Abstract
Neonicotinoid insecticides are agonists of insect nicotinic acetylcholine receptors (AChRs) and show selective toxicity for insects over vertebrates. To elucidate the molecular basis of the selectivity, amino acid residues influencing neonicotinoid sensitivity were investigated by site-directed mutagenesis of the chicken α7 nicotinic AChR subunit, based on the crystal structure of an ACh binding protein (AChBP).
In the ligand binding site of AChBP, Q55 in loop D is close to Y164 in loop F that corresponds to G189 of the α7 nicotinic receptor. Since Q55 of AChBP is preserved as Q79 in the α7 nicotinic receptor and the G189D and G189E mutations have been found to reduce the neonicotinoid sensitivity, we investigated effects of Q79E, Q79K and Q79R mutations on the neonicotinoid sensitivity of the α7 receptor expressed in Xenopus laevis oocytes to evaluate contributions of the glutamine residue to nicotinic AChR–neonicotinoid interactions.
The Q79E mutation markedly reduced neonicotinoid sensitivity of the α7 nicotinic AChR whereas the Q79K and Q79R mutations increased sensitivity, suggesting electronic interactions of the neonicotinoids with the added residues.
By contrast, the Q79E mutation scarcely influenced responses of the α7 nicotinic receptor to ACh, (−)-nicotine and desnitro–imidacloprid (DN–IMI), an imidacloprid derivative lacking the nitro group, whereas the Q79K and Q79R mutations reduced the sensitivity to these ligands. The results indicate that the glutamine residue of the α7 nicotinic receptor is likely to be located close to the nitro group of the insecticides in the nicotinic receptor–insecticide complex.
Keywords: Imidacloprid, nitenpyram, neonicotinoid, desnitro-imidacloprid, nicotinic acetylcholine receptor, chicken α7 subunit, loop D, loop F, Xenopus laevis oocyte, two-electrode voltage-clamp
Introduction
Imidacloprid and related insecticides are referred to as neonicotinoids and their use for crop protection is widespread and increasing. Imidacloprid (Figure 1), the neonicotinoid with the largest sales to date, is similar to classical nicotinoids, in possessing a pyridine ring and in acting on nicotinic acetylcholine receptors (nAChRs) (Matsuda et al., 2001). Unlike nicotine, however, neonicotinoids exhibit selective toxicity toward insects based, at least in part, on their higher affinity for insect nicotinic AChRs (Liu & Casida, 1993; Zwart et al., 1994, Matsuda et al., 1998). Although many nicotinic AChRs are pentameric glycoproteins with two α and three non-α subunits (Karlin & Akabas, 1995; Itier & Bertrand, 2001, Karlin, 2002), some subunits such as α7, α8 and α9 (Couturier et al., 1990; Gerzanich et al., 1994; Elgoyhen et al., 1994) of vertebrates, and αL1 (Marshall et al., 1990) of the locust (Shistocerca gregaria) and ACR-16 of the nematode (Caenorhabditis elegans) (Ballivet et al., 1996; Raymond et al., 2000) are capable of forming homo-pentamers when expressed in Xenopus laevis oocytes. Residues contributing to ACh binding sites have been identified by site-directed mutagenesis and photoaffinity labelling experiments and are referred to as loops A–F (Arias, 1997; Corringer et al., 2000; Itier & Bertrand, 2001). Loop F is located either on non-α subunits of heteromeric receptors or on the α7–α9 subunits forming homo-oligomers, and is considered to interact electronically with the quaternary ammonium nitrogen of ACh, being designated as the negative subsite (Karlin & Akabas, 1995). We have previously investigated the role of loop F in nicotinic AChR interactions with neonicotinoids (Matsuda et al., 2000). At that time, it was designated as loop D by the nomenclature of Arias (1997) but is now universally referred to as loop F (Corringer et al., 2000). The homomer-forming chicken α7 subunit was deployed in conjunction with site-directed mutagenesis and two-electrode voltage-clamp electrophysiology and it was found that G189D and G189E mutations in loop F markedly reduced sensitivity to neonicotinoids of the α7 nicotinic AChR expressed in Xenopus oocytes (Matsuda et al., 2000). By contrast, these mutations scarcely influenced the agonist profile for a denitrated derivative of imidacloprid (DN–IMI) as well as ACh, (−)-nicotine and (+)-epibatidine, demonstrating electronic interactions of the added aspartate and glutamate residues with the nitro group of imidacloprid.
Figure 1.
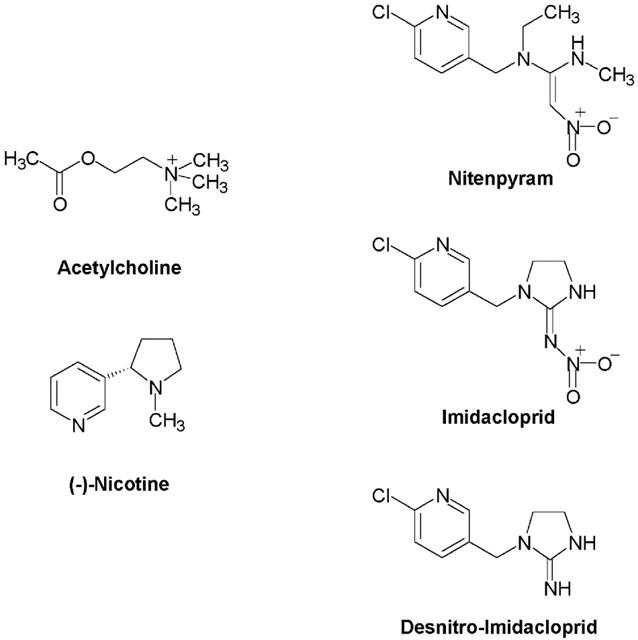
Chemical structures of acetylcholine (ACh), imidacloprid (IMI), nitenpyram and desnitro-imidacloprid (DN–IMI).
Recently the crystal structure of the acetylcholine binding protein (AChBP) secreted by the glia cells of the mollusc, Lymnaea stagnalis, has been reported (Brejc et al., 2001). The AChBP shows high homology (23.9% identity) to the N-terminal, extracellular region of the α7 subunit and forms a homo-pentamer (Smit et al., 2001). Since loops A–F are all seen in AChBP, amino acid residues contributing to neonicotinoid binding may be identified by referring to the crystal structure. The three-dimensional structure of AChBP (Figure 2) showed that Q55 in loop D is located close to Y164, the residue corresponding to G189 of the α7 subunit. Because Q55 is preserved as Q79 in the α7 subunit, we have studied by site-directed mutagenesis the role of loop D in the interactions with neonicotinoids and a denitrated derivative as well as the natural ligands, ACh and (−)-nicotine. The results suggest a close proximity between Q79 and the nitro group of neonicotinoids when the α7 nicotinic receptor binds to neonicotinoids, and therefore corresponding residues in insect nicotinic receptors may contribute to the high affinity of the insecticides for the receptors.
Figure 2.
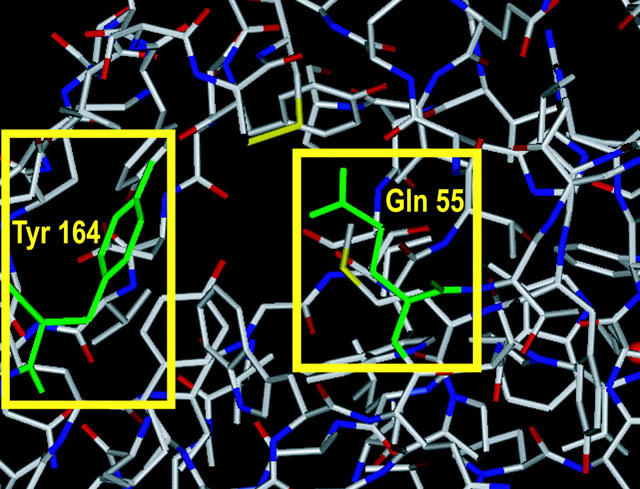
Crystal structure of the agonist binding domain of acetylcholine binding protein from the snail Lymnaea stagnalis. The figure was generated based on the PDB files (Nos. 1I9BA, 1I9BB, 1I9BC, 1I9BD and 1I9BE) using SYBYL software version 6.8 (Tripos, St. Louis, MO, U.S.A.). Q55 and Y164 of AChBP, which are coloured green, correspond to Q79 and G189, respectively, of the chicken α7 subunit.
Methods
Preparation of DNAs encoding mutant α7 subunits
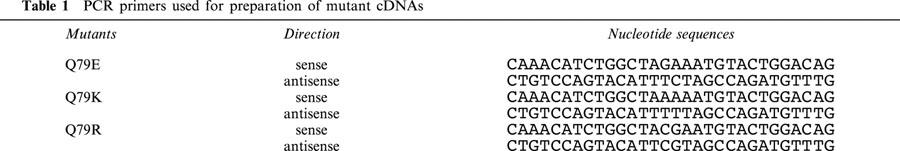
The chicken nicotinic α7 subunit cDNA (Couturier et al., 1990) in the pMT3 vector (Swick et al., 1992) was used as a template for mutagenesis. A series of mutations were introduced by PCR. An example for preparation of the Q79E mutant cDNA is as follows. Oligonucleotides for mutagenesis Q79E sense (5′-CAAACATCTGGCTAGAAATGTACTGGACAG-3′) and Q79E antisense (5′-CTGTCCAGTACATTTCTAGCCAGATGTTTG-3′) were based on the α7 subunit containing the Q79E mutation. Forward primer KM001 (5′-TGTCCACTCCCAGGTCCAACTG-3′) was based on the sequence flanking the multiple cloning site of the pMT3 vector; reverse primer KM012 (5′-CTCCATGCTTGACAGGCTGCATC-3′) was based on α7 cDNA about 1.1 kb downstream of the start codon. A pair of the first round PCRs were carried out using 1.25 U of LA-Taq (Takara, Kusatsu, Shiga, Japan), 100 ng of the wild-type pMT3-α7 as template, 0.3 μM primers (KM001 and Q79E antisense; KM012 and Q79E sense) and 0.4 mM dNTP mixture in a 50 μl solution for 30 cycles of 98°C 30 s, 50°C 30 s, 72°C 60 s. The second round PCR was performed using 1.25 U of LA-Taq, 20 ng each of the first round PCR products and 0.3 μM primers (KM001 and KM012) mixture in a 50 μl solution for 30 cycles of 98°C 30 s, 60°C 30 s, 72°C 90 s, yielding a single band of the predicted size. After gel purification using a low melting point agarose gel, the isolated fragment was digested by PstI and EcoRI and subcloned into PstI and EcoRI sites of pMT3-α7. This plasmid was cut with EcoRI and ligated with a 1.2 kbp EcoRI fragment of pMT3-α7 to complete the full-length mutant α7. Orientation of the 1.2 kbp insert was confirmed by BglII mapping. Other DNA constructs encoding the Q79K and Q79R mutants were prepared in the same manner and the entire sequences of all mutants were confirmed by automated DNA sequencing. Oligonucleotides used as primers for the mutagenesis are listed in Table 1.
Table 1.
PCR primers used for preparation of mutant cDNAs
Preparation and nuclear injection of Xenopus oocytes
Mature Xenopus laevis females were anaesthetized by immersion in 1.5 g l−1 tricaine for 30–45 min, depending on body weight, before surgical removal of part of the ovary. Oocytes at stage V or VI of development were separated from the follicle cell layer by treatment with 2 mg ml−1 collagenase Type IA (Sigma-Aldrich Japan, Tokyo, Japan) in calcium-free standard oocyte saline (see below for composition) for 15 min at room temperature. The follicle cell layer was removed manually using fine forceps. The nucleus of each defolliculated oocyte was injected with 20 nl of cDNA in distilled water (0.1 ng nl−1) and incubated at 16°C in standard oocyte saline (SOS) of the following composition (in mM); NaCl 100, KCl 2.0, CaCl2 1.8, MgCl2 1.0 and HEPES 5.0, pH 7.6, supplemented with penicillin (100 units ml−1), streptomycin (100 μg ml−1), gentamycin (20 μg ml−1) and 2.5 mM sodium pyruvate. The incubation medium was changed daily. Electrophysiology was performed 3–6 days after nuclear injection.
Electrophysiology
Xenopus oocytes were secured in a Perspex recording chamber (80 μl volume) with a Sylgard base and perfused continuously with normal oocyte saline (7–10 ml min−1) by a gravity-fed system (Buckingham et al., 1994). To suppress any endogenous muscarinic responses, 0.5 μM atropine was included in the saline. Membrane currents were recorded by the two-electrode voltage-clamp method using 2.0 M KCl-filled electrodes (resistances 0.5–5.0 MΩ) and a Geneclamp 500 (Axon Instruments, Union City, CA, U.S.A.) amplifier. The oocyte membrane was clamped at −100 mV. Current signals were recorded by a pen recorder.
To prepare test solutions, stock solutions of ligands were diluted with SOS containing 0.5 μM atropine. Stock solutions of (−)-nicotine (100 mM), nitenpyram (100 mM) and DN-IMI (100 mM) in SOS were stored at 4°C, whereas solutions of ACh in SOS were prepared immediately prior to experiments. Stock solutions of imidacloprid (300 mM) were prepared in DMSO, and diluted with SOS prior to tests. DMSO at concentrations lower than 1% (v v−1) had no effect on the responses. Oocytes were challenged with compounds for 1 s at concentrations higher than EC50, the concentration giving half the maximum response, and for a longer period (2 –5 s) at lower concentrations to ensure that the response attained its peak or steady-state. Each challenge was 3–5 min apart to minimize reduction of the peak height resulting from the slow desensitization of the receptor. (−)-Nicotine and DN–IMI often resulted in desensitization that was not fully reversible at concentrations close to or higher than 10 mM. However, at all lower doses tested, stable responses were repeatedly observed after rebathing oocytes in SOS. Dose–response data were obtained by challenging oocytes with increasing concentrations of an agonist and the maximum amplitude of the current was recorded in response to each challenge was normalized to the maximum amplitude of the saturating or sub-saturating response to ACh. Data from the wild-type receptor and Q79E mutant were normalized by the response to 1 mM and 3 mM ACh, respectively, while those from the Q79K and Q79R mutants were normalized by the response to 10 mM ACh. Using GraphPad ‘Prism' (GraphPad Software, San Diego, CA, U.S.A.), normalized data were fitted to the following equation:
where ψ is the normalized response of a compound applied at logarithmic concentration [A]. Imax and Imin are the maximum and the minimum normalized responses respectively, [EC50] is the concentration giving half the maximum normalized response and nH is the Hill coefficient. Experiments were performed at room temperature (19–25°C). Differences of the Imax and pEC50 (−log EC50) values determined for each agonist between the wild-type and mutants were statistically examined by one-way ANOVA (Dunnett's multiple comparison test). Imidacloprid and DN-IMI were synthesized de novo and nitenpyram was donated by Takeda Chemical Industries. ACh (chloride salt) were obtained from Wako Pure Chemical Industries (Osaka, Japan). (−)-Nicotine (free base) and atropine sulphate were obtained from Sigma-Aldrich Japan (Tokyo, Japan). Structures of chemicals tested in this study are shown in Figure 1.
Results
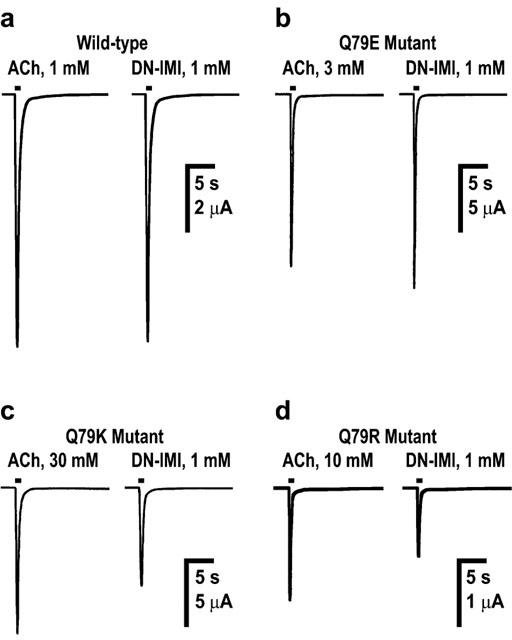
Imidacloprid (Figure 3a) and nitenpyram both rapidly activated and desensitized the recombinant α7 nicotinic AChR with the Imax value of 0.49±0.03 (n=8) and 0.20±0.01 (n=8), respectively (Table 2). Thus these two insecticides were partial agonists. On the other hand, (−)-nicotine (n=6) and DN–IMI (n=6, Figure 4a) acted as full agonists with the Imax values similar to that of ACh (n=12, Figure 5a). The rank order of the agonist potency in terms of pEC50 values was DN–IMI>(−)-nicotine>ACh>imidacloprid>nitenpyram.
Figure 3.
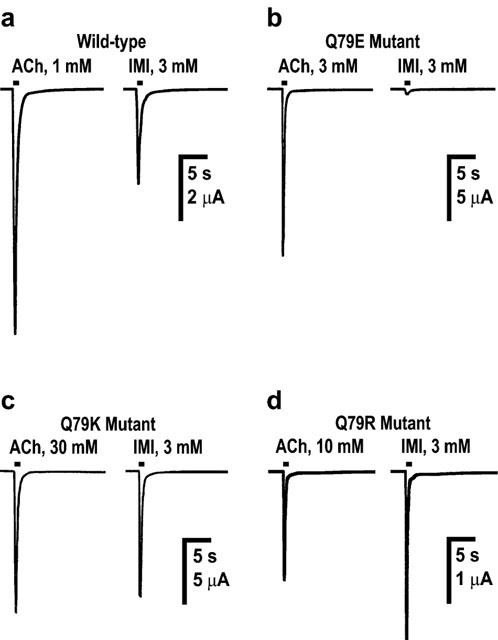
Actions of acetylcholine (ACh) and imidacloprid (IMI) on wild-type (a) and mutant (Q79E (b), Q79K (c) and Q79R (d)) chicken α7 nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. In (a)–(d), examples of inward currents recorded from the same oocyte in response to ACh and imidacloprid (applied for 1 s) are shown. Imidacloprid was a partial agonist of the wild-type receptor (n=8) when compared with the maximum response to ACh (n=12). The Q79E mutation markedly reduced the peak current amplitude of the maximum response to imidacloprid (n=8), whereas the Q79K (n=12) and Q79R (n=13) mutations significantly increased the amplitude of imidacloprid-induced currents compared to those recorded in response to ACh (Q79K, n=11; Q79R, n=8).
Table 2.
pEC50 and Imax values of neonicotinoid insecticides and related ligands acting on wild-type and mutant α7 receptors
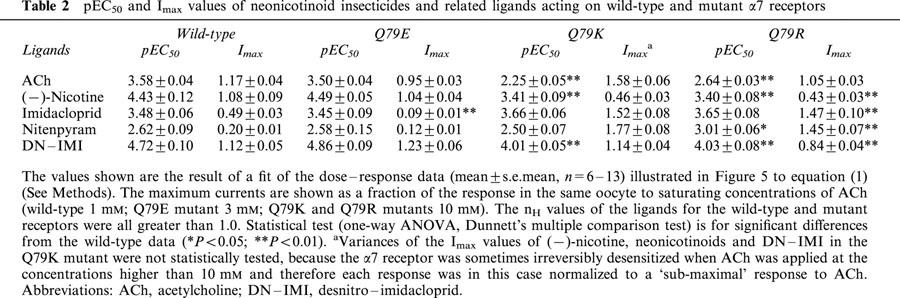
Figure 4.
Actions of acetylcholine (ACh) and desnitro-imidacloprid (DN-IMI) on wild-type (a) and mutant (Q79E (b), Q79K (c) and Q79R (d)) chicken α7 nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. In (a)–(d), examples of inward currents recorded in response to ACh and DN–IMI (applied for 1 s) are shown. In the wild-type α7 receptor, DN–IMI was a full agonist (n=6). The Q79E mutation scarcely influenced the amplitude of the maximum response to DN–IMI (n=12), whereas in the Q79K (n=10) and Q79R (n=9) mutations, the amplitude of the maximum response was reduced significantly compared to that observed for ACh. As a result, DN–IMI became a partial agonist.
Figure 5.
Dose–response relationships for nicotinic ligands obtained for wild-type (a) and mutant (Q79E (b), Q79K (c) and Q79R (d)) α7 nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Abbreviation: DN–IMI, desnitro–imidacloprid.
The normalized Imax values of DN–IMI (n=12, Figure 4b) and (−)-nicotine (n=10) were 1.23±0.06 and 1.04±0.04, respectively, in the Q79E mutant (Table 2). By contrast, the maximum responses to imidacloprid (n=8, Figure 3b, significant P<0.01) and nitenpyram (n=8, Figure 5b) were both markedly reduced by the Q79E mutation. The Q79E mutation did not significantly shift the pEC50 values and therefore the rank of the agonist potency in terms of the pEC50 value was DN–IMI>(−)-nicotine>ACh>imidacloprid>nitenpyram as was the case for the wild-type receptor.
When the glutamine residue was replaced by either lysine or by arginine, the normalized Imax values of imidacloprid (n=12 for Q79K; n=13 for Q79R, Figure 3c,d and Table 2) and nitenpyram (n=11 for Q79K; n=11 for Q79R, Table 2) were markedly increased when compared with those measured in the wild-type receptor (Figure 5c,d vs 5a). In addition to this, the dose–response curves for these insecticides were slightly shifted to the left by these two mutations, with pEC50 values of 3.66±0.06 (Q79K) and 3.65±0.08 (Q79R) for imidacloprid and of 2.50±0.07 (Q79K) and 3.01±0.06 (Q79R, significant P<0.05) for nitenpyram (Table 2). In contrast to the effects on the insecticides, the Q79K and Q79R mutations reduced not only the Imax values (Figure 4c,d for DN–IMI) but also the pEC50 values of DN–IMI and (−)-nicotine for the α7 receptor (Figure 5c,d). The normalized Imax values of ACh (n=11), DN–IMI (n=10) and (−)-nicotine (n=6) were 1.58±0.06, 1.14±0.04 and 0.46±0.03 in the Q79K mutant (Table 2), thus the ratio of the Imax values of DN–IMI and (−)-nicotine compared with the value of ACh were 0.72 and 0.29, respectively, in the Q79K mutant. Similarly, the ratio of the normalized Imax values of DN–IMI (n=9) and (−)-nicotine (n=8) relative to the Imax value of ACh (n=8) were 0.80 and 0.41, respectively, in the Q79R mutant. The dose–response curves for ACh, (−)-nicotine and DN–IMI were slightly but significantly (P<0.01 for these natural agonists) shifted to the right by these two mutations (Figure 5c,d). The rank order of the agonist potency in terms of the pEC50 values were DN–IMI>imidacloprid>(−)-nicotine>nitenpyram>ACh in the Q79K and Q79R mutants. The pEC50 and Imax values along with their standard errors obtained by fitting of the dose–response curves for the ligands tested in the wild-type and mutants are summarized in Table 2.
The Imax values (nA) for ACh, which were measured 4 days after cDNA injection to oocytes, were 4712±640 (n=25), 3313±521 (n=33), 2295±342 (n=29), 708±81 (n=29) for the wild-type, Q79E, Q79K and Q79R α7 nicotinic AChRs, respectively. The reduction in efficacy of ACh for the α7 nicotinic receptor by the Q79K and Q79R mutations was significant (P<0.01).
Discussion
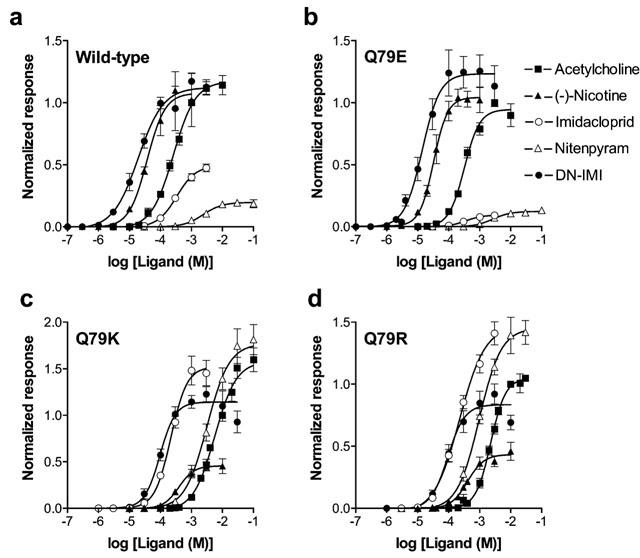
To evaluate roles of non-α subunit residues in the nicotinic receptor–neonicotinoid interactions, we have utilized the α7 subunit as a convenient model, because it is capable of forming a homo-pentamer and therefore possesses not only loops A–C of the ACh binding site, characteristic of all α subunits, but also loops D–F, normally located on non-α subunits in the heteromeric nicotinic AChRs. The invertebrate homomer-forming nicotinic AChR subunit, ACR-16, does not show such robust expression in every batch of oocytes. Although from a vertebrate species, the α7 nicotinic AChR shows moderate sensitivity to imidacloprid, enabling us to investigate, by site-directed mutagenesis, residues influencing sensitivity to the neonicotinoid. We have shown that imidacloprid and nitenpyram are partial agonists of the α7 nicotinic AChR. However, smaller Imax values of the insecticides compared to the value of ACh might be due to their open channel blocking effects. If this is the case, then the peak current amplitude as well as the fast desensitization (decay) rate of responses should be reduced by prolonging duration of the drug application. As illustrated in Figure 6 (n=4), both duration of the drug application at high doses and the Q79E mutation scarcely influenced the rate of fast desensitization of responses to imidacloprid. Furthermore, the desensitization rate (half decay time) of responses to other agonists including ACh did not vary significantly with mutations (data not shown). Thus, it is unlikely that the partial agonist actions of neonicotinoids as well as variations in the agonist dose–response curves resulting from mutations are primarily the result of open channel blocking effects.
Figure 6.
Inward currents evoked in response to imidacloprid applied for 1 s and 5 s in oocytes expressing wild-type (a) and Q79E mutant (b) α7 nicotinic acetylcholine receptors. A pair of currents in (a) and (b) were recorded from the same oocyte expressing the wild-type and mutant receptors, respectively.
We have found that the G189D and G189E mutations in loop F of the α7 nicotinic receptor markedly suppress the agonist actions of imidacloprid and nitenpyram on the receptor, whereas G189N and G189Q were ineffective in reducing neonicotinoid sensitivity (Matsuda et al., 2000). In addition, the G189D and G189E as well as G189N and G189Q mutations scarcely affected the dose–response curves for DN–IMI, which lacks the nitro group (Matsuda et al., 2000). Although these earlier findings suggested a close proximity between G189 and the nitro group of the neonicotinoids in the α7 receptor, it was difficult to explain the selectivity of neonicotinoids based on the interactions with loop F since the amino acid residues in insect non-α subunits that correspond to G189 of the α7 subunit are either aspartate or glutamate. Nevertheless, this apparent contradiction may be resolved by the present findings, when considered alongside the change in electronic charges of imidacloprid by hydrogen bonding of the nitro group calculated using a semi-empirical molecular orbital method (Matsuda et al., 2001).
ACh has a quaternary nitrogen with a positive charge, whereas imidacloprid has no such nitrogen. Yamamoto et al. (1995) has proposed that the bridge-head nitrogen possessing slightly positive character in imidacloprid corresponds to the quaternary nitrogen of acetylcholine and plays an important role in the insecticide–nicotinic receptor interactions. Even though Tomizawa et al. (2000) suggested that the electronic charge of this nitrogen is negligible, we found that by taking into account hydrogen bonding of the nitro group of imidacloprid, the bridge-head nitrogen can have a positive charge as shown by calculations using the AMSOL program (Matsuda et al., 2001). The calculations suggest that lysine or arginine residues in insect nAChR subunits are likely to increase affinity for neonicotinoids via electronic attraction of the nitro group and subsequent formation of hydrogen bonding.
Reduction of the neonicotinoid sensitivity of the α7 receptor by the Q79E mutation is attributable to an electronic repulsion between the nitro group of the insecticides and the negative glutamate residues, while enhancement of the neonicotinoid sensitivity by the Q79K and Q79R mutations seems to be due to an increase in the positive charge of the bridge-head nitrogen in the insecticides by formation of hydrogen bonding of the added lysine or arginine residue with the nitro group. It is of interest that the Imax values of imidacloprid and nitenpyram were greater than that of ACh in the Q79R mutant. Thus these insecticides appear to act as ‘super agonists' on this mutant if ACh is defined as a full agonist. However, this could also be explained if ACh becomes a partial agonist as a result of the Q79R mutation, as indicated by the reduction of the maximum response (4712±640 for the wild-type vs 708±81 for the Q79R mutant). In contrast to the effects on the insecticide pharmacology, the Q79K and Q79R mutations reduced sensitivity of the α7 nicotinic receptor to DN–IMI, the imidacloprid derivative lacking the nitro group, as well as ACh and (−)-nicotine. This is reasonable because these positively-charged ligands can undergo electronic repulsion by the positive amino acid residues.
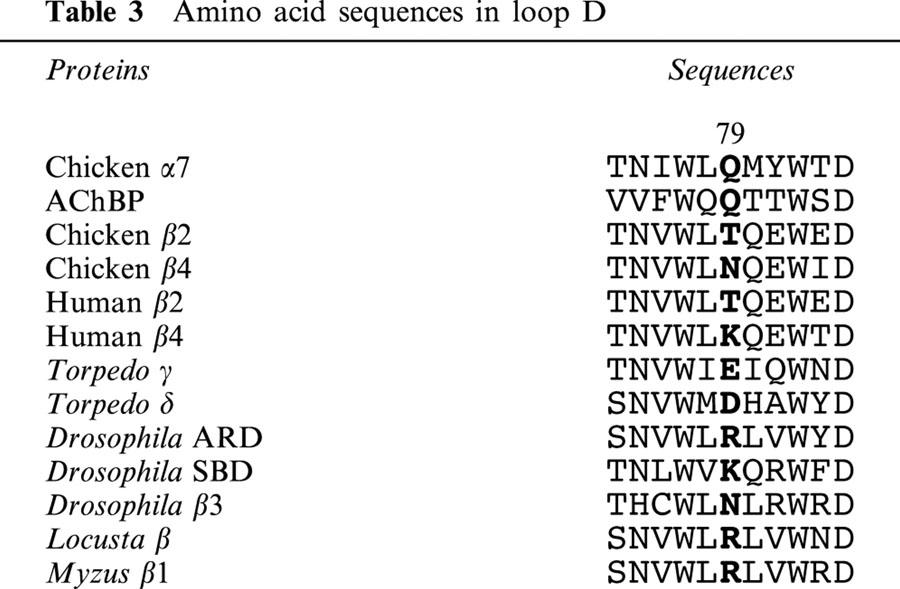
As shown in Table 3, lysine or arginine residues of insect non-α subunits except for the Drosophila β3 subunit are aligned at the equivalent position to the Q79 residue in the α7 subunit and the Q55 residue of AChBP. By contrast, most of the vertebrate non-α subunits possess neutral or acidic residues at this position. Although we have shown that amino acid residues on the α subunits of insect nicotinic receptors have critical roles in determining the affinity of neonicotinoids (Matsuda et al., 1998), the lysine or arginine residues in insect non-α subunits corresponding to Q79 of the α7 nicotinic receptor may also contribute to strengthening interactions with the insecticide.
Table 3.
Amino acid sequences in loop D
In conclusion we have for the first time identified a glutamine residue in loop D in the α7 subunit that can interact with the nitro group of neonicotinoids, using a combination of site-directed mutagenesis and voltage-clamp electrophysiology. We therefore suggest that residues in both the α subunit site and the complementary site (from α or non-α subunit) contribute to neonicotinoid sensitivity of nAChRs. In most of the insect non-α subunits, amino acid residues corresponding to Q79 of the α7 subunit are lysine or arginine residues. These basic residues probably interact with the nitro group of neonicotinoids through electrostatic force and, possibly, hydrogen bonding, strengthening the nicotinic receptor-insecticide interactions. We have shown that substitution of these basic residues can result in reduction of the insecticide sensitivity of nicotinic receptors. It will be of interest in future to examine nicotinic AChR subunit sequences in this region in insects intrinsically less sensitive to neonicotinoids.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (C) from the Japan Society for Promotion of Science (No. 13660113) and the program for promotion of Basic Research Activities for Innovative Biosciences (Bio-oriented Technology Research Advancement Institution) to K. Matsuda and The Medical Research Council of the UK (D.B. Sattelle).
Abbreviations
- ACh
acetylcholine
- AChR
acetylcholine receptor
- DN–IMI
desnitro–imidacloprid (a denitrated derivative of imidacloprid)
- EC50
concentration giving half the maximum normalized response
- Imax
maximum normalized response
- Imin
minimum normalized response
References
- ARIAS H.R. Topology of ligand binding sites on the nicotinic acetylcholine receptor. Brain Res. Rev. 1997;25:133–191. doi: 10.1016/s0165-0173(97)00020-9. [DOI] [PubMed] [Google Scholar]
- BALLIVET M., ALLIOD C., BERTRAND S., BERTRAND D. Nicotinic acetylcholine receptor in the nematode Caenorhabditis elegans. J. Mol. Biol. 1996;258:261–269. doi: 10.1006/jmbi.1996.0248. [DOI] [PubMed] [Google Scholar]
- BREJC K., VAN DIJK W.J., KLAASSEN R.V., SCHUURMANS M., VAN DER OOST J., SMIT A.B., SIXMA T.K. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM S.D., HOSIE A.M., ROUSH R.T., SATTELLE D.B. Actions of agonists and convulsant antagonists on a Drosophila melanogaster GABA receptor (Rdl) homo-oligomer expressed in Xenopus oocytes. Neurosci. Lett. 1994;181:137–140. doi: 10.1016/0304-3940(94)90578-9. [DOI] [PubMed] [Google Scholar]
- CORRINGER P.J., LE NOVÈRE N., CHANGEUX J.P. Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- COUTURIER S., BERTRAND D., MATTER J.-M., HERNANDEZ M.-C., BERTRAND S., MILLAR N., VALERA S., BARKAS T., BALLIVET M. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- ELGOYHEN A.B., JOHNSON D.S., BOULTER J., VETTER D.E., HEINEMANN S. α9: An acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- GERZANICH V., ANAND R., LINDSTROM J. Homomers of α8 and α7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties. Mol. Pharmacol. 1994;45:212–220. [PubMed] [Google Scholar]
- ITIER V., BERTRAND D. Neuronal nicotinic receptors: from protein structure to function. FEBS Lett. 2001;504:118–125. doi: 10.1016/s0014-5793(01)02702-8. [DOI] [PubMed] [Google Scholar]
- KARLIN A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- KARLIN A., AKABAS M.H. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- LIU M.-Y., CASIDA J.E. High affinity binding of [3H]imidacloprid in the insect acetylcholine receptor. Pestic. Biochem. Physiol. 1993;46:40–46. [Google Scholar]
- MARSHALL J., BUCKINGHAM S.D., SHINGAI R., LUNT G.G., GOOSEY M.W., DARLISON M.G., SATTELLE D.B., BARNARD E.A. Sequence and functional expression of a single α subunit of an insect nicotinic acetylcholine receptor. EMBO J. 1990;9:4391–4398. doi: 10.1002/j.1460-2075.1990.tb07889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUDA K., BUCKINGHAM S.D., FREEMAN J.C., SQUIRE M.D., BAYLIS H.A., SATTELLE D.B. Effects of the α subunit on imidacloprid sensitivity of recombinant nicotinic acetylcholine receptors. Br. J. Pharmacol. 1998;123:518–524. doi: 10.1038/sj.bjp.0701618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUDA K., SHIMOMURA M., KONDO Y., HASHIGAMI K., YOSHIDA N., RAYMOND V., MONGAN N.P., FREEMAN J.C., KOMAI K., SATTELLE D.B. Role of loop D of the α7 nicotinic acetylcholine receptor in its interaction with the insecticide imidacloprid and related neonicotinoids. Br. J. Pharmacol. 2000;130:981–986. doi: 10.1038/sj.bjp.0703374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUDA K., BUCKINGHAM S.D., KLEIER D., RAUH J.J., GRAUSO M., SATTELLE D.B. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001;22:573–580. doi: 10.1016/s0165-6147(00)01820-4. [DOI] [PubMed] [Google Scholar]
- RAYMOND V., MONGAN N.P., SATTELLE D.B. Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: chicken α7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience. 2000;101:785–791. doi: 10.1016/s0306-4522(00)00279-7. [DOI] [PubMed] [Google Scholar]
- SMIT A.B., SYED N.I., SCHAAP D., VAN MINNEN J., KLUMPERMAN J., KITS K.S., LODDER H., VAN DER SCHORS R.C., VAN ELK R., SORGEDRAGER B., BREJC K., SIXMA T.K., GERAERTS W.P.M. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature. 2001;411:261–268. doi: 10.1038/35077000. [DOI] [PubMed] [Google Scholar]
- SWICK A.G., JANICOT M., CHENEVAL-KASTELIC T., MCLENITHAN J.C., LANE M.D. Promoter-cDNA-directed heterologous protein expression in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1812–1816. doi: 10.1073/pnas.89.5.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMIZAWA M., LEE D.L., CASIDA J.E. Neonicotinoid insecticides: Molecular features conferring selectivity for insect versus mammalian nicotinic receptor. J. Agric. Food Chem. 2000;48:6016–6024. doi: 10.1021/jf000873c. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO I., YABUTA G., TOMIZAWA M., SATO T., MIYAMOTO T., KAGABU S. Molecular mechanism for selective toxicity of nicotinoids and neonicotinoids. J. Pestic. Sci. 1995;20:33–40. [Google Scholar]
- ZWART R., OORTGIESEN M., VIJVERBERG H.P.M. Nitromethylene heterocycles: Selective agonists of nicotine receptors in locust neurons compared to mouse N1E-115 and BC3H1 cells. Pestic. Biochem. Physiol. 1994;48:202–213. [Google Scholar]


