Abstract
We have previously demonstrated that β3-adrenoceptor (β3-AR) stimulation induces endothelium-dependent vasorelaxation in rat aorta through the activation of an endothelial NO synthase associated with an increase in intracellular cGMP. The aim of the present study was to localise β3-AR to confirm our functional study and to complete the signalling pathway of β3-AR in rat aorta.
By RT–PCR, we have detected β3-AR transcripts both in aorta and in freshly isolated endothelial cells. The absence of markers for adipsin or hormone-sensitive lipase in endothelial cells excluded the presence of β3-AR from adipocytes. The localization of β3-AR in aortic endothelial cells was confirmed by immunohistochemistry using a rat β3-AR antibody.
To identify the G protein linked to β3-AR, experiments were performed in rat pre-treated with PTX (10 μg kg−1), a Gi/0 protein inhibitor. The blockage of Gi/0 protein by PTX was confirmed by the reduction of vasorelaxation induced by UK 14304, a selective α2-AR agonist. The cumulative concentration-response curve for SR 58611A, a β3-AR agonist, was not significantly modified on aorta rings from PTX pre-treated rats.
At the same level of contraction, the relaxations induced by 10 μM SR 58611A were significantly reduced in 30 mM-KCl pre-constricted rings (Emax=16.7±8.4%, n=5), in comparison to phenylephrine (0.3 μM) pre-constricted rings (Emax=49.11±11.0%, n=5, P<0.05). In addition, iberotoxin (0.1 μM), glibenclamide (1 μM) and 4-aminopyridine (1 mM), selective potassium channels blockers of KCa, KATP, and Kv respectively, decreased the SR 58611A-mediated relaxation.
We conclude that β3-AR is preferentially expressed in rat aortic endothelial cells. β3-AR-mediated aortic relaxation is independent of Gi/0 proteins stimulation, but results from the activation of several potassium channels, KCa, KATP, and Kv.
Keywords: β3-adrenoceptor, rat aorta, endothelial cells, potassium channels, Gi/0 protein
Introduction
Beta-adrenoceptors (β-AR) of the β1 and β2 subtypes classically mediate the effects of catecholamines on the relaxation of vascular smooth muscle, β2-AR subtype being the most important (Guimaraes & Moura, 2001). Recently, the use of β1/β2-AR antagonists and β3-AR agonists in several studies in vivo and in vitro provided evidence for the functional expression of β3-AR in some vascular beds in addition to β1- and β2-AR subtypes (Gauthier et al., 2000). As for other tissues, the effects of β3-AR stimulation in vessels vary across species. In rat isolated common carotid arteries, BRL 37344, a preferential β3-AR agonist, produced a significant relaxation, but with a slower kinetic than that induced by isoprenaline, a non selective β-AR agonist (Oriowo, 1994). We have obtained similar results in rat thoracic aorta where isoprenaline produced a rapid relaxation whereas SR 58611A, another preferential β3-AR agonist, caused slowly developing relaxations (Trochu et al., 1999). However, to our knowledge, expression of β3-AR in rat arteries has not been investigated.
The β3-AR-signalling pathway in vessels is partially characterized and shows apparent variability according to the vascular bed studied. In rat carotid arteries, β3-AR-induced relaxation was mainly endothelium-independent (Oriowo, 1994), whereas it was strongly reduced by endothelium removal in the rat thoracic aorta, suggesting a predominant localization of β3-AR on the endothelial cells (Trochu et al., 1999). In this latter model, the β3-AR stimulation activated an endothelial NO synthase leading to an increase in intracellular cGMP (Trochu et al., 1999). The G protein linked to β3-AR in this model is not yet determined. It is now acknowledged that β3-AR can be coupled with Gs or Gi/0 protein (Strosberg, 1997). However, the type of G protein depends on tissues studied and/or the pharmacological profile of β3-AR (Gauthier et al., 2000).
Effectors involved in the signalling pathway subsequent to the stimulation of β3-AR have not yet been identified in the rat thoracic aorta. The vascular tone is regulated by activation of several potassium (K+) channels (for review, see Sobey, 2001). In perfused rat lungs, β3-AR agonists induced vasorelaxant effects on the vasoconstriction elicited by hypoxia (Dumas et al., 1999). This vasorelaxation resulted from the activation of low and high conductance Ca2+-dependent K+ channels, SKCa and BKCa, respectively (Dumas et al., 1999).
The aim of the present study was to characterize β3-AR in rat aorta. The first objective was to determine the expression and the localization of β3-AR. The expression of mRNA encoding for this receptor was studied by RT–PCR in intact tissue and in endothelial cells isolated from aorta. Then, the localization of the β3-AR in this vessel was determined by immunohistochemistry. The second objective was to examine the signalling pathway of β3-AR i.e. the potential involvement of the pertussis toxin (PTX)-sensitive G protein and the type of potassium channels (BKCa, KATP, Kv) involved in the β3-AR-induced vasorelaxation.
Methods
Animals
Male Wistar rats (200–270 g) were purchased from Janvier (Le Genest St Isle, France). They were housed at 18°C and submit to 0700–1900 h light–dark cycle. Rats were anaesthetized with pentobarbital (30 mg kg−1, i.p.).
Tissue preparation
The thoracic aorta was dissected as previously described (Trochu et al., 1999). The descending thoracic aorta was cleared of fat and connective tissues.
Isolation of rat aortic endothelial cells
Rat aortic endothelial cells (RAEC) were dissociated as described by Battle et al. (1994). RAEC were isolated by collagenase digestion. Thoracic aorta was removed and rinsed with PSS (Physiological Salt Solution) having the following composition (in mM): NaCl, 130; KCl, 5.6; MgCl2, 1; HEPES, 10; pH adjusted to 7.4 with NaOH. The adventitia was removed. The remaining media and intima were cut in thin rings (1 mm thick). Endothelial cells were obtained by incubating these rings for 30 min in PSS containing 1248 u ml−1 collagenase type 1 (Worthington Biochemial Corporation, Lakewood) followed by a mechanical dispersion. The cell suspension was centrifuged (500×g, 5 min) and RNAs were extracted from the cell pellet. Endothelial cells were identified first by their morphology and secondly by using a rabbit anti-human von Willebrand factor antibody (vWf Ab–DAKO, Trappes). The vWf is a glycoprotein involved in primary hemostasis and synthesized by endothelial cells (Wagner, 1990). All the isolated cells have the endothelial morphology and were immunoreactive (data not shown).
RNA preparation
Total RNAs were extracted from aorta or endothelial cells by a modification of the acid guanidium-thiocyanate-phenol-chloroform method as described by Chomczynski & Sacchi (1987). Briefly, the vessels were homogenized with an ultra-turrax homogenizer in Trizol solution (Gibco Brl, France). After centrifugations and precipitation by addition of isopropanol, the RNA pellets were washed with 75% ethanol and resuspended in RNAse-free water. The concentrations were determined spectrophotometrically at 260 nm and the integrity of the RNA was verified on 0.8% TAE agarose gels.
Oligonucleotide primers and RT–PCR experiments
The oligonucleotides were purchased from Genosis (U.K.). Sequences and positions of the β3-AR, adipsin and GAPDH oligoprobes on the rat forms and the number of PCR cycles have been described in Table 1. First strand synthesis of cDNA from aorta or endothelial total RNAs was carried out using moloney murine leukaemia virus reverse transcriptase (Gibco BRL, France) with oligo(dT) (Sigma, France). The single strand cDNAs were consequently amplified by PCR using Taq DNA polymerase (Pharmacia, France). As well as sample cDNAs, each PCR experiment included a negative control consisting of a RT reaction containing no added reverse transcriptase (NRT on Figure 1) and positive control corresponding to fat cDNA. Following initial heating of samples at 94°C for 3 min, each cycle of amplification consisted of 30 s at 94°C, 45 s at annealing temperature appropriate for the primers used and 30 s at 72°C. Individual annealing temperatures were 65°C for β3-AR and GAPDH, and 55°C for adipsin primers. Following 35 cycles of amplification, the PCR products were separated through a 2% TAE agarose gels and visualized under UV after staining with ethidium bromide.
Table 1.
Oligonucleotides used as primers for PCR reactions
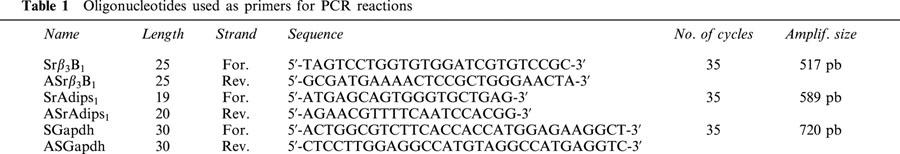
Figure 1.
Detection by RT–PCR of β3-AR and adipsin gene transcripts in white adipose tissue, heart, thoracic aorta and freshly isolated aortic endothelial cells. Amplified cDNA fragments were separed on 2% agarose gels and visualized by staining with ethidium bromide. Sizes of the PCR products are indicated on the left and right of the panels. M: 100 pb ladder (Biolabs, New England); NRT: none reverse transcription; RT: reverse transcription; β3-AR: β3-adrenoceptors; WAT: white adipose tissue; Thor. Aorta: thoracic aorta; Endo. Cell: endothelial cells.
Immunohistochemistry analysis
Fixation and tissue processing protocols were performed as described elsewhere (Bittencourt et al., 1992). After freezing in 2-methyl butane solution (−50°C), 10 micrometers sections of thoracic aorta were cut on cryostat microtome and stored at −20°C until using. The quality of the endothelial layer of the preparations was performed by incubation with a vWf Ab (DAKO, Trappes, France). The rat β3-AR antibody (rβ3-AR Ab) (Santa Cruz Biotechnology Inc., U.S.A.) is an affinity-purified goat polyclonal antibody raised against a peptide mapping at the carboxy terminus of the β3-AR of mouse origin. This antibody reacts with β3-AR of mouse and rat origin, without cross-reactive signal with β1- and β2-AR. After the secondary hybridization with an anti-goat Ig G peroxidase conjugated developed in rabbit (Sigma, France), the antibody complexes were revealed for sensitive detection of the enzymatic activity with peroxydase substrate kit AEC (SK4200–Vector, France). Controls for specificity of the rβ3-AR antiserum involved incubating primary antiserum overnight at 4°C with a five excess by weight of blocking peptide, the sequence used for the immunization procedure without the immunogenic carrier.
Pertussis toxin pre-treatment in vivo
Rats were anaesthetized with a mixture of pentobarbital (50 mg kg−1, i.p.) and ketamine (25 mg kg−1, i.m.). A bolus injection of pertussis toxin (PTX, 10 μg kg−1, i.v.) was done in the jugular vein with a 30 gauge needle. Sham rats were injected with the same volume of salt solutions. Seventy-two hours after the PTX injection, animals were sacrificed, and the thoracic aorta was removed (Komatsu et al., 1995; Kost et al., 1999).
Tension recording
Aorta were cut in 4–5 mm long rings. These rings were suspended in stainless wire in 10 ml organ bath containing Krebs solution composed as follows (mM): NaCl 118.3, KCl 4.7, MgSO4 1.2, NaHCO3 20, glucose 11.1, EDTA (ethylenediaminetetraacetic acid) 0.016 and CaCl2 2.5. The medium was maintained at 37°C and continuously gassed with 95% O2 and 5% CO2. The rings were progressively stretched in order to get a resting tension of 2 g. Functional endothelium was checked by the presence of at least 70% relaxation to acetylcholine (1 μM) in rings pre-contracted with phenylephrine (0.3 μM).
Cumulative concentration-response curves
Some rings were equilibrated in Krebs solution containing potassium channels inhibitors (iberotoxin 0.1 μM, glibenclamide 1 μM, and 4-aminopyridine 1 mM) whereas control rings were not treated during this period. Then, aortic rings were contracted with phenylephrine at concentrations ranging between 0.3 μM and 0.7 μM in order to obtain a similar value of the sustained tension for each ring studied (6.62±0.61 g). Then, cumulative concentration-response curves to SR 58611A or UK 14304 were conducted. For the experiments performed on rings pre-contracted with KCl, only two concentrations of SR 58611A were applied.
As SR 58611A induced a long lasting relaxation (Trochu et al., 1999), spontaneous, time-dependent relaxation was evaluated in control rings and subtracted to the corresponding percentage of relaxation induced by SR 58611A. As iberiotoxin, a specific BKCa potassium channel blocker, was dissolved in bovine serum albumin solution (BSA). The control concentration-response curves of SR 58611A for this experimental protocol were performed in the presence of BSA.
Drugs
Phenylephrine hydrochloride, acetylcholine chloride (Sigma-Aldrich, St Louis, MO, U.S.A.), pertussis toxin (Calbiochem, La Jolla, CA, U.S.A.), 4-aminopyridine (Research Biochemical International, U.S.A.) and SR 58611A (SR 58611A, ethyl [(7)S)-((2R)2-(3-chlorophenyl)-2-hydroxyethyl]amino]-5,6,7,8-tetrahydronaphtyl2-yl] oxyacetate hydrochloride) (a generous gift from Sanofi Recherche; Montpellier, France) were dissolved in distilled water. Glibenclamide, tolbutamide (Sigma Aldrich) were dissolved in dimethylsulphoxide (DMSO). Iberiotoxin and UK 14304 (Tocris, Oxford, U.K.) were respectively dissolved in BSA 0.1% and ethanol. The final concentration of the solvents in the organ bath was less than 0.1% v v−1.
Statistical analysis
Results are expressed as the means±s.e.mean of n experiments. The statistical significance of a drug effect was assessed using one way analysis of variance (ANOVA) followed by a Dunnett's test. Comparison of the different cumulative concentration-response curves was performed by a two-way ANOVA. To determine agonist potencies from cumulative concentration-response curves, concentrations producing 50% of maximal effect (EC50) were calculated by fitting curves with the Boltzmann's equation. pD2 values were then determined according to the equation pD2=−log (molar EC50) and compared using student's t-test (P<0.05 being considered as significant).
Results
β3-AR mRNA in rat aorta
The RT–PCR analysis shown in Figure 1, indicates that an expected amplified product of 517 bp with the rβ3-AR primer couple could be identified by ethidium bromide staining of the amplified samples of rat adipose tissue (Granneman et al., 1991), which was our positive control, and thoracic aorta RNAs (Figure 1A). Moreover, to exclude a non-specific amplification, an experiment was carried out in the same conditions on heart extract (Figure 1C) which is described to lack of expression for β3-AR transcripts (Gauthier et al., 1999). As expected, no transcript was detected for β3-AR despite the integrity of our reverse transcriptase which was performed by an amplification with GAPDH primer couple. No signal was also observed when the RNA samples were not reverse transcripted into cDNA and directly used for the different PCR experiments (lane NRT for all conditions). As this receptor is highly abundant in the rat adipose tissues, adipsin marker was developed to check for fat contamination in our different preparations. Expression of serine protease adipsin is confined to adipose tissue and the myelin sheath of nerves which are tissues active in lipid metabolism (Cook et al., 1987). The RT–PCR analysis with the adipsin primer couple indicated an expected PCR product of 589 bp for amplified samples of adipose tissue but also for thoracic aorta mRNAs (Figure 1B). So the possibility of an adipose contamination in the preparations, prevented us to conclude at a real expression of β3-AR transcript in vascular cells. However, the same procedure was realized on mRNAs obtained from freshly endothelial cells after enzymatic dissociation. Integrity of our reverse transcriptase was performed by an amplification with GAPDH primer couple (Figure 1D). Under these conditions, the rβ3-AR mRNA was still present (Figure 1E). The lack of amplification for adipsin transcript (Figure 1F) or other fat markers like the hormone-sensitive lipase (data not shown) suggests an absence of adipose contamination.
Immunohistochemistry
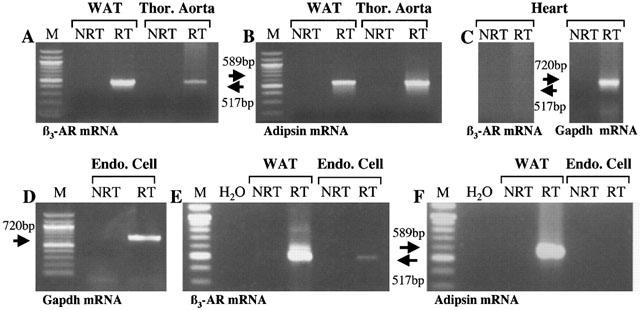
This RT–PCR analysis prompted us to search for rβ3-AR protein expression in the endothelial layer of rat thoracic aorta. An antiserum raised against the β3-AR was used in immunohistochemical analysis. The pattern of rβ3-AR immunoreactivity was compared with the vWf expression profile (Figure 2). The rβ3-AR Ab highly stained cells from the endothelial layer (Figure 2B) in a similar distribution and form to that revealed with the vWf antiserum (Figure 2A). A light and diffuse signal was also observed in the smooth muscle layer. Furthermore, the pre-absorption of rβ3-AR antiserum with the synthetic peptide, used for the procedure of immunisation, totally abolished the staining observed for the endothelial layer and only partially in the smooth muscle layer (Figure 2C).
Figure 2.
Comparison of von Willebrand factor (A) and β3-AR expression in rat thoracic aorta (B). Adjacent 10 μm thick sections were incubated with either von Willebrand antibody (vWf Ab; A) or rat β3-AR antibody (rβ3-AR Ab; B) revealed by peroxydase-conjugated second antiserum. Black arrowhead shows endothelium intensively stained with vWf and rβ3-AR Ab (A–B). Same staining after pre-absorption of rβ3-AR Ab with the blocking peptide (rβ3-AR Ab +B pept.; C). Micrographs X100.
β3-AR mediated relaxation in rat aorta
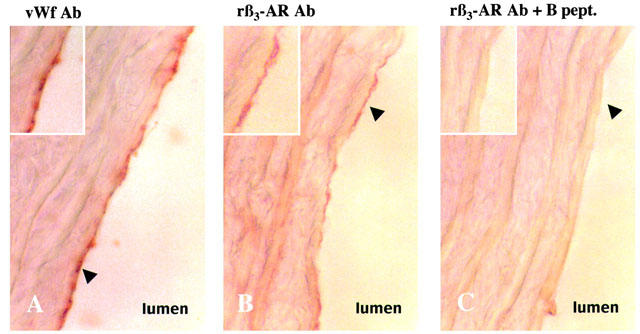
SR 58611A, a preferential β3-AR agonist, for concentration ranging from 0.1 to 30 μM, induced a concentration-dependent vasorelaxation in rat thoracic aorta. This relaxation was characterised by a long lasting response (Figure 4A). The spontaneous relaxation obtained in control rings (10.0±2.1% relaxant effect at the end of the experiment; n=20) was subtracted from the corresponding relaxant effect of SR 58611A. In these conditions, the pD2 value was 5.08±0.02 (n=21) and the Emax value obtained for a concentration of 30 μM was 55.4±1.6% (n=21).
Figure 4.
Effects of PTX pre-treatment on β3-AR-induced relaxation in rat aorta. The rats were injected i.v. with saline (sham) or PTX 10 μg kg−1 (pre-treated) 3 days before experiments. Typical recordings of relaxant effects induced by SR 58611A for concentrations ranging from 0.1 to 30 μM on aortic rings from sham operated rats (A) and PTX pre-treated rats (B). (C) Concentration-response curves for SR 58611A in aortic rings from sham operated rats (n=7) and PTX pre-treated rats (n=8). The mean curves resulting from subtraction of the spontaneous relaxation of control vessels pre-treated or not with PTX are shown. Results are expressed as the percentage of relaxation from the steady-state contraction level induced by phenylephrine. Each point is the mean of n experiments, and the vertical lines show the s.e. of the mean. When no error bar is shown, the error is smaller than the symbol. *P<0.05 and **P<0.01 indicate significant differences from control.
Effect of PTX pre-treatment on β3-AR induced relaxation
In order to identify the G protein linked to β3-AR, we performed experiments in rat pre-treated with PTX (10 μg kg−1), a Gi/0 protein inhibitor. In our experimental conditions, amplitude of contraction in response to phenylephrine was not significantly different in PTX-treated rings (4.39±0.24 g; n=16) compared to sham rings (4.74±0.31 g; n=14). As the endothelial α2-AR is known to be coupled with PTX sensitive G-protein in arteries (Vanhoutte, 1997), we evaluated the α2-AR-induced relaxation in order to verify the PTX pre-treatment efficiency. In isolated aortic rings obtained from sham rats, UK 14304, a selective α2-AR agonist, produced a concentration-dependent relaxation for concentrations ranging from 0.01 to 1 μM (Figure 3). The Emax value obtained at a concentration of 1 μM, was 62.1±6.5% (n=7). In aortic rings isolated from PTX pre-treated rats, the response to UK 14304 was blunted (P<0.01 versus sham rats) and the maximal relaxation induced by 1 μM UK 14304 was significantly reduced (Emax=36.7± 3.2%; n=8, P<0.05).
Figure 3.
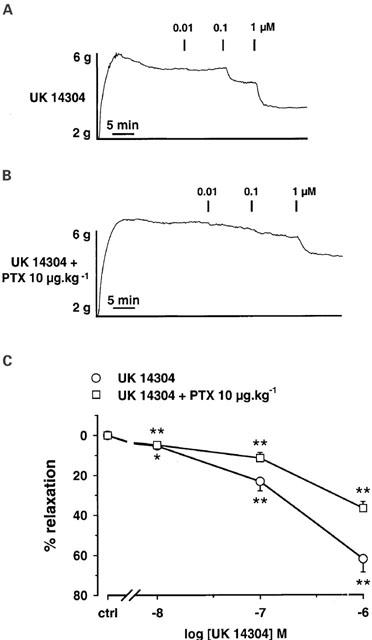
Inhibition of α2-AR induced relaxation, in thoracic aorta rings from PTX pre-treated rats. The rats were injected i.v. with saline (sham) or PTX 10 μg kg−1 (pre-treated) 3 days before experiments. Typical recordings of relaxant effects of UK 14304 for concentrations ranging from 0.01 to 1 μM in aortic rings from sham operated rats (A) and PTX pre-treated rats (B). (C) Concentration-response curves for UK 14304 in aortic rings from sham operated rats (n=7) and PTX pre-treated rats (n=8). Results are expressed as the percentage of relaxation from the steady-state contraction level induced by phenylephrine. Each point is the mean of n experiments, and the vertical lines show the s.e. of the mean. When no error bar is shown, the error is smaller than the symbol. *P<0.05 and **P<0.01 indicate significant differences from control. Both curves are significantly different (two-way ANOVA).
At the opposite, the cumulative concentration-response curve for SR 58611A was not significantly modified in aorta rings isolated from PTX pre-treated rats (Figure 4). The potencies of SR 58611A (sham rats: pD2=5.06±0.07, n=7; PTX pre-treated rats: pD2=4.96±0.06, n=8) and the maximal relaxation induced by 30 μM SR 58611A (sham rats: Emax=63.4±5.3%; PTX pre-treated rats: Emax=57.2±5.8%) were similar in both conditions.
Involvement of potassium channels in β3-adrenergic mediated vasorelaxation in rat aorta
The role of potassium channels in mediating relaxation to SR 58611A were evaluated in vessels pre-constricted with 30 mM KCl before performing a concentration-response curve to SR 58611A. This concentration of KCl blocked potassium efflux and prevented relaxation mediated by opening of potassium channels. The amplitude of contraction was not significantly different in rings constricted with KCl or phenylephrine (5.0±0.4 g and 4.5±0.4 g, respectively; n=5). The decreased driving force for potassium inhibited the relaxant effect induced by 30 μM SR 58611A (n=5; P<0.05), the maximal relaxation being only 16.7±3.7% (n=5) instead of 49.1±9.8% in phenylephrine-constricted rings (n=5; Figure 5).
Figure 5.
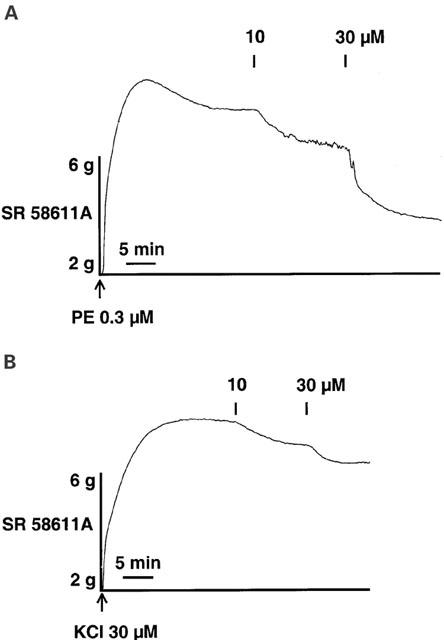
Inhibition of β3-AR-induced relaxation in rat aorta by high extra-cellular potassium concentration. Typical recordings of SR 58611A for concentrations ranging from 10 to 30 μM on rings pre-constricted with either phenylephrine (PE) (A) or KCl (B). The recordings are representative in each case of five experiments.
In order to determine which types of potassium channels are involved in the β3-AR-induced relaxation, aortic rings were pre-treated with a variety of selective potassium channel blockers before applying cumulative concentrations of SR 58611A (Figure 6). In the presence of 0.1 μM iberiotoxin, a specific blocker of BKCa (Nelson et al., 1990), the relaxant effects of SR 58611A were significantly reduced. SR 58611A had a lower potency (pD2=4.86±0.02; n=8; P<0.01) than in the absence of iberiotoxin (pD2=5.13±0.11, n=9) and the maximal effect produced by 30 μM SR 58611A was significantly reduced by 13.5% (n=9; P<0.01 versus SR 58611A alone; Figure 6A). Glibenclamide (1 μM), an inhibitor of KATP (Waldron & Cole, 1999), shifted to the right the concentration-relaxation curve for SR 58611A (SR 58611A alone: pD2=5.08±0.02; n=21; SR 58611A+glibenclamide: pD2=4.40±0.08; n=6; P<0.01 versus SR 58611A alone) and reduced the maximal relaxant effect obtained at 30 μM by 14.5% (P<0.01 versus SR 58611A alone). Similar effects were obtained in the presence of another KATP blocker, tolbutamide (100 μM). The potency of SR 58611A (pD2=4.46±0.10; P<0.01 versus SR 58611A alone) and its maximal relaxant effect obtained at a concentration of 30 μM (Emax=43.6±3.5%; n=6; P<0.01 versus SR 58611A alone) were significantly reduced (Figure 6B). In arteries, 4-aminopyridine (4-AP) inhibited at millimolar concentrations voltage-dependent potassium channels (Kv). In aortic rings pre-treated with 1 mM 4-AP, the effects of SR 58611A were decreased only at highest concentrations (10 and 30 μM; Figure 6C). In addition, the potency of SR 58611A was reduced (pD2=4.76±0.09; n=7; P<0.01 versus SR 58611A alone).
Figure 6.
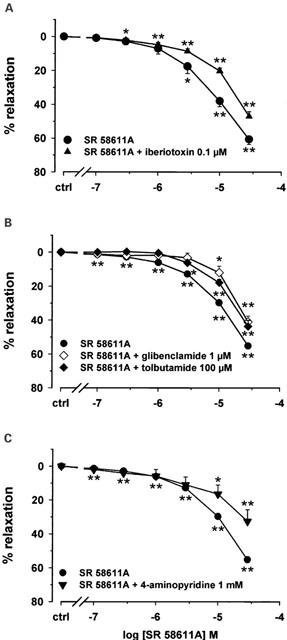
Inhibition of β3-adrenergic induced relaxation in rat aorta by potassium channel blockers. The mean curves resulting from subtraction of the spontaneous relaxation of control vessel pre-treated or not with potassium channels blockers are shown. (A) Concentration-response curves for SR 58611A in the absence (n=9) and presence of 0.1 μM iberiotoxin (n=8) in rat thoracic aortic rings constricted with phenylephrine. (B) Concentration response curves for SR 58611A in the absence (n=21) and presence of 1 μM glibenclamide (n=6) or 100 μM tolbutamide (n=6) in rat thoracic aortic rings constricted with phenylephrine. (C) Concentration response curves for SR 58611A in the absence (n=21) and presence of 1 mM 4-aminopyridine (n=7) in rat thoracic aortic rings constricted with phenylephrine. Results are expressed as the percentage of relaxation from the steady-state contraction level induced by phenylephrine. Each point is the mean of n experiments, and the vertical lines show the s.e. of the mean. When no error bar is shown, the error is smaller than the symbol. *P<0.05 and **P<0.01 indicate significant differences from control.
Discussion
In this study, we provided molecular and biochemical evidence for the expression of β3-AR in endothelial cells of rat thoracic aorta. In addition, in this vascular bed, we demonstrated that β3-AR-mediated relaxation did not result from the activation of PTX-sensitive G proteins but rather involved the activation of several potassium channel subtypes: BKCa, KATP and KV.
In a previous study, (Trochu et al., 1999), we have shown by a pharmacological approach that β3-AR stimulation produced a relaxation of rat thoracic aorta mediated mainly by endothelium-derived nitric oxide. However, no molecular and biochemical data concerning the expression of this receptor in endothelial cells were available. By RT–PCR, we have detected β3-AR transcripts both in thoracic aorta and in freshly isolated endothelial cell preparation. No transcript was detected for β3-AR on heart extract, which is described to lack of expression for β3-AR transcripts (Gauthier et al., 1999). This observation excludes a non-specific amplification and makes more relevant the result for the freshly isolated endothelial cell preparation. In this latter preparation, we have looking for adipose tissue markers (adipsin and hormone-sensitive lipase) because rat adipocytes are known to express a high level of β3-AR (Lafontan, 1994). In our conditions, no specific products corresponding to adipsin and hormone-sensitive lipase were detected on reverse transcription performed by a GAPDH primer amplification. Thus, these results indicate that the expression of the β3-AR in rat thoracic aorta was not due to the presence of adipocytes. Although we have verified the nature of our endothelial cells by a vWf immunocytochemical analysis in order to exclude a putative expression of β3-AR mRNA from contaminating smooth muscle cells. Immunohistochemical studies performed on intact aorta demonstrated a strong stain in endothelial layer with rβ3-AR Ab which disappeared when the antiserum was pre-absorbed with the blocking peptide. By contrast, the light staining observed in smooth muscle layer was not totally competed by the antigenic peptide, suggesting a non-specific signal in smooth muscle cells, and/or the presence of a very low expression of β3-AR in smooth muscle cells, at the limit of detection of this procedure. These results are in agreement with our previous pharmacological study (Trochu et al., 1999). To date, although several studies have been performed to characterize the presence of β3-AR in vessels, only one study reports the expression of β3-AR mRNA expression in vessels. In rat portal vein, β3-AR transcripts have been detected in primary culture of smooth muscle cells (Viard et al., 2000). This discrepancy could be explained by the type of vessels studied (artery versus veins). In the study of Viard et al. (2000), a potential contamination by adipocytes has not been evaluated. However, immunohistochemistry with an anti-peptide polyclonal antibody revealed a β3-AR expression in human gastrointestinal arteries. In this vascular bed, β3-AR have been localized in smooth muscle cells but not in endothelial cells (Anthony et al., 1998). These results suggest that the localization of β3-AR in vascular wall varies across species. These data corroborate the pharmacological results showing an endothelium-dependent vasorelaxation or not according to the vascular bed or the species studied.
In the rat thoracic aorta, we have previously shown that β3-AR stimulation produced an endothelium-dependent vasorelaxation by activation of an endothelial NO synthase leading to an increase in intracellular cGMP. This effect is not modified by nadolol, a β1- and β2-AR blocker but was abolished in the presence of SR 59230A, a β3-AR antagonist (Trochu et al., 1999). However, the signalling pathway was incomplete, and especially the type of G protein involved was missing. In human cardiac ventricle where β3-AR are also linked to the NO pathway, we have demonstrated that β3-AR are coupled to Gi/0 proteins (Gauthier et al., 1996). In the present study, the vasorelaxation induced by β3-AR stimulation was not modified in PTX-pre-treated rats, suggesting that in rat thoracic aorta, β3-AR are not linked to Gi/0 proteins. Indeed, in our experimental conditions, we have verified that Gi/0 proteins were significantly inhibited by PTX because the relaxant effect induced by UK 14304, a selective α2-AR agonist, which is known to activate Gi/0 proteins (Vanhoutte, 1997), was blunted in aorta rings isolated from PTX-pre-treated rats. In native and recombinant systems, it has been shown that β3-AR could be linked to different types of G proteins and activated different signalling pathways (Strosberg, 1997; Gauthier et al., 2000). In rat portal vein myocytes, β3-AR are linked to Gs proteins (Viard et al., 2000). However, in this study, the compound used to stimulated β3-AR was CGP 12177, which is known to also activate the state of low affinity of β1-AR (Granneman, 2001). This atypical β-AR is described to activated cAMP pathway through stimulation of Gs proteins. Clearly, further experiments are needed to identify the type of G proteins involved in the vasorelaxation induced by β3-AR in rat thoracic aorta.
Potassium channels play an important role in the regulation of calcium influx and vascular tone by affecting the membrane potential in smooth muscle (Sobey, 2001). Activation of potassium channels and subsequent membrane hyperpolarisation contribute to vasorelaxation. The role of the activation of potassium channels in β-AR agonist-induced dilation of mammalian blood vessels has been recently described by a number of research groups. However, only one study performed in rat isolated perfused lung has determined the role of potassium channels in the β3-AR-induced vasorelaxation (Dumas et al., 1999). In the present study, the relaxant effect of SR 58611A was strongly reduced by elevated concentration of extracellular KCl (decreasing the driving force for potassium ions, thereby decreasing potassium efflux and subsequent hyperpolarisation) suggesting the involvement of potassium channels in this effect. This result was confirmed by the use of specific potassium channels blockers. In the presence of iberiotoxin, glibenclamide, tolbutamide or 4-AP, the SR 58611A-induced vasorelaxation was significantly reduced, suggesting that BKCa, KATP and Kv respectively, are effectors of the vasorelaxation produced by β3-AR stimulation in rat thoracic aorta. However, this inhibition was not complete in each experimental condition suggesting the participation of several classes of potassium channels in this global effect. In this model of rat thoracic aorta, it has been shown that the relaxation induced by isoprenaline resulted from the activation of KCa channels mainly by activation of β2-AR and Kv channels by activation of β1-AR (Satake et al., 1996). However, as isoprenaline activated all types of β-AR, the analysis of this study is difficult because the effects of β3-AR were not evaluated. The involvement of potassium channels in the vasorelaxation-induced by β3-AR stimulation seems different according the vascular bed in one species. Indeed, on the rat hypoxic pulmonary pressure, the activation of BKCa and SKCa participated to the vasorelaxation but not KATP; the involvement of Kv in this effect was not investigated in this study (Dumas et al., 1999). The activation of a Kv channel by β3-AR stimulation has been previously shown in a recombinant expression system (Xenopus oocytes) in which β3-AR stimulation produced by isoprenaline, activated the cardiac KvLQT1/MinK potassium channel through Gs proteins (Kathofer et al., 2000). With our technique of organ bath, we cannot distinguish the involvement of endothelial and/or smooth muscle cell potassium channels in the β3-AR-induced vasorelaxation. For exemple, BKCa are expressed both in smooth muscle cells and in endothelial cells. In endothelial cells, they regulate the membrane potential and the intracellular calcium level (Marchenko & Sage, 1996). Thus, their activation results in an hyperpolarization of endothelial cells, which increases the electrochemical gradient for calcium and leads to increase intracellular calcium, a prerequisite for the synthesis/release of relaxant factors including NO (Mayer et al., 1989). Hence, to determine the localisation of potassium channels involved in the β3-AR relaxation in rat aorta, further investigations are needed.
Taken together with our previous data, this study demonstrated that functional β3-AR are mainly localized in rat aorta endothelial cells. Their activation produced a vasorelaxation through an independent activation of Gi/0 proteins but involved at least three types of potassium channels, BKCa, KATP and Kv. As endothelium could be altered in some diseases, this study opens new fields of investigations. In particular, future work will be needed to determine whether β3-AR stimulation may present a potential interest in hypertension.
Acknowledgments
This work was partially supported by grants from the ‘Fédération Française de Cardiologie' and the ‘Fondation de France'. Y. Rautureau was supported by a grant from ‘La Communauté Urbaine de Nantes' and S. Serpillon by a grant from ‘Le Conseil Général de Loire Atlantique'. We are grateful to Agnès Hivonnait for animal care.
Abbreviations
- 4-AP
4-aminopyridine
- β-AR
β-adrenoceptor
- BSA
bovine serum albumin
- cDNA
complementary DNA
- Emax
maximal relaxant response
- KATP
ATP dependent potassium channels
- BKCa
calcium-dependent potassium channels of big conductance
- Kv
voltage-dependent potassium channels
- PTX
pertussis toxin
- RAEC
rat aortic endothelial cells
- rβ3-AR Ab
rat β3-AR antibody
- RT–PCR
reverse transcription-polymerase chain reaction
- SR 58611A
ethyl [(7)S)-((2R)2-(3-chlorophenyl)-2-hydroxyethyl]amino]-5,6,7,8-tetrahydronaphtyl2-yl]oxyacetate hydrochloride
- TAE
tris acetate EDTA buffer
- UK 14304
5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline
- vWf Ab
von Willebrand factor antibody
References
- ANTHONY A., SCHEPELMANN S., GUILLAUME J.L., STROSBERG A.D., DHILLON A.P., POUNDER R.E., WAKEFIELD A.J. Localization of the beta(beta)3-adrenoceptor in the human gastrointestinal tract: an immunohistochemical study. Aliment. Pharmacol. Ther. 1998;12:519–525. doi: 10.1046/j.1365-2036.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- BATTLE T., ARNAL J.F., CHALLAH M., MICHEL J.B. Selective isolation of rat aortic wall layers and their cell types in culture-application to converting enzyme activity measurement. Tissue Cell. 1994;26:943–955. doi: 10.1016/0040-8166(94)90043-4. [DOI] [PubMed] [Google Scholar]
- BITTENCOURT J.C., PRESSE F., ARIAS C., PETO C., VAUGHAN J., NAHON J.L., VALE W., SAWCHENKO P.E. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J. Comp. Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate- phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- COOK K.S., MIN H.Y., JOHNSON D., CHAPLINSKY R.J., FLIER J.S., HUNT C.R., SPIEGELMAN B.M. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- DUMAS J.P., GOIRAND F., BARDOU M., DUMAS M., ROCHETTE L., ADVENIER C., GIUDICELLI J.F. Role of potassium channels and nitric oxide in the relaxant effects elicited by beta-adrenoceptor agonists on hypoxic vasoconstriction in the isolated perfused lung of the rat. Br. J. Pharmacol. 1999;127:421–428. doi: 10.1038/sj.bjp.0702575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUTHIER C., LANGIN D., BALLIGAND J.L. Beta3-adrenoceptors in the cardiovascular system. Trends Pharmacol. Sci. 2000;21:426–431. doi: 10.1016/s0165-6147(00)01562-5. [DOI] [PubMed] [Google Scholar]
- GAUTHIER C., TAVERNIER G., CHARPENTIER F., LANGIN D., LE MAREC H. Functional beta3-adrenoceptor in the human heart. J. Clin. Invest. 1996;98:556–562. doi: 10.1172/JCI118823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUTHIER C., TAVERNIER G., TROCHU J.N., LEBLAIS V., LAURENT K., LANGIN D., ESCANDE D., LE MAREC H. Interspecies differences in the cardiac negative inotropic effects of beta(3)-adrenoceptor agonists. J. Pharmacol. Exp. Ther. 1999;290:687–693. [PubMed] [Google Scholar]
- GRANNEMAN J.G. The putative beta4-adrenergic receptor is a novel state of the beta1- adrenergic receptor. Am. J. Physiol. Endocrinol. Metab. 2001;280:E199–E202. doi: 10.1152/ajpendo.2001.280.2.E199. [DOI] [PubMed] [Google Scholar]
- GRANNEMAN J.G., LAHNERS K.N., CHAUDHRY A. Molecular cloning and expression of the rat beta 3-adrenergic receptor. Mol. Pharmacol. 1991;40:895–899. [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- KATHOFER S., ZHANG W., KARLE C., THOMAS D., SCHOELS W., KIEHN J. Functional coupling of human beta 3-adrenoreceptors to the KvLQT1/MinK potassium channel. J. Biol. Chem. 2000;275:26743–26747. doi: 10.1074/jbc.M003331200. [DOI] [PubMed] [Google Scholar]
- KOMATSU M., MCDERMOTT A.M., GILLISON S.L., SHARP G.W. Time course of action of pertussis toxin to block the inhibition of stimulated insulin release by norepinephrine. Endocrinology. 1995;136:1857–1863. doi: 10.1210/endo.136.5.7720630. [DOI] [PubMed] [Google Scholar]
- KOST C.K., JR, HERZER W.A., LI P.J., JACKSON E.K. Pertussis toxin-sensitive G-proteins and regulation of blood pressure in the spontaneously hypertensive rat. Clin. Exp. Pharmacol. Physiol. 1999;26:449–455. doi: 10.1046/j.1440-1681.1999.03058.x. [DOI] [PubMed] [Google Scholar]
- LAFONTAN M. Differential recruitment and differential regulation by physiological amines of fat cell beta-1, beta-2 and beta-3 adrenergic receptors expressed in native fat cells and in transfected cell lines. Cell Signal. 1994;6:363–392. doi: 10.1016/0898-6568(94)90085-x. [DOI] [PubMed] [Google Scholar]
- MARCHENKO S.M., SAGE S.O.Calcium-activated potassium channels in the endothelium of intact rat aorta J. Physiol. 199649253–60.(Pt 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER B., SCHMIDT K., HUMBERT P., BOHME E. Biosynthesis of endothelium-derived relaxing factor: a cytosolic enzyme in porcine aortic endothelial cells Ca2+-dependently converts L- arginine into an activator of soluble guanylyl cyclase. Biochem. Biophys. Res. Commun. 1989;164:678–685. doi: 10.1016/0006-291x(89)91513-1. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., PATLAK J.B., WORLEY J.F., STANDEN N.B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- ORIOWO M.A. Atypical beta-adrenoceptors in the rat isolated common carotid artery. Br. J. Pharmacol. 1994;113:699–702. doi: 10.1111/j.1476-5381.1994.tb17049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATAKE N., SHIBATA M., SHIBATA S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by beta 1- and beta 2-adrenoceptor activation in rat aortic rings. Br. J. Pharmacol. 1996;119:505–510. doi: 10.1111/j.1476-5381.1996.tb15700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBEY C.G. Potassium channel function in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2001;21:28–38. doi: 10.1161/01.atv.21.1.28. [DOI] [PubMed] [Google Scholar]
- STROSBERG A.D. Structure and function of the beta 3-adrenergic receptor. Annu. Rev. Pharmacol. Toxicol. 1997;37:421–450. doi: 10.1146/annurev.pharmtox.37.1.421. [DOI] [PubMed] [Google Scholar]
- TROCHU J.N., LEBLAIS V., RAUTUREAU Y., BEVERELLI F., LE MAREC H., BERDEAUX A., GAUTHIER C. Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br. J. Pharmacol. 1999;128:69–76. doi: 10.1038/sj.bjp.0702797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHOUTTE P.M. Endothelial dysfunction and atherosclerosis. Eur. Heart J. 1997;18 Suppl. E:E19–E29. doi: 10.1016/s0195-668x(97)90005-1. [DOI] [PubMed] [Google Scholar]
- VIARD P., MACREZ N., COUSSIN F., MOREL J.L., MIRONNEAU J. Beta-3 adrenergic stimulation of L-type Ca(2+) channels in rat portal vein myocytes. Br. J. Pharmacol. 2000;129:1497–1505. doi: 10.1038/sj.bjp.0703187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER D.D. Cell biology of von Willebrand factor. Annu. Rev. Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- WALDRON G.J., COLE W.C. Activation of vascular smooth muscle K+ channels by endothelium-derived relaxing factors. Clin. Exp. Pharmacol. Physiol. 1999;26:180–184. doi: 10.1046/j.1440-1681.1999.03006.x. [DOI] [PubMed] [Google Scholar]


