Abstract
GABAA receptor agonists have previously been characterized at human GABAA receptors expressed in Xenopus oocytes. The correlation between these data and functional in vivo data of 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) has shown that THIP is 100 fold more potent in clinical studies than in oocytes.
THIP and a series of agonists (GABA, Isoguvacine), partial agonists (Imidazole acetic acid; P4S, 4-PIOL, thio-4-PIOL) and one antagonist (SR95531) were characterized in the rat cortical wedge preparation using inhibition of spontaneous activity in Mg++ free medium as the measurable parameter.
Agonists were in general 40 times more potent in the wedge preparation than at α1β3γ2s containing receptors expressed in Xenopus oocytes, whereas the antagonist was equipotent under these two conditions.
Partial agonists with responses above 6% at α1β3γ2s containing receptors were full agonists in the rat cortical wedge preparation, whereas partial agonists with maximum responses below 6% behaved as partial agonists in the rat cortical wedge preparation.
These data suggest that only a small fraction of the GABAA receptors in the rat cortical wedge needs to be activated by GABAA agonists in order to obtain a maximum response. Results therefore indicate a significant contribution of extrasynaptic receptors to pharmacological activity of exogenous applied GABAA agonists in this system.
Keywords: GABAA receptor, α1β3γ2s, Xenopus oocyte, cortex, wedge, slice, partial agonist, agonist, THIP, P4S, 4-PIOL
Introduction
In the mammalian brain the inhibitory neurotransmitter γ-aminobutyric acid (GABA) plays a key role in the control of synaptic activity. Several studies have demonstrated the involvement of GABA and GABAA receptors in diseases like anxiety, depression, seizures, schizophrenia and sleep disorders (recent review: Thomsen & Ebert, 2002). Thus, much research has focused on the development of ligands for the GABAA receptor complex selective for certain parts of the brain or certain receptor subtypes.
The GABAA receptor system is formed by a pentameric assembly of protein subunits, which all are divided into subfamilies (Olsen & Tobin, 1990). In the frontal cortex, the most abundant synaptically located GABAA receptors are composed of α1β2/3γ2S containing receptors and α3β2/3γ2S subunits (McKernan & Whiting, 1996).
In order to understand the functional significance of these receptors, we and others have carried out pharmacological characterization of human α1β3γ2s GABAA receptors expressed in Xenopus oocytes (Ebert et al., 1994; 1997; 2001; Maksay & McKernan, 2001; Maksay et al., 2000). The conclusion of these studies has been that compounds like piperidin-4-ylsulphonic acid (P4S), 5-(4-piperidyl)isoxazol-3-ol (4-PIOL), and 5-(4-piperidyl)isothiazol-3-ol (thio-4-PIOL) may act as low efficacy partial agonists with maximal responses ranging from 1 to 30%, whereas compounds like Isoguvacine and 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) are full agonists with maximal responses quite similar to that of GABA (Ebert et al., 2001).
In contrast to the synaptic receptors, extrasynaptic receptors in the neocortex contain α4 and δ subunits. In thalamus and other brain regions where α4 is more abundant than in neocortex and thus easier to investigate, α4 and δ subunits are reported to co-localize (Wisden et al., 1991; 1992; Sur et al., 1999; Pirker et al., 2000). Since α4βyδ receptors have proven difficult to express in recombinant systems, this subunit composition has not been characterized in oocytes so far. However, in a very recent study, a novel mouse Ltk cell line capable of expressing receptors composed of α4β3δ subunits has been created. The pharmacology of GABA, THIP and muscimol on this receptor subtype was compared to that of α4β3γ2 containing receptors. All agonists exhibited very high potencies at α4β3δ, GABA being the most potent. In addition, THIP displayed super agonist behaviour at this subunit assembly with an Emax of approximately 160% (Adkins et al., 2001).
Since clinical studies with THIP have indicated that sleep quality improving effects have been obtained at plasma concentrations around 1 μM (Madsen et al., 1983; Faulhaber et al., 1997) and the potency at α1β3γ2s containing receptors expressed in Xenopus oocytes is 350 μM (Ebert et al., 1994), these data clearly suggest that a significant part of the activity of THIP in a complex system may be mediated via receptors others than the synaptic α1β3γ2s containing receptors.
In order to shed further light on this discrepancy we have now characterized THIP and a series of direct acting GABAA ligands in the rat cortical wedge preparation. Within the neocortex, the major neuronal cell types are pyramidal cells and non-pyramidal cells. Most pyramidal cells are projection neurons that are thought to use excitatory amino acids as neurotransmitters. Projection neurons have long axons and mediate the output from neocortex to other cortical areas and to subcortical structures. The pyramidal cell bodies are located in layer II-VI (Zilles, 1990). From the soma of the cell body, a single apical dendrite rises that ascends vertically towards layer I. In addition, from the cell soma, an array of short dendrites spread laterally. Of particular importance to the cortical wedge preparation are the corticostriatal projection neurons, which provide a massive glutamatergic input from the neocortex to the striatum (Ottersen et al., 1995). In contrast, most non-pyramidal cells are local-circuit neurons, which use GABA as neurotransmitter. These cells have short axons and do not project to other cortical areas or subcortical structures. The physiological function of the GABAergic neurons is to focus and refine the firing pattern of the excitatory projection neurons. Thus, neuronal excitability is under GABAergic control. Immunocytochemical studies of GABAA receptor subunit localization have revealed that α1, β2, and β3 subunits are equally distributed throughout the six cortical layers, whereas α2, α4, γ2 and δ are enriched in the outermost layers. In contrast, α3, α5 and β1 subunits are concentrated in the inner layers (α3 predominantly in layer V/VI, α5 in IV/VI and β1 and IV) (Pirker et al., 2000).
As previously mentioned, the corticostriatal projection neurons provide a dense glutamatergic innervation within the neocortical region used for the cortical wedge preparation. Therefore, originally, this method was used for pharmacological characterization of compounds interacting with the glutamatergic system as described by Harrison & Simmonds (1985). Since application of excitatory amino acids and glutamate analogues to the cortical brain slices gives rise to a depolarization of the brain tissue, Harrison & Simmonds (1985) quantified the functional outcome by measuring the height of the peak recorded on a chart recorder. In addition, in the original work, it was described that application Mg2+-free Krebs superfusion medium to the brain slices resulted in development of spontaneous activity (seen as fast spikes) within 30 min in some of the slices. The spike frequency increased upon application of glutamate receptor agonists and vanished upon application of the NMDA receptor selective antagonist, (−)-aminophosphonovaleric acid (Harrison & Simmonds, 1985). Thus, the spontaneous activity has NMDA receptor origin. Under physiological conditions where the extracellular concentration of Mg2+ is approximately 1 mM, extracellular Mg2+ ions voltage-dependently block the open NMDA receptor channel thereby providing a very important inhibition on NMDA receptor activation (Mayer et al., 1984). This inhibitory mechanism is lost when Mg2+-free medium is applied to the cortical slices as the Mg2+ present in the brain tissue gradually washes out giving rise to development of spontaneous activity.
In order to obtain a high variation in the maximum response at α1β3γ2S containing receptors, known to be present at the synapses in the neocortex, the following compounds were selected: GABA and Isoguvacine as full agonists, THIP, P4S, IAA (imidazole-acetic acid), thio-4-PIOL and 4-PIOL as partial agonists, and SR95531, (2-(3-carboxypropyl)-3-amino-6-methoxyphenyl-pyradizinum bromide) as competitive antagonist (Ebert et al., 1994; 2001). These structurally diverse compounds seen in Figure 1 exhibit very different potencies and maximal responses when acting via GABAA receptors.
Figure 1.

Structures of compounds used in the present study.
Methods
Compounds were obtained through normal commercial sources except for 4-PIOL and thio-4-PIOL, which were synthesized according to previously published methods (Frølund et al., 1995; Byberg et al., 1987).
The rat cortical wedge preparation was carried out according to previously published methods (Harrison & Simmonds, 1985; Ebert et al., 1997). In short: an adult male Sprague–Dawley rat (150–175 g; M&B A/S, Ry, Denmark) was decapitated and the brain rapidly removed and placed in ice-cold O2/CO2 (95%/5%) saturated Ca-Krebs medium ((mM) NaCl 118; KCl 2.1; KH2PO4 1.2; D-glucose 11; NaHCO3 25; CaCl2 2.5). With a coronal section, the cerebellum was freed from the brain, using a hand-held razor blade. The cut surface of the remaining block of the brain was fixed to a Teflon pad with a cyanoacrylate glue (Loctite 401, Loctite Ltd., Dublin, Ireland) (the anterior part of the brain pointing upwards). The pad was mounted in a vibratome chamber which was immediately filled with ice-cold Ca-Krebs medium. Using a vibratome (Vibroslice model HA752, Campden Instrument, Ltd., Leicester, U.K.), successive coronal sections were made and discarded until the corpus callosum appeared as an unbroken line connecting the two hemispheres of the brain. Subsequently, 3–4 slices (500 μm thick) were cut and carefully transferred to a Petri dish containing ice-cold Ca-Krebs medium. With two hand-held razor blades, the slices were divided at the midline, thereby separating the hemispheres. Parallel with the midline, a rod (‘wedge') of brain tissue, approximately 1.5 mm thick, was cut from each hemisphere and the striatal tissue was trimmed off.
The recording arrangement consisted of a stand holding a slide (angled 45°) containing four two-compartment baths. The two compartments of each bath were separated by a wall supplied with a slot for placement of the wedge. Along the wall in each compartment, a strip of double-layered, non-woven dishcloth tissue was placed, which when wetted provided contact to the Ag/AgCl electrodes (Dri-Ref™, World Precision Instruments, Sarasota, Florida, U.S.A.) situated in the top of the slide. The electrical potential between the two compartments was measured by the electrodes and displayed on a Yokogawa LR 4220E chart recorder (Yokogawa Electric Corporation, Tokyo, Japan).
With a pair of forceps, the wedge was mounted across the slot, with the cortex part of the wedge situated in the left compartment and the corpus callosum part in the right compartment. The gap between the two compartments was insulated with grease (a mixture of high vacuum silicone grease, Wacker-Chemie, Munich, Germany, and heavy white mineral oil, Sigma Diagnostics, Inc., St. Louis, Missouri, U.S.A.) beneath and above the wedge traversing the slot. In order to prevent the wedge from drying out, another wetted strip of dishcloth tissue was placed on top of the wedge. The two compartments were independently and continuously superfused with Krebs medium at 1 ml min−1 (provided by a peristaltic pump model 110, Ole Dich Instrumentmakers, Hvidovre, Denmark). In order to ensure that the impulses would propagate only from the left to the right, Ca-Krebs was supplied to the left compartment and Krebs medium devoid of Ca2+ to the right compartment.
The wedges were left for development of spontaneous activity for 2–3 h. Characterization of effects on the spontaneous activity was initiated when the spontaneous activity was more than 30 spikes per 12 min and stable over a 30 min period. The number of population responses (spikes) per 12 min was counted. Compounds were applied in superfusion buffer and the wedges were superfused for 20 min. The number of spikes during the last 12 min of the drug application were counted and the frequency (spikes per min) were calculated. The relative frequency, calculated as the ratio between frequencies in the presence and absence, respectively, of compound was calculated. Dose response curves were constructed using cumulative doses with increase in dose every 20 min. Obtained data were analysed using the non-linear curve fitting programme GraFit 4.0 (Erithacus Software) and the logistic equation: Response=Max effect ×[Agonist]n / (EC50n+[Agonist]n).
Results
In initial experiments the inhibitory effect of Isoguvacine, THIP and GABA was compared using either cumulative curves, with an increase in concentration every 20 min or a paradigm where every dose of compound was followed by a 20 min washout period. No differences in the activities were detected (data not shown), wherefore cumulative curves were used in the remaining study.
As illustrated in Figure 2a, Isoguvacine dose dependently inhibited the spontaneous activity. Analysis of the concentration response curve gave an IC50 value (hereafter EC50) of 5.2 μM. In order to test whether Isoguvacine was a substrate for the GABA uptake systems, co-application with the GABA uptake inhibitor Tiagabine was carried out. As shown in Table 1, 10 μM Tiagabine increased the potency of Isoguvacine approximately 3 fold, suggesting that Isoguvacine is indeed a substrate for the GABA uptake system.
Figure 2.
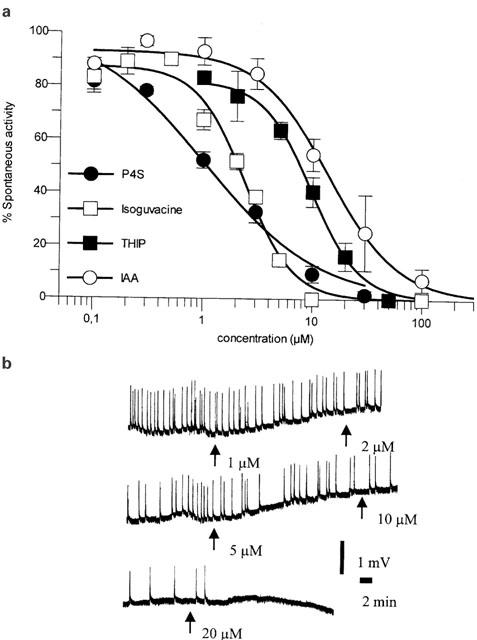
(A) Dose response curves for P4S, Isoguvacine, THIP and IAA obtained in the rat cortical wedge preparation. Curves were obtained in the presence of 10 μM Tiagabine and carried out at not less than four different wedges on at least 2 different days. Values are mean values±s.e.mean. (B) Trace from the rat cortical wedge preparation illustrating the dose dependent inhibition of spontaneous activity with increasing concentrations of THIP.
Table 1.
Pharmacological parameters determined in the rat cortical wedge preparation

GABA was first characterized as an inhibitor of spontaneous activity. The relatively high EC50 value (Table 1: 143 μM) suggested that GABA might be taken up by the transport system. A dose response curve in the presence of Tiagabine subsequently confirmed this. Thus, 10 μM Tiagabine parallel shifted the dose-response curve to GABA 7 fold to the left, corresponding to an EC50 value of 21 μM. In order to ensure that the used concentration of Tiagabine blocked the uptake of GABA without affecting other systems, concentrations of 0.1, 1, 10, 30 and 100 μM Tiagabine were used. These data showed that at a concentration below 1 μM of Tiagabine the EC50 value increased, whereas concentrations of 30 μM and above did not further affect the EC50 value significantly (data not shown). We therefore decided to use 10 μM Tiagabine in the subsequent experiments.
THIP, which at oocytes containing α1β3γ2S receptors is a partial agonist with a maximum response of 70% of that of GABA (Ebert et al., 1994), behaved as a full agonist in the rat cortical wedge preparation (Figure 2a and b). In agreement with previous data no difference in the potency of THIP in the presence and absence, respectively, of Tiagabine was seen, indicating that THIP is not a substrate for transporters in the synapse.
IAA was subsequently characterized. At human α1β3γ2S containing GABAA receptors expressed in oocytes, IAA is a partial agonist with a maximum response of 24% of that of GABA (Ebert et al., 2001). It was therefore very surprising that IAA in the wedge preparation behaved as a full agonist. Like Isoguvacine, the potency of IAA was increased 2–3 fold by Tiagabine, so that the EC50 value of IAA in the presence of Tiagabine was 12 μM (Figure 2a).
Data in the rat cortical wedge preparation for P4S, which at human α1β3γ2S containing receptors is a weak partial agonist with a maximum response of 21% (Ebert et al., 1994), were in agreement with data for IAA. Thus, P4S was as a full agonist and with an EC50 value of 0.9 μM (measured in the presence of Tiagabine), the most potent compound tested (Figure 2a). The relatively shallow curve for P4S (Figure 2a) is in agreement with findings at neuronal cultures, where the slope reflects the interaction with receptor populations with different pharmacological profile (Hansen et al., 2001). However, the full agonism of IAA and P4S prompted us to investigate if any degree of partial agonism could be detected in the cortical wedge model.
We therefore in the presence of Tiagabine characterized the competitive antagonist SR95531 as inhibitor of THIP or GABA responses. As shown in Table 1, SR95531 behaved as a competitive antagonist with a potency corresponding to what has previously been determined at GABA receptors expressed in the oocytes (Ebert et al., 1997). By mixing fixed ratios of THIP and SR95531 we were able to predict and subsequently determine which level the maximum response would reach as a function of the ratio. As shown in Figure 3 partial agonism at different levels at GABAA receptors is obtainable in the rat cortical wedge preparation.
Figure 3.

Dose response curves for different fixed ratios of THIP and SR95531 in the rat cortical wedge preparation. Curves were obtained in the presence of 10 μM Tiagabine and carried out at not less than four different wedges on at least 2 different days. Dotted lines are fitted curves using the waud equation (Lazareno & Birdsall, 1993) using fixed ratios of agonist and antagonist and a fixed value of Ki for SR95531. Values are mean values±s.e.mean.
We therefore continued with compounds, which in the oocyte system had agonist responses lower than 20% of the maximum response obtained with GABA. Thio-4-PIOL (Maksay et al., 2000) and 4-PIOL (Kristiansen et al., 1991) have in different systems previously been shown to be low efficacy partial agonists. At α1β3γ2S containing receptors thio-4-PIOL with maximum responses of 8% and 4-PIOL with a maximum response of 3% both are partial agonists with very low efficacy. 4-PIOL with a maximum response of 30% and thio-4-PIOL with a maximum response of 50%, were indeed partial agonists in the rat cortical wedge preparation (Figure 4).
Figure 4.

Dose dependent inhibition of spontaneous activity by 4-PIOL and Thio-4-PIOL in the rat cortical wedge preparation. Curves were obtained in the presence of 10 μM Tiagabine and carried out at not less than four different wedges on at least 2 different days. Values are mean values±s.e.mean.
Discussion
The rat cortical wedge preparation has previously been used to characterize GABAA receptor ligands. In one of these studies, Harrison & Simmonds (1985) showed that muscimol, as expected, is able to inhibit spontaneous activity, but no attempts were made to use this for more quantitative studies. Others have used very high concentrations of GABA to induce a depolarization of the slice (e.g. Phillips et al., 1998) a method, which can be used in quantitative studies. However, the determined potencies of GABA are in the mM range, which, since the changes in ionic strength may start to play a role for the response, makes full dose-response correlations for compounds weaker than GABA difficult to produce.
In order to be able to determine the functional consequences of GABAA receptor activation at physiological and–in the case of THIP–at clinical relevant concentrations, we decided to use the indirect modulation of NMDA receptor mediated spontaneous activity as a measurement.
This may be a more physiologically relevant method, since inhibitory neurons determine the excitability of a certain tissue. The advantage of this method is that it supposedly reflects the in vivo situation well, however, the major disadvantage is that the system is like a black box, since the individual cell and network parameters contributing to the overall membrane potential and thus the spike frequency is only sparsely understood. However, it is known that GABAA receptors (primarily α1β3γ2S) are located at the synapses, whereas a certain number of α5 and α4 containing receptors are located at dendrites and extrasynaptic sites.
In the present study GABA was initially characterized as a weak agonist (Table 1). Without Tiagabine, EC50 values of 143 μM and 6.8 μM for GABA and THIP, respectively, were established. Upon co-application with 10 μM Tiagabine, the potency of GABA was enhanced 7 fold (EC50=21 μM) whereas THIP essentially was unaffected (4.5 μM) confirming that only GABA is substrate for re-uptake. In neuronal cell cultures, it has been estimated that GAT-1 contributes to approximately 70% of the re-uptake. The remaining 30% is mediated via GABA transporters other than GAT-1 (Borden et al., 1995). Therefore, since Tiagabine only inhibits a fraction of the total re-uptake, it is likely that the true EC50 value of GABA in the rat cortical wedge preparation is lower than 21 μM.
It is interesting that THIP and other GABAA agonists are more than 50 times more potent in the wedge than at the cloned α1β3γ2S GABA receptors expressed in oocytes. This observation is in agreement with data by Kemp et al. (1986), who in the hippocampal slice showed that, when measured with extracellular electrodes, lower potencies were seen. Similarly, in isolated neurons, Kristiansen et al. (1991) and Hansen et al. (2001) have shown that the potencies of agonists are much lower than in the cortical preparation, suggesting that at the single neuron, when measuring Cl− fluxes, the sensitivity towards GABAA agonist is similar to that seen in to Xenopus oocytes. However, it must be emphasised that whereas oocytes express receptors with a confined subunit composition (in this case α1β3γ2S), a wide variety of different subunit assemblies are present in neocortex. In situ hybridization and immunoprecipitation techniques have revealed that α1, β2, β3, and γ2S are the most abundant subunits in neocortex whereas α2, α3, α4, α5, γ3 and δ are present in lower amounts (Fritschy & Möhler, 1995; Pirker et al., 2000; Wisden et al., 1991; 1992).
One possible reason why THIP acts much more potently in the rat cortical wedge preparation may be therefore that the effect of THIP in this system is not mediated primarily via α1β3γ2S containing receptors although these receptor subunits (along with β2) are the most predominant in neocortex. A previous study (Ebert et al., 1994) in Xenopus oocytes expressing GABAA receptors composed of various combinations of α1, α3, α5, β1, β2, β3, γ1, γ2 and γ3 has revealed that THIP exerts the greatest potency at α5β3γ2 and α5β3γ3 receptors (with EC50 values of 40 μM and 29 μM, respectively). At both these receptor subtypes THIP acts as a full agonist (showing Emax values of 99 and 93%, respectively) (Ebert et al., 1994). The higher potency of THIP and the behaviour as a full agonist in the rat cortical wedge preparation may therefore be ascribed to a preferential activation of α5β3γ2/3 receptors.
Another likely explanation for the high potency of THIP observed in the rat cortical wedge preparation is that the effect of THIP to a large extent may arise from activation of extrasynaptically located α4 containing receptors. At α4β3δ containing receptors muscimol, Isoguvacine and THIP all exhibited very high potencies. In addition, THIP displayed superagonist behaviour at this subunit assembly with an Emax of approximately 160% (Adkins et al., 2001). The physiological role of extrasynaptic receptors is presumably to respond to synaptic spill-over of neurotransmitter (Brickley et al., 1996) and must therefore be sensitive to low concentrations of transmitter. A preferential localization of α4β3δ receptors at extrasynaptic sites in neocortex, would implicate that this receptor subtype is more readily accessible to exogenous GABAA receptor ligands, since a diffusion of the ligand into the synaptic cleft prior to interaction with the receptor is not necessary. This, and in particular the fact that GABAA agonists act very potently at α4β3δ receptors suggest that the effects of GABA and THIP in the rat cortical wedge preparation are mainly mediated via this receptor subtype. This assertion, however, raises the question; Why is the potency of GABA established from experiments using the rat cortical wedge model lower than that observed in Xenopus oocytes expressing GABAA receptors composed of α1β3γ2S subunits?
Several lines of evidence have shown that GAT-1 and GAT-3 are located in and around axon terminals (GAT-3 exclusively in glial cells and GAT-1 both in glial cells and presynaptically (Minelli et al., 1995; 1996; Pietrini et al., 1994)). In contrast, it has been suggested that BGT-1 is distributed at neuronal dendrites (Pietrini et al., 1994).
Thus, in the rat cortical wedge preparation, inhibition of GAT-1 mediated GABA reuptake with Tiagabine will prevent the exogenous GABA from being cleared from the synaptic cleft, thereby increasing the effective concentration of GABA in the synapse.
Assuming that the exogenous GABA will cause a drug–receptor interaction with synaptic α1β2/3γ2 as well as extrasynaptic α4β3δ receptors (at which the potency of GABA is ∼8 fold that of α1β2/3γ2), GAT-1 inhibition will affect only synaptic α1β2/3γ2 receptors, which will be activated to a larger extent than in the absence of Tiagabine, thereby lowering the apparent EC50 value. However, since Tiagabine does not inhibit BGT-1 transporters, the re-uptake mechanism on the dendrites remains intact and accordingly extrasynaptic receptors will not be additionally activated. Thereby, a very effective component of the integral response is precluded. For THIP which is substrate of neither of the GABA transporters, interaction with extrasynaptic α4β3δ receptors is not prevented by re-uptake mechanisms. Therefore, a full-scale activation of the highly sensitive extrasynaptic receptors is possible. Had all re-uptake mechanisms been inhibited completely, GABA might very well have exhibited a potency higher than that observed for THIP, as is the case at recombinant α4β3δ receptors.
Alternatively, the voltage clamp condition may in part explain the discrepancy. Under voltage clamp conditions the membrane potential is by default kept constant, allowing a direct correlation between chloride conductance and channel openings to be determined. In contrast under non-voltage clamp conditions a complex correlation between the membrane potential and the chloride conductance is present. Thus, a minor activation of the GABAA receptor, may lead to a minor change in the chloride conductance, which, in contrast to the voltage clamp condition, subsequently will largely affect the membrane potential thereby leading to a very steep dose response curve for the inhibition of the spontaneous activity mediated via membrane depolarisations. Although this explanation may account for the increased potency and to some degree the increased maximum response for the partial agonists no steep dose response correlation is found.
Alternatively, if one assumes that the hyperpolarizing response and subsequent inhibition at spontaneous activity is a consequence of an integration of small contributions from the whole network, the level of activation at the individual sites can be predicted. Thus, under the very unlikely assumption of a homogenous receptor population, where all receptors contribute equally only 3–5% of the receptors need to be activated in order to obtain a maximum inhibition of the spontaneous activity. If this is true, any agonist with an Emax above 3–5% will behave as a full agonist, whereas only low efficacy partial agonists like 4-PIOL and thio-4-PIOL will behave as partial agonists. Our data therefore clearly suggests that in terms of spontaneous activity a large functional receptor reserve is present. It still needs to be clarified if this receptor population is formed by extrasynaptic receptors containing α4β3δ subunits.
In line with this are the functional partial agonism data for THIP and SR95531. If the interaction between THIP and the GABAA receptor antagonist SR95531 is purely competitive it should be possible to mix the two compounds in fixed ratios and thereby determine the maximum response prior to the experiment. The parameters, EC50 and Ki value for THIP and SR95531, respectively, were estimated from dose-response curves and parallelshifts of GABA dose-response curves and then inserted into the waud equation (Lazareno & Birdsall, 1993), which allows prediction of maximum responses as function of mixtures of agonists and antagonists. As illustrated in Figure 3, a high correlation between predicted and obtained values is seen, indicating that the compounds are interacting competitively. Furthermore, as the potency of SR95531 and other antagonists is similar to those determined at oocytes and single cells, there is no indication of differences within the GABA receptor populations in the two systems. The differences in potency of the agonists in the two systems must therefore originate from differences in the measured endpoint, and therefore the number of receptors activated.
In agreement with the idea of the extrasynaptic receptor reserve are findings for the partial agonists. THIP, IAA and P4S, which all are partial agonists with maximum responses ranging from 80% to 25% of that of GABA at α1β3γ2S receptors, are full agonists in the wedge preparation. However, when low efficacy partial agonists with 3–6% are tested these compounds still behave as partial agonists, but with much higher maximum responses.
Since this high efficacy partial agonism is obtainable as a consequence of altered measurement of endpoint it clearly suggests a large functional receptor reserve.
If indeed GABAA receptor agonists only need to activate a small fraction of the GABAA receptor ligands in order to induce a large response, this will have large consequences for the mode of action. If only a minor fraction of the receptors are activated this will give a very reduced risk of tolerance. THIP, which is the only GABAA receptor agonist in clinical development, has been characterized in animals during 5 days of treatment (Lancel & Langebartels, 2000). In these studies, where benzodiazepines normally induce tolerance after 4–6 days of treatment no fading in response was seen. In addition to this one would predict that the interaction between THIP or other GABAA agonists and benzodiazepines or barbiturates would be very low in complex systems whereas an interaction similar to that of GABA would be seen in e.g. oocytes. (Maksay et al., 2000; Skerritt & Macdonald, 1984).
In conclusion the present study suggests that extra-synaptic receptors may play an important role for the activity of partial and full GABAA receptor agonists in the rat cortical wedge preparation. The correlation with the in vivo situation must be evaluated before conclusions on the consequences can be drawn.
Acknowledgments
Drs Uffe Kristiansen and Christian Thomsen are thanked for constructive comments.
Abbreviations
- IAA
imidazole-acetic acid
- P4S
piperidin-4-ylsulphonic acid
- 4-PIOL
5-(4-piperidyl)isoxazol-3-ol
- SR95531
2-(3-carboxypropyl)-3-amino-6-methoxyphenyl-pyradizinum bromide
- thio-4-PIOL
5-(4-piperidyl)isothiazol-3-ol
- THIP
4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol
References
- ADKINS C.E., PILLAI G.V., KERBY J., BONNERT T.P., HALDON C., MCKERNAN R.M., GONZALEZ J.E., OADES K., WHITING P.J., SIMPSON P.B. alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J. Biol. Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- BORDEN L.A., SMITH K.E., VAYSSE P.J., GUSTAFSON E.L., WEINSHANK R.L., BRANCHEK T.A. Re-evaluation of GABA transport in neuronal and glial cell cultures: correlation of pharmacology and mRNA localization. Receptors Channels. 1995;3:129–146. [PubMed] [Google Scholar]
- BRICKLEY S.G., CULL-CANDY S.G., FARRANT M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. 1996;497 Pt 3:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYBERG J.R., LABOUTA I.M., FALCH E., HJEDS H., KROGSGAARD-LARSEN P., CURTIS D.R., GYNTHER B.D. Synthesis and biological activity of a GABAA agonist which has no effect on benzodiazepine binding and of structurally related glycine antagonists. Drug Des. Deliv. 1987;1:261–274. [PubMed] [Google Scholar]
- EBERT B., MIKKELSEN S., THORKILDSEN C., BORGBJERG F.M. Norketamine, the main metabolite of ketamine, is a non competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur. J. Pharmacol. 1997;333:99–104. doi: 10.1016/s0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- EBERT B., MORTENSEN M., THOMPSON S.A., KEHLER J., WAFFORD K.A., KROGSGAARD-LARSEN P. Bioisosteric determinants for subtype selectivity of ligands for heteromeric GABA(A) receptors. Bioorg. Med. Chem. Lett. 2001;11:1573–1577. doi: 10.1016/s0960-894x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- EBERT B., THOMPSON S.A., SAOUNATSOU K., MCKERNAN R., KROGSGAARD-LARSEN P., WAFFORD K.A. Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human γ-aminobutyric acid type A receptors. Mol. Pharmacol. 1997;52:1150–1156. [PubMed] [Google Scholar]
- EBERT B., WAFFORD K.A., WHITING P.J., KROGSGAARD-LARSEN P., KEMP J.A. Molecular pharmacology of γ-aminobutyric Acid type A receptor agonists and partial agonists in oocytes injected with different alpha, beta and gamma receptor subunit combinations. Mol. Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- FAULHABER J., STEIGER A., LANCEL M. The GABAA agonist THIP produces slow wave sleep and reduces spindling activity in NREM sleep in humans. Psychopharmacology (Berl). 1997;130:285–291. doi: 10.1007/s002130050241. [DOI] [PubMed] [Google Scholar]
- FRITSCHY J.M., MÖHLER H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- FRØLUND B., KRISTIANSEN U., BREHM L., HANSEN A.B., KROGSGAARD-LARSEN P., FALCH E. Partial GABAA receptor agonists. Synthesis and in vitro pharmacology of a series of nonannulated analogs of 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol. J. Med. Chem. 1995;38:3287–3296. doi: 10.1021/jm00017a014. [DOI] [PubMed] [Google Scholar]
- HANSEN S.L., EBERT B., FJALLAND B., KRISTIANSEN U. Effects of GABA(A) receptor partial agonists in primary cultures of cerebellar granule neurons and cerebral cortical neurons reflect different receptor subunit compositions. Br. J. Pharmacol. 2001;133:539–549. doi: 10.1038/sj.bjp.0704121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON N.L., SIMMONDS M.A. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br. J. Pharmacol. 1985;84:381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP J.A., MARSHALL G.R., WOODRUFF G.N. Quantitative evaluation of the potencies of GABA-receptor agonists and antagonists using the rat hippocampal slice preparation. Br. J. Pharmacol. 1986;87:677–684. doi: 10.1111/j.1476-5381.1986.tb14585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISTIANSEN U., LAMBERT J.D., FALCH E., KROGSGAARD-LARSEN P. Electrophysiological studies of the GABAA receptor ligand, 4-PIOL, on cultured hippocampal neurones. Br. J. Pharmacol. 1991;104:85–90. doi: 10.1111/j.1476-5381.1991.tb12389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEL M., LANGEBARTELS A. γ-aminobutyric aidA (GABAA) agonist 4,5,6, 7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol persistently increases sleep maintenance and intensity during chronic administration to rats. J. Pharmacol. Exp. Ther. 2000;293:1084–1090. [PubMed] [Google Scholar]
- LAZARENO S., BIRDSALL N.J. Estimation of antagonist Kb from inhibition curves in functional experiments: alternatives to the Cheng-Prusoff equation. Trends Pharmacol Sci. 1993;6:237–239. doi: 10.1016/0165-6147(93)90018-f. [DOI] [PubMed] [Google Scholar]
- MADSEN S.M., LINDEBURG T., FØLSGÅRD S., JACOBSEN E., SILLESEN H. Pharmacokinetics of the gamma-aminobutyric acid agonist THIP (Gaboxadol) following intramuscular administration to man, with observations in dog. Acta. Pharmacol. Toxicol. (Copenh). 1983;53:353–357. doi: 10.1111/j.1600-0773.1983.tb03434.x. [DOI] [PubMed] [Google Scholar]
- MAKSAY G., MCKERNAN R. Entropy as the predominant driving force of binding to human recombinant alpha(x)beta(3)gamma(2) GABA(A) receptors. Eur. J. Pharmacol. 2001;411:55–60. doi: 10.1016/s0014-2999(00)00898-0. [DOI] [PubMed] [Google Scholar]
- MAKSAY G., THOMPSON S.A., WAFFORD K.A. Allosteric modulators affect the efficacy of partial agonists for recombinant GABA(A) receptors. Br. J. Pharmacol. 2000;129:1794–1800. doi: 10.1038/sj.bjp.0703259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER M.L., WESTBROOK G.L., GUTHRIE P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- MCKERNAN R.M., WHITING P.J. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- MINELLI A., BRECHA N.C., KARSCHIN C., DEBIASI S., CONTI F. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J. Neurosci. 1995;15:7734–7746. doi: 10.1523/JNEUROSCI.15-11-07734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINELLI A., DEBIASI S., BRECHA N.C., ZUCCARELLO L.V., CONTI F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J. Neurosci. 1996;16:6255–6264. doi: 10.1523/JNEUROSCI.16-19-06255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSEN R.W., TOBIN A.J. Molecular biology of GABAA receptors. FASEB J. 1990;5:1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- OTTERSEN O.P., HJELLE O.P., OSEN K.K., LAAKE J.H.Amino acid transmitters The rat nervous system 1995San Diego: Academic Press, Inc; 1017–1037.ed. Paxinos, G. pp [Google Scholar]
- PHILLIPS I., MARTIN K.F., THOMPSON K.S., HEAL D.J. GABA-evoked depolarisations in the rat cortical wedge: involvement of GABAA receptors and HCO3- ions. Brain Res. 1998;798:330–332. doi: 10.1016/s0006-8993(98)00479-x. [DOI] [PubMed] [Google Scholar]
- PIETRINI G., SUH Y.J., EDELMANN L., RUDNICK G., CAPLAN M.J. The axonal gamma-aminobutyric acid transporter GAT-1 is sorted to the apical membranes of polarized epithelial cells. J. Biol. Chem. 1994;269:4668–4674. [PubMed] [Google Scholar]
- PIRKER S., SCHWARZER C., WIESELTHALER A., SIEGHART W., SPERK G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- SKERRITT J.H., MACDONALD R.L. Diazepam enhances the action but not the binding of the GABA analog, THIP. Brain Res. 1984;297:181–186. doi: 10.1016/0006-8993(84)90557-2. [DOI] [PubMed] [Google Scholar]
- SUR C., FARRAR S.J., KERBY J., WHITING P.J., ATACK J.R., MCKERNAN R.M. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acid A receptor in rat thalamus. Mol. Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- THOMSEN C., EBERT B.Modulators of the GABAA receptor complex: novel therapeutic prospects Glutamate and GABA receptors and transporters 2002London, UK: Taylor & Francis; 407–427.eds. Egebjerg, J., Schousboe, A., Krogsgaard-Larsen, P. pp [Google Scholar]
- WISDEN W., HERB A., WIELAND H., KEINÄNEN K., LÜDDENS H., SEEBURG P.H. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor alpha 4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- WISDEN W., LAURIE D.J., MONYER H., SEEBURG P.H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZILLES K.Anatomy of the neocortex: cytoarchitecture and myeloarchitecture The cerebral cortex of the rat 1990Cambridge: MIT Press; 76–95.Kolb, B, Tees, RC, eds [Google Scholar]


