Abstract
Sodium hydrogen sulphide (NaHS), a donor of hydrogen sulphide (H2S), produced dose-related relaxation of the rabbit isolated ileum (EC50, 76.4±7.9 μM) and rat vas deferens (EC50, 64.8±5.4 μM) and reduced ACh-mediated contraction of the guinea-pig isolated ileum.
NaHS also reduced the response of the guinea-pig (EC50, 80.0±5.7 μM) and rat (EC50, 108.2±11.2 μM) ileum preparations to electrical stimulation of the intramural nerves. In guinea-pig ileum this effect was spontaneously reversible and mimicked by sodium nitroprusside (SNP, EC50, 2.1 μM). Combination of NaHS (20 μM) with SNP (0.5 μM) produced a greater than additive inhibition of the twitch response of the ileum to electrical stimulation.
The inhibitory effect of NaHS on the field-stimulated guinea-pig ileum was unaffected by pretreatment with L-NAME (100 μM), indomethacin (10 μM), naloxone (1 μM) or glibenclamide (100 μM). Furthermore, NaHS (200 μM) did not affect the contractile response of the ileum to KCl (10 to 60 mM).
Propargylglycine (PAG, 1 mM) and β-cyanoalanine (BCA, 1 mM) (inhibitors of cystathionine-γ-lyase) but not aminooxyacetic acid (AOAA, 1 mM) (inhibitor of cystathionine-β-synthetase) caused a slowly developing increase in the contraction of the guinea-pig ileum to field stimulation. This effect was reversed by cysteine (1 mM).
These results show that NaHS relaxes gastrointestinal and urogenital smooth muscle and suggest that H2S is responsible for these effects. The possibility that endogenous H2S, formed as a consequence of activation of intramural nerves, plays a part in controlling the contractility of the guinea-pig ileum is discussed.
Keywords: Hydrogen sulphide, ileum, acetylcholine, vas deferens, propargylglycine, β-cyanoalanine, cysteine
Introduction
Whilst the pharmacology of gaseous mediators such as nitric oxide (NO) and carbon monoxide (CO) has been extensively studied over many years, the profile of biological activity of another such biologically active gas, hydrogen sulphide (H2S), has received scant attention.
H2S is found in the atmosphere (about 1 μg m−3, Air Quality Guidelines for Europe, World Health Organization, 1987) as well as in foodstuffs such as dairy products and cooked meats (Kraft et al., 1956). In the environment, H2S is a chemical hazard associated with natural gas production, sewage treatment and is a bi-product of certain types of paper and pulp production (Guidotti, 1996). It is a product of bacterial (Pitcher et al., 2000) and helminth (Goffredi et al., 1997) metabolism and, perhaps more significantly, also occurs naturally in mammals. Thus, H2S can be found in (concentration of 1.1 μM in man, Suarez et al., 1998), and indeed accounts for, the characteristic odour of flatus. H2S also occurs in human faeces (e.g. Florin et al., 1991). In both cases, the H2S detected is probably of bacterial origin. However, the in situ formation of H2S by mammalian cells has also been noted. For example, relatively high concentrations of H2S occur in rat (50 μM; Zhao et al., 2001) and human (10–100 μM; Richardson et al., 2000) blood and in rat brain homogenates (60–150 μM, Warenycia et al., 1989). Furthermore, both renal cortical tubule cells (Stipanuk et al., 1990) and colonic enterocytes (Coloso & Stipanuk, 1989) synthesise H2S.
The formation of H2S by mammalian cells is most probably accounted for by two enzymes, cystathionine-γ-lyase (CSE) and cystathionine-β-synthetase (CBS). These occur in bacteria and mammals and have been known for many years to catalyse the final step in a transsulphuration pathway by which methionine and cysteine are metabolically interchanged (Finkelstein & Martin, 1984). Two decades ago, Stipanuk & Beck (1982) reported that CSE was able to utilize cysteine (in addition to cystathionine) as a substrate to form thiocysteine and H2S. Since CSE occurs in several mammalian (including human) cells and tissues it seems reasonable to propose that this enzyme may account for, or at least contribute to, the presence of H2S in mammalian blood and organ homogenates.
The profile of pharmacological activity of H2S is not entirely clear. Administered acutely, and at high concentrations, H2S is toxic by complexing with the Fe3+ of mitochondrial cytochrome oxidase thereby blocking cellular oxidative metabolism (Gosselin et al., 1984). Other possible targets include carbonic anhydrase (Nicholson et al., 1998) and tyrosine aminotransferase (Hargrove, 1988), both of which are inhibited by H2S. Interestingly, at lower (more physiological) concentrations, H2S has recently been shown to exert a range of biological effects. For example, it relaxes isolated vascular (Zhao et al., 2001), reproductive (Hayden et al., 1989; Sidhu et al., 2001) and gastrointestinal (Hosoki et al., 1997) smooth muscle preparations in vitro. In the central nervous system, H2S also enhances NMDA receptor-mediated currents and facilitates long-term potentiation (LTP) in the hippocampus (Abe & Kimura, 1996) as well as hyperpolarizing brainstem neurones (Kombian et al., 1993) and inhibiting corticotrophin releasing factor (CRF) efflux from the hypothalamus (Navarra et al., 2000).
With this in mind, we have now investigated the effect of H2S (as sodium hydrogen sulphide, NaHS) on contractions of guinea-pig, rat and rabbit ileum and rat vas deferens to appropriate drugs and/or to electrical (field) stimulation of the intramural nerves. In addition, using inhibitors of CSE and CBS, we have obtained evidence of a role for endogenous H2S in the regulation of gut contractility in the guinea-pig ileum in vitro.
Methods
Guinea-pigs (male, Dunkin-Hartley, 500–700 g), rats (Wistar, male, 200–250 g) and rabbits (New Zealand White, male 2.5–3.5 kg) were used in this study. Guinea-pigs and rats were killed by cervical dislocation and exsanguination. Rabbits were killed by an overdose of sodium pentobarbitone (60 mg kg−1) administered via a marginal ear vein. All experiments were conducted under the authority of the UK Animals (Scientific Procedures) Act (1986).
Pharmacological preparations were rapidly dissected and mounted in 25 ml organ baths containing warmed (37°C) and oxygenated (95% O2: 5% CO2) Krebs' solution (composition, mM: NaCl 118, KCl 5.4, NaHCO3 25, MgSO4 1.2, CaCl2, 2.5, glucose 11.1, pH 7.4). Changes in tension were recorded using Grass-FT03 force transducers connected to a Devices pen recorder.
After equilibration (60 min), preparations were exposed to ACh (88 nM, EC70 from preliminary experiments, not shown), KCl (10 or 60 mM) or were electrically stimulated by means of parallel platinum electrodes positioned on either side of the tissue and connected to a square wave stimulator (Harvard Apparatus Ltd.). For guinea-pig ileum and rat vas deferens, the following stimulation parameters were used: 0.1 Hz (frequency), 0.5 ms (pulse width) and 80 V. A higher frequency of stimulation (5 Hz) was used for rat ileum preparations due to the spontaneous activity of these preparations which obscured contractions at lower frequencies.
In some experiments, field-stimulated guinea-pig ileum preparations were exposed to graded concentrations of sodium hydrogen sulphide (NaHS, 1–1000 μM) or sodium nitroprusside (SNP, 0.1–100 μM) in the presence or absence of propargylglycine (PAG, 1 mM), β-cyanoalanine (β-CA, 1 mM; both inhibitors of cystathionine-γ-lyase, Reed, 1995; Uren et al., 1978), amino-oxyacetic acid (AOAA, 1 mM; inhibitor of cystathionine-β-synthetase, Braunstein et al., 1971), cysteine hydrochloride or base (1 mM) or appropriate concentrations (derived from similar tissue bath experiments reported in the literature, see references indicated below) of the nitric oxide synthase inhibitor (L-NG nitroarginine methyl ester, L-NAME, 100 μM; Moore et al., 1990), cyclo-oxygenase inhibitor, indomethacin (10 μM; Rodriguez-Martinez et al., 1998), opioid antagonist, naloxone (1 μM; Rizzi et al., 2001), or the KATP channel blocker, glibenclamide (100 μM; Zhao et al., 2001). In all cases, tissues were exposed to the inhibitor for 60 min prior to further testing. Control tissues were exposed to an appropriate volume (1.0 ml per l Krebs) of vehicle (0.9% w v−1 NaCl i.e. saline or DMSO) for the same time period. In some experiments, electrically stimulated guinea-pig ileum preparations were exposed to a mixture of NaHS (20 μM) and SNP (0.5 μM). In this case, drugs were added to the bath simultaneously.
Results show mean±s.e.mean with the number of observations indicated in parenthesis. Statistical analysis of differences between multiple sets of data was determined using ANOVA followed by post-hoc Dunnett's test. For comparison between two data sets unpaired Student's t-test was used. In both cases, a P value less than 0.05 was taken to indicate statistical significance. All drugs were purchased from Sigma-Aldrich Ltd. Stock solutions of indomethacin and glibenclamide were dissolved in DMSO. All other drugs were dissolved in saline. Solutions of NaHS and SNP were prepared fresh on the morning of each experiment and kept stoppered on ice.
Results
Effect of NaHS on gastrointestinal and urogenital smooth muscle in vitro
Addition of NaHS (10–2000 μM) to the bath produced dose-related inhibition of the spontaneous, pendular contractions of the isolated rabbit ileum preparation (Figure 1A). The EC50 for NaHS was 76.4±7.9 μM with an Emax of 70.0±6.5% (both n=5). NaHS (80 μM) exhibited no demonstrable effect on resting tone of the guinea-pig, but did reduce the contractile response of this tissue to exogenous ACh (88 nM; Figure 1B). The smooth muscle relaxant effect of NaHS (determined as inhibition of the ACh-induced contraction) in the guinea-pig ileum was time-dependent, with a decline in activity as the pre-incubation period was increased (Figure 1B).
Figure 1.
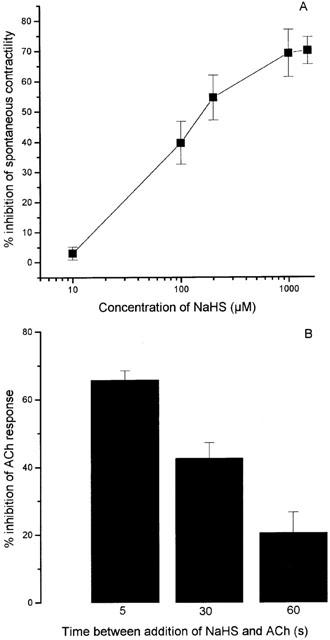
(A) Relaxant effect of NaHS on the rabbit isolated ileum. Results show per cent inhibition of spontaneous contractility and are mean±s.e.mean, n=5. (B) Time-dependent inhibition of the ACh-induced contraction of the guinea-pig isolated ileum to NaHS. ACh (88 nM, EC70 determined in preliminary experiments) was added to the organ bath at timed intervals (5–60 s) after prior application of NaHS (80 μM). Results show per cent reduction in ACh response and are mean±s.e.mean.
NaHS (1–1000 μM) also produced a rapidly developing (commencing within a few seconds) and dose-dependent inhibition of the response of the guinea-pig ileum to electrical field stimulation (Figure 2A). The twitch response was completely abolished at a concentration of 400 μM and the calculated EC50 was 62.6±4.7 μM (n=8). In some experiments, the effect of NaHS (80 μM) on the twitch response was monitored in the absence of drug washout in order to determine the longevity of the response. The inhibitory effect of NaHS was observed to be relatively short-lived reversing spontaneously within 150–180 s. For comparison, sodium nitroprusside (SNP) produced qualitatively similar results but was approximately 30 times more potent than NaHS (EC50, 2.1±0.08 μM, n=6) (Figure 2A). Interestingly, the combination of low concentrations of NaHS (20 μM) and SNP (0.5 μM) resulted in a degree of inhibition of the twitch response of the guinea-pig ileum which was greater than that expected from the simple additive effect of the two drugs (Figure 2B).
Figure 2.
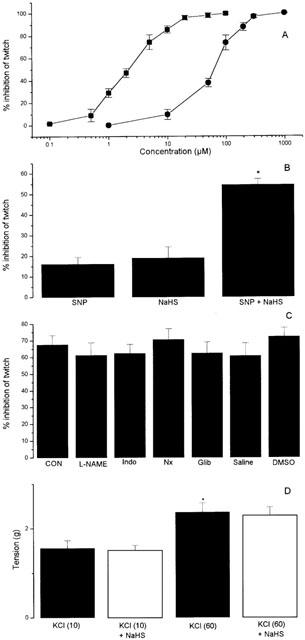
(A) Inhibition of the twitch response of the guinea-pig isolated ileum to electrical stimulation by NaHS and SNP. Results show peak relaxant effect and are mean±s.e.mean, n=8 (NaHS) and n=6 (SNP). (B) Effect of SNP (0.5 μM), NaHS (20 μM) alone and in combination (SNP+NaHS) on the response to electrical stimulation of the guinea-pig ileum. Results show per cent inhibition of twitch response and are mean±s.e.mean, n=6, *P<0.05 c.f. either SNP or NaHS (ANOVA plus post-hoc Dunnett's test). (C) Effect of NaHS (80 μM) on the response of the guinea-pig ileum to electrical stimulation in the presence of L-NAME (100 μM), indomethacin (Indo, 10 μM), naloxone (Nx, 1 μM), glibenclamide (Glib, 100 μM), saline (1 ml l−1 or DMSO (1 ml l−1). CON represents the response to NaHS prior to drug or vehicle administration. Inhibitors were left in contact with the tissue for 60 min before further testing. Results show per cent inhibition of twitch response and are mean±s.e.mean, n=4–8, (P>0.05). (D) Effect of a high concentration of NaHS (200 μM) on contractions of the guinea-pig ileum due to KCl at ‘low' concentration (10 mM) or ‘high' concentration (60 mM). The interval between administration of NaHS and KCl was 30 s. Results show tension developed (g) and are mean±s.e.mean, n=5, *P>0.05, c.f. KCl (10 mM).
A number of experiments were carried out in an attempt to determine the mechanism of action of NaHS in the guinea-pig ileum. Thus, the inhibitory effect of NaHS in the field-stimulated guinea-pig ileum was unaltered by preincubation of tissues for 60 min with L-NAME (100 μM), indomethacin (10 μM), naloxone (1 μM) or glibenclamide (100 μM) or the appropriate vehicle (saline or DMSO) (Figure 2C). In separate experiments, the effect of a concentration of NaHS (200 μM), which produced >90% inhibition of the response to electrical stimulation, was assessed on contractions of the guinea-pig ileum to ‘low' (10 mM) and ‘high' (60 mM) concentrations of KCl. Preincubation of tissues with NaHS for 30 s did not affect the responsiveness of the tissue to either concentration of KCl (Figure 2D).
NaHS (10–1000 μM) also produced dose-dependent inhibition of the contractile response of the field-stimulated rat vas deferens and rat ileum (Figure 3). The EC50 values for NaHS in these tissues were 108.2±11.2 μM (n=5) and 64.8±5.4 μM (n=8) respectively. Like the guinea-pig ileum, responses to NaHS were rapid in onset and spontaneously reversible (about 180 s) without washout. Unlike the guinea-pig ileum preparation, high concentrations of NaHS failed to bring about complete abolition of the response to field stimulation in these tissues (Emax values of 60.0±10.2% and 60.6±6.8% inhibition, n=5–8, respectively).
Figure 3.
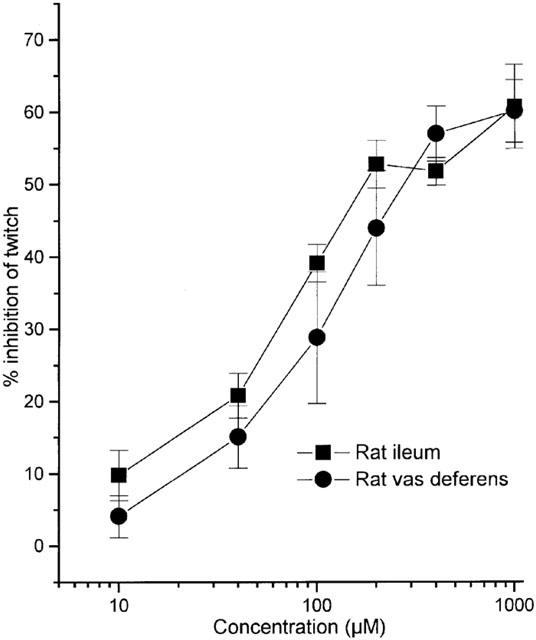
Relaxant effect of NaHS on the rat isolated ileum and vas deferens. Results show per cent inhibition of response to electrical stimulation at a frequency of either 0.1 Hz (vas deferens) or 5 Hz (rat ileum) and are mean±s.e.mean, n=5–7.
Effect of inhibitors of H2S synthesis on responses of the field-stimulated guinea-pig ileum
Preincubation of field-stimulated guinea-pig ileum preparations with propargylglycine (PAG; 1 mM) or β-cyanoalanine (BCA; 1 mM) led to a slowly developing increase in the twitch contraction (Figure 4). The enhanced response was first apparent following exposure to either drug for 15 min, and thereafter reached a plateau at 60 min, at which time the contraction to field stimulation was increased by 43.0±3.0%, (PAG, n=11) or 31.2±8.1%, (BCA, n=6). Exposure (60 min) of ileum preparations to BCA (1 mM) did not affect contractions due to exogenous ACh (inset to Figure 4). In addition, no significant increase in twitch response was observed in the 60 min period after injection into the bath of either saline (0.1 ml) or amino-oxyacetic acid (AOAA, 1 mM) (Figure 4).
Figure 4.
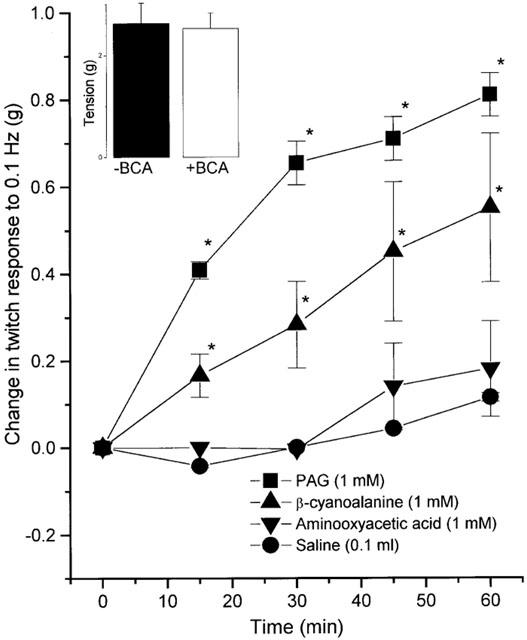
Effect of BCA and PAG (both 1 mM, inhibitors of cystathione-γ-lyase, CES), aminooxyacetic acid (1 mM, inhibitor of cystathionine-β-synthetase, CBS) and vehicle (saline, 0.1 ml) on the twitch response of the isolated guinea-pig ileum to electrical stimulation. Results show per cent change in response to electrical stimulation (0.1 Hz) with time and are mean±s.e.mean, n=6 (BCA) and n=11 (PAG), *P<0.05 (c.f. prior to drug treatment, ANOVA plus post-hoc Dunnett's test). Inset shows the effect of pretreatment (60 min) of the guinea-pig ileum with BCA (1 mM;+BCA) or saline (0.1 ml; −BCA) on the response to ACh (88 nM, EC70). Results show tension developed (g) and are mean±s.e.mean, n=5–11, P>0.05, Student's t-test).
In preliminary experiments, cysteine hydrochloride (1 mM) added to the organ bath resulted in a prompt (within 10 s) and complete inhibition of the twitch response of the guinea-pig isolated ileum to field stimulation. Subsequent investigation revealed that this effect was due to the highly acid pH (i.e. 1.5) of the stock drug solution used and did not occur when pH-neutral cysteine base (1 mM) was substituted.
In subsequent work the ability of cysteine base to reverse the pro-contractile effect of PAG (1 mM) and β-CA (1 mM) in the field-stimulated guinea-pig ileum preparation was assessed. For these experiments, cysteine (1 mM) or saline (0.1 ml) was added to field-stimulated guinea-pig ileum preparations which had been pre-exposed (60 min) to PAG or β-CA and in which contractility was consequently increased by about 50%. Addition of cysteine (but not saline) in these circumstances resulted in a slowly developing decline in the size of the β-CA-augmented twitch response with complete reversal to control levels occurring within 60 min (Figure 5). It should be noted that cysteine did not affect responses of the electrically stimulated guinea-pig ileum in the absence of CSE inhibitor. Identical treatment of guinea-pig ileum preparations with either cysteine (1 mM) or saline did not significantly reverse the pro-contractile effect of PAG (1 mM) (Figure 5).
Figure 5.
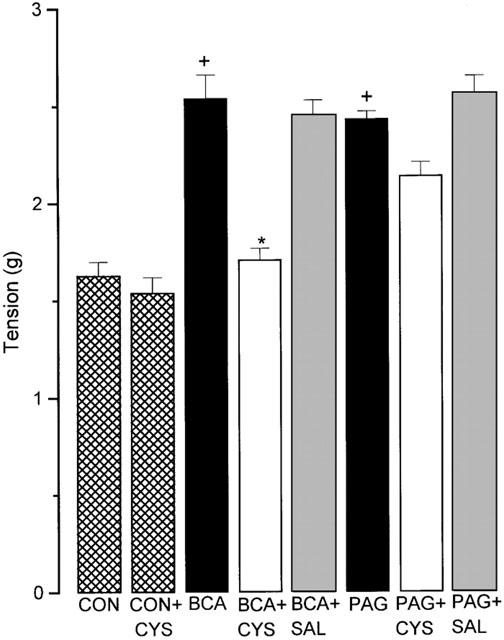
Effect of cysteine base (CYS, 1 mM); or saline (SAL; 0.1 ml) on the response of the isolated guinea-pig ileum to electrical stimulation before (hatched columns) and after exposure (60 min) to either BCA or PAG (both 1 mM). CON represents data from tissues prior to drug exposure. CON+CYS indicates contractions following 60 min incubation with cysteine. Results show tension developed (g) per twitch and are mean±s.e.mean, n=6, +P<0.05 c.f. CON, *P<0.05 c.f. BCA.
Discussion
Recent studies have revealed that NaHS relaxes precontracted rat aortic rings (Zhao et al., 2001) and uterine strips (Hayden et al., 1989; Sidhu et al., 2001). NaHS was used in these particular studies, and in the present experiments, since it dissociates into hydrosulphide anion (HS−) which then reacts with H+ to form H2S. As such, NaHS provides a useful experimental source of H2S.
We report here that NaHS causes dose-related inhibition of the response of the rat and guinea-pig ileum and the rat vas deferens to electrical stimulation, reduces the spontaneous contractility of the rabbit ileum and inhibits contractions of the guinea-pig ileum due to ACh. Importantly, the smooth muscle relaxant effect of exogenous NaHS in these various preparations occurs, for the most part, over a dose range which is similar to the H2S concentration found naturally in both rat (i.e. 50 μM; Zhao et al., 2001) and human (Richardson et al., 2000; 10–100 μM) blood. It should be noted that, at physiological pH, H2S in solution is approximately 30% intact with the remaining 70% present as HS− anion (US National Research Council, 1979). Whether HS− anions contribute to the pharmacological effects of NaHS observed in this, or other studies, is not known. However, assuming that H2S is the pharmacologically active moiety it is conceivable that the present experiments may underestimate the potency of NaHS in molar terms.
The ability of NaHS to inhibit the response of the ileum to both electrical stimulation and to exogenous ACh is relatively short-lived. It is possible that this reflects either an inherent instability of H2S in the experimental conditions employed, or its escape from the organ bath by diffusion, or rapid enzymatic catabolism of H2S. In the latter case, both thiol S-methyltransferase and rhodanese occur in human small intestine (Picton et al., 2002) and have been reported to break down H2S (Weisiger et al., 1980).
An additional point of interest in the present study is that NaHS mimics the effect of SNP (albeit with reduced potency) in producing spontaneously reversible and dose-related inhibition of contractions of the guinea-pig ileum to field stimulation. The combination of low doses of NaHS and SNP resulted in a disproportionately greater inhibition of the response to electrical stimulation than might be expected from addition of either drug alone. A synergistic vasorelaxant effect of NaHS and NO has also been noted in isolated rat aorta (Hosoki et al., 1997). This aspect of the effect of H2S on gastrointestinal smooth muscle requires further study.
The mechanism of the smooth muscle relaxant effect of H2S in the field-stimulated guinea-pig ileum has been examined in some detail. Pretreatment of ileum preparations with indomethacin, L-NAME or naloxone at doses used previously by other researchers to inhibit cyclo-oxygenase, nitric oxide synthase (NOS) enzyme activity or antagonize opioid receptors in isolated tissues failed to influence the effect of NaHS on contractions due to electrical stimulation. Thus, it may be concluded that the release of prostanoids, NO or endogenous opioids does not contribute to the inhibitory effect of NaHS in the field-stimulated guinea-pig ileum.
In a recent study, Zhao et al. (2001) reported that glibenclamide antagonized the relaxant effect of NaHS in the phenylephrine-precontracted rat aorta thereby concluding that H2S opens KATP channels in vascular smooth muscle cells to bring about smooth muscle relaxation. A similar mechanism may therefore underlie the relaxant effect of H2S in nonvascular smooth muscle. Indeed, cromakalim has previously been shown to relax guinea-pig ileum in a glibenclamide-sensitive manner (Sun & Benishin, 1994) indicating the presence of operational KATP channels in this preparation. However, in the present experiments, glibenclamide did not affect the response of the electrically stimulated ileum to NaHS suggesting that KATP channels are most probably not involved. To investigate this possibility in more detail, additional experiments were carried out in which the relaxant effect of NaHS was assessed on contractions of the guinea-pig ileum to both low (10 mM) and high (60 mM) concentrations of KCl. Activation of smooth muscle KATP channels would be expected to result in an inhibition of the response to low (but not high) concentrations of KCl whereas inhibition of L-type Ca2+ channels would be expected to reduce the response to both concentrations of KCl. NaHS, at a concentration (200 μM), which caused near maximal inhibition of the response to electrical stimulation in this preparation, failed to affect the contractile response to KCl at either concentration. Again, in an analogous experiment, Zhao et al. (2001) noted that NaHS preferentially inhibited contractions of the rat aorta to a ‘low' concentration of KCl (in this case, 20 mM) with very much less effect on the response to ‘high' KCl (in this case, 100 mM).
Accordingly, we consider it unlikely that activation of KATP channels (or indeed inhibition of L-type Ca2+ channels) accounts for the relaxant effect of NaHS in the guinea-pig ileum. It is clear that H2S relaxes guinea-pig ileum (this study) and rat aorta (Zhao et al., 2001) by different mechanisms. The precise nature of these mechanisms and the manner by which H2S and NO interact at the cellular level remains to be clarified.
In addition to characterizing the effect of exogenous H2S on the response of several nonvascular smooth muscle preparations, the present study also provides evidence for endogenous production of this mediator by the electrically stimulated guinea-pig ileum. To the best of our knowledge this is the first demonstration of a biological effect of naturally produced H2S and, as such, the results on which this conclusion is based warrant very careful scrutiny.
The profile of biological activity of the CSE and CBS inhibitors used in this study is of particular interest. Of the inhibitors employed, PAG has been most studied. This compound causes an irreversible, mechanism-based inhibition of CSE enzyme activity in vitro (Johnston et al., 1979) and, when administered to rats, produces an almost complete inhibition of liver CSE enzyme activity (measured ex vivo) (Porter et al., 1996; Uren et al., 1978) as well as elevated brain cystathionine concentration (Yu et al., 2000). Despite assertions to the contrary (e.g. Hosoki et al., 1997) it would therefore appear that PAG is well absorbed and readily crosses biological membranes (see also review by Reed, 1995). Of the other compounds used in the present experiments, AOAA is a potent and reversible inhibitor of CBS (Braunstein et al., 1971) but, in addition, also inhibits other pyridoxal phosphate-dependent enzymes notably GABA transaminase (Loscher, 1981) and glutamic acid decarboxylase (Hamel et al., 1982). Less is known of the pharmacology of β-CA although this compound has been reported to cause potent and reversible inhibition of CSE activity (Uren et al., 1978; Pfeffer & Ressler, 1967).
Of the drugs tested, the CSE inhibitors, PAG and β-CA, but not the CBS inhibitor, AOAA, produced a slowly-developing increase in contractile response of the ileum to field stimulation. Bearing in mind the relative lack of information concerning the pharmacology of these inhibitors (see above) the possibility that one or more of these compounds may affect the guinea-pig ileum by mechanism(s) which are unrelated to inhibition of CSE or CBS (and thus of H2S) cannot be excluded. However, the finding that BCA exposure does not influence responses of the ileum to applied ACh excludes, for example, a non-selective post-junctional effect of this compound to augment smooth muscle responsiveness as well as an anti-cholinesterase action.
Perhaps more convincingly, cysteine (precursor for H2S biosynthesis by CSE) completely reversed the BCA-mediated but failed to affect the PAG-mediated increase in contractile response of the ileum to electrical stimulation. The difference in susceptibility of the two inhibitors with respect to cysteine reversal may reflect the manner in which they interact with CSE i.e. competitive (with substrate) for BCA, irreversible for PAG. In separate experiments, cysteine (at the same concentration and over the same time course) did not affect the contractility of the ileum to electrical stimulation in the absence of H2S biosynthesis.
Based on these various experimental observations, we therefore propose the following working hypothesis: (1) electrical stimulation of the guinea-pig ileum triggers the formation of H2S by CSE (but not CBS); and (2) the H2S which is formed, possibly acting in concert with NO, acts on ileal smooth muscle cells to cause relaxation by an, as yet, unidentified mechanism.
Clearly, a number of questions remain to be resolved. For example, information about the precise cellular site of H2S biosynthesis in the ileum (e.g. smooth muscle cells, nerves) is lacking. That BCA does not affect the response of the ileum to exogenous ACh suggests that CSE activity is a direct consequence of electrical activity in intramural nerves in this tissue rather than a result of smooth muscle contraction per se. This would imply a neuronal localization of CSE. In this context, (Eto et al., 2002) have recently reported that electrical stimulation of mouse cerebral cortical slices also triggers H2S formation suggesting that H2S is formed within neurones. In this case, the particular enzyme involved is likely to be CBS and not CSE. It is also not clear whether analogous release of H2S occurs in vivo and, if it does, to what extent (if any) does H2S contribute to the regulation of gastrointestinal contractility in the whole animal?
Whilst the present data go some way towards characterizing the pharmacological effects of H2S, further research will clearly be required to determine the precise physiological significance (if any) of this novel, gaseous mediator. Nevertheless, the present data suggest a hitherto unappreciated role for, most probably neuronally-derived H2S, in the regulation of autonomic transmission in the guinea-pig ileum.
Abbreviations
- BCA
β-cyanoalanine
- CBS
cystathionine-β-synthetase
- CO
carbon monoxide
- CSE
cystathionine-γ-lyase
- H2S
hydrogen sulphide
- NaHS
sodium hydrogen sulphide
- NO
nitric oxide
- PAG
propargylglycine
References
- ABE K., KIMURA H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUNSTEIN A.E., GORYACHENKOVA E.V., TOLOSA E.A., WILLHARDT I.H., YEFREMOVA L.L. Specificity and some other properties of liver serine sulphhydrase: evidence for its identity with cystathionine-β-synthase. Biochim. Biophys. Acta. 1971;242:247–260. doi: 10.1016/0005-2744(71)90105-7. [DOI] [PubMed] [Google Scholar]
- COLOSO R.M., STIPANUK M.H. Metabolism of cysteine in rat enterocytes. J. Nutr. 1989;119:1914–1924. doi: 10.1093/jn/119.12.1914. [DOI] [PubMed] [Google Scholar]
- ETO K., OGASAWARA M., UMEMURA K., NAGAI Y., KIMURA H. Hydrogen sulphide is produced in response to neuronal excitation. J. Neurosci. 2002;22:3386–3391. doi: 10.1523/JNEUROSCI.22-09-03386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- FINKELSTEIN J.D., MARTIN J.J. Methione metabolism in mammals. Distribution of homocysteine between competing pathways. Biochem. Biophys. Res. Commun. 1984;118:14–19. [PubMed] [Google Scholar]
- FLORIN T.H. Hydrogen sulfide and total acid-volatile sulfides in faeces, determined with a direct spectrophotometric method. Clin. Chim. Acta. 1991;196:127–134. doi: 10.1016/0009-8981(91)90065-k. [DOI] [PubMed] [Google Scholar]
- GOFFREDI S.K., CHILDRESS J.J., DESAULNIERS N.T., LALLIER F.H. Sulfide acquisition by the vent worm Riftia Pachyptila appears to be via uptake of HS- rather than H2S. J. Exp. Biol. 1997;200:2609–2616. doi: 10.1242/jeb.200.20.2609. [DOI] [PubMed] [Google Scholar]
- GOSSELIN R.E., SMITH R.P., HODGE H.C. Clinical Toxicology of Commercial Products. Baltimore, USA: Williams and Wilkins Ltd; 1984. Hydrogen sulfide. [Google Scholar]
- GUIDOTTI T.L. Hydrogen sulphide. Occup. Med. 1996;46:367–371. doi: 10.1093/occmed/46.5.367. [DOI] [PubMed] [Google Scholar]
- HAMEL E., KRAUSE D.N., ROBERTS E. Characterization of glutamic acid decarboxylase activity in cerebral blood vessels. J. Neurochem. 1982;39:842–849. doi: 10.1111/j.1471-4159.1982.tb07969.x. [DOI] [PubMed] [Google Scholar]
- HARGROVE J.L. Persulfide generated from L-cysteine inactivates tyrosine aminotransferase. Requirement for a protein with cysteine oxidase activity and gamma cysthionase. J. Biol. Chem. 1988;263:17262–17269. [PubMed] [Google Scholar]
- HAYDEN L.J., FRANKLIN K.J., ROTH S.H., MOORE G.J. Inhibition of oxytocin-induced but not angiotensin-induced rat uterine contractions following exposure to sodium sulfide. Life Sci. 1989;45:2557–2560. doi: 10.1016/0024-3205(89)90239-7. [DOI] [PubMed] [Google Scholar]
- HOSOKI R., MATSUKI N., KIMURA H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- JOHNSTON M., JANKOWSKI D., MARCOTTE P., TANAKA H., ESAKI N., SODA K., WALSH C. Suicide inactivation of bacterial cystathionine gamma synthase and methionine gamma lyase during processing of L-propargylglycine. Biochemistry. 1979;18:4690–4701. doi: 10.1021/bi00588a033. [DOI] [PubMed] [Google Scholar]
- KOMBIAN S.B., REIFFENSTEIN R.J., COLMERS W.F. The actions of hydrogen sulphide on dorsal raphe serotonergic neurons in vitro. J. Neurophysiol. 1993;70:81–96. doi: 10.1152/jn.1993.70.1.81. [DOI] [PubMed] [Google Scholar]
- KRAFT A.A., BRANT A.W., AYRES J.C. Detection of hydrogen sulphide in packaged meats and in broken-out shell eggs. Food Technology. 1956;10:443–444. [Google Scholar]
- LOSCHER W. Effect of inhibitors of GABA aminotransferase on the metabolism of GABA in brain tissue and synaptosomal fractions. J. Neurochem. 1981;36:1521–1527. doi: 10.1111/j.1471-4159.1981.tb00595.x. [DOI] [PubMed] [Google Scholar]
- MOORE P.K., AL-SWAYEH O.A., CHONG N.W.S., EVANS R.A., GIBSON A. L-NG-nitro arginine (L-NOARG)- a novel, L-arginine reversible inhibitor of endothelium-dependent vasodilatation. Br. J. Pharmacol. 1990;99:408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAVARRA P., DELLO RUSSO C., MANCUSO C., PREZIOSI P., GROSSMAN A. Gaseous neurotransmitters in the control of the neuroendocrine stress axis. Ann. N.Y. Acad. Sci. 2000;917:638–646. doi: 10.1111/j.1749-6632.2000.tb05429.x. [DOI] [PubMed] [Google Scholar]
- NICHOLSON R.A., RITH S.H., ZHANG A., BROCKES J., SKRAJNY B., BENNINGTON R. Inhibition of respiratory and bioenergetic mechanisms by hydrogen sulphide in mammalian brain. J. Toxicol. Environ. Health. 1998;54:491–507. doi: 10.1080/009841098158773. [DOI] [PubMed] [Google Scholar]
- PFEFFER M., RESSLER C. Beta-cyanoalanine, an inhibitor of rat liver cystathionase. Biochem. Pharmacol. 1967;242:2299–2308. doi: 10.1016/0006-2952(67)90217-1. [DOI] [PubMed] [Google Scholar]
- PICTON R., EGGO M.C., MERRILL G.A., LANGMAN M.J.S., SINGH S. Mucosal protection against sulphide: importance of the enzyme rhodanese. Gut. 2002;50:201–205. doi: 10.1136/gut.50.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITCHER M.C.L., BEATTY E.R., CUMMINGS J.H. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000;46:64–72. doi: 10.1136/gut.46.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER D.W., NEALLEY E.W., BASKIN S.L. In vivo detoxification of cyanide by cystathionase gamma-lyase. Biochem. Pharmacol. 1996;27:941–944. doi: 10.1016/0006-2952(96)00466-2. [DOI] [PubMed] [Google Scholar]
- REED D.J. Cystathionine. Meth. In Enzymol. 1995;252:92–102. doi: 10.1016/0076-6879(95)52012-0. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C.J., MAGEE E.A., CUMMINGS J.H. A new method for the determination of sulphide in gastrointestinal contents and whole blood by microdistillation and ion chromatography. Clin. Chim. Acta. 2000;293:115–125. doi: 10.1016/s0009-8981(99)00245-4. [DOI] [PubMed] [Google Scholar]
- RIZZI D., BIGONI R., RIZZI A., JENCK F., WICHMANN J., GUERRINI R., REGOLI D., CALO G. Effects of Ro 64-6198 in nociceptin/orphanin FQ-sensitive isolated tissues. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363:551–555. doi: 10.1007/s002100100399. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-MARTINEZ M.A., GARCIA-COHEN E.C., BAENA A.B., GONZALEZ R., SALAICES M., MARIN J. Contractile responses elicited by hydrogen peroxide in aorta from normotensive and hypertensive rats. Endothelial modulation and mechanisms involved. Br. J. Pharmacol. 1998;125:1329–1335. doi: 10.1038/sj.bjp.0702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIDHU R., SINGH M., SAMIR G., CARSON R.J. L-cysteine and sodium hydrosulphide inhibit spontaneous contractility of isolated pregnant rat uterine strips in vitro. Pharmacol. Toxicol. 2001;88:198–203. doi: 10.1034/j.1600-0773.2001.d01-104.x. [DOI] [PubMed] [Google Scholar]
- STIPANUK M.H., BECK P.W. Characterisation of the enzymatic capacity for cysteine desulphydration in liver and kidney of the rat. Biochem. J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STIPANUK M.H., DE LA ROSA J., HIRSCHBERGER L.L. Catabolism of cysteine by rat renal cortical tubules. J. Nutr. 1990;120:450–458. doi: 10.1093/jn/120.5.450. [DOI] [PubMed] [Google Scholar]
- SUAREZ F.L., SPRINGFIELD J., LEVITT M.D. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut. 1998;43:100–104. doi: 10.1136/gut.43.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN Y.D., BENISHIN C.G. K+ channel openers relax longitudinal muscle of guinea pig ileum. Eur. J. Pharmacol. 1994;271:453–459. doi: 10.1016/0014-2999(94)90806-0. [DOI] [PubMed] [Google Scholar]
- UREN J.R., RAGIN R., CHAYKOVSKY M. Modulation of cysteine metabolism in mice–effects of propargylglycine and L-cysteine-degrading enzymes. Biochem. Pharmacol. 1978;27:2807–2814. doi: 10.1016/0006-2952(78)90194-6. [DOI] [PubMed] [Google Scholar]
- US NATIONAL RESEARCH COUNCIL . Subcommittee on hydrogen sulphide. Baltimore, USA: University Park Press; 1979. [Google Scholar]
- WARENYCIA M.W., STEELE J.A., KARPINSKI E., REIFFENSTEIN R.J. Hydrogen sulfide in combination with taurine or cysteic acid reversibly abolishes sodium currents in neuroblastoma cells. Neurotoxicology. 1989;10:191–199. [PubMed] [Google Scholar]
- WEISIGER R.A., PINKUS L.M., JAKOBY W.B. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem. Pharmacol. 1980;29:2885–2887. doi: 10.1016/0006-2952(80)90029-5. [DOI] [PubMed] [Google Scholar]
- WORLD HEALTH ORGANIZATION . Air Quality Guidelines for Europe. Copenhagen: WHO Regional Office for Europe; 1987. [Google Scholar]
- YU S., SUGAHARA K., NAKAYAMA K., AWATA S., KODAMA H. Accumulation of cystathionine, cystathionine ketimine, and pehydro-1,4-thiazepine-3,5-dicarboxylic acid in whole brain and various regions of the brain of D,L-propylargylglycine-treated rats. Metabolism. 2000;49:1025–1029. doi: 10.1053/meta.2000.7705. [DOI] [PubMed] [Google Scholar]
- ZHAO W., ZHANG J., LU Y., WANG R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]


