Abstract
The present study was performed to determine the role of Rho-Rho kinase signalling pathway in smooth muscle cells from both healthy and varicose human saphenous vein.
The Rho kinase inhibitor Y-27632 inhibited the noradrenaline (NA)-induced contraction in human saphenous veins with IC50 corresponding to 0.5 μM and 10.9 μM in control and varicose veins, respectively. The maximal amplitude of the NA-induced contraction was smaller in varicose vein compared to control (1263±172 mg versus 1974±245 mg, P<0.05).
In β-escin permeabilized strips, GTPγS induced a rise in tension that was inhibited by Y-27632. The amplitude of the GTPγS-induced contraction was smaller in varicose compared to control veins (23.1±2.4% versus 41.3±2.2%, P<0.002).
In smooth muscle cells, Y-27632 induced disassembly of both actin cytoskeleton and extracellular fibronectin matrix. In comparison to control cells, varicose vein smooth muscle cells show decreased actin cytoskeleton organization and reduction of fibronectin matrix deposition.
The Rho proteins Rnd1 and RhoA, and Rho kinase 1 are expressed in human saphenous veins. A 2.6 fold reduction of Rho kinase expression was found in varicose veins.
These results indicate that RhoA-Rho kinase mediated Ca2+ sensitization of the contraction and regulated actin cytoskeleton and extracellular fibronectin matrix assembly in human saphenous smooth muscle. The decrease of Rho kinase expression and Rho kinase-dependent functions detected in smooth muscle from varicose veins supports a role of this signalling pathway in the functional alterations of the vein wall occurring in the course of the disease.
Keywords: Smooth muscle cell, Rho proteins, contractility, actin cytoskeleton, Ca2+ sensitization
Introduction
The small G proteins of the Rho family, initially described as key regulators of the actin cytoskeleton are now known to control many biological processes including cell contraction, migration, adhesion, gene transcription, proliferation and transformation (Bar-Sagi & Hall, 2000; Bishop & Hall, 2000). During the past 4 years, Rho proteins have been recognized as major regulators of smooth muscle cell functions in arteries. In response to G-protein coupled receptor activation, RhoA acting through its effector Rho kinase, inhibits myosin phosphatase, favouring accumulation of phosphorylated myosin light chain and enhanced contraction (Somlyo & Somlyo, 2000). This RhoA-Rho kinase mediated Ca2+ sensitization of arterial contraction contributes to arterial blood pressure regulation (Uehata et al., 1997). The Rho-Rho kinase signalling pathway is also involved in proliferation and migration of arterial smooth muscle cells (Seasholtz et al., 1999; Sawada et al., 2000; 2001). Data are now accumulating regarding the involvement of Rho proteins and Rho kinase in arterial disorders associated with arterial wall remodelling, altered cell contractility and cell migration such as hypertension (Uehata et al., 1997; Seasholtz et al., 2001), atherosclerosis (Morishige et al., 2001) and restenosis (Sawada et al., 2000; Shibata et al., 2001). In contrast to this abundance of data regarding Rho-Rho kinase-dependent functions in arteries, little is known about the role of this signalling pathway in veins. Only one recent report had suggested a role for Rho kinase-dependent pathways in the adaptation of human saphenous vein to circumferential deformations (McGregor et al., 2002).
Contractile properties of human saphenous smooth muscle are essential to maintain the required tone in the vein wall as for adaptive responses to standing and exposure to cold (Flavahan & Vanhoutte, 1986). Impaired contractile responses and alteration of the vein wall with disturbance of smooth muscle cell/extracellular matrix organization are associated with the development of varicose veins. However, signalling pathways that regulate saphenous vein smooth muscle contraction have only been investigated marginally and are still poorly understood.
The present investigation was undertaken to determine the role of Rho-Rho kinase signalling pathway in smooth muscle cells from both healthy and varicose human saphenous vein. Our data demonstrate that the RhoA-Rho kinase signalling pathway mediated Ca2+ sensitization of the contraction and regulated actin cytoskeleton and extracellular fibronectin matrix assembly. RhoA-Rho kinase-dependent smooth muscle functions are down regulated in varicose vein, which is correlated with significant decrease in Rho kinase expression. Taken together these results suggest that Rho kinase signalling is as important in veins as in arteries and that alterations of this transduction pathway could be involved in venous diseases.
Methods
Tissue Preparation
Two groups of human internal saphenous veins (proximal segments) were used. Fourteen control veins were obtained from patients undergoing coronary bypass surgery (11 men aged 44–66 years; three women aged 28–63 years). Preoperative examination attested the absence of varicose disease. Seventeen varicose veins were obtained during saphenectomy (five men aged 48–62 years; 12 women aged 33–59 years). All patients were at stage III of venous disease with permanent retrograde flow and varicosities all along the venous axis. All samples were collected in ice-cold sterile physiological saline solution (PSS, in mM: NaCl 130, KCl 5.6, MgCl2 1, CaCl2 2, glucose 11, Tris 10, pH 7.4 with HCl) in the operating room and rapidly transported to the laboratory where they were cleaned of adherent connective tissue.
Tension measurements in intact fibres
The cleaned veins were cut into rings (5–7 mm in length). The endothelium was carefully removed by gently rubbing the intimal surface with the tip of small forceps. Smooth muscle rings were then suspended under isometric conditions and connected to a force transducer (Pioden Controls Ltd, Canterbury, U.K.) in organ baths filled with Krebs–Henseleit solution (in mM: NaCl 118.4, KCl 4.7, CaCl2 2, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11) maintained at 37°C, and equilibrated with 95% O2-5% CO2. The preparations were initially placed under a resting tension of 1500 mg, left to equilibrate for 1 h and washed at 20 min intervals. The absence of endothelium was confirmed in each ring by the inability of carbachol (10 μM) to relax phenylephrine (PE, 1 μM)-induced contraction. Successive applications of agonist were separated by a time-interval of 1 h. Concentration-response curves were obtained by increasing the concentration of the tested substance in the organ chamber.
Isometric tension measurement in skinned fibres
Small muscle strips (approximately 200 μm wide and 4 mm long) were isolated from the media of the vein and tied at each end with a single silk thread to the tips of two needles, one of which was connected to a force transducer (AE 801, SensoNor, Horten, Norway). Strips were placed in a well on a bubble plate filled with PSS (Horiuti, 1988) and stretched to about 1.3 resting length. The solution was rapidly changed by sliding the plate to an adjacent well. After measuring contraction evoked by high-K+ solution, the strips were incubated in the normal relaxing solution (in mM: KCl 85, MgCl2 5, Na2ATP 5, creatine phosphate 5, EGTA 2 and Tris-maleate 20, brought to pH 7.1 at 25°C with KOH) for a few minutes, followed by treatment with β-escin (50–70 μM) in the relaxing solution for 35 min at 25°C as previously described (Loirand et al., 1999). The skinned muscle strip was then washed several times with fresh relaxing solution containing 10 mM EGTA. Calmodulin (1.5 μM) was added to the bathing solutions throughout the experiments. Tension developed by permeabilized muscle strips was measured in activating solutions, containing 10 mM EGTA and a specified amount of CaCl2 to give a desired concentration of free Ca2+ (Loirand et al., 1999).
Smooth muscle cell culture
The veins were cleaned of adherent connective and adventicial tissue, and the endothelium was removed. The remaining tissue, essentially containing smooth muscle cells, was cut into small pieces (1×1 mm) which were placed into collagen precoated cultured dishes in SMBM (Biowhittaker). The medium was changed every 2 or 3 days. When cells reached confluence, subculture was obtained by harvesting the cells with 0.2% ethylenediaminetetra-acetic acid and 0.25% trypsin. Cells were characterized using a monoclonal antibody against smooth muscle α-actin. Cells were used at passage two to four. For all experiments, cells were seeded at 104 cells (cm2)−1 in culture plate without precoating.
Estimation of intracellular Ca2+ concentration
Human saphenous vein smooth muscle cells were loaded with indo-1 by incubation in PSS containing 1 μM indo-1 penta-acetoxymethyl ester for 25 min at room temperature. Intracellular Ca2+ concentration (Cai) was estimated as described previously from the fluorescence ratio at 405 and 480 nm (Pacaud et al., 1993).
Plasmid constructions and TAT-C3 protein purification
cDNA encoding for Clostridium botulinum C3 exoenzyme was cloned in frame, C-terminal of the HIV TAT protein transduction domain (AA 47–57) in vector pTAT-HA (kindly provided by S. Dowdy). Recombinant TAT-C3 protein was produced in E. coli and purified basically as described (Nagahara et al., 1998).
Actin and fibronectin staining
Cells were then fixed for 30 min in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, and then rinsed in phosphate-buffered saline (PBS). For polymerized (F) actin staining, cells were incubated with FITC-conjugated phalloidin (5 μg ml−1) for 45 min at room temperature then washed with PBS. Actin staining was also performed with a monoclonal anti-α-smooth muscle actin antibody followed by FITC-conjugated anti-mouse antibody, which gave results similar to those obtained with FITC-conjugated phalloidin. When dual labelling was performed, cells were simultaneously stained with FITC-conjugated phalloidin and Texas red-labelled DNAse I (10 μg ml −1) to localize monomeric G-actin (Knowles & McCulloch, 1992), and then washed in PBS. Coverslips were mounted on a glass slide and examined with a fluorescence microscope (Eclipse E-600, Nikon, Champigny-sur-Marne, France). The background fluorescence signal was estimated by collecting planes from areas of the slide without cells and was electronically subtracted before analysis. Images were collected with a cool-SNAP camera (Princeton Instruments, Evry, France) and stored and analysed using Metamorph software (Universal Imaging, West Chester, PA, USA). For each area examined, images of FITC-phalloidin and Texas Red-DNase I fluorescence were collected. The time of measurements and image capturing and the image intensity gain at both wavelengths were optimally adjusted and kept constant. The ratio of fluorescence of FITC-phalloidin and Texas Red-DNase I (F- to G-actin ratio), used to quantify actin cytoskeleton organization was calculated for at least 20 cells in each experimental condition and expressed as percentage of the ratio obtained under control condition. A decrease in the F- to G-actin ratio was assumed to represent depolymerization of actin filaments.
Dual labelling was also performed to simultaneously stained F-actin with FITC-conjugated phalloidin, and fibronectin using with a rabbit anti-fibronectin antibody (1/750, overnight, 4°) revealed with FITC-conjugated goat anti-rabbit antibody.
Western blot analysis
Media of veins were homogenized in lysis buffer containing (in mM): HEPES-NaOH 20, KCl 10, NaCl 10, MgCl2 5, DTT and Complete (Boehringer 1 tablet 50 ml−1). Nuclei and unlysed cells were removed by low speed centrifugation. Protein concentration was measured and adjusted, then Laemmli sample buffer was added and equal amounts of protein were loaded in each lane of polyacrylamide/SDS gels, which were then electrophoresed and transferred to nitrocellulose. The amount of protein was checked by staining with ponceau red and by reprobing the membranes with anti-β-actin antibody. Before immunoblotting, the membrane was blocked with 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% tween 20, 5% non-fat milk for 1 h at room temperature and then probed with a mouse monoclonal anti-RhoA antibody (2 μg ml −1) for 3 h at room temperature. After three washes, membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-mouse antibody (16 ng ml −1).
Expression of Rnd1 and Rho kinase was analysed by Western blot using rabbit polyclonal anti-RndI antibody (1/600) (Loirand et al., 1999) and goat polyclonal anti-Rho kinase antibody (2 μg ml −1), respectively, then revealed with horseradish peroxidase conjugated anti-rabbit or anti-goat antibodies. The immunoreactive bands were detected by ECL (Amersham Pharmacia, Orsay, France), and quantified using ImageQuant (Molecular Dynamics, Sunnyvale, CA, U.S.A.).
Statistics
All results are expressed as the mean±s.e.mean, where n is the sample size. Significance was tested by means of Student's t-test. Probabilities <5% (P<0.05) were considered significant. Concentration-response curves were fitted to a logistic equation using Origin software (Dipsi, Chatillon, France).
Chemicals and drugs
Texas Red-DNase I was obtained from Molecular Probe (Leiden, The Netherlands). Mouse monoclonal RhoA antibody (26C4) and rabbit polyclonal Rho kinase antibody (C9) were purchased from Santa Cruz Biotechnology (Inc., California, U.S.A.). The vector used for TAT-C3 was kindly provided by Dr Steve Dowdy (University of California, LaJolla). Indo-1 penta-acetoxymethyl ester and RGD were purchased from Calbiochem (Francebiochem, France). The Rho kinase inhibitor Y-27632 was a gift from Institut International de Recherche Servier (Courbevoie, France). All other reagents were purchased from Sigma (Saint Quentin Fallavier, France).
Results
NA-induced contraction was reduced in varicose veins without change in Ca2+ signalling
Stimulation of endothelium-denuded rings of human internal saphenous veins with 60 mM KCl caused a rise in tension, the maximal amplitude of which corresponded to 847±149 mg and 1010±215 mg in control and varicose veins, respectively (P>0.5). Application of 10 μM NA induced a maintained tension the amplitude of which was significantly higher in control than in varicose samples (1974±245 mg versus 1263±172 mg, P<0.05) (see Figure 2a). However, the rises in Cai induced by KCl and by NA were of similar amplitude in healthy and in varicose veins (Figure 1). In addition, rises in Cai induced by Ca2+ entry through P2X receptor ion channels in response to ATP (Loirand & Pacaud, 1995) and Ca2+ release from intracellular Ca2+ store in response to caffeine were also similar in cells from healthy and varicose veins (Figure 1). These results thus indicate that Ca2+ handling mechanisms were not modified in varicose veins, suggesting that the reduction of the NA-induced contraction was not related to alteration of Ca2+ signalling. Therefore, we next analysed the RhoA-Rho kinase mediated Ca2+ sensitization, shown to play a major role in G protein-coupled receptor-induced contraction in other smooth muscles including arterial smooth muscle.
Figure 2.
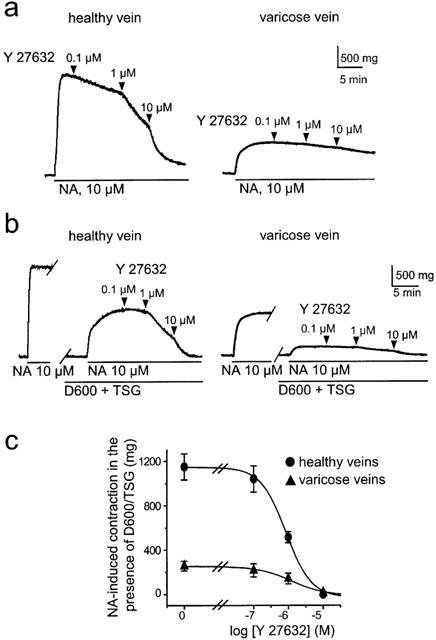
Contracting effect of NA in human saphenous vein smooth muscle is inhibited by Y-27632. (a) Typical traces showing the inhibition of NA-induced contraction by increasing concentrations of Y-27632 in healthy and varicose vein. (b) Y-27632 inhibited the sustained NA-induced rise in tension in the presence of TSG (2 μM) and D600 (20 μM). Typical traces showing the inhibition of the D600/TSG-resistant component of the NA-induced contraction by increasing concentration of Y-27632 in healthy (left) and varicose vein (right). (c) Concentration-response curves show inhibitory effect of Y-27632 on tension rise induced by 10 μM NA in the presence of D600 and TSG in healthy and varicose veins.
Figure 1.
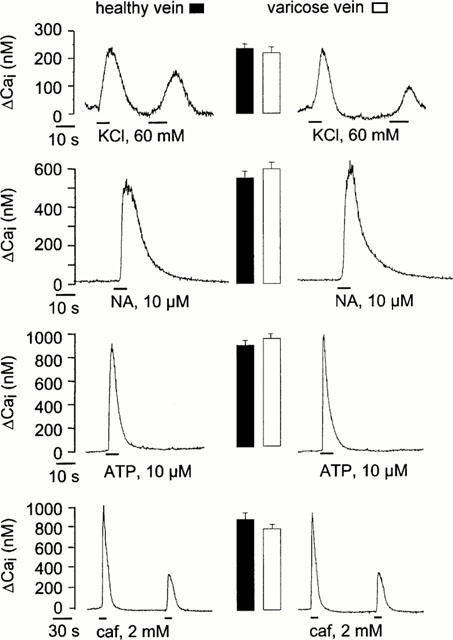
Cai rise in smooth muscle cells from healthy and varicose veins. Typical traces showing the rise in Cai recorded in cells stimulated by KCl, ATP, noradrenaline (NA), and caffeine (caf). The mean of maximal amplitude of Cai rise recorded under each condition was represented as columns near the corresponding traces (15–20 cells from 5–6 different healthy and varicose veins).
Y-27632 inhibited NA-induced contraction
The Rho kinase inhibitor Y-27632 (0.1–10 μM) inhibited NA-induced contraction in a concentration-dependent manner in control veins but had only a weak effect on NA-induced contraction (Figure 2a), in comparison to sham-treated veins (not shown).
To further analyse the Rho kinase-dependent component of the NA-induced contraction in saphenous vein, we performed experiments under conditions in which the NA-induced rise in intracellular Ca2+ concentration was suppressed. This was achieved with the use of the voltage-gated Ca2+ channel inhibitor methoxyverapamil (D600, 20 μM) and the Ca2+ store depleting agent thapsigargin (TSG, 2 μM) as previously described (Sauzeau et al., 2000). Under these conditions, NA still induced contraction, dependent on Ca2+-sensitizing mechanism, that corresponded to 59±10% (n=5) of control the response in control vein (Figure 2b). The TSG/D600 resistant component of the NA-induced contraction was significantly smaller in varicose veins where it represented only 22±9% (n=5, P<0.01) of the control response (Figure 2b). The TSG/D600 resistant component of the NA-induced contraction was dose-dependently inhibited by Y-27632 with similar IC50 values of 0.9 and 1.2 μM in control and varicose veins, respectively (Figure 2).
These results indicate that NA-induced contraction in saphenous vein involved both a rise in intracellular Ca2+ concentration and Rho kinase-dependent Ca2+ sensitization. In addition, the lower sensitivity of the NA-induced contraction to inhibition by Y-27632 as well as the reduced amplitude of the TSG/D600 resistant component in varicose veins suggest that the Rho kinase-dependent component of the contraction was smaller in varicose veins.
Rho kinase mediated Ca2+ sensitization in permeabilized muscle strips of human saphenous veins
β-escin-permeabilized smooth muscle strips of human saphenous vein have been used to more directly analyse G-protein-dependent Ca2+ sensitizing mechanism. Ca2+-dependent contractions and Ca2+ sensitization of contractile proteins could be independently evoked by an increase in Ca2+ concentration (maximal pCa (-log [Ca2+]) 4.5) and by addition of TPGγS (10 μM) at submaximal Ca2+ concentration (pCa 6.5), respectively. GTPγS induced a rise in tension that was concentration-dependently inhibited by Y-27632 (IC50=6 μM), indicating that the Ca2+ sensitization mediated by GTPγS depended on Rho kinase activation (Figure 3a,b). In contrast, the Ca2+-induced rise in tension recorded at pCa 6 was not affected by Y-27632, indicating that Rho kinase did not participate to Ca2+-dependent contraction (Figure 3a). Similar effects of Y-27632 were found on smooth muscle strips from varicose veins. However, the amplitude of the GTPγS-induced contraction was significantly smaller in varicose veins compared to control veins (23.1±2.4% of the maximal tension induced at pCa 4.5 (n=4) versus 41.3±2.2% (n=4, P<0.002), respectively (Figure 3c). The amplitude of the maximal tension induced at pCa 4.5 was similar in varicose (74.2±14 mg, n=4) and in control rings (68.5±19 mg, n=4, P>0.5) (Figure 3c). Similarly, the amplitude of the contraction induced at pCa 6.5 was not different in varicose and control veins (32.7±2.5%, n=4 versus 35.6±3.2%, n=4, P>0.5) thus showing that the Ca2+-dependent regulation of the contraction directly controlled by myosin light chain kinase activity was not affected in varicose vein.
Figure 3.
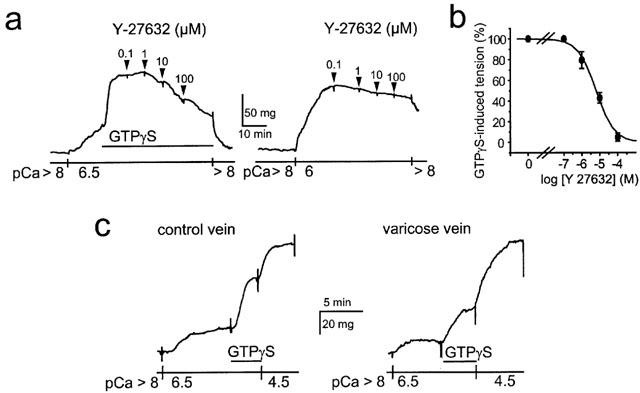
GTPγs-induced Ca2+ sensitization in β-escin-permeabilized saphenous smooth muscle. (a) Typical traces showing the Ca2+ sensitization induced by GTPγS (10 μM) at pCa 6.5 (left) and pCa 6-induced contraction (right). GTPγS-induced Ca2+ sensitization was concentration-dependently inhibited by Y-27632 whereas the Rho kinase inhibitor had no effect on the pCa 6-induced contraction. (b) Concentration-response curve showing the inhibitory effect of Y-27632 on tension rise induced by 10 μM GTPγS at pCa 6.5. (c) Typical traces showing the Ca2+ sensitization induced by 10 μM GTPγS at pCa 6.5 in permeabilized muscle from healthy (left) and varicose vein (right). pCa 4.5 was used to evoke maximal tension.
These results indicate that Rho kinase mediates Ca2+ sensitization of contractile proteins in human saphenous smooth muscle and that the Rho kinase-mediated Ca2+ sensitization is reduced in varicose veins.
Rho-Rho kinase pathway controls actin cytoskeleton and extracellular fibronectin assembly in cultured human saphenous vein smooth muscle cells
Various pharmacological tools have been used to analyse the role of Rho-Rho kinase signalling in actin cytoskeleton organization and extracellular assembly of endogenous fibronectin, secreted by saphenous vein smooth muscle cells (Figure 4). The involvement of RhoA has been analysed by treatment of the cells with the membrane permeant RhoA inhibitor TAT-C3 (Sauzeau et al., 2001). In the presence of TAT-C3, (10 μg ml −1, 4 h), the actin cytoskeleton, organized in thick stress fibres under control conditions, was almost completely disorganized (Figure 4a). Figure 4c shows quantification of actin cytoskeleton using F- to G-actin ratio. The TAT-C3 treatment also led to the loss of the dense extracellular fibronectin network covering the cells under control conditions (Figure 4a). Y-27632 (10 μM, 2 h) had effect similar to TAT-C3, inhibiting both actin cytoskeleton and extracellular fibronectin matrix assembly (Figure 4a,c). To examine whether a causal relationship existed between actin cytoskeleton organization and extracellular fibronectin matrix assembly, we used cytochalasin D to depolarize F-actin. Cytochalasin D (2 μM, 2 h) efficiently disorganized actin cytoskeleton, which caused disassembly of extracellular fibronectin matrix (Figure 4a,c). In the opposite direction, RGD peptide has been used to inhibit fibronectin binding to its membrane binding sites. In the presence of RGD peptide (1 mM, 1 h), extracellular fibronectin network was completely disorganized, without significant effect on actin fibre organization (Figure 4a,c).
Figure 4.
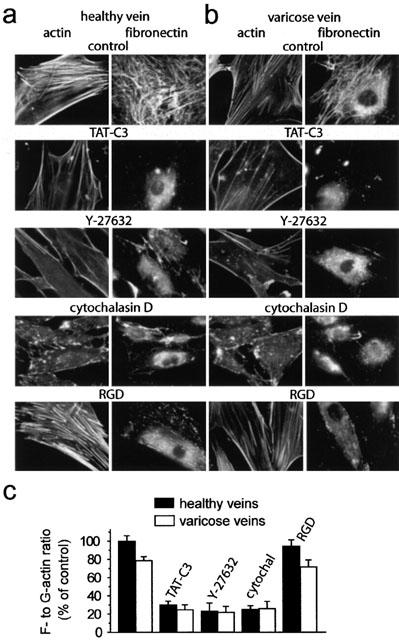
Rho-Rho kinase signalling pathway controlled actin cytoskeleton and extracellular fibronectin matrix assembly in human saphenous vein smooth muscle cells. F-actin (left) and fibronectin staining (right) of smooth muscle cells from healthy (a) and varicose veins (b) under control condition or treated with the indicated inhibitors: TAT-C3 (10 μm ml −1), Y-27632 (10 μM), cytochalasin D (2 μM), RGD peptide (1 mM). (c) Effects of indicated inhibitors on actin cytoskeleton organization quantified by the F- to G-actin ratio in smooth muscle cells from healthy and varicose veins. Results were expressed as percentage of control values (designated 100%) determined in the absence of inhibitor.
These results show that in human saphenous vein smooth muscle cells, Rho-Rho kinase pathway controls both actin cytoskeleton and extracellular fibronectin matrix organization. The latter being indirectly regulated through the Rho-Rho kinase mediated actin cytoskeleton organization.
Similar results were found in smooth muscle cells from varicose veins, indicating that the Rho-Rho kinase signalling pathway plays a similar role (Figure 4b,c). However, the actin cytoskeleton organization, quantified by the F- to G-actin ratio in smooth muscle cells from varicose veins corresponded to 79±4% (n=15, P<0.005) of that of control cells. This decrease of actin cytoskeleton organization observed in varicose smooth muscle cells was associated to a reduced extracellular fibronectin matrix (Figure 4c). These results are in agreement with the contraction measurements showing a reduced Rho-Rho kinase-dependent component of the contraction and the smaller Rho-Rho kinase-mediated Ca2+ sensitization in varicose veins compared to control veins.
Expression of RhoA, Rnd1 and Rho kinase in human saphenous vein smooth muscle
The above results, based on functional data, suggest that Rho-Rho kinase signalling pathway was down regulated in varicose vein smooth muscle. We therefore performed Western blot experiments to assess the expression of proteins involved in Rho-Rho kinase signalling, both in control and varicose veins. Rnd1 is a constitutively active member of Rho proteins (Nobes et al., 1998) that inhibits Rho-Rho kinase-dependent cell functions and the expression of which is regulated by steroid hormones (Loirand et al., 1999). The significant role of hormonal factors in the development of varicose veins prompted us to analyse Rnd1 expression in varicose veins (Cordts & Gawley, 1996), in addition to RhoA, the well-defined upstream activator of Rho kinase, and Rho kinase 1, the major isoform of the enzyme. The expression of both Rnd1 and RhoA was similar in control and in varicose veins (Figure 5). In contrast, a 2.6 fold decrease in Rho kinase 1 expression was observed in the media in varicose veins (Figure 5).
Figure 5.
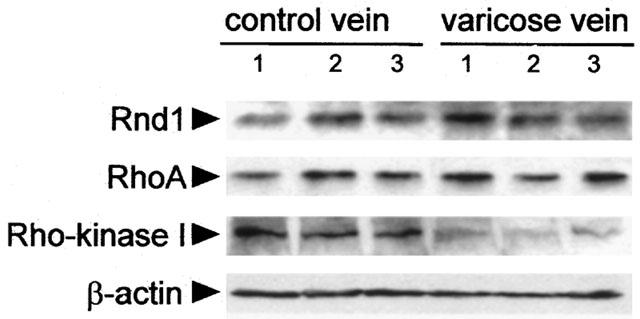
Expression of Rnd1, RhoA and Rho kinase 1 in human saphenous smooth muscle. Western blot analysis of Rnd1, RhoA and Rho kinase 1 in three representative samples of healthy (left) and varicose veins (right). β-actin expression was used to check the amount of protein in each lane.
Discussion
The present study shows that the Rho-Rho kinase signalling pathway mediates Ca2+ sensitization of the contraction in human saphenous vein and controls actin cytoskeleton and extracellular matrix assembly in saphenous vein smooth muscle cells. In addition, our data indicate that Rho kinase 1 expression is reduced in varicose vein smooth muscle, which is correlated with a decrease in Rho kinase-dependent cell functions, including Ca2+ sensitization of the contraction, actin cytoskeleton organization and fibronectin matrix assembly.
Beside the extensive bibliography regarding the role of Rho-Rho kinase pathway in arterial smooth muscle, the knowledge of this signalling mechanism and its involvement in the control of venous smooth muscle functions was nearly absent. Recent report had suggested a role for Rho kinase-dependent pathways in the adaptation of human saphenous vein to circumferential deformations (McGregor et al., 2002). By contraction measurements in both intact and permeabilized smooth muscle, we demonstrate that Rho kinase mediates Ca2+ sensitization of the contractile proteins in human saphenous vein. In healthy saphenous vein, the large amplitude of the D600/TSG-resistant component of the NA-induced contraction suggests that Rho-Rho kinase dependent Ca2+ sensitization plays a major role in the establishment of a maintained tone in response to vasoconstrictors. In varicose veins, the contractile response to NA was reduced, which confirmed previous data showing that varicose veins were characterized by a loss of contractility in response to various agonists such as NA, angiotensin II and endothelin 1 (Rizzi et al., 1998; Lowell et al., 1992). Although these observations suggested that the decrease in the response to these contracting agents might result from alteration of convergent signalling pathways shared by these agonists, the mechanisms involved in this functional alteration associated with the development of varicose veins have not been elucidated. Here we show that the D600/TSG-resistant component of the NA-induced contraction was strongly reduced in varicose veins, suggesting that the decreased response to vasoconstrictors in varicose veins resulted from a reduction of the Rho-Rho kinase-mediated Ca2+ sensitization. This was confirmed by the decrease in the amplitude of the Y-27632-sensitive GTPγS-induced Ca2+ sensitization observed in permeabilized smooth muscle, which is correlated with a decrease in the Rho kinase 1 expression in smooth muscle of varicose saphenous veins. These results are also in agreement with the absence of change in the amplitude of the Ca2+-dependent K+-induced contraction, which does not involve Ca2+ sensitization, and in Ca2+ signalling assessed in both healthy and varicose smooth muscle cells by Indo1 measurements.
In addition to the regulation of contractility, Rho-Rho kinase signalling controlled actin cytoskeleton organization in saphenous smooth muscle cells, as shown by the inhibitory action of the Rho inhibitor TAT-C3 and Y-27632. This finding is in agreement with the observation that the decreased Rho kinase expression in varicose vein smooth muscle is associated with a reduced actin cytoskeleton organization. Our results show that Rho or Rho kinase inhibition also led to inhibition of endogenous fibronectin matrix assembly by saphenous vein smooth muscle cells. This effect was mimicked by cytochalasin D-induced actin cytoskeleton depolymerization, suggesting that the disassembly of fibronectin induced by Rho kinase inhibition was secondary to Y-27632-induced actin cytoskeleton disorganization. Experiments using the RGD peptide to inhibit fibronectin matrix assembly by preventing its binding to integrins indicated that in the opposite direction, fibronectin matrix did not control actin organization. It has been demonstrated in fibroblasts that Rho-mediated actin cytoskeleton organization allowed the generation of tension on fibronectin fibrils, which has a critical role in the assembly of the fibronectin matrix (Zhong et al., 1998). Our results showing that a similar mechanism exists in venous smooth muscle cells allow the establishment of a relationship between contractility and extracellular matrix organization.
This relationship between Rho-Rho kinase-mediated actin cytoskeleton organization and extracellular fibronectin matrix assembly is in agreement with the reduction of extracellular fibronectin matrix associated with the decrease in Rho kinase expression and actin cytoskeleton organization in smooth muscle cells from varicose veins. In addition to the loss in contractility, alteration of extracellular matrix organization and composition characterized varicose vein wall. The available literature on the subject reports conflicting data regarding collagen and elastin content in the varicose vein wall (Gandhi et al., 1993; Venturi et al., 1996; Travers et al., 1996). Concerning fibronectin, our results agree with previous work showing that smooth muscle cells from varicose veins deposit less fibronectin compared to control cells, despite a similar level of fibronectin mRNA, suggesting dysregulation at a post-translational level in smooth muscle cells from varicose veins (Sansilvestri-Morel et al., 1998). In addition, the fact that alteration of Rho kinase-dependent functions was retained in cultured cells could indicate that alteration of Rho kinase expression could result from genetic defect affecting either Rho kinase gene itself or another gene that secondarily controlled Rho kinase expression.
In conclusion, the present study demonstrates the key role of Rho-Rho kinase signalling pathway in the control of venous smooth muscle contractility and fibronectin matrix organization. In addition, it suggests that a decrease in Rho kinase expression in varicose veins could participate to both decrease in contractility and extracellular matrix assembly associated with the development of the venous disease. Several origins could lead to this down regulation of Rho kinase, and it has been previously suggested that alterations in smooth muscle properties could be the result of changes in endothelium functions induced by hypoxia during venous stasis (Michiels et al., 1996). Whatever the cause of these changes was, our findings confirm the major role of smooth muscle cells which, being able to control both venous wall tone and extracellular matrix organization, could be ultimately responsible for the varicose process occurring in the vein wall.
Acknowledgments
This work is supported by grants from INSERM and Institut International de Recherche Servier (Courbevoie, France). We gratefully acknowledge all the members of the Service de Chirurgie Cardiaque (Hôpital Laennec, Nantes, France) and the Service de Chirurgie Vasculaire (Clinique Saint Augustin, Nantes, France) in supplying us with human saphenous vein samples.
Abbreviations
- Cai
intracellular Ca2+ concentration
- D600
methoxyverapamil
- NA
noradrenaline
- PBS
phosphate-buffered saline
- pCa
-log [Ca2+]
- PE
phenylephrine
- PSS
physiological saline solution
- TSG
thapsigargin
References
- BAR-SAGI D., HALL A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- BISHOP A.L., HALL A. Rho GTPases and their effector proteins. Biochem. J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- CORDTS P.R., GAWLEY T.S. Anatomic and physiologic changes in lower extremity venous hemodynamics associated with pregnancy. J. Vasc. Surg. 1996;24:763–767. doi: 10.1016/s0741-5214(96)70010-1. [DOI] [PubMed] [Google Scholar]
- FLAVAHAN N.A., VANHOUTTE P.M. Sympathetic purinergic vasoconstriction and thermosensitivity in a canine cutaneous vein. J. Pharmacol. Exp. Ther. 1986;239:784–789. [PubMed] [Google Scholar]
- GANDHI R.H., IRIZARRY E., NACKMAN G.B., HALPERN V.J., MULCARE R.J., TILSON M.D. Analysis of the connective tissue matrix and proteolytic activity of primary varicose veins. J. Vasc. Surg. 1993;18:814–820. [PubMed] [Google Scholar]
- HORIUTI K. Mechanism of contracture on cooling of caffeine-treated frog skeletal muscle fibres. J. Physiol. 1988;398:131–148. doi: 10.1113/jphysiol.1988.sp017034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOWLES G.C., MCCULLOCH C.A.G. Simultaneous localization and quantification of relative G and F actin content: optimization of fluorescence labeling methods. J. Histochem. Cytochem. 1992;40:1605–1612. doi: 10.1177/40.10.1527379. [DOI] [PubMed] [Google Scholar]
- LOIRAND G., CARIO-TOUMANIANTZ C., CHARDIN P., PACAUD P. The Rho-related protein Rnd1 inhibits Ca2+ sensitization of rat smooth muscle. J. Physiol. 1999;516:825–834. doi: 10.1111/j.1469-7793.1999.0825u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOIRAND G., PACAUD P. Mechanism of the ATP-induced rise in cytosolic Ca2+ in freshly isolated smooth muscle cells from human saphenous vein. Pflugers Arch. 1995;430:429–436. doi: 10.1007/BF00373919. [DOI] [PubMed] [Google Scholar]
- LOWELL R.C., GLOVICZKI P., MILLER V.M. In vitro evaluation of endothelial and smooth muscle function of primary varicose veins. J. Vasc. Surg. 1992;16:679–686. [PubMed] [Google Scholar]
- MICHIELS C., ARNOULD T., JANSSENS D., BAJOU K., GERON I., REMACLE J. Interactions between endothelial cells and smooth muscle cells after their activation by hypoxia. A possible etiology for venous disease. Int. Angiol. 1996;15:124–130. [PubMed] [Google Scholar]
- MCGREGOR E., GOSLING M., BEATTIE D.K., RIBBONS D.M., DAVIES A.H., POWELL J.T. Circumferential stretching of saphenous vein smooth muscle enhances vasoconstrictor responses by Rho kinase-dependent pathways. Cardiovasc. Res. 2002;53:219–226. doi: 10.1016/s0008-6363(01)00436-9. [DOI] [PubMed] [Google Scholar]
- MORISHIGE K., SHIMOKAWA H., ETO Y., KANDABASHI T., MIYATA K., MATSUMOTO Y., HOSHIJIMA M., KAIBUCHI K., TAKESHITA A. Adenovirus-mediated transfer of dominant-negative rho-kinase induces a regression of coronary arterosclerosis in pigs in vivo. Arterioscler. Thromb. Vas. Biol. 2001;21:548–554. doi: 10.1161/01.atv.21.4.548. [DOI] [PubMed] [Google Scholar]
- NAGAHARA H., VOCERO-AKBANI A.M., SNYDER E.L., HO A., LATHAM D.G., LISSY N.A., BECKER-HAPAK M., EZHEVSKY S.A., DOWDY S.F. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- NOBES C.D., LAURITZEN I., MATTEI M.G., PARIS S., HALL A., CHARDIN P. New member of the Rho family, Rnd1, promotes disassembly of actin filament structure and loss of cell adhesion. J. Cell. Biol. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACAUD P., LOIRAND G., GREGOIRE G., MIRONNEAU C., MIRONNEAU J. Noradrenaline-activated heparin-sensitive Ca2+ entry after depletion of intracellular Ca2+ store in portal vein smooth muscle cells. J. Biol. Chem. 1993;268:3866–3872. [PubMed] [Google Scholar]
- RIZZI A., QUAGLIO D., VASQUEZ G., MASCOLI F., AMADESI S., CALO G., REGOLI D., ZAMBONI P. Effects of vasoactive agents in healthy and diseased human saphenous veins. J. Vasc. Surg. 1998;28:855–861. doi: 10.1016/s0741-5214(98)70061-8. [DOI] [PubMed] [Google Scholar]
- SANSILVESTRI-MOREL P., NONOTTE I., FOURNET-BOURGUIGNON M.P., RUPIN A., FABIANI J.N., VERBEUREN T.J., VANHOUTTE P.M. Abnormal deposition of extracellular matrix proteins by cultured smooth muscle cells from human varicose veins. J. Vasc. Res. 1998;35:115–123. doi: 10.1159/000025573. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE JEUNE H., CARIO-TOUMANIANTZ C., VAILLANT N., GADEAU A.P., DESGRANGES C., SCALBERT E., CHARDIN P., PACAUD P., LOIRAND G. P2Y(1), P2Y(2), P2Y(4), and P2Y(6) receptors are coupled to Rho and Rho kinase activation in vascular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1751–1761. doi: 10.1152/ajpheart.2000.278.6.H1751. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE MELLIONNEC E., BERTOGLIO J., SCALBERT E., PACAUD P., LOIRAND G. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ. Res. 2001;88:1102–1104. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- SAWADA N., ITOH H., UEYAMA K., YAMASHITA J., DOI K., CHUN T.H., INOUE M., MASATSUGU K., SAITO T., FUKUNAGA Y., SAKAGUCHI S., ARAI H., OHNO N., KOMEDA M., NAKAO K. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- SEASHOLTZ T.M., MAJUMDAR M., KAPLAN D.D., BROWN J.H. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ. Res. 1999;84:1186–1193. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- SEASHOLTZ T.M., ZHANG T., MORISSETTE M.R., HOWES A.L., YANG A.H., BROWN J.H. Increased expression and activity of RhoA are associated with increased DNA synthesis and reduced p27(Kip1) expression in the vasculature of hypertensive rats. Circ. Res. 2001;89:488–495. doi: 10.1161/hh1801.096337. [DOI] [PubMed] [Google Scholar]
- SHIBATA R., KAI H., SEKI Y., KATO S., MORIMATSU M., KAIBUCHI K., IMAIZUMI T. Role of Rho-associated Kinase in Neointima Formation After Vascular Injury. Circulation. 2001;103:284–289. doi: 10.1161/01.cir.103.2.284. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAVERS J.P., BROOKES C.E., EVANS J., BAKER D.M., KENT C., MAKIN G.S., MAYHEW T.M. Assessment of wall structure and composition of varicose veins with reference to collagen, elastin and smooth muscle content. Eur. J. Vasc. Endovasc. Surg. 1996;11:230–237. doi: 10.1016/s1078-5884(96)80058-x. [DOI] [PubMed] [Google Scholar]
- UEHATA M.L., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- VENTURI M., BONAVINA L., ANNONI F., COLOMBO L., BUTERA C., PERACCHIA A., MUSSINI E. Biochemical assay of collagen and elastin in the normal and varicose vein wall. J. Surg. Res. 1996;60:245–248. doi: 10.1006/jsre.1996.0038. [DOI] [PubMed] [Google Scholar]
- ZHONG C., CHRZANOWSKA-WODNICKA M., BROWN J., SHAUB A., BELKIN A.M., BURRIDGE K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell. Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]


