Abstract
In guinea-pig sino-atrial (SA) node cells the delayed rectifier K+ current (IK) is composed of rapidly and slowly activating components of IK (IKr and IKs, respectively). The present study was undertaken to characterize the blocking action of the chromanol derivative 293B on IKs in guinea-pig SA node cells using whole-cell patch-clamp technique.
Bath application of 293B blocked IKs, elicited by 4-s depolarizing voltage pulses from a holding potential of −50 mV, under conditions in which the L-type Ca2+ current (ICa,L) and IKr were inhibited; the effect was concentration-dependent with an IC50 of 5.3 μM, when evaluated by the decrease in the amplitude of IKs tail current following 4-s depolarizing voltage steps to +50 mV.
The 293B block of IKs progressed with time during depolarizing voltage steps with a more rapid block at higher concentrations.
The block of IKs by 293B was fully reversed within a few minutes after washing off the drug, even when a maximal effect (a nearly full block) was achieved at high drug concentration (50 μM).
Bath application of 293B at 50 μM greatly and reversibly reduced the amplitude of IKs which is maximally stimulated by β-adrenergic agonist isoprenaline (1 μM), while the degree of 293B block of the isoprenaline-stimulated IKs was slightly but significantly smaller than that of non-stimulated IKs (94.0±0.98% block, n=6 vs 99.4±0.45% block, n=6; P<0.01).
We conclude that, in guinea-pig SA node cells (i) 293B is a potent and fully reversible blocker of IKs in control and during β-adrenergic stimulation and (ii) block with 293B occurs in a time-dependent manner during depolarizing voltage steps.
Keywords: Delayed rectifier K+ current, IKs, chromanol 293B, isoprenaline, sino-atrial node, time-dependent block
Introduction
The delayed rectifier K+ current (IK) is gradually activated during the plateau phase of action potentials and provides an outward current which facilitates phase 3 repolarization in various cardiac cell types, including sino-atrial (SA) node, atrial and ventricular cells. In addition, a deactivation of IK during membrane repolarization in pacemaker SA node cells is considered to be involved in the onset of a net inward current at the maximum diastolic potential (MDP) that precedes the slow diastolic depolarization (for a review see Irisawa et al., 1993). It has been demonstrated in many cardiac cells that IK can be separated into two distinct components on the basis of the differential sensitivity to block by methanesulphonanilide class III antiarrhythmic drugs such as E-4031, d-sotalol and dofetilide (Sanguinetti & Jurkiewicz, 1990; 1991; Carmeliet, 1992; Jurkiewicz & Sanguinetti, 1993; Wang et al., 1994; Liu & Antzelevitch, 1995; Li et al., 1996; Gintant, 1996); the drug-sensitive current activates rapidly and exhibits a marked inward rectification (designated as IKr) whereas the drug-resistant current displays slower activation kinetics and a minimal rectification (designated as IKs). Subsequent molecular biological studies have identified the distinct proteins underlying IKr and IKs channels: the potassium channel protein KVLQT1 associates with minK (IsK) protein to produce IKs channels (Barhanin et al., 1996; Sanguinetti et al., 1996), while human ether-á-go-go related gene (HERG) protein forms the pore-forming subunit of IKr channels (Curran et al., 1995; Sanguinetti et al., 1995; Trudeau et al., 1995).
The chromanol derivative 293B was found to be a potent blocker not only for IKs in guinea-pig ventricular cells but also for the membrane current through the IsK proteins heterologously expressed in Xenopus oocytes (Busch et al., 1996; Suessbrich et al., 1996). Subsequent studies have confirmed the highly selective blocking action of 293B on IKs in many types of isolated cardiac cells (Bosch et al., 1998; Fujisawa et al., 2000; Ono et al., 2000; Lei et al., 2002). As for molecular basis for 293B action on IKs channels, it has been suggested that KvLQT1 protein represents a relevant target for the actions of 293B (Loussouarn et al., 1997) while minK protein also acts allosterically to facilitate 293B binding to KvLQT1 protein (Busch et al., 1997; Lerche et al., 2000).
In the present study we characterized the blocking action of 293B on IKs under control conditions and during exposure to β-adrenergic agonist isoprenaline in isolated guinea-pig SA node cells using the whole-cell patch-clamp technique. The results demonstrate that 293B is a potent and fully reversible blocker of IKs in the absence and presence of isoprenaline and that 293B preferentially affects IKs channels in an open state.
Methods
Isolation of SA node cells
Single SA node cells were obtained from guinea-pig hearts using an enzymatic dissociation procedure similar to that previously described by Guo et al. (1997). In brief, guinea-pigs (250–400 g body weight) were deeply anaesthetized by an intraperitoneal injection of sodium pentobarbitone (80 mg kg−1), and then were ventilated through a tracheotomy. The chest cavity was opened and the ascending aorta was exposed and cannulated in situ to start coronary perfusion of the heart. The heart was then excised and retrogradely perfused through the aortic cannula on a Langendorff perfusion apparatus at 37°C, initially for 4 min with normal Tyrode solution, and then for 4 min with nominally Ca2+-free Tyrode solution, and finally for 6–10 min with nominally Ca2+-free Tyrode solution containing 0.4 mg ml−1 collagenase (Wako Pure Chemical Industries, Osaka, Japan). All the above solutions were oxygenated with 100% O2. The digested heart was then removed from the Langendorff perfusion apparatus and the SA node region was dissected out and was cut perpendicular to the crista terminalis into small strips measuring about 0.5 mm in width. These SA node tissue strips were further incubated for 16–20 min at 37°C in nominally Ca2+-free Tyrode solution containing 1.0 mg ml−1 collagenase and 0.1 mg ml−1 elastase (Roche, Mannheim, Germany) while being agitated in a shaking water bath. Finally, the enzyme-digested SA node strips were mechanically dissociated in a high-K+, low-Cl− Kraftbrühe (KB) solution (Isenberg & Klöckner, 1982). Isolated cells thus obtained were then stored at 4°C in KB solution until required for experimental use.
All these experimental procedures were reviewed and approved by the Shiga University of Medical Science Animal Care Committee, Japan.
Voltage-clamp technique
Isolated SA node cells, which were typically spindle-shaped and were spontaneously beating when superfused with normal Tyrode solution, were voltage-clamped using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) with a patch-clamp amplifier (CEZ-2400, Nihon Kohden, Tokyo, Japan). Patch pipettes were made from glass capillaries (o.d., 1.5 mm; i.d., 0.9 mm; Narishige Scientific Instrument Laboratory, Tokyo, Japan) using a horizontal microelectrode puller (P-97; Sutter Instrument Co., Novato, CA, U.S.A.), and the tips were then fire-polished using a microforge. Patch pipettes had a resistance of 2.0–3.0 MΩ when filled with the control pipette solution. An aliquot of the KB solution containing dissociated cells was transferred to a recording chamber (0.5 ml in volume) mounted on the stage of an inverted Nikon Diaphot microscope (Tokyo, Japan). The chamber was maintained at 34–36°C and continuously perfused at a rate of about 2 ml min−1 with normal Tyrode solution. A tight seal (resistance, 5 to 50 GΩ) was formed between the electrode tip and the cell membrane by gentle suction (−20 to −40 cm H2O). The patch membrane was then ruptured by a brief period of more vigorous suction, controlled manually with a 2.5-ml syringe.
IKs was activated by depolarizing voltage steps to various test potentials, under conditions where the Na+ current (INa) was inactivated by setting the holding potential to −50 mV and the L-type Ca2+ current (ICa,L) and IKr were blocked by adding 0.4 μM nisoldipine and 5 μM E-4031 to normal Tyrode solution, respectively. Nisoldipine was shown to have no effect on cardiac IK at this concentration (Sanguinetti & Jurkiewicz, 1991). Under these experimental conditions IKs was monitored by measuring the amplitudes of outward tail currents elicited on repolarization to a holding potential of −50 mV following a 4 s depolarizing step to +30 mV every 15 or 30 s and was found to reach a steady level (about 60–80% of the initial value) within 5–7 min of patch rupture. Accordingly, IKs was measured after this stable baseline current level had been established.
The current and voltage signals as well as trigger pulses were stored on digital audiotape (DM120, Hitachi Maxell, Tokyo, Japan) using a PCM data recorder (RD-120TE, TEAC, Tokyo, Japan). The current and voltage records were fed to a computer (PC98RL, NEC, Tokyo, Japan) every 0.2–1 ms through a low-pass 3 kHz filter (48 dB per octave, E-3201A, NF, Tokyo, Japan) and then were analysed using in-house programmes.
In all current traces demonstrated in Figures 1, 2, 5 and 6 the zero-current level is indicated to the left of the traces by a horizontal line.
Figure 1.
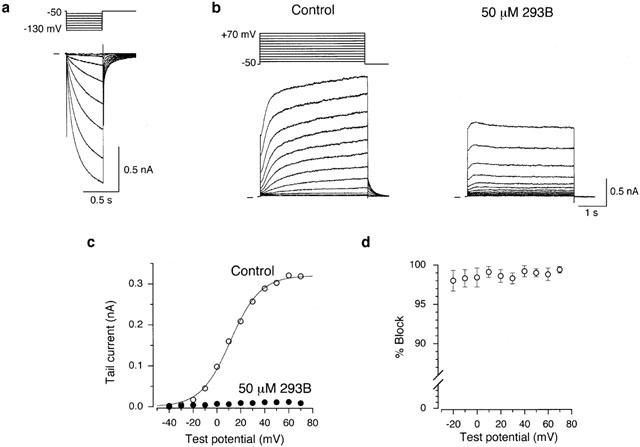
Block of IKs by 293B in an isolated guinea-pig SA node cell. (a) An SA node cell was hyperpolarized every 8 s from a holding potential of −50 mV to test potentials of −60 to −130 mV for 500 ms in 10 mV steps during superfusion with normal Tyrode solution. (b) A 4-s depolarizing test pulse was applied every 15 s from a holding potential of −50 mV to membrane potentials of −40 to +70 mV in 10 mV steps before (left-hand panel) and 3 min after exposure to 50 μM 293B (right-hand panel). These records were obtained from the cell shown in (a) but in the presence of 0.4 μM nisoldipine and 5 μM E-4031 to block ICa,L and IKr, respectively. A schematic diagram of the voltage-clamp protocol is given above the control traces. (c) I-V relationships for the tail currents measured upon return to a holding potential of −50 mV following 4-s depolarizing clamp pulses to various test potentials under control conditions and during exposure to 50 μM 293B, from the data shown in (b). A continuous curve through the control data represents the least-squares fit of a Boltzmann equation, yielding V1/2 of 11.4 mV and k of +12.6 mV. (d) Percentage block of IKs by 50 μM 293B measured in the tail currents elicited upon return to a holding potential of −50 mV following a 4 s depolarizing voltage step to various test potentials between −20 and +70 mV (n=6). IKs tail current at a test potential of −30 and −40 mV were too small (⩽10 pA) to accurately measure the reduction by 50 μM 293B.
Figure 2.
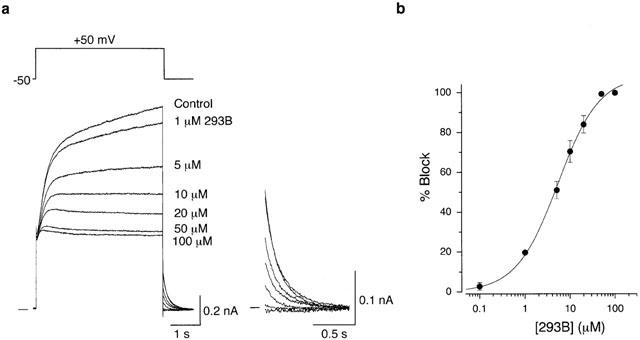
Concentration-dependent block of IKs by 293B. (a) Superimposed current traces in response to a 4-s depolarizing voltage step to a test potential of +50 mV before (Control) and during exposure to 293B at a concentration of 1, 5, 10, 20, 50 and 100 μM in a same cell. The cell was exposed to each concentration of 293B for 2–3 min. All tail currents were illustrated on an expanded scale (inset). (b) Concentration-response relationship for the block of IKs tail current by 293B. Percentage block represents the fraction of IKs tail current at −50 mV reduced by 293B at each concentration with reference to the control amplitude of the tail current. The continuous curve was drawn by a least-squares fit of a Hill equation, yielding Imax of 100%, IC50 of 5.3 μM, and nH of 0.94 (see text). Data points represent mean±s.e.mean of six different cells.
Figure 5.
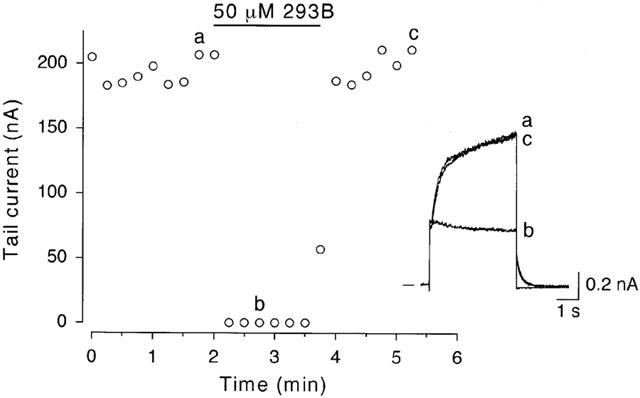
Reversibility of 293B block of IKs. An SA node cell was repetitively depolarized every 30 s from a holding potential of −50 mV to +50 mV for 4 s. The amplitude of tail current elicited upon return to the holding potential was plotted as a function of time for the entire experiment. 293B at a concentration of 50 μM was added to the bath during the period indicated by the horizontal bar. The inset illustrates examples of the original current traces recorded at the time points indicated on the graph.
Figure 6.
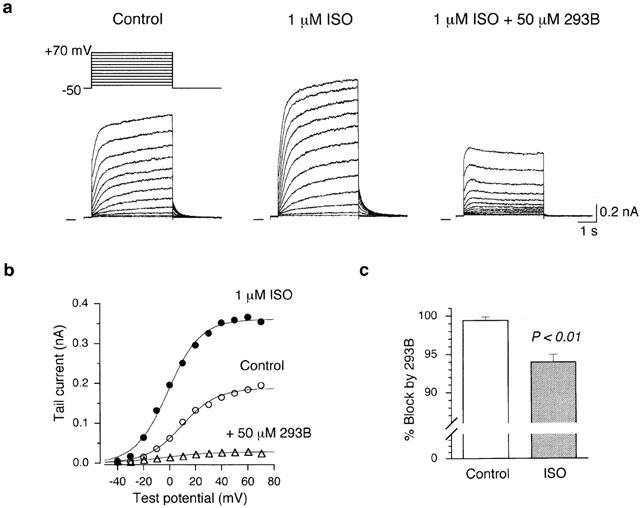
Blockade of isoprenaline-stimulated IKs by 293B. (a) Superimposed current traces during 4-s voltage-clamp pulses to test potentials of −40 to +70 mV in 10 mV steps applied from a holding potential of −50 mV, under control conditions (left-hand panel), 4 min after exposure to 1 μM isoprenaline (middle panel), and 3 min after further addition of 50 μM 293B in the presence of isoprenaline (right-hand panel). A schematic diagram of voltage-clamp protocol is shown above the control traces. (b) I-V relationships for IKs tail currents obtained from the records in (a), under control conditions, during application of isoprenaline, and after addition of 293B. The least-squares fit of the data points to a Boltzmann equation provides V1/2 and k (see text). Control: V1/2=9.3 mV, k=13.5 mV; Isoprenaline: V1/2=−1.3 mV, k=12.5 mV; Isoprenaline+293B: V1/2=−3.6 mV, k=18.1 mV. Since the amplitude of IKs tail current in the presence of isoprenaline+293B was relatively large (∼30 pA at a test potential of +70 mV), these data points were fitted to a Boltzmann equation. (c) Percentage block of IKs, measured upon return to the −50 mV holding potential following 4-s depolarization to +70 mV, under control conditions and in the presence of 1 μM isoprenaline. The columns and bars represent the mean and s.e.mean, respectively. A significant difference (P<0.01; Student's unpaired t-test) can be detected between these two groups (Control, 99.4±0.45% block, n=6; Isoprenaline, 94.0±0.98% block, n=6).
Solutions and drugs
Normal Tyrode solution contained (in mM): NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 0.5, NaH2PO4 0.33, glucose 5.5, and N - (2 - hydroxyethyl)piperazine -N′ - (2-ethanesulphonic acid) (HEPES) 5.0 (pH adjusted to 7.4 with NaOH). The nominally Ca2+-free Tyrode solution used for the cell isolation procedure was prepared by simply omitting CaCl2 from the normal Tyrode solution. Normal Tyrode solution supplemented with 0.4 μM nisoldipine (a generous gift from Bayer, Germany) and 5 μM E-4031 (N-(4-((1-(2-(6-methyl-2-pyridinyl)ethyl)-4-piperidinyl)carbonyl)phenyl)methanesulphonamide dihydrochloride dihydrate; Wako Pure Chemical Industries, Osaka, Japan) was used as the standard external solution for measuring whole-cell IKs. Nisoldipine was prepared as a 1-mM stock solution in ethanol and then was added to normal Tyrode solution to achieve a final concentration of 0.4 μM. E-4031 was dissolved in distilled water to make a 1-mM stock solution and then was diluted in normal Tyrode solution to give a concentration of 5 μM. Isoprenaline ((−)-isoproterenol, Sigma Chemical Co., MO, U.S.A) was dissolved in distilled water to make a 1-mM stock solution together with the same concentration of ascorbic acid and then was diluted in the external solution to achieve a concentration of 1 μM. The chromanol derivative 293B (trans-6-cyano-4-(N-ethylsulphonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane; a generous gift from Aventis Pharma Deutschland GmbH, Frankfurt, Germany) was dissolved in dimethyl sulphoxide (DMSO, Sigma) to make a 100 mM stock solution and was diluted at a final concentration of 0.1–100 μM in the external solution (DMSO concentrations ⩽0.1%). In preliminary experiments, we confirmed that DMSO alone did not have any appreciable effect on IKs at concentrations of up to 0.5%. The control pipette solution consisted of (in mM): potassium aspartate 70, KCl 50, KH2PO4 10, MgSo4 1, Na2-ATP 3, Li2-GTP 0.1, ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) 5, CaCl2 2, HEPES 5 (pH adjusted to 7.2 with KOH). The concentration of free Ca2+ in the pipette solution was calculated to be approximately 10−7 M (Fabiato & Fabiato, 1979; Tsien & Rink, 1980). The Ca2+-free pipette solution, which was made by omitting CaCl2 from the control pipette solution, was used in some experiments. The free Ca2+ in this Ca2+-free pipette solution was estimated to be approximately 10−10 M (Fabiato & Fabiato, 1979; Tsien & Rink, 1980). The KB solution for cell preservation contained (in mM): potassium glutamate 70, KCl 30, KH2PO4 10, MgCl2 1, taurine 20, EGTA 0.3, glucose 10, and HEPES 10 (pH adjusted to 7.2 with KOH).
Data analysis and statistics
Group data are presented as the mean±s.e.mean, and n indicates the number of cells studied. A statistical comparison was made using Student's t-test for paired or unpaired data where appropriate, and differences were considered to be statistically significant for P⩽0.05.
Results
Blocking action of 293B on IKs
Spontaneously and regularly contracting cells when superfused with normal Tyrode solution were regarded as representing SA node cells and were used for all the electrophysiological experiments. These SA node cells were typically spindle-shaped with faint striations and were small in size (diameter, 5 to 8 μm; length, 60 to 80 μm) and only comprised less than 5% of the cells isolated from SA node region of guinea-pig heart (Guo et al., 1997; Matsuura et al., 2002). After establishing a whole-cell voltage-clamp mode, an isolated SA node cell was held at −50 mV to allow a complete deactivation of IKs and initially hyperpolarized to test potentials of −60 to −130 mV for 500 ms in 10 mV steps to confirm the presence of the hyperpolarization-activated cation current (If). As demonstrated in Figure 1a, a time-dependent inward current was activated during the hyperpolarizing voltage steps to potentials ⩽−70 mV and its amplitude, measured as the difference between the initial and late current levels during hyperpolarizing steps, was increased with increasing the magnitude of hyperpolarization. Based on these characteristic voltage-and time-dependent activation properties, this inward current can be identified as If. It should also be noted that an instantaneous current jump in the inward direction upon hyperpolarization was small in amplitude (Figure 1a), indicating that the inwardly rectifying K+ current (IK1) was either absent or, if present at all, very small in guinea-pig SA node cells, as has been described previously (Guo et al., 1997). These spontaneously active SA node cells showing an obvious If were then exposed to normal Tyrode solution supplemented with 0.4 μM nisoldipine and 5 μM E-4031 to isolate IKs from ICa,L and IKr during depolarizing voltage steps.
Figure 1b shows superimposed current traces in response to 4-s depolarizing pulses to test potentials of −40 to +70 mV in 10 mV steps applied from a holding potential of −50 mV under control conditions (left-hand panel) and 3 min after exposure to 50 μM 293B (right-hand panel). Under control conditions, the depolarizing pulses to potentials ⩾−20 mV elicited a slowly activating outward current which was followed by an outward tail current upon repolarization to the −50 mV holding potential (Figure 1b, left-hand panel). We have previously shown, using an envelope of tails test (Noble & Tsien, 1969), that the time-dependent increase in outward current during depolarizations primarily represents an activation of IKs while a decaying outward tail current elicited upon return to the holding potential arises from deactivation of IKs, when an isolated guinea-pig SA node cell was superfused with normal Tyrode solution supplemented with 0.4 μM nisoldipine and 5 μM E-4031 (Matsuura et al., 2002). The blocking action of 293B on IKs was therefore characterized in guinea-pig SA node cells using this external solution. Bath application of 50 μM 293B almost completely suppressed IKs tail current elicited upon return to the −50 mV holding potential following depolarizations to all test potentials, although a transient increase in the outward current was detected during an initial part of the depolarizing voltage steps to potentials⩾approximately+10 mV (Figure 1b, right-hand panel).
The inhibitory effect of 293B on IKs was evaluated by measuring the amplitude of the tail current elicited upon return to a holding potential of −50 mV (Figure 1c), which reflects the degree of IKs activation at the preceding depolarizing test potential. Under control conditions, the relationship between the amplitude of IKs tail current and the test potential was reasonably well described by a Boltzmann equation:
where IK,tail,max is the fitted maximal tail current amplitude, V1/2 is the voltage at which the activation is half-maximal, Vm is the test potential and k is the slope factor. In a total of six cells, values for V1/2 and k averaged 12.3±2.4 mV and 13.2±0.4 mV, respectively. The amplitude of IKs tail current in the presence of 50 μM 293B was mostly too small (⩽∼20 pA) to accurately measure these parameters by fitting to a Boltzmann equation (equation 1). Figure 1d represents the percentage block of IKs by 50 μM 293B at each test potential, obtained by normalizing the amplitude of IKs tail current blocked by 293B with reference to the control amplitude of IKs tail current (n=6). There was no statistically significant difference between any two of the data points, thus suggesting that the 293B block of IKs was largely independent of the membrane potential.
We then evaluated the blocking effect of 293B at various concentrations between 0.1 and 100 μM on IKs in an isolated SA node cell. IKs was evoked every 15 s by 4-s depolarizing voltage steps to +50 mV before and during exposure to increasing concentrations of 293B in a cumulative manner after the inhibition due to the previous concentration reached a steady state (Figure 2a). Extracellular 293B reduced a time-dependent outward current during a depolarizing voltage step as well as decaying outward tail current upon repolarization in a concentration-dependent manner. An almost full inhibition of the tail current was obtained by 293B at concentrations ⩾50 μM (inset in Figure 2a). Interestingly, the initial time course of IKs activation elicited by a depolarizing voltage step was little affected by 293B applied at a concentration of 1 μM, which suggests that 293B scarcely affected IKs channels at a holding potential of −50 mV. Figure 2b demonstrates the concentration-response relationship for the block of IKs tail current by 293B, obtained from six different cells. The amplitude of IKs tail current was measured upon return to a holding potential of −50 mV following depolarization to +50 mV in the absence and the presence of each concentration of 293B and percentage block by each concentration of 293B was determined as the fractional reduction in the amplitude of IKs tail current evoked by 293B. The mean data (obtained from six cells) were reasonably well fitted with a Hill equation:
where Imax is the maximum degree of block expressed as a percentage, IC50 is the concentration of 293B causing a half-maximal block, and nH is the Hill coefficient. The values for IC50 and nH are 5.3 μM and 0.94, respectively. These values were comparable to those for the 293B block of IKs in native cardiac cells (guinea-pig ventricular cells, IC50=2.1 μM, Busch et al., 1996; IC50=1.02 μM, Bosch et al., 1998; IC50=2.11–3.54 μM, nH=0.91–1.27, Fujisawa et al., 2000; porcine SA node cells, IC50=8.79 μM, nH=1.22, Ono et al., 2000; rabbit SA node cells, IC50=1.35 μM, Lei et al., 2002) or KvLQT1/minK current heterologously expressed in Xenopus oocytes (IC50=2.6 μM, Lerche et al., 2000) or mammalian cells (IC50=9.8 μM, nH=0.9, Loussouarn et al., 1997).
Time course of IKs block by 293B
As is evident in Figure 2a, the initial rate of developing outward current which primarily represents the activation of IKs (Matsuura et al., 2002) was little affected by lower concentrations (1 μM) of 293B, while a substantial decrease in the outward current was observed near the end of the depolarizing pulse at the same concentrations, showing the presence of a time-dependent block of IKs by 293B. To analyse the time course of block development during depolarization, the time-dependent outward currents in response to depolarizing steps to +50 mV recorded under control conditions and during exposure to 293B at a concentration of 1, 5, 10, 20, 50 and 100 μM were superimposed after digital subtraction of an initial outward current jump upon depolarizations (Figure 3a). The amplitude of the outward current jump was almost the same in each current trace (approximately 0.4 nA). The 293B-sensitive currents during depolarizing steps to +50 mV were obtained by digitally subtracting the current in the presence of various concentrations of 293B from the control trace and then were superimposed with the control trace (Figure 3b). The current ratios representing the 293B block were obtained by normalizing the current component blocked by 293B at each concentration with reference to the control current and then were plotted as a function of time during a depolarizing voltage step, which represents the time dependence for 293B block (Figure 3c). It is evident that the IKs block by 293B at all concentrations tested progressed with time during the depolarizing pulse. The time course of 293B block of IKs was approximated by fitting a single exponential function to the current ratios at an initial 1000 ms (Figure 3d) and the time constants (τ) of 1045, 291, 252, 201, 126 and 99 ms were obtained for the block by 293B at a concentration of 1, 5, 10, 20, 50 and 100 μM, respectively. 293B was thus found to block IKs in a time-dependent manner with a more rapid block at higher concentrations.
Figure 3.
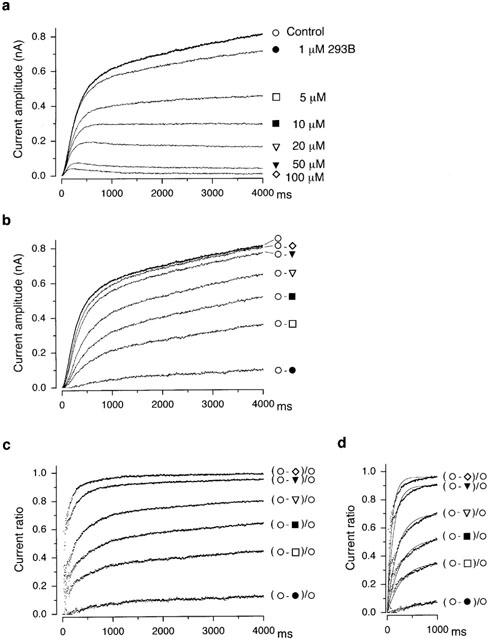
Time course of 293B block of IKs. (a) Time-dependent outward currents of IKs during 4-s depolarizing steps to +50 mV under control conditions and during exposure to 293B at a concentration of 1, 5, 10, 20, 50 and 100 μM, obtained from the data shown in Figure 2a. These currents were averages of 3–4 traces recorded under each condition. For demonstration purpose, these current traces were superimposed after subtraction of an initial outward current jump (approximately 0.4 nA) upon depolarizations. (b) Membrane current components blocked by 293B at a concentration of 1, 5, 10, 20, 50 and 100 μM, obtained by digital subtraction of the current trace in the presence of 293B at each concentration from the control current trace, as indicated. Control current trace was also superimposed after subtraction of an initial outward current jump (approximately 0.4 nA) upon depolarization. (c) Time-dependent block of IKs by 293B, represented by the ratio of the membrane current component blocked by 293B at each concentration to the control current, as indicated. (d) Single exponential fit (continuous line) to the current ratios representing the 293B block (shown in panel c) during the initial 1000 ms of depolarizing steps, yielding a time constant (τ) of 1045, 291, 252, 201, 126 and 99 ms for the block by 293B at a concentration of 1, 5, 10, 20, 50 and 100 μM, respectively.
It should be noted that a similar transient increase in outward current was observed during moderate or strong depolarizations in the presence of higher concentrations (⩾20 μM) of 293B in SA node cells dialyzed with Ca2+-free pipette solution (data not shown). It has been demonstrated in guinea-pig cardiac cells that an activity of Na+/Ca2+ exchange is dependent upon intracellular Ca2+ levels and that an outward Na+/Ca2+ exchange current is practically abolished in the absence of intracellular Ca2+ (Kimura et al., 1986). It is therefore reasonable to assume that a transient increase in outward current in the presence of 293B (Figures 1 and 2) is practically uncontaminated by the Na+/Ca2+ exchange current.
If the 293B block of IKs progresses with time during a depolarizing pulse, the block is expected to be potentiated with lengthening the pulse duration. The degree of 293B block of IKs following brief (400 ms) and long (4000 ms) depolarizing pulses was evaluated by measuring the fractional decrease in IKs tail current elicited upon repolarization to a holding potential of −50 mV in the presence of 293B. An isolated SA node cell was depolarized from a holding potential of −50 mV to +50 mV for 400 ms without and then with 293B at a concentration of 1, 10 or 100 μM and the percentage block was measured as the fractional decrease in the amplitude of IKs tail current. After allowing a nearly full recovery from the block by washing the 293B off (see Figure 5), the same cell was then depolarized to +50 mV for 4000 ms, first without and then with 293B, and the percentage block was again estimated as the differences in IKs tail currents in the absence and the presence of 293B. As demonstrated in Figure 4, the percentage block was significantly potentiated at each concentration of 293B with lengthening the pulse duration from 400 ms to 4000 ms, which is consistent with the observations showing the time-dependent block of IKs by 293B (Figures 2 and 3).
Figure 4.
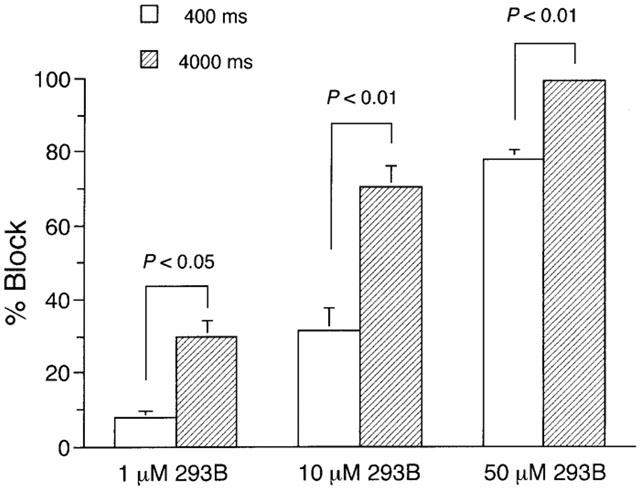
Dependence of IKs blockade by 293B upon pulse duration. An SA node cell was depolarized every 10 s from a holding potential of −50 mV to a test potential of +50 mV for 400 ms before and during exposure to 293B at a given concentration (1, 10 or 100 μM). The cell was usually exposed to 293B for 2–3 min, which evoked a steady-state block. After washing 293B off for more than 3 min, the same cell was then depolarized every 20 s to +50 mV for 4000 ms and was again exposed to 293B for more than 2 min to attain a steady-state block. Percentage block was measured as the fractional decrease in the amplitude of IKs tail current elicited upon return to a holding potential of −50 mV in the presence of 293B. The columns and bars represent the mean and s.e.m., respectively (n=6 at each group). There was a statistically significant difference between the two groups at each concentration of 293B (Student's paired t-test).
Reversible block of IKs by 293B
We next investigated whether the blocking effect of 293B on IKs was reversible by using a maximally effective concentration (50 μM). Figure 5 shows a representative example of the time course of changes in amplitude of IKs tail current elicited upon repolarization to −50 mV following 4-s depolarization to +50 mV during exposure to 50 μM 293B. IKs tail current was almost totally abolished by exposure to 293B within 30 s after the application. The blocking effect of 293B on IKs was fully reversed within approximately 1 min following superfusion with drug-free external solution. Such a complete recovery of IKs tail current after a washout of 50 μM 293B was observed in most of the cells examined (9/11). 293B was thus found to be a fully and rapidly reversible blocker of IKs in guinea-pig SA node cells, as has been previously described in human (Bosch et al., 1998) and guinea-pig ventricular cells (Busch et al., 1996).
Block of isoprenaline-stimulated IKs by 293B
It has been demonstrated in various types of cardiac myocytes that β-adrenergic agonist isoprenaline potentiates IKs through a pathway that involves the activation of adenylyl cyclase (AC) and adenosine 3′5′ cyclic monophosphate (cAMP)-dependent protein kinase (PKA; Harvey & Hume, 1989; Yazawa & Kameyama, 1990; Sanguinetti et al., 1991). We examined the interaction of 293B and isoprenaline on IKs in the experiments represented in Figure 6. In guinea-pig SA node cells, increasing the concentration of isoprenaline beyond 1 μM produced no further increase in the amplitude of IKs tail current elicited upon return to −50 mV following 4-s depolarizations to various test potentials of up to +70 mV (data not shown). Isoprenaline at 1.0 μM was thus found to evoke a maximal enhancement of IKs, which is in good agreement with the observations in guinea-pig atrial (Matsuura et al., 1996) and ventricular cells (Yazawa & Kameyama, 1990). An isolated SA node cell was initially exposed to 1.0 μM isoprenaline, which produced a marked increase in IKs (Figure 6a, left-hand and middle panels). After the stimulatory response to isoprenaline reached the steady state, 50 μM 293B was further added to the bath in the presence of isoprenaline. The subsequently applied 293B greatly reduced the amplitude of IKs (Figure 6a, right hand panel), thus showing that 293B is also effective in blocking IKs during β-adrenergic stimulation. Since an initial rise of IKs activation was still observed upon depolarizations in the presence of 293B, the blocking action of 293B on isoprenaline-stimulated IKs appears to develop in a time-dependent manner, qualitatively similar to that on IKs under control conditions (Figures 2, 3 and 4).
To quantitatively evaluate the stimulatory effect of isoprenaline and the blocking action of 293B on IKs, the amplitudes of tail currents elicited upon return to −50 mV following various depolarizing pulses were measured. Figure 6b illustrates I-V relationships for the tail currents recorded under control conditions, during exposure to 1 μM isoprenaline, and after addition of 50 μM 293B in the presence of isoprenaline. Isoprenaline evoked an increase in IKs tail current by a factor of approximately 2, when measured upon repolarization following strong depolarizations (⩾+40 mV). This degree of maximal enhancement (approximately 2.0 fold) of IKs tail current by isoprenaline in SA node cells is comparable to that observed in guinea-pig atrial and ventricular cells (2.5–3 fold; Matsuura et al., 1996; Yazawa & Kameyama, 1990). The smooth curves represent the least-squares fit of the data to a Boltzmann equation (equation 1). In a total of four cells, V1/2 and k values respectively averaged 10.4±3.7 mV and 12.3±1.2 mV for control, −1.9±1.7 mV and 12.8±0.8 mV for isoprenaline, and −4.0±9.7 mV and 17.9±3.3 mV for isoprenaline+293B. The voltage-dependence of IKs activation was shifted by isoprenaline to a more negative direction by 12.2±2.4 mV (n=4), and this result is consistent with previous observations regarding the effect of cAMP-PKA-activating agents on IKs in guinea-pig ventricular myocytes (Harvey & Hume, 1989; Yazawa & Kameyama, 1990; Walsh & Kass, 1991).
293B at 50 μM caused a nearly complete block of IKs maximally stimulated by 1.0 μM isoprenaline (94.0±0.98% block, n=6), when evaluated by the decrease in IKs tail current elicited following depolarization to +70 mV. Block of control IKs by the same concentration of 293B (99.4±0.45% block, n=6) was slightly but significantly larger than that of isoprenaline (1 μM)-stimulated IKs (P<0.01, Figure 6c). However, 293B at a concentration of 100 μM blocked the isoprenaline-stimulated IKs by 98.8±1.23% (n=3), a value similar to the block observed in control IKs by the same concentration (100 μM) of 293B (99.7±0.16% block, n=6), showing that a similar maximal response (a nearly full block) was achieved by 293B for basal and isoprenaline-stimulated IKs by increasing its concentration to 100 μM.
After washing off 50 μM 293B in the presence of 1 μM isoprenaline, the amplitude of IKs tail current was restored by more than 90% at all test potentials between −40 and +70 mV (data not shown), thus suggesting that blocking action of 293B on isoprenaline-stimulated IKs is also reversible. It was shown in guinea-pig ventricular cells that isoprenaline not only increases the amplitude of IKr but also reduces its E-4031 sensitivity through a mechanism that involves an activation of protein kinase C (Heath & Terrar, 2000). This observation was reported to be only detected when IKr is recorded using a perforated patch-clamp method and in the absence of blockers for ICa,L. Since the present experiments were not conducted in such a way, it is unlikely that a small amplitude of the tail current observed in the presence of isoprenaline and 293B (Figure 6a, right-hand panel) is related to deactivation of IKr.
Absence of effect of 293B on If
The effect of 293B on If was examined in guinea-pig SA node cells. In these experiments cells were held at −40 mV to minimize the activation of If at the holding potential and then hyperpolarized every 12 s to test potentials of −50 to −120 mV for 2 s in 10 mV steps. The amplitude of If at each test potential was determined by digitally subtracting the current level measured after decay of the capacitance transient from that at the end of the hyperpolarizing pulse. Since an obvious If (amplitude⩾∼30 pA) was evoked during hyperpolarizations to test potentials ⩽−70 mV, the effect of 293B was evaluated at these negative potentials. The amplitude of If measured 5 min after exposure to 50 μM 293B was more than 95% of that recorded before its exposure at each test potential, thus suggesting that 293B did not appreciably affect If in guinea-pig SA node cells. This observation is similar to a recent report in isolated rabbit SA node cells (Lei et al., 2002).
Discussion
The present investigation demonstrates (i) that IKs in an isolated guinea-pig SA node cell is highly sensitive to block by the chromanol derivative 293B (IC50=5.3 μM; Figure 2); (ii) that IKs block with 293B progresses with time during a depolarizing step with a more rapid block at higher concentrations (Figures 3 and 4); (iii) that the blockade of IKs by 293B is fully and rapidly reversible with a washout of the drug even after a maximal effect (a nearly full inhibition) is achieved (Figure 5); and (iv) that 293B also exerts a potent blocking action on IKs which is maximally stimulated by β-adrenergic agonist isoprenaline (Figure 6).
We recently demonstrated that IK in guinea-pig SA node cells is divided into two distinct components by the sensitivity to E-4031; an E-4031-sensitive IK activates rapidly and at potentials positive to −40 mV and displays a marked inward rectification whereas an E-4031-resistant IK activates more slowly and at more depolarized potentials and shows a minimal inward rectification (Matsuura et al., 2002). These results provide functional evidence for the presence of IKr and IKs in guinea-pig SA node cells. In addition, an envelope of tails test performed in the presence of 5 μM E-4031 showed that the ratio of time-dependent outward current during depolarization to the tail current elicited on hyperpolarization is almost constant and is close to the predicted ratio of the driving force for a minimally rectifying ionic conductance (see Figure 2, Matsuura et al., 2002). Thus, there is practically no clear evidence for the activation of ionic current systems other than IKs in guinea-pig SA node cells when depolarized from a holding potential of −50 mV in the presence of E-4031 and nisoldipine. It has also been reported that the transient outward current (Ito) is hardly detected in guinea-pig SA node cells (Guo et al., 1997). It is therefore reasonable to regard the membrane current changes elicited by depolarizing voltage steps in the absence or presence of 293B as representing the time course of IKs activation under the present experimental conditions.
The present experiments consistently detected a characteristic transient increase in the outward current during moderate or strong depolarizations in the presence of 293B at concentrations ⩾20 μM (Figures 1 and 2), which indicates the time-dependent development of IKs block with 293B. A similar response to 293B during a depolarizing voltage step has also been noted for IKs in guinea-pig ventricular cells (Fujisawa et al., 2000) and KvLQT1/minK current heterologously expressed in Xenopus oocytes (Seebohm et al., 2001) or mammalian cells (Loussouarn et al., 1997). The degree of IKs block by 293B during a depolarizing voltage step, measured as the ratio of amplitude of IKs blocked by 293B to baseline IKs, was found to progress in a time-dependent manner with faster time constants at higher concentrations (Figure 3). Block of IKs by higher concentrations (⩾50 μM) of 293B reached a peak level within a 4-s voltage step, while a block by lower concentrations (⩽20 μM) of 293B still did not reach a steady state level even at the end of 4-s step, when evaluated during depolarizations to +50 mV (Figure 3C). The observation that the degree of IKs block was significantly potentiated by lengthening the duration of depolarizing voltage steps (Figure 4) is also consistent with the time-dependent development of 293B block during depolarizations. An initial rise of time-dependent outward current which represents an activation of IKs was found to be almost unchanged by the addition of 1 μM 293B while the reduction of IKs became evident towards the end of depolarizing voltage steps (Figure 2a and 3a), which is consistent with the hypothesis that 293B exerts little or no appreciable effect on IKs channels in a closed state but produces a time-dependent block at the channels in an open state. Taken together, it is reasonable to suggest that 293B preferentially affects IKs channels in an open state in guinea-pig SA node cells. Fujisawa et al. (2000) proposed that 293B acts as an open channel blocker of IKs channels in guinea-pig ventricular cells with relatively small blocking rates, compared to those so far reported for other open channel blockade of various ionic currents.
It has been shown in cardiac myocytes of many mammalian species including humans that the two components of IK (IKr and IKs) not only play a dominant role in repolarization process of action potentials but also represent a potentially relevant target for the actions of neurotransmitters and drugs. The methanesulphonanilide class III antiarrhythmic drugs selectively inhibit IKr and thereby prolong the action potential duration which is an important determinant of the effective refractory period in cardiac muscle. On the other hand, β-adrenergic stimulation has been demonstrated to preferentially potentiate IKs in guinea-pig ventricular cells (Sanguinetti et al., 1991) and human atrial cells (Wang et al., 1994), while in recent years it was shown in guinea-pig ventricular cells that β-adrenoceptor stimulation potentiates IKr through a pathway that involves the activation of protein kinase C (Heath & Terrar, 2000). These drugs and neurotransmitters thus can affect the relative contribution of IKr and IKs to repolarizing outward currents in cardiac muscle. The prolongation of ventricular action potential duration by E-4031 or dofetilide was shown to be reversed by the presence of isoprenaline (Sanguinetti et al., 1991; Schreieck et al., 1997), whereas isoprenaline was demonstrated to fail to antagonize the prolongation of ventricular action potential duration induced by 293B (Schreieck et al., 1997; Shimizu & Antzelevitch, 1998). The present experiments clearly demonstrate that IKs which is maximally stimulated by isoprenaline is almost totally blocked by the subsequently applied 293B (Figure 6), which may underlie the ineffectiveness of isoprenaline in antagonizing the 293B-induced prolongation of ventricular action potential (Schreieck et al., 1997; Shimizu & Antzelevitch, 1998). The present results further indicate that 293B can still effectively prolong the action potential duration and the refractory period during elevated sympathetic tone in vivo, which may clearly contrast with the effect of IKr-blocking class III antiarrhythmic drugs (Sanguinetti et al., 1991; Schreieck et al., 1997). Although the blocking effect of 50 μM 293B on IKs was slightly but significantly reduced after exposure to 1 μM isoprenaline (Figure 6c), increasing the concentration of 293B to 100 μM resulted in a similar degree of IKs block in the absence and presence of 1 μM isoprenaline (99.7±0.16%, n=6 vs 98.8±1.2%, n=3). These results might be accounted for by assuming that the sensitivity of IKs to 293B was somewhat reduced by phosphorylation of channel proteins by PKA. It will be interesting to examine the possible alteration of the 293B sensitivity of IKs channels after phosphorylation by PKA.
Since the chromanol derivative 293B has been demonstrated to block IKs without appreciably affecting other ionic currents in the heart, when used at concentrations less than approximately 50 μM (Bosch et al., 1998), 293B is expected to represent a valuable pharmacological tool to evaluate the functional role of IKs in cardiac electrical activity. The full reversibility of 293B effect on IKs (Figure 4) may also help these investigations. We previously examined the effect of 293B at 30 μM on spontaneous action potentials in isolated guinea-pig SA node cells and found that 293B slowed the repolarization process at potentials negative to about −20 mV and induced moderate depolarization of MDP leading to the arrest of electrical activity (Matsuura et al., 2002). These results strongly indicate that IKs also plays an indispensable role in pacemaker activity in guinea-pig heart.
In summary, the present study demonstrates that 293B potently and reversibly blocks not only control IKs but also β-adrenergically stimulated IKs in guinea-pig SA node cells and that its blocking action progresses in a time-dependent manner with a more rapid block at higher concentrations.
Acknowledgments
The authors thank Dr Hans-J. Lang (Aventis Pharma Deutschland GmbH, Frankfurt, Germany) for kindly providing the chromanol 293B. This study was supported by Grants-in-Aid for Scientific Research (Nos. 11670040 and 13670042) from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- 293B
trans-6-cyano-4-(N-ethylsulphonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane
- cAMP
adenosine 3′5′ cyclic monophosphate
- E-4031
N-(4-((1-(2-(6-methyl-2-pyridinyl)ethyl)-4-piperidinyl)carbonyl)phenyl)methanesulphonamide
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- HEPES
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid)
- HERG
human ether-á-go-go related gene
- IC50
half-maximally inhibitory concentration
- ICa,L
L-type Ca2+ current
- If
hyperpolarization-activated cation current
- IK
delayed rectifier K+ current
- IKr
rapidly activating component of delayed rectifier K+ current
- IKs
slowly activating component of delayed rectifier K+ current
- IK1
inwardly rectifying K+ current
- INa
Na+ current
- KB solution
Kraft-Brühe solution
- PKA
adenosine 3′5′ cyclic monophosphate-dependent protein kinase
- SA node
sino-atrial node
References
- BARHANIN J., LESAGE F., GUILLEMARE E., FINK M., LAZDUNSKI M., ROMEY G. KVLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- BOSCH R.F., GASPO R., BUSCH A.E., LANG H.J., LI G.-R., NATTEL S. Effects of the chromanol 293B, a selective blocker of the slow component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc. Res. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- BUSCH A.E., BUSCH G.L., FORD E., SUESSBRICH H., LANG H.-J., GREGER R., KUNZELMANN K., ATTALI B., STÜHMER W. The role of the IsK protein in the specific pharmacological properties of the IKs channel complex. Br. J. Pharmacol. 1997;122:187–189. doi: 10.1038/sj.bjp.0701434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSCH A.E., SUESSBRICH H., WALDEGGER S., SAILER E., GREGER R., LANG H.-J., LANG F., GIBSON K.J., MAYLIE J.G. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflügers Arch. 1996;432:1094–1096. doi: 10.1007/s004240050240. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J. Pharmacol. Exp. Ther. 1992;262:809–817. [PubMed] [Google Scholar]
- CURRAN M.E., SPLAWSKI I., TIMOTHY K.W., VINCENT G.M., GREEN E.D., KEATING M.T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- FABIATO A., FABIATO F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- FUJISAWA S., ONO K., IIJIMA T. Time-dependent block of the slowly activating delayed rectifier K+ current by chromanol 293B in guinea-pig ventricular cells. Br. J. Pharmacol. 2000;129:1007–1013. doi: 10.1038/sj.bjp.0703126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINTANT G.A. Two components of delayed rectifier current in canine atrium and ventricle: Does IKs play a role in the reverse rate dependence of class III agents. Circ. Res. 1996;78:26–37. doi: 10.1161/01.res.78.1.26. [DOI] [PubMed] [Google Scholar]
- GUO J., MITSUIYE T., NOMA A. The sustained inward current in sino-atrial node cells of guinea-pig heart. Pflügers Arch. 1997;433:390–396. doi: 10.1007/s004240050293. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARVEY R.D., HUME J.R. Autonomic regulation of delayed rectifier K+ current in mammalian heart involves G proteins. Am. J. Physiol. 1989;257:H818–H823. doi: 10.1152/ajpheart.1989.257.3.H818. [DOI] [PubMed] [Google Scholar]
- HEATH B.M., TERRAR D.A. Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J. Physiol. (Lond.) 2000;522:391–402. doi: 10.1111/j.1469-7793.2000.t01-2-00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRISAWA H., BROWN H.F., GILES W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- ISENBERG G., KLÖCKNER U. Calcium tolerant ventricular myocytes prepared by preincubation in a ‘KB medium'. Pflügers Arch. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- JURKIEWICZ N.K., SANGUINETTI M.C. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ. Res. 1993;72:75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- KIMURA J., NOMA A., IRISAWA H. Na-Ca exchange current in mammalian heart cells. Nature. 1986;319:596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- LEI M., COOPER P.J., CAMELLITI P., KOHL P. Role of the 293b-sensitive, slowly activating delayed rectifier potassium current, iKs, in pacemaker activity of rabbit isolated sino-atrial node cells. Cardiovasc. Res. 2002;53:68–79. doi: 10.1016/s0008-6363(01)00459-x. [DOI] [PubMed] [Google Scholar]
- LERCHE C., SEEBOHM G., WAGNER C.I., SCHERER C.R., DEHMELT L., ABITBOL I., GERLACH U., BRENDEL J., ATTALI B., BUSCH A.E. Molecular impact of MinK on the enantiospecific block of IKs by chromanols. Br. J. Pharmacol. 2000;131:1503–1506. doi: 10.1038/sj.bjp.0703734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI G.-R., FENG J., YUE L., CARRIER M., NATTEL S. Evidence of two components of delayed rectifier K+ current in human ventricular myocytes. Circ. Res. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- LIU D.-W., ANTZELEVITCH C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes: A weaker IKs contributes to the longer action potential of the M cell. Circ. Res. 1995;76:351–365. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- LOUSSOUARN G., CHARPENTIER F., MOHAMMAD-PANAH R., KUNZELMANN K., BARÓ I., ESCANDE D. KvLQT1 potassium channel but not IsK is the molecular target for trans-6-Cyano-4-(N -ethylsulfonyl-N-methylamino) - 3 - hydroxy - 2,2-dimethyl-chromane. Mol. Pharmacol. 1997;52:1131–1136. doi: 10.1124/mol.52.6.1131. [DOI] [PubMed] [Google Scholar]
- MATSUURA H., EHARA T., DING W.G., OMATSU-KANBE M., ISONO T. Rapidly and slowing activating components of delayed rectifier K+ current in guinea-pig sinoatrial node pacemaker cells. J. Physiol. (Lond.) 2002;540:815–830. doi: 10.1113/jphysiol.2001.016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUURA H., TSURUHARA Y., SAKAGUCHI M., EHARA T. Enhancement of delayed rectifier K+ current by P2-purinoceptor stimulation in guinea-pig atrial cells. J. Physiol. (Lond.) 1996;490:647–658. doi: 10.1113/jphysiol.1996.sp021174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOBLE D., TSIEN R.W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J. Physiol. (Lond.) 1969;200:205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONO K., SHIBATA S., IIJIMA T. Properties of the delayed rectifier potassium current in porcine sino-atrial node cells. J. Physiol. (Lond.) 2000;524:51–62. doi: 10.1111/j.1469-7793.2000.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., CURRAN M.E., ZOU A., SHEN J., SPECTOR P.S., ATKINSON D.L., KEATING M.T. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JIANG C., CURRAN M.E., KEATING M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current: Differential sensitivity to block by class III antiarrhythmic Agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. Am. J. Physiol. 1991;260:H393–H399. doi: 10.1152/ajpheart.1991.260.2.H393. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K., SCOTT A., SIEGL P.K.S. Isoproterenol antagonizes prolongation of refractory period by the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Mechanism of action. Circ. Res. 1991;68:77–84. doi: 10.1161/01.res.68.1.77. [DOI] [PubMed] [Google Scholar]
- SCHREIECK J., WANG Y., GJINI V., KORTH M., ZRENNER B., SCHÖMIG A., SCHMITT C. Differential efect of β-adrenergic stimulation on the frequency-dependent electrophysiologic actions of the new class III antiarrhythmic dofetilide, ambasilide, and chromanol 293B. J. Cardiovasc. Electr. 1997;8:1420–1430. doi: 10.1111/j.1540-8167.1997.tb01039.x. [DOI] [PubMed] [Google Scholar]
- SEEBOHM G., LERCHE C., PUSCH M., STEINMEYER K., BRÜGGEMANN A., BUSCH A.E. A kinetic study on the stereospecific inhibition of KCNQ1 and IKs by the chromanol 293B. Br. J. Pharmacol. 2001;134:1647–1654. doi: 10.1038/sj.bjp.0704421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMIZU W., ANTZELEVITCH C. Cellular basis for the ECG Features of the LQT1 form of the long-QT syndrome. Effects of β-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and Torsade de Pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- SUESSBRICH H., BLEICH M., ECKE D., RIZZO M., WALDEGGER S., LANG F., SZABO I., LANG H.-J., KUNZELMANN K., GREGER R., BUSCH A.E. Specific blockade of slowly activating IsK channels by chromanols – impact on the role of IsK channels in epithelia. FEBS Lett. 1996;396:271–275. doi: 10.1016/0014-5793(96)01113-1. [DOI] [PubMed] [Google Scholar]
- TRUDEAU M.C., WARMKE J.W., GANETZKY B., ROBERTSON G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- TSIEN R.Y., RINK T.J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim. Biophys. Acta. 1980;599:623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- WALSH K.B., KASS R.S. Distinct voltage-dependent regulation of a heart-delayed IK by protein kinases A and C. Am. J. Physiol. 1991;261:C1081–C1090. doi: 10.1152/ajpcell.1991.261.6.C1081. [DOI] [PubMed] [Google Scholar]
- WANG Z., FERMINI B., NATTEL S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc. Res. 1994;28:1540–1546. doi: 10.1093/cvr/28.10.1540. [DOI] [PubMed] [Google Scholar]
- YAZAWA K., KAMEYAMA M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J. Physiol. (Lond.) 1990;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]


