Abstract
Orexin-A and orexin-B (also known as hypocretin-1 and hypocretin-2) are hypothalamic peptides and regulate feeding behaviour, energy metabolism and the sleep-wake cycle. Orexin-A binds equally to both orexin-1 and orexin-2 receptors, while orexin-B has a preferential affinity for orexin-2 receptors.
Orexins are also known to be concentrated in superficial laminae of the spinal dorsal horn, and orexin-A and orexin-1 receptors are found in the dorsal root ganglion cells.
In the present study, the authors examined the effect of intrathecal injection of either orexin-A or orexin-B in the rat formalin test (a model of inflammatory pain) and in the rat hot plate test. The paw formalin injection induces biphasic flinching (phase 1: 0–6 min; phase 2: 10–60 min) of the injected paw.
Intrathecal injection of orexin-A, but not orexin-B, decreased the sum of flinches in phases 1 and 2 in the formalin test and increased the hot plate latency. These effects of orexin-A were completely antagonized by pre-treatment with SB-334867, a selective orexin-1 receptor antagonist. Intrathecal injection of SB-334867 alone had no effect in the formalin test or in the hot plate test.
Intrathecal injection of orexin-A suppressed the expression of Fos-like immunoreactivity (Fos-LI), induced by paw formalin injection, in laminae I-II of L4–5 of the spinal cord.
These data suggest that the spinal orexin-1 receptor is involved in the nociceptive transmission and that the activation of the spinal orexin-1 receptor produces analgesic effects in the rat formalin test and in the rat hot plate test.
Keywords: Formalin test, hot plate test, hypocretin-1, hypocretin-2, orexin-A, orexin-B, orexin-1 receptor, SB-334867
Introduction
Orexin-A and orexin-B (also known as hypocretin-1 and hypocretin-2) are novel hypothalamic peptides of 33 and 28 amino acids, respectively, and have a role in the regulation of feeding behaviour, energy metabolism, and the sleep-wake cycle. Orexin-A and orexin-B act via G-protein coupled receptors named orexin-1 receptor and orexin-2 receptor (Sakurai et al., 1998). Orexin-A binds equally to both orexin-1 and orexin-2 receptors, while orexin-B has a preferential affinity for orexin-2 receptors (Sakurai et al., 1998).
Van den Pol (1999) demonstrated the presence of robust projection of orexin-B from the hypothalamus to lamina I of the spinal cord. Moreover, Date et al. (2000) reported that both orexin-A and orexin-B were distributed throughout the spinal cord and that, in the spinal cord, orexin fibres were concentrated in lamina I of the dorsal horn and in lamina X surrounding the central canal and were moderately abundant in laminae II–VII in the dorsal horn. Hervieu et al. (2001) reported that orexin-1 receptor is localized on C-fibres in the spinal cord. Both orexin-A and orexin-1 receptors were found in dorsal root ganglion cells (Bingham et al., 2001). These data strongly suggested that spinal orexin-1 and orexin-2 receptors are involved in nociceptive transmission.
Formalin injection into the rat hindpaw induces agitation behaviour, and this formalin-induced agitation behaviour has been used as a model of inflammatory pain (the formalin test). If orexin-1 and orexin-2 receptors are involved in regulating nociceptive transmission in the spinal cord during inflammation, intrathecal injection of orexin-A or orexin-B may have some effect on the formalin-induced agitation behaviour. In the present study, to define the role of orexin-1 and orexin-2 receptors in nociceptive transmission in the spinal cord, the authors examined the effect of intrathecal administration of orexin-A and orexin-B on the formalin test and the thermal nociceptive hot plate test in the rat.
Expression of Fos, which is the protein product of the immediate-early protooncogene c-fos, has been widely used to identify populations of neurons that are activated by noxious stimuli (Hunt et al., 1987) and to concomitantly examine the ability of drugs to suppress the expression of Fos-like immunoreactivity (Fos-LI) in the spinal cord in the formalin test (Yamamoto et al., 2000; Hammond et al., 1998). In the present study, we also examined the effect of intrathecally administered orexin-A on the expression of Fos-LI induced by paw formalin injection.
Methods
The following investigations were performed according to a protocol approved by the Institutional Animal Care Committee of Chiba University, Chiba, Japan. Male Sprague-Dawley rats weighing 250–300 g were fitted with long-term intrathecal catheters and examined for the effects of the agents on the formalin test and the hot plate test.
Intrathecal catheters
Chronic intrathecal catheters were inserted during halothane anaesthesia by passing a PE-10 catheter through an incision in the atlanta-occipital membrane to a position 8 cm caudal to the cisterna at the level of lumbar enlargement (Yaksh & Rudy, 1976). The catheter was externalized on the top of the skull, sealed with a steel wire, and the wound was closed with 3-0 silk sutures. The animals were allowed to recover for 1 week before experimental use. All animals displayed normal feeding and drinking behaviour post-operatively. About 10% of rats operated upon showed neurological deficits and rats showing neurological deficits were not studied.
Formalin test
To carry out the formalin test, the animal was briefly anaesthetized with halothane (4%). When there was momentary loss of spontaneous movement with preservation of the deep spontaneous respiration, blink and pinnae reflexes, 50 μl of 5% formalin was injected subcutaneously into the dorsal surface of the right hindpaw with a 27-gauge needle. Immediately after the formalin injection, the animal was placed in an open Plexiglas box (10×20×24 cm), which permitted observation. Within 1 min after the formalin injection, the rat displayed the behaviour typical of this model, i.e. it held the injected paw just off the floor. During this period, spontaneous flinching of the injected paw could also be observed. Flinching is readily discriminated and is characterized as a rapid and brief withdrawal or flexion of the injected paw. This pain-related behaviour was quantified by counting the number of flinches for 1 min periods at 1–2 and 5–6 min, and then for 1 min periods at intervals during the period from 10 to 60 min after the injection. Two phases of spontaneous flinching behaviour were observed. An initial acute phase (phase 1, during the first 6 min after the formalin injection) was followed by a relative short quiescent period and then by a prolonged tonic response (phase 2, beginning about 10 min after the formalin injection). After the observation period, the animals were immediately killed with an overdose of barbiturate.
Hot plate test
The hot plate test was carried out to assess the effects of agents on the thermal nociceptive threshold. Rats were placed on 52.5°C hot plate. The response latency to either a hind-paw lick or jump was recorded. In the absence of a response, the animals were removed from the hot plate at 60 s to avoid tissue injury, and a 60 s latency was assigned as the response.
Immunohistochemistry
After anaesthetizing the rats with 60 mg kg−1 of sodium pentobarbitone intraperitoneally, surgery proceeded with sternotomy, transcardiac aortic needle cannulation, and perfusion with 50 ml of saline, followed by 500 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH=7.4). Spinal cords were removed and postfixed in the same fixative solution overnight at 4°C. After storing in 0.01 M phosphate buffered saline (PBS) containing 20% sucrose for 20 h at 4°C, the L 4–5 spinal cord was sectioned at 40 μm thickness on a cryostat. Sections of the lumbar spinal cord were collected in PBS. After immersion in PBS containing 5% normal goat serum and 0.3% Triton X-100 for 1 h, the sections were processed for Fos immunohistochemistry by a free-floating ABC technique using rabbit antibody to Fos (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) diluted with PBS containing 5% normal goat serum and 0.3% Triton X-100 for 20 h at 4°C. The sections were then incubated at room temperature for 90 min with a biotinylated goat anti-rabbit immunogloblin G (1 : 100; Vector Labs, Burlingame, CA, U.S.A.) in PBS containing 5% normal goat serum and 0.3% Triton X-100. The sections were incubated at room temperature for 1 h in avidin-biotin complex (1 : 100; Vector Labs) and visualized with diaminobenzidine (DAB) and ammonium nickel sulphate. The tissue sections were mounted onto gelatin-coated slides, air dried, dehydrated in alcohol in a graded manner, cleared in xylene and coverslipped.
Behavioural analysis
The general behaviour of each rat was carefully observed and tested. Motor functions were evaluated by the performance of two specific behavioural tasks, as follows. (1) The placing/stepping reflex: this response was evoked by drawing the dorsum of either hindpaw over the edge of a table top. In normal animals, this stimulus elicits an upward lifting of the paw onto the surface of the table, called stepping. Animals with any degree of hind limb flaccidity will demonstrate an altered or absent reflex. (2) The righting reflex: an animal placed horizontally with its back on the table will normally show an immediate coordinated twisting of the body around its longitudinal axis to regain its normal position on its feet. Animals displaying ataxic behaviour will show a decreased ability to right themselves. To quantify the evaluation of motor functions, both tasks were scored on a scale of 0–2 in which 0=absence of function and 2=normal motor functions. Animals that were able to perform the motor tasks but did so more slowly than normal animals were assigned a score of 1.
Drugs
The intrathecally administered drugs were delivered in a total volume of 10 μl followed by 10 μl of saline to flush the catheter. The intraperitoneally administered drugs were delivered in a total volume of 1 ml. The agents used in this study were orexin-A (molecular weight=3561, Peptide Institute, Osaka, Japan), orexin-B (molecular weight=2936, Peptide Institute), SB-334867 (1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride, molecular weight=356, GlaxoSmithKline, Herts, U.K.), a selective orexin-1 receptor antagonist (Smart et al., 2001; Porter et al., 2001) and naloxone hydrochloride (molecular weight=364, Sigma, St. Louis, MO, U.S.A.).
Experimental protocol
Formalin test
For the dose-response study, agents were administered intrathecally 10 min before the formalin injection. To obtain control data, the vehicle (saline) was injected intrathecally. In a separate group of rats, to verify whether the effects of intrathecally administered orexin-A on the formalin test were mediated by the action of the drugs in the spinal cord, a most-effective dose (0.3 nmol) of orexin-A was administered intraperitoneally 10 min before the foramlin injection and the effect on the formalin test was examined (intraperitoneal injection study). To obtain control data, the vehicle (saline) was injected intraperitoneally. To verify that the effect of intrathecally administered orexin-A on the formalin test was produced by an interaction between orexin-A and spinal orexin-1 receptor, 30 nmol of SB-334867 were administered intrathecally 5 min before the intrathecal injection of 0.3 nmol of orexin-A and formalin was injected subcutaneously into the right hindpaw 10 min after the orexin-A administration. The effect of intrathecal administration of 30 nmol of SB-334867 on the formalin test was also examined. To verify that the effect of intrathecally administered orexin-A is due to the modulation of release of endogenous opioids at the spinal level, 28 nmol of naloxone were administered intrathecally 5 min before the intrathecal injection of 0.3 nmol of orexin-A and formalin was injected subcutaneously 10 min after the orexin-A injection.
Hot plate test
Two baseline measurements were made before the drug injection. For the dose-response study, agents were administered intrathecally and the hot plate latency was measured at 5, 15, 30 and 60 min after the drug injection. To obtain control data, the vehicle (saline) was injected intrathecally. In a separate group of rats, to verify whether the effects of intrathecally administered orexin-A on the hot plate test were mediated by the action of the drugs in the spinal cord, a most-effective dose (0.1 nmol) of orexin-A was administered intraperitoneally and the effect on the hot plate test was examined (intraperitoneal injection study). To obtain control data, vehicle (saline) was injected intraperitoneally. To verify that the effect of intrathecally administered orexin-A on the hot plate test was produced by an interaction between orexin-A and spinal orexin-1 receptor, 10 nmol of SB-334867 was administered intrathecally 5 min before the intrathecal injection of 0.1 nmol of orexin-A and the effect of 0.1 nmol of orexin-A on the hot plate test was examined. The effect of intrathecal injection of 10 nmol of SB-334867 on the hot plate test was also examined.
Immunohistochemical study
A most-effective dose (0.3 nmol) of orexin-A or saline was administered intrathecally 10 min before the formalin injection, and the expression of Fos-LI was examined 2 h after the formalin injection.
For the quantitation of Fos-LI, five sections from the L4 and L5 segments of spinal cord of each rat were randomly selected. The number of Fos-LI positive neurons in the superficial laminae (laminae I and II) and the nucleus proprius (laminae III and IV) and the neck of dorsal horn (lamina V) on the side of the spinal cord ipsilateral to the site of formalin injection were counted. Laminae borders were identified by use of anatomical landmarks in grey matter and standard anatomical drawings. A Fos-LI positive nucleus was identified by a round or an oval profile with darker than background staining visible by light microscopy at 50× magnification, and Fos positive neurons were counted from printed images with respect to each lamina. The investigator responsible for counting the Fos-LI positive neurons was blind to the drug treatment of each animal. The average of the number of Fos-LI positive neurons in five slices was defined as the number of Fos-LI positive neurons.
Statistical analysis
Formalin test
The time-response data are presented as the mean flinches (±s.e.m.) per minute for the periods of 1–2 min and 5–6 min and then for 1 min periods at 5 min intervals up to 60 min. For the dose-response analysis, data from phase 1 (0–6 min) and phase 2 (10–60 min) observations were considered separately. In each case, the cumulative instances of formalin-evoked flinches during the phase 1 and phase 2 were calculated for each rat. These individual rat data were then used to construct phase 1 and phase 2 dose-response curves. To evaluate the dose-dependence, one way analysis of variance (ANOVA) was used. For multiple comparison, the Dunnett's test was used. In the intraperitoneal study, to compare the cumulative instances of formalin-evoked flinches during phase 1 and phase 2 in the orexin-A treated rats with those in the saline treated rats, respectively, the unpaired t-test (two-tailed) was used. In the antagonist study, to compare the cumulative instances of formalin-evoked flinches during phase 1 and phase 2 in the orexin-A+SB-334867 treated rats or the orexin-A+naloxone treated rats with those in the orexin-A treated rats, respectively, the unpaired t-test (two-tailed) was used.
Hot plate test
To compare the base-line hot-plate latencies between groups, ANOVA with the Dunnett's multiple comparison test was used. To analyse the effects of intrathecally administered orexin-A and orexin-B on the hot plate test, the per cent maximum possible effect (%MPE) was calculated, where %MPE=([post-drug maximum response latency–pre-drug response latency]/[cut-off time (60 s)–pre-drug response latency]) ×100. The post drug maximum response latency was defined as the single longest response latency during the first 30 min after the intrathecal drug injection. To evaluate the dose-dependence, ANOVA was used. For multiple comparison, the Dunnett's test was used. In the intraperitoneal injection study, to compare the level of %MPE in the orexin-A treated rats with that in the saline treated rats, the unpaired t-test (two-tailed) was used. In the antagonist study, to compare the level of %MPE in the orexin-A+SB-334867 treated rats with that in the orexin-A treated rats, the unpaired t-test (two-tailed) was used.
Immunohistochemical study
In the comparison of the number of Fos-LI positive neurons between the orexin-A treated group and the saline treated group, the unpaired t-test (two-tailed) was used.
Wherever appropriate, results are expressed as mean±s.e.m. Critical values that reached a P<0.05 level of significance were considered statistically significant.
Results
Behavioural analysis
After the intrathecal injection of orexin-A, orexin-B or SB-334867, all animals scored 2 (normal motor function) in the placing/stepping reflex and righting reflex tests. The rats treated with orexin-A, orexin-B or SB-334857 showed no change in overall level of motor activity.
Formalin test
In the saline-treated rats, a subcutaneous injection of formalin resulted in a highly reliable biphasic display of flinching of the injected paw (Figure 1), and this behaviour was comparable to that previously reported (Yamamoto & Yaksh, 1992; Wheeler-Aceto et al., 1990).
Figure 1.
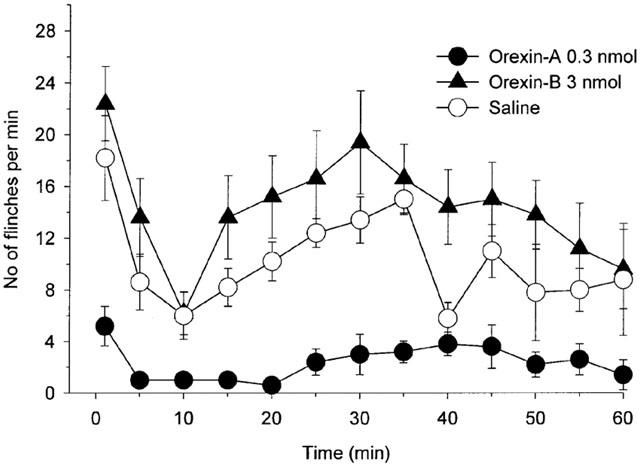
Effect of intrathecal injection of 0.3 nmol of orexin-A, 3 nmol of orexin-B and saline on the time course of the flinches observed after the formalin injection into the dorsal surface of the right rat hind-paw. Drugs were administered intrathecally 10 min before the formalin injection. The number of flinches/min is plotted vs time after the formalin injection. Each line represents the group mean and s.e.m. of five rats.
Intrathecal injection of 0.3 and 3 nmol of orexin-A decreased the sum of flinches in phase 1 as compared with saline treated rats (Figure 2, P<0.001 by ANOVA) and intrathecal injection of 0.3 nmol of orexin-A decreased the sum of flinches in phase 2 as compared with saline treated rats (Figure 2, P<0.001 by ANOVA). Intrathecal injection of orexin-B had no effect on the sum of flinches in both phase 1 and phase 2 at a dose between 0.03 and 3 nmol as compared with saline treated rats (Figure 2, phase 1: P>0.2; phase 2: P>0.2 by ANOVA).
Figure 2.
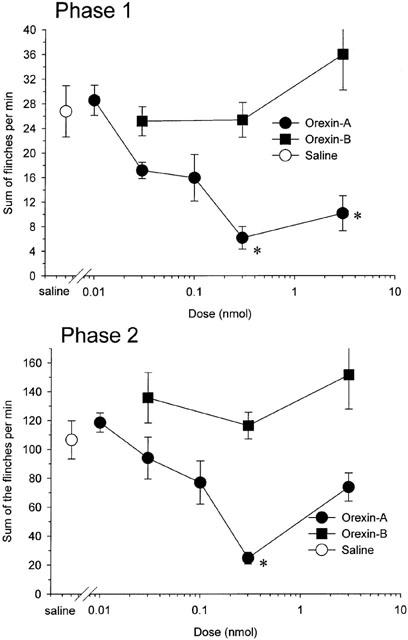
Dose-response curves for intrathecal injection of orexin-A and orexin-B presenting the cumulative instances of formalin evoked flinches during phase 1 (top) and phase 2 (bottom). Drugs were administered intrathecally 10 min before the formalin injection. Each point represents the mean and s.e.m. of five rats. Orexin-A, but not oreixn-B, reduced the number of both phase 1 and phase 2 flinching behaviour. *P<0.05 as compared with the phase 1 or phase 2 responses of saline treated rats.
In the intraperitonenal injection study, 0.3 nmol of orexin-A had no effect on phases 1 and 2 flinching behaviour as compared with the saline treated rats (Figure 3, phase 1: P>0.8; phase 2: P>0.9, by t-test).
Figure 3.
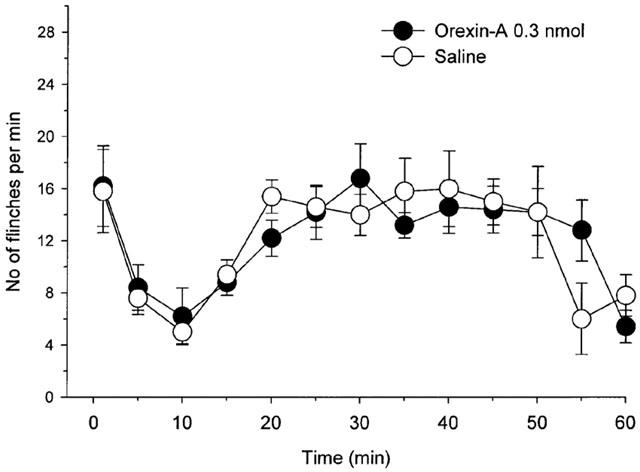
Effect of intraperitoneal injection of 0.3 nmol of orexin-A and saline on the time course of the flinches observed after formalin injection into the dorsal surface of the right rat hind-paw. Drugs were administered intraperitoneally 10 min before the formalin injection. The number of flinches/min is plotted vs time after the formalin injection. Each line represents the group mean and s.e.m. of five rats. Intraperitoneal injection of 0.3 nmol of orexin-A had no effect on the formalin test as compared with saline treated rats.
In the antagonist study, pre-treatment with 30 nmol of SB-334867 antagonized the effect of 0.3 nmol of orexin-A on the phase 1 and phase 2 flinching behaviour (Figure 4, phase 1: P<0.001; phase 2: P<0.001, by t-test). Intrathecal injection of 30 nmol of SB-334867 had no effect on phase 1 and phase 2 flinching behaviour as compared with the saline treated rats (Figure 4, phase 1: P>0.9; phase 2: P>0.7, by t-test). Pre-treatment with 28 nmol of naloxone had no effect on the analgesic effect of 0.3 nmol of orexin-A (Figure 4, phase 1: P>0.6; phase 2: P>0.1, by t-test).
Figure 4.
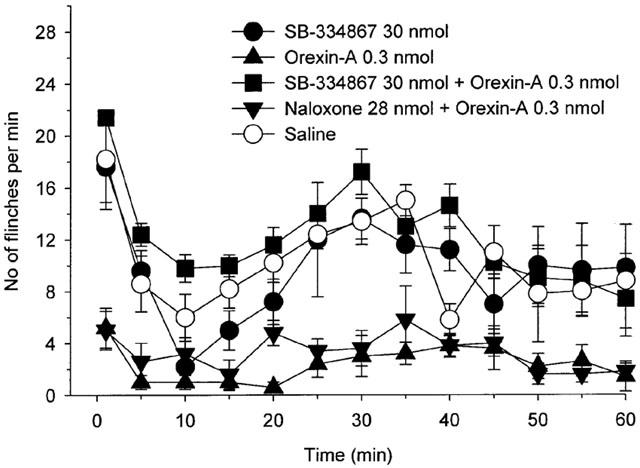
Effects of intrathecal injection of 30 nmol of SB-334867, 0.3 nmol of orexin-A, 30 nmol of SB-334867+0.3 nmol of orexin-A, 28 nmol of naloxone+0.3 nmol of orexin-A and saline on the time course of the flinches observed after formalin injection into the dorsal surface of the right rat hind-paw. Drugs were administered intrathecally 10 min before the formalin injection, except in the study for 30 nmol of SB-334867+0.3 nmol of orexin-A treated rats and 28 nmol of naloxone+0.3 nmol of orexin-A treated rats (SB-334867 or naloxone was administered intrathecally 5 min before the orexin-A administration and formalin was injected 10 min after the orexin-A administration). The number of flinches/min is plotted vs time after the formalin injection. Each line represents the group mean and s.e.m. of five rats. Pre-treatment of 30 nmol of SB-334867, but not 28 nmol of naloxone, antagonized the effect of 0.3 nmol of orexin-A in the rat formalin test. Intrathecal injection of 30 nmol of SB-334867 had no effect on the formalin test as compared with saline treated rats.
Hot plate test
No difference was apparent between base-line hot plate latencies in each group (3 nmol of orexin-A treated rats (n=5): 13.1±1.5 s; 0.3 nmol of orexin-A treated rats (n=5): 13.2±2.2 s; 0.1 nmol of orexin-A treated rats (n=5): 14.0±2.2 s; 0.03 nmol of orexin-A treated rats (n=5): 14.0±1.3 s; 0.01 nmol of orexin-A treated rats (n=5): 10.9±0.9 s; 3 nmol of orexin-B treated rats (n=5): 13.9±1.7 s; 0.3 nmol of orexin-B treated rats (n=4): 16.1±2.3 s; 0.03 nmol of orexin-B treated rats (n=4): 17.0±4.2 s; 0.1 nmol of orexin-A+10 nmol of SB-334867 treated rats (n=5): 16.4±4.1 s; 10 nmol of SB-334867 treated rats (n=5): 17.0±4.8; saline treated rats (n=5): 15.1±1.7 s; P>0.4 by ANOVA). Intrathecal injection of 0.1 and 0.3 nmol of orexin-A increased the level of %MPEs as compared with saline treated rats (Figure 5, P<0.01 by ANOVA). Intrathecal injection of orexin-B had no effect on the level of %MPE at a dose between 0.03 and 3 nmol as compared with saline treated rats (Figure 5, P>0.4 by ANOVA).
Figure 5.
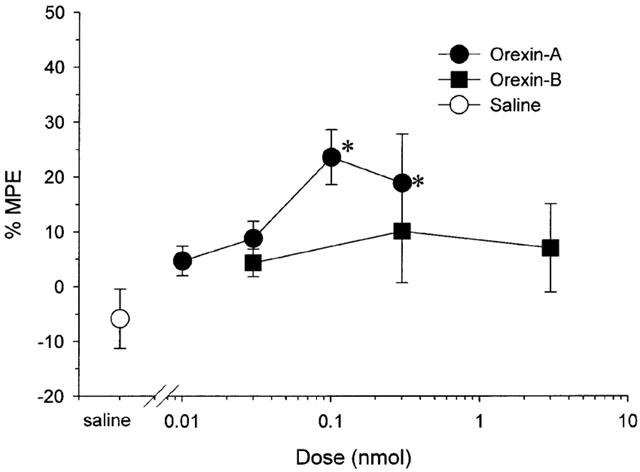
Dose-response curves for intrathecal injection of orexin-A and orexin-B in the hot plate test presenting the per cent maximum possible effect (%MPE) where %MPE=([post-drug maximum response latency–pre-drug response latency]/[cut-off time (60 s)–pre-drug response latency])×100. Each point represents the mean and s.e.m. of five rats. Orexin-A, but not oreixn-B, increased the level of %MPE. *P<0.05 as compared with the %MPE of saline treated rats.
Intraperitoneal injection of 0.1 nmol of orexin-A had no effect on the level of %MPE as compared with the saline treated rats (0.1 nmol of orexin-A treated rats (n=5): −0.9±8.5; saline treated rats (n=5): 1.3±7.3; P>0.7 by t-test).
In the antagonist study, pre-treatment with 10 nmol of SB-334867 antagonized the effect of 0.1 nmol of orexin-A on the level of %MPE (0.1 nmol of orexin-A treated rats (n=5): 23.6±5.0; 0.1 nmol of orexin-A+10 nmol of SB-334867 treated rats (n=5): 1.24±4.4; P<0.01 by t-test). Intrathecal injection of 10 nmol of SB-334867 had no effect on the level of %MPE as compared with the saline treated rats (10 nmol of SB-334867 treated rats (n=5): 5.5±9.7; saline treated rats (n=5): −5.9±5.4; P>0.3 by t-test).
Immunohistochemical study
When 0.3 nmol of orexin-A was administered intrathecally 10 min before the formalin injection, the number of the Fos-LI positive neurons in the laminae I–II was significantly smaller than that of the saline treated group (P<0.005 by t-test), but, in the laminae III–IV and lamina V, there were no differences between the numbers of the Fos-LI positive neurons in the orexin-A treated group and those in the saline treated group (laminae III–IV: P>0.1; lamina V: P>0.1 by t-test, Figures 6 and 7).
Figure 6.
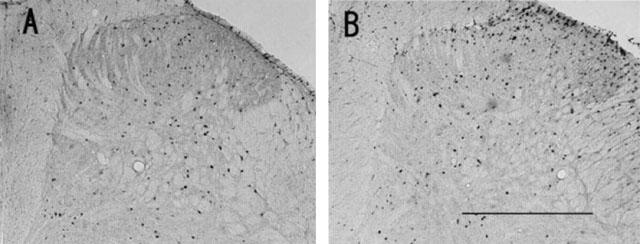
Photomicrographs of transverse sections of the L4 or L5 segments of the spinal cord ipsilateral to the site of formalin injection illustrating the distribution of Fos-LI neurons induced by paw formalin injection in rats with intrathecal injection of 0.3 nmol of orexin-A (A) and in those with intrathecal injection of saline (B). Scale bar, 500 μm.
Figure 7.
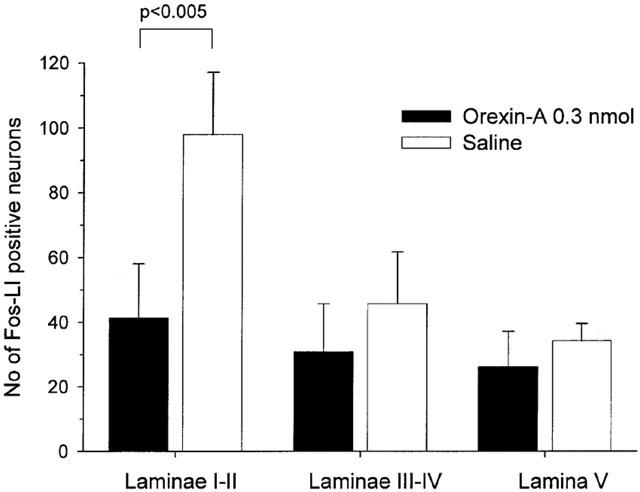
Effect of 0.3 nmol of orexin-A on the number of Fos-LI positive neurons in laminae I–II, laminae III–IV and lamina V on the L4 or L5 segments of the spinal cord ipsilateral to the site of formalin injection. Orexin-A decreased the number of Fos-LI positive neurons in laminae I–II, but not in laminae III–V. Each bar represents the group mean and s.d. of six rats.
Discussion
In the present study, the authors have clearly demonstrated that intrathecal injection of 0.3 nmol of orexin-A significantly depressed both phase 1 and phase 2 flinching behaviour, and that intrathecal administration of orexin-B had no effect on the phase 1 and phase 2 flinching behaviour at a dose between 0.03 and 3 nmol. Intraperitoneal injection of 0.3 nmol of orexin-A had no effect on the phase 1 and phase 2 flinching behaviour and this suggested that the effect of intrathecally administered orexin-A on the flinching behaviour induced by paw formalin injection was mediated by spinal mechanisms. Moreover, intrathecal injection of 0.1 and 0.3 nmol of orexin-A produced a mild, but a distinct analgesic effect in the hot plate test and intrathecal injection of orexin-B had no effect on the hot plate latency at a dose between 0.03 and 3 nmol. Intraperitoneal injection of 0.1 nmol of orexin-A had no effect on the hot plate latency and this suggested that the analgesic effect of intrathecally administered orexin-A in the hot plate test was also mediated by spinal mechanisms. Orexin-A binds equally to both orexin-1 and orexin-2 receptors and orexin-B has a preferential affinity for orexin-2 receptors (Sakurai et al., 1998). Moreover, SB-334867, a selective orexin-1 receptor antagonist, antagonized an analgesic effect of orexin-A in both the formalin test and the hot plate test. These data strongly suggest that an analgesic effect of spinally applied orexin-A is mediated by the activation of spinal orexin-1 receptor and spinal orexin-2 receptor is not involved in the spinal nociceptive transmission during either the formalin test or the hot plate test. It has been reported that intravenous injection of orexin-A produces an analgesic effect in the mouse hot plate test and an anti-hyperalgesic effect in the mouse carrageenan test and that these effects of orexin-A are mediated by the activation of orexin-1 receptor at the peripheral terminals of the primary afferent (Bingham et al., 2001). Moreover, intracerebroventricular injection of orexin-A has been reported to produce an analgesic effect in the rat hot plate test (Bingham et al., 2001). Our data are the first to indicate that the activation of spinal orexin-1 receptors produces an analgesic effect.
In the present study, pre-treatment of 28 nmol of naloxone had no effect on the analgesic effect of 0.3 nmol of orexin-A in the formalin test. The authors had previously found that 28 nmol of naloxone fully reversed the analgesic effect of 17 nmol of dynorphin-A on the formalin test (Yamamoto et al., 1997). This suggested that intrathecal injection of 28 nmol of naloxone is an adequate dose to antagonize the activation of naloxone sensitive spinal opioid receptors. This suggested that the analgesic effect of orexin-A is not caused by modulation of release of opioids at the spinal cord.
The immunohistochemical study revealed that intrathecal injection of 0.3 nmol of orexin-A reduced the number of the Fos-LI positive neurons in laminae I–II and that 0.3 nmol of orexin-A had no effect on the number of the expression of the Fos-LI positive neurons in laminae III–V. These data strongly suggested that activation of spinal orexin-1 receptor suppresses the nociceptive input to the laminae I–II and produces an analgesic effect in the rat formalin test. As indicated in the introduction, orexin-A immunoreactivity is concentrated in the superficial laminae of spinal dorsal horn (Date et al., 2000; Bingham et al., 2001) and our finding is consistent with these reports.
Intrathecal injection of SB-334867 itself had no effect in both the formalin test and the hot plate test at doses which antagonized the analgesic effect of orexin-A in the formalin test and the hot plate test. These data suggest that a tonic orexin-1 receptor mediated inhibitory system does not exist in the spinal cord during either the formalin test or the hot plate test. On the other hand, it has been reported that intraperitoneal injection of SB-334867 produces pro-hyperalgesic effect in the mouse carrageenan-induced thermal hyperalgesia test (Bingham et al., 2001) and these data suggest that a tonically activated orexin-1 receptor mediated inhibitory system is present during a carrageenan-induced thermal hyprealgesia test in mice. Several possibilities present themselves. First, although both subcutaneous formalin injection and subcutaneous carrageenan injection induce localized inflammation, there are several differences between the carrageenan hyperalgesia test and the formalin test. Paw formalin injection induces biphasic spontaneous nociceptive behaviour, such as flinching, and the nociceptive behaviour lasts about 1 h (Wheeler-Aceto et al., 1990; Yamamoto & Yaksh, 1992). However, paw carrageenan injection induces no flinching response but induces a much more severe paw oedema than does formalin and the maximum thermal hyperalgesia occurs about 2 h after the carrageenan injection (Wheeler-Aceto et al., 1990; Yamamoto et al., 1993). The difference in sensitivity produced by SB-334867 between the formalin test and the carrageenan test may reflect the different characteristics of these two tests. Second, in the current studies, SB-334867 was administered intrathecally, and SB-334867 may act in the spinal cord. On the other hand, Bingham et al. (2001) administered SB-334867 intraperitoneally and the pro-hyperalgesic effect of SB-334867 in the mouse carrageenan-induced thermal hyperalgesia test may be mediated by the interaction between SB-334867 and the orexin-1 receptor located in the outside of spinal cord. Third, the current studies were carried out in rat, and the possibility of a species difference cannot be excluded.
There are some hypothalamic neuropeptides known to modulate response to noxious stimuli, such as corticotropin-releasing factor, pituitary adenylate cyclase activating polypeptide, oxytocin and neuropeptide FF (Lariviere & Melzack, 2000; Sakashita et al., 2001; Yamamoto & Tatsuno, 1995; Xu & Wiezenfeld-Hallin, 1994; Panula et al., 1999). Orexin-A is a new member of the hypothalamic neuropeptides to produce an analgesic effect.
In conclusion, intrathecal injection of orexin-A, but not orexin-B, depressed the phase 1 and phase 2 flinching behaviour in the rat formalin test and increased the hot plate latency. These effects of orexin-A are mediated by the activation of spinal orexin-1 receptors. These data suggest a new potential therapeutic approach to treating pain.
Acknowledgments
We thank professor Takashi Nishino, Department of Anesthesiology, Chiba University Hospital for his generous support of our study. This study was supported in part by a Grant-in-Aid for Scientific Research (B) 12470315, Japan.
Abbreviations
- ANOVA
one way analysis of variance
- Fos-LI
Fos-like immunoreactivity
- %MPE
per cent maximum possible effect
References
- BINGHAM S., DAVEY P.T., BABBS A.J., IRVING E.A., SAMMONS M.J., WYLES M., JEFFREY P., CUTLER L., RIBA I., JOHNS A., PORTER R.A., UPTON N., HUNTER A.J., PARSONS A.A. Orexin-A, an hypothalamic peptide with analgesic properties. Pain. 2001;92:81–90. doi: 10.1016/s0304-3959(00)00470-x. [DOI] [PubMed] [Google Scholar]
- DATE Y., MONDAL M.S., MATSUKURA S., NAKAZATO M. Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord. Neurosci. Lett. 2000;288:87–90. doi: 10.1016/s0304-3940(00)01195-2. [DOI] [PubMed] [Google Scholar]
- HAMMOND D.L., WANG H., NAKASHIMA N., BASBAUM A.I. Differential effects of intrathecally administered delta and mu opioid receptor agonist on formalin-evoked nociception and on the expression of Fos-like immunoreacrivity in the spinal cord of the rat. J. Pharmacol. Exp. Ther. 1998;284:378–387. [PubMed] [Google Scholar]
- HERVIEU G.J., CLUDERAY J.E., HARRISON D.C., ROBERTS J.C., LESLIE R.A. Gene expression and protein distribution of the orexin-1 receptor in the rats brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- HUNT S.P., PINI A., EVAN G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- LARIVIERE W.R., MELZACK R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84:1–12. doi: 10.1016/S0304-3959(99)00193-1. [DOI] [PubMed] [Google Scholar]
- PANULA P., KALSO E., NIEMINEN M.L., KONTINEN V.K., BRANDT A., PERTOVAARA A. Neuropeptide FF and modulation of pain. Brain Res. 1999;848:191–196. doi: 10.1016/s0006-8993(99)02044-2. [DOI] [PubMed] [Google Scholar]
- PORTER R.A., CHAN W.N., COULTON S., JOHNS A., HADLEY M.S., WIDDOWSON K., JERMAN J.C., BROUGH S.J., COLDWELL M., SMART D., JEWITT F., JEFFREY P., AUSTIN N. 1,3-biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg. Med. Chem. Lett. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- SAKASHITA Y., KURIHARA T., UCHIDA D., TATSUNO I., YAMAMOTO T. Involvement of PACAP receptor in primary afferent fibre-evoked responses of ventral roots in the neonatal rat spinal cord. Br. J. Pharmacol. 2001;132:1769–1776. doi: 10.1038/sj.bjp.0703980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURAI T., AMEMIYA A., ISHII M., MATSUZAKI I., CHEMELLI R.M., TANAKA H., WILLIAMS S.C., RICHARDSON J.A., KOZLOWSKI G.P., WILSON S., ARCH J.R.S., BUCKINGHAM R.E., HAYNES A.C., CARR S.A., ANNAN R.S., MCNULTY D.E., LIU W.-S., TERRETT J.A., ELSHOURBAGY N.A., BERGSMA D.J., YANAGISAWA M. Orexin and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- SMART D., SABIDO-DAVID C., BROUGH S.J., JEWITT F., JOHNS A., PORTER R.A., JERMAN J.C. SB-334867-A: the first selective orexin-1 receptor antagonist. Br. J. Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEN POL A.N. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J. Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELER-ACETO H., PORRECA F., COWAN A. The rat formalin test: comparison of noxious agents. Pain. 1990;40:229–238. doi: 10.1016/0304-3959(90)90073-M. [DOI] [PubMed] [Google Scholar]
- XU X.-J., WIEZENFELD-HALLIN Z. Is systemically administered oxytocin an analgesic. Pain. 1994;57:193–196. doi: 10.1016/0304-3959(94)90223-2. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., RUDY T.A. Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., OHTORI S., CHIBA T. Inhibitory effect of intrathecally administered nociceptinon the expression of Fos-like immunoreactivity in the rat formalin test. Neurosci. Lett. 2000;284:155–158. doi: 10.1016/s0304-3940(00)01003-x. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., SHIMOYAMA N., MIZUGUCHI T. The effects of morphine, MK-801, an NMDA antagonist, and CP-96,345, an NK1 antagonist, on the hyperesthesia evoked by carrageenan injection in the rat paw. Anesthesiology. 1993;78:124–133. doi: 10.1097/00000542-199301000-00018. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., TATSUNO I. Antinociceptive effect of intrathecally administered pituitary adenylate cyclase activating polypeptide (PACAP) on the rat formalin test. Neurosci. Lett. 1995;184:32–35. doi: 10.1016/0304-3940(94)11161-b. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., YAKSH T.L. Comparison of the antinociceptive effects of pre- and posttreatment with intrathecal morphine and MK801, an NMDA antagonist, on the formalin test in the rat. Anesthesiology. 1992;77:757–763. doi: 10.1097/00000542-199210000-00021. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI-TAGUCHI N., KIMURA S. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like receptor agonist, in the rat formalin test. Neuroscience. 1997;81:249–254. doi: 10.1016/s0306-4522(97)00166-8. [DOI] [PubMed] [Google Scholar]


