A highly selective β3-AR agonist CL 316243 (Yoshida et al., 1994) induced a small increase in GT, but only at the highest concentration used (Table 1, Figure 1c).
Figure 1.
(a) Undifferentiated L6 cells. Scale bar=45 μm. (b) L6 myotubes differentiated for 7 days. (c) CR curves for accumulation of [3H]-2-DG were constructed for insulin, BRL 37344, CL 316243, zinterol and isoprenaline, in L6 myotubes. The graph shows mean±s.e.mean, n=6–10.
Table 1.
Effects of the β-AR agonists on [3H]-2DG transport in L6 cells
The effect of antagonists on β-AR stimulated glucose transport
To characterize the receptor subtype stimulated by the β-AR agonists, antagonists selective for each receptor subtype (β1-, β2- and β3-ARs) were employed. Analysis of the data by two-way ANOVA (P<0.05) showed that the β3-AR selective antagonist SR 59230A and the β1-AR selective antagonist CGP 20712A caused significant dextral shifts in the CR curves to zinterol and isoprenaline (Figure 2b,c,e,f), but not BRL37344 (Figure 2a,d). However, application of Student's t-test showed that the differences in the pEC50 values for isoprenaline in the presence and absence of SR59230A or CGP20712A were not statistically significant. Since dextral shifts in the CR curve to zinterol in the presence of SR59230A and CGP20712A were not observed in all individual experiments, pKB values for the antagonists were calculated from the mean pEC50 values of zinterol. The pKB values calculated for CGP20712A (5.9) and SR59230A (6.0) were not appropriate for an action at β1- or β3-ARs respectively (Table 2). In contrast, the β2-AR selective antagonist ICI 118551 caused marked and significant dextral shifts in the CR curves to BRL 37344, isoprenaline and zinterol (Figure 3a–c). The non-selective β-AR antagonist propranolol was used to further assess the receptor subtype stimulated by these agonists. In the presence of propranolol significant shifts to the right in the CR curves to all three β-AR agonists were observed (Table 2, Figure 3d–f). The pKB values calculated for ICI118551 and propranolol using all three agonists were appropriate for an action at β2-ARs.
Figure 2.

[3H]-2-DG transport in response to BRL 37344, zinterol and isoprenaline in the absence or presence of 10−7 M of the β3-AR selective antagonist SR 59230A (a, b and c), or the β1-AR selective antagonist CGP 20712A (d, e and f) in L6 myotubes. The graphs show mean±s.e. of the mean, n=4–5.
Table 2.
Effects of β-AR agonists on the CR curves for stimulation [3H]-2-DG transport in L6 cells by β-AR agonists
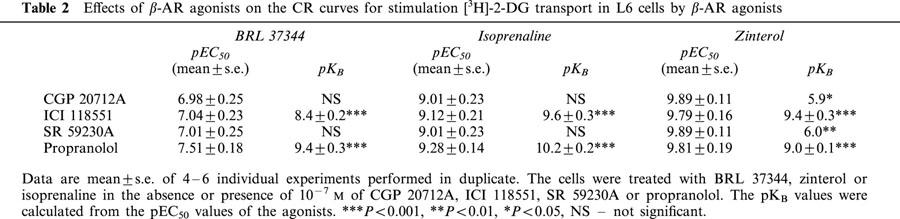
Figure 3.

[3H]-2-DG transport in response to BRL 37344, zinterol and isoprenaline in the absence or presence of 10−7 M of the β2-AR selective antagonist ICI 118551 (a, b and c) or the non-selective β-AR antagonist propranolol (d, e, and f) in L6 myotubes. The graphs show mean±s.e.mean, n=4–6.
The effects of β-AR antagonists on insulin-stimulated glucose transport
To test whether CGP 20712A, ICI 118551, SR 59230A or propranolol have any effect on GT other than by their effects on the β2-AR, L6 cells were treated with insulin in the presence or absence of these antagonists (10−7 M). Analysis using two-way ANOVA showed that the response to insulin was significantly affected by the presence of SR59230A (P<0.05). However, application of Student's t-test demonstrated no significant difference between the pEC50 values or the maximal responses to insulin in the presence or absence of any of the four antagonists (Table 3).
Table 3.
Effects of β-AR antagonists on the insulin stimulated [3H]-2-DG transport in L6 cells
Measurement of β-AR subtype mRNA expressionby RT/PCR
RT/PCR experiments were conducted to examine whether β1, β2 or β3-ARs are expressed in L6 cells differentiated for 7 days. β2-AR mRNA was expressed at high levels, whereas β3-AR mRNA was not expressed at all, and β1-AR mRNA showed negligible expression (Figure 4). Positive controls for all three β-AR subtypes were included in the RT/PCR experiment. Readily detectable β3-AR mRNA in rat WAT and β1-AR mRNA in rat heart indicates that the absence or low expression levels of β1- and β3-AR mRNA in L6 cells was not due to a failure of the RT/PCR reaction.
Figure 4.
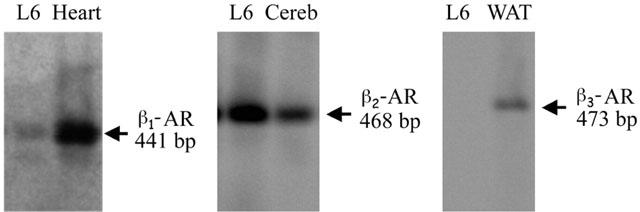
The figure shows β1- (441 bp), β2- (468 bp) and β3-AR (473 bp) PCR products from rat heart, cerebellum and WAT, and β2-AR PCR product from L6 myotubes. Levels of β1-, β2- and β3-ARs expression were measured using RT/PCR analysis.
The effect of forskolin and dibutyryl cyclic AMP on glucose transport
Further experiments were carried out to elucidate the possible mechanisms by which BRL 37344, isoprenaline and zinterol stimulated GT in L6 cells. The classical pathway stimulated by β2-AR activates adenylate cyclase and increases cellular cyclic AMP levels. Forskolin directly stimulates adenylate cyclase and increases production of cyclic AMP, whereas dibutyryl cyclic AMP is a cell-permeable analogue of cyclic AMP. CR curves to forskolin and dibutyryl cyclic AMP were constructed to examine whether cyclic AMP is involved in stimulation of GT in L6 cells (Figure 5c). Although GT did appear to be increased by both forskolin (141±13% at 10−6 M, n=4) and dibutyryl cyclic AMP (144±8% at 10−5 M, n=5), comparison of the responses with the basal rate of GT showed that the increases were not statistically significant (one-way ANOVA, P>0.05), suggesting that the ability of forskolin to increase cyclic AMP levels or the direct application of a cyclic AMP analogue may not be directly coupled to facilitation of GT.
Figure 5.
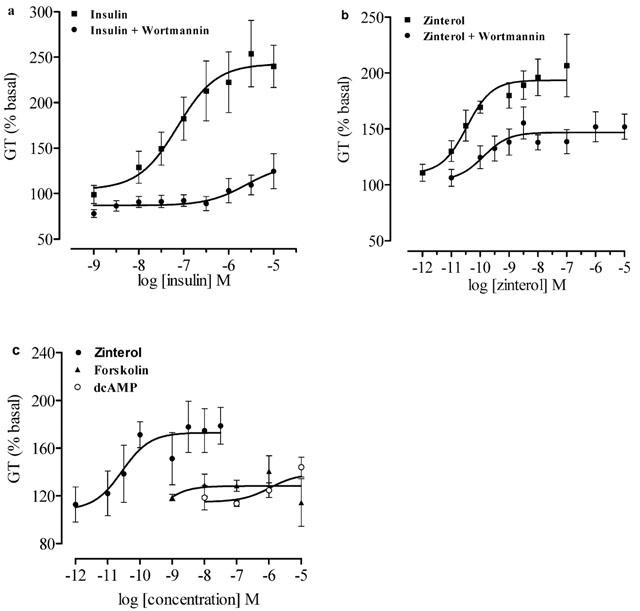
(a,b) [3H]-2-DG transport in response to insulin and zinterol in the absence or presence of PI3K inhibitor wortmannin (10−7 M) in L6 myotubes. (c) CR curves for accumulation of [3H]-2-DG by forskolin and dibutyryl cyclic AMP in L6 myotubes. The graphs show mean±s.e.mean, n=4–6.
The effect of wortmannin on insulin- andzinterol-stimulated glucose transport
Wortmannin is an inhibitor of phosphatydylinositol-3 kinase (PI3K) and inhibits insulin stimulated GT and GLUT translocation to the plasma membrane (Srivastava, 1998). CR curves to insulin and zinterol were constructed in the absence or presence of wortmannin to test whether β-AR agonists cause an increase in GT via a pathway similar to that of insulin (Figure 5a,b). The maximal responses to insulin (10−5 M, 236±20%) and zinterol (10−7 M, 200±25%) without wortmannin were compared with the responses in the presence of wortmannin (insulin, 120±17%; zinterol, 142±10%). Inhibition of responses to insulin and zinterol by wortmannin were statistically significant (insulin, n=7, P<0.001; zinterol, n=7, P<0.05). The basal level of GT was not affected by wortmannin (P=0.9, n=6).
The effect of combining insulin and the β-AR agonists on glucose transport
To examine the possible interaction between the insulin and β-AR agonist pathways, L6 cells were treated with the agonists in the absence or presence of insulin at 10−5 M. In the presence of insulin the responses to BRL 37344, isoprenaline and zinterol were significantly potentiated (Table 4 and Figure 6).
Table 4.
Effects of insulin on the β-AR stimulated [3H]-2DG transport in L6 cells
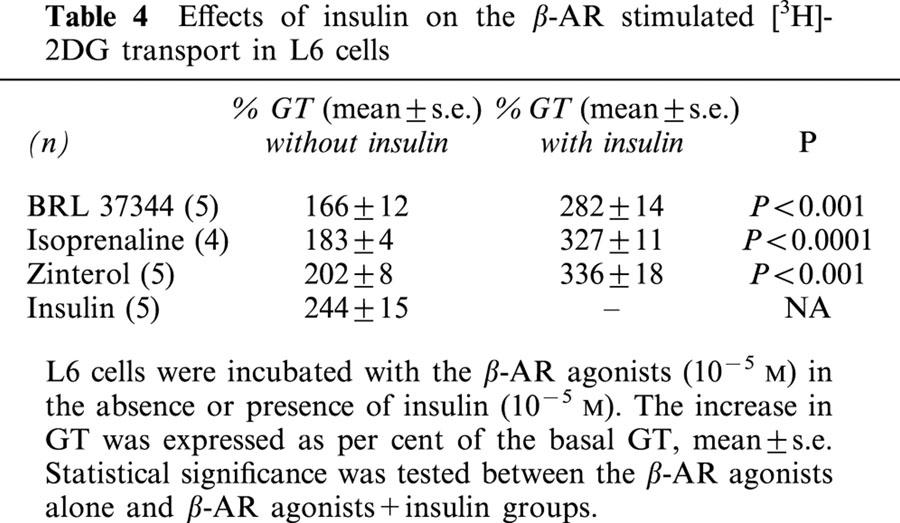
Figure 6.
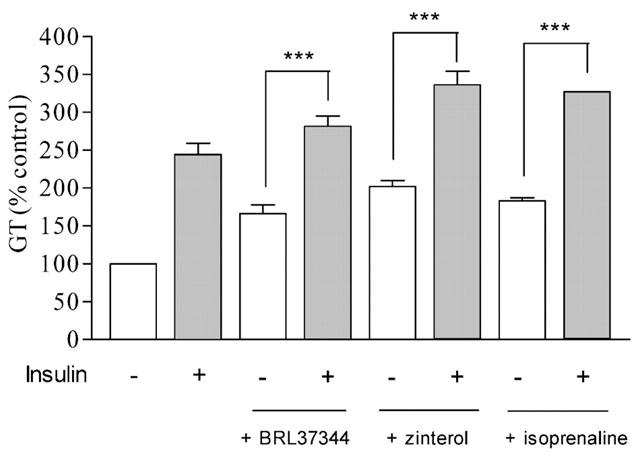
[3H]-2-DG transport in response to 10−5 M of BRL 37344, zinterol and isoprenaline in the absence or presence of insulin (10−5 M). The histograms are mean±s.e.mean, n=4–5.
Discussion
The present study provides strong evidence that the increase in GT stimulated by the β3-AR agonist BRL 37344 in L6 cells is mediated via β2-AR. Although it is known that BRL37344 can activate β2-AR (Board et al., 2000) several previous reports have shown that BRL 37344 stimulates GT in rat isolated skeletal muscle and in the rat skeletal muscle cell line L6 and suggested that the effect was mediated by atypical β-AR or β3-AR (Liu et al., 1996; Tanishita et al., 1997). GT in differentiated L6 myotubes was studied using the selective β3-AR agonists, BRL 37344 and CL 316243, the selective β2-AR agonist zinterol, and the non subtype-selective β-AR agonist isoprenaline. Selective antagonists for each β-AR subtype and the non-selective β-AR antagonist propranolol were employed to characterise the receptor subtype mediating increase in GT in these cells.
Although BRL 37344 stimulated GT in the absence of insulin in accord with the previous findings (Tanishita et al., 1997), another potent and highly selective β3-AR agonist CL 316243 had only a very weak effect on GT in L6 cells. Previous studies have shown that CL 316243 is some 10,000 fold selective for β3-ARs vs β1- and β2-AR, is active in the nanomolar range (Dolan et al., 1994), and is ineffective in β3-AR knockout animals (Cohen et al., 1999). Both the β2-AR selective agonist zinterol and non-selective agonist isoprenaline were much more potent than BRL 37344 in stimulating GT. The selective β2-AR antagonist ICI 118551 and non-selective β-AR antagonist propranolol were highly effective in inhibiting BRL 37344, zinterol and isoprenaline stimulated GT. The calculated pKB values for both the antagonists were as expected for β2-AR blockade (Roberts & Summers, 1998) against all of the agonists. Molecular analysis of β2-AR mRNA expression revealed that β2-AR but not β1- or β3-AR mRNA is expressed at high levels in L6 cells as in a previous study in rat soleus muscle (Roberts et al., 1999), and in a more recent study in L6 cells (Nagase et al., 2001). The β3-AR selective antagonist SR 59230A failed to inhibit the response to the non-selective β-AR agonist, isoprenaline, indicating that in these cells, isoprenaline did not act at β3-ARs. SR 59230A caused a small shift in the CR curve to the β2-AR selective agonist zinterol, but the shift was not consistent. The pKB value for this antagonist calculated from the mean pEC50 values of zinterol was much lower (6.0) than the pA2 value for SR59230A at the β3-AR which has been reported as 8.76 (Manara et al., 1996), indicating that the agonist was acting at β2- and not β3-ARs. Stimulation of GT by β-AR agonists did not involve β1-AR since the β1-AR selective antagonist CGP 20712A failed to block the responses to BRL 37344 and isoprenaline. Although there was a small shift in the CR curve to zinterol it was not consistent for all individual experiments, and the pKB value was much lower (5.9) than expected for the action at the β1-ARs. If zinterol were acting at β1-ARs in L6 cells, CGP 20712A at 10−7 M would have a pKB value close to 9.6 (Kaumann, 1997). These results suggest that GT stimulated by BRL 37344, isoprenaline and zinterol is mediated neither by β1- nor by β3-ARs. Rather, the high potency of zinterol and isoprenaline, blockade by ICI 118551 and propranolol, presence of high levels of β2-AR mRNA together with the poor or non-existent antagonism by β1- or β3-AR antagonists and lack of expression of β1- or β3-AR mRNA provide strong indications that GT responses in L6 cells to BRL 37344, isoprenaline and zinterol are mediated entirely by β2-ARs.
In all systems examined, including soleus muscle (Roberts & Summers, 1998), β2-ARs couple to the stimulatory G protein (Gs), which activates adenylate cyclase and causes an increase in intracellular cyclic AMP. It was therefore important to establish whether stimulation of GT by β2-ARs could be mimicked by increased cyclic AMP levels in L6 cells. GT was not significantly increased by high concentrations of the direct adenylate cyclase stimulant forskolin, or the cell permeable analogue dibutyryl cyclic AMP, suggesting that cyclic AMP may have little direct role in stimulation of GT in L6 cells. However, it should be borne in mind that forskolin has been shown to inhibit GT in cultured fibroblast cells (Tokuda et al., 1994), possibly through a direct action on glucose transporters (Sergeant & Kim, 1985; Klip et al., 1988). It has also been shown that treatment of placental cells with an analogue of cyclic AMP (8-bromo-cyclic AMP), an activator of Gs protein (cholera toxin), or forskolin results in inhibition of GT (Sakata et al., 1996), which suggests that elevation of cyclic AMP can inhibit GT. In rat soleus muscle, zinterol and isoprenaline, but not BRL37344, cause accumulation of cyclic AMP (Roberts & Summers, 1998), and all three agonists increase cyclic AMP in L6 cells (Nevzorova et al., unpublished observations). BRL37344 thus increases GT in both rat soleus muscle (Abe et al., 1993; Liu et al., 1996) and L6 cells (Tanashita et al., 1997 and present results) yet increased cyclic AMP was observed only in L6 cells. This lack of correlation between cyclic AMP levels and GT suggests that β2-AR might also couple to a pathway not involving cyclic AMP, and further experiments employed an inhibitor of PI3K, wortmannin, to determine if this pathway was utilised by zinterol to increase GT in L6 cells.
Wortmannin inhibits insulin-stimulated GT and translocation of glucose transporters to the plasma membrane (Shepherd et al., 1997; Tsakiridis et al., 1995). In the present study, wortmannin inhibited GT stimulated by both insulin and zinterol. Similar effects on insulin-stimulated GT are observed with another inhibitor of PI3K, LY 294002 (Srivastava, 1998), and preliminary experiments in our laboratory show that LY 294002 inhibits both insulin and zinterol stimulated GT in L6 cells (data not shown). In contrast, BRL 37344-stimulated GT in L6 cells was reported to be unaffected by wortmannin (Tanishita et al., 1997). However, as this study used a single concentration of BRL 37344, it was not possible to determine whether wortmannin produced a shift in the CR curve to BRL 37344. Our findings suggest that the pathway downstream of PI3K may be common for insulin and β2-ARs. There is evidence that the β2-AR can form signalling complexes with receptor tyrosine kinases, including the epidermal growth factor receptor (Maudsley et al., 2000), and the insulin receptor (Wang et al., 2000). In addition, the insulin and insulin-like growth factor-1 receptors have been shown to interact with components of G-proteins (Dalle et al., 2001). Thus there is a possibility that the β2-AR can directly interact with the insulin receptor in the stimulation of GT. There is some evidence that β-arrestins, that couple to phosphorylated β-AR, can act as adaptors between G-protein coupled receptors and other signalling pathways (Miller & Lefkowitz, 2001).
To further examine whether insulin and β-AR agonists stimulated GT in L6 cells through distinct mechanisms, cells were incubated with supramaximal concentrations of the β-AR agonists alone or in the presence of insulin. The results show that the addition of insulin significantly increases responses to BRL 37344, isoprenaline and zinterol compared to responses to the agonists alone. However, the responses were not additive probably indicating that insulin and zinterol utilise two pathways which overlap, probably at the level of PI3K.
In conclusion, the present study demonstrates that the selective β3-AR agonist, BRL 37344, the β2-AR agonist, zinterol, and the non-selective β-AR agonist, isoprenaline, all stimulate GT in differentiated rat skeletal muscle L6 cells via β2-ARs. Molecular analysis of β-AR expression in L6 cells provided further support for these findings, demonstrating that only β2-AR mRNA is highly expressed in these cells. The present study contrasts with previous findings that suggest that stimulation of GT in L6 cells by the β3-AR agonist, BRL 37344, is mediated via atypical or β3-ARs. The experiments with forskolin and dibutyryl cyclic AMP to investigate the role of cyclic AMP in GT were inconclusive and the role of cyclic AMP needs to be investigated further. In addition, the use of PI3K inhibitor, wortmannin, suggested that the mechanisms of both insulin and β2-AR stimulated GT in L6 cells involve PI3K. Further characterization of β2-AR mediated GT will assist in understanding the mechanisms involved.
Acknowledgments
Supported by a project grant from the National Health and Medical Research Council. J. Nevzorova is a Monash University Research Scholar. The authors would like to thank Dr Peter Little of the Baker Institute for helpful discussions.
Abbreviations
- [3H]-2-DG
2-deoxy-[3H]-D-glucose; ATCC, American Type Culture Collection
- CR
concentration-response curve
- DMEM
Dulbecco's modified Eagle's medium
- FBS
foetal bovine serum
- Gi proteins
inhibitory guanosine triphosphatases
- GLUT
glucose transporter
- GS proteins
stimulatory guanosine triphosphatases
- GT
glucose transport
- HBS
HEPES buffered saline
- pEC50
negative log EC50
- P13K
phosphatidyl inositol-3 kinase
- pKB
negative log KB
- RT
reverse transcription
- WAT
white adipose tissue
References
- ABE H., MINOKOSHI Y., SHIMAZU T. Effect of a beta 3-adrenergic agonist, BRL35135A, on glucose uptake in rat skeletal muscle in vivo and in vitro. J. Endocrinol. 1993;139:479–486. doi: 10.1677/joe.0.1390479. [DOI] [PubMed] [Google Scholar]
- BIRNBAUM M.J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- BOARD M., DOYLE P., CAWTHORNE M.A. BRL37344, but not CGP12177, stimulates fuel oxidation by soleus muscle in vitro. Eur. J. Pharmacol. 2000;406:33–40. doi: 10.1016/s0014-2999(00)00671-3. [DOI] [PubMed] [Google Scholar]
- CHAMBERLAIN P.D., JENNINGS K.H., PAUL F., CORDELL J., BERRY A., HOLMES S.D., PARK J., CHAMBERS J., SENNITT M.V., STOCK M.J., CAWTHORNE M.A., YOUNG P.W., MURPHY G.J. The tissue distribution of the human β3-adrenoceptor studied using a monoclonal antibody: direct evidence of the β3-adrenoceptor in human adipose tissue, atrium and skeletal muscle. Int. J. Ob. 1999;23:1057–1065. doi: 10.1038/sj.ijo.0801039. [DOI] [PubMed] [Google Scholar]
- COHEN M.L., BLOOMQUIST W., ITO M., LOWELL B.B. Beta3 mediate relaxation in stomach fundus whereas a fourth beta receptor mediates tachycardia in atria from transgenic beta3 receptor knockout mice. Rec. Chan. 1999;7:17–23. [PubMed] [Google Scholar]
- DALLE S., RICKETTS W., IMAMURA T., VOLLENWEIDER P., OLEFSKY J.M. Insulin and insulin-like growth factor I receptors utilise different G protein signalling components. J. Biol. Chem. 2001;276:15688–15695. doi: 10.1074/jbc.M010884200. [DOI] [PubMed] [Google Scholar]
- DEFRONZO R.A., JACOT E., JEQUIER E., MAEDER E., WAHREN J., FELBER J.P. The effect of insulin on the disposal of intravenous glucose. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DOLAN J.O.A., MUENKEL H.A., BURNS M.G., PELLEGRINO S.M., FRASER C.M., PIETRI F., STROSBERG A.D., LARGIS E.E., DITUA M.D., BLOOM J.D., BASS A.S., TANIKELLA T.K., COBUZZI A., LAI F.M., CLAUS T.H. Beta-3 adrenoceptor selectivity of the dioxolane dicarboxylate phenethanolamines. J. Pharmacol. Exp. Ther. 1994;269:1000–1006. [PubMed] [Google Scholar]
- EWART H.S., SOMWAR R., KLIP A. Dexamethasone stimulates the expression of GLUT1 and GLUT4 proteins via different signalling pathways in L6 skeletal muscle cells. FEBS Lett. 1998;425:178–183. [PubMed] [Google Scholar]
- GASTER M., HANDBERG A., BECK-NIELSEN H., SCHRØDER H.D. Glucose transporter expression in human skeletal muscle fibres. Am. J. Physiol. 2000;279:E529–E538. doi: 10.1152/ajpendo.2000.279.3.E529. [DOI] [PubMed] [Google Scholar]
- HAGSTRÖM-TOFT E., ENOKSSON S., MOBERG E., BOLINDER J., ARNER P. β-adrenergic regulation of lipolysis and blood flow in human skeletal muscle in vivo. Am. J. Physiol. 1998;275:E909–E916. doi: 10.1152/ajpendo.1998.275.6.E909. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Four β-adrenoceptor subtypes in the mammalian heart. Trends Pharamcol. Sci. 1997;18:70–76. doi: 10.1016/s0165-6147(96)01033-4. [DOI] [PubMed] [Google Scholar]
- KLIP A., RAMLAL T., DOUEN A.G., BILAN P.J., SKORECKI K.L. Inhibition by forskolin of insulin-stimulated glucose transport in L6 muscle cells. Biochem. J. 1988;255:1023–1029. doi: 10.1042/bj2551023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y.L., CAWTHORNE M.A., STOCK M.J. Biphasic effects of the beta-adrenoceptor agonist, BRL 37344, on glucose utilization in rat isolated skeletal muscle. Br. J. Pharmacol. 1996;117:1355–1361. doi: 10.1111/j.1476-5381.1996.tb16736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y.L., STOCK M.J. Acute effects of the beta 3-adrenoceptor agonist, BRL 35135, on tissue glucose utilisation. Br. J. Pharmacol. 1995;114:888–894. doi: 10.1111/j.1476-5381.1995.tb13287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANARA L., BADONE D., BARONI M., BOCCARDI G., CECCHI R., CROCI T., GIUDICE A., GUZZI U., LANDI M., LE FUR G. Functional identification of rat atypical β-adrenoceptors by the first β3-selective antagonists, aryloxypropanolaminotetralins. Br. J. Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUDSLEY S., PIERCE K.L., ZAMAH A.M., MILLER W.E., AHN S., DAAKA Y., LEFKOWITZ R.J., LUTTRELL L.M. The β2-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J. Biol. Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- MILLER W.E., LEFKOWITZ R.J. Expanding roles for β-arrestins as scaffolds and adapters in GPCR signaling and trafficking [Review] Curr. Opin. Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- MITSUMOTO Y., BURDETT E., GRANT A., KLIP A. Differential expression of the GLUT1 and GLUT4 glucose transporters during differentiation of L6 muscle cells. Biochem. Biophys. Res. Comm. 1991;175:652–659. doi: 10.1016/0006-291x(91)91615-j. [DOI] [PubMed] [Google Scholar]
- NAGASE I., YOSHIDA T., SAITO M. Up-regulation of uncoupling proteins by β-adrenergic stimulation in L6 myotubes. FEBS Lett. 2001;494:175–180. doi: 10.1016/s0014-5793(01)02341-9. [DOI] [PubMed] [Google Scholar]
- NIKAMI H., SHIMIZU Y., SUMIDA M., MINOKOSHI Y., YOSHIDA T., SAITO M., SHIMAZU T. Expression of β3-adrenoceptor and stimulation of glucose transport by β3-agonists in brown adipocyte primary culture. J. Biochem. 1996;119:120–125. doi: 10.1093/oxfordjournals.jbchem.a021196. [DOI] [PubMed] [Google Scholar]
- ROBERTS S.J., PAPAIOANNOU M., EVANS B.A., SUMMERS R.J. Characterization of β-adrenoceptor mediated smooth muscle relaxation and the detection of mRNA for β1-, β2- and β3-adrenoceptors in rat ileum. Br. J. Pharmacol. 1999;127:949–961. doi: 10.1038/sj.bjp.0702605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS S.J., SUMMERS R.J. Cyclic AMP accumulation in rat soleus muscle:stimulation by β2- but not β3-adrenoceptors. Eur. J. Pharmacol. 1998;348:63–70. doi: 10.1016/s0014-2999(98)00021-1. [DOI] [PubMed] [Google Scholar]
- SAKATA M., YAMAGUCHI M., IMAI T., TADOKORO C., YOSHIMOTO Y., OKA Y., KURACHI H., MIYAKE A. 8-Bromo-cAMP inhibits glucose transport activity in mouse placental cells in culture. J. Endocrinol. 1996;150:319–327. doi: 10.1677/joe.0.1500319. [DOI] [PubMed] [Google Scholar]
- SCHALIN-JANTTI C., YKI-JARVINEN H., KORANYI L., BOUREY R., LINDSTROM J., NIKULA-IJAS P., FRANSSILA-KALLUNKI A., GROOP L.C. Effect of insulin on GLUT-4 mRNA and protein concentrations in skeletal muscle of patients with NIDDM and their first-degree relatives. Diabetologia. 1994;37:401–407. doi: 10.1007/BF00408478. [DOI] [PubMed] [Google Scholar]
- SERGEANT S., KIM H.D. Inhibition of 3-O-methylglucose transport in human erythrocytes by forskolin. J. Biol. Chem. 1985;260:14677–14682. [PubMed] [Google Scholar]
- SHEPHERD P.R., NAVE B.T., RINCON J., HAIGH R.J., FOULSTONE E., PROUD C., ZIERATH J.R., SIDDLE K., WALLBERG-HENRIKSSON H. Involvement of phosphoinositide 3-kinase in insulin stimulation of MAP-kinase and phosphorylation of protein kinase-B in human skeletal muscle: implications for glucose metabolism. Diabetologia. 1997;40:1172–1177. doi: 10.1007/s001250050803. [DOI] [PubMed] [Google Scholar]
- SRIVASTAVA A.K. Use of pharmacological agents in elucidating the mechanisms of insulin action. Trends Pharamcol. Sci. 1998;19:205–209. doi: 10.1016/s0165-6147(98)01208-5. [DOI] [PubMed] [Google Scholar]
- TANISHITA T., SHIMIZU Y., MINOKOSHI Y., SHIMAZU T. The beta3-adrenergic agonist BRL37344 increases glucose transport into L6 myocytes through a mechanism different from that of insulin. J. Biochem. 1997;122:90–95. doi: 10.1093/oxfordjournals.jbchem.a021744. [DOI] [PubMed] [Google Scholar]
- TOKUDA H., SATO E., KUROKAWA T., KUSUNOKI N., ISHIBASHI S. Dual effects of forskolin on glucose utilization in cultured fibroblasts, as observed with the use of glucose analogs. Biochem. Mol. Biol. Int. 1994;34:653–659. [PubMed] [Google Scholar]
- TSAKIRIDIS T., MCDOWELL H.E., WALKER T., DOWNES C.P., HUNDAL H.S., VRANIC M., KLIP A. Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose transport, amino acid transport, and glucose transporters in L6 skeletal muscle cells. Endocrinology. 1995;136:4315–4322. doi: 10.1210/endo.136.10.7664650. [DOI] [PubMed] [Google Scholar]
- WANG H., DORONIN S., MALBON C.C. Insulin activation of mitogen-activated protein kinases Erk1,2 is amplified via beta-adrenergic receptor expression and requires the integrity of the Tyr350 of the receptor. J. Biol. Chem. 2000;275:36086–36093. doi: 10.1074/jbc.M004404200. [DOI] [PubMed] [Google Scholar]
- YOSHIDA T., SAKANE N., WAKABAYASHI Y., UMEKAWA T., KONDO M. Anti-obesity and anti-diabetic effects of CL 316,243, a highly specific β3-adrenoceptor agonist, in yellow KK mice. Life Sci. 1994;54:491–498. doi: 10.1016/0024-3205(94)00408-0. [DOI] [PubMed] [Google Scholar]


