Abstract
The ability of the nitric oxide (NO)-releasing aspirin, NCX 4016, to control vasoconstrictor responses induced by electrical field stimulation (TNS) or by exogenous norepinephrine (NE) was investigated in perfused rat tail artery with intact endothelium.
NCX 4016 (25, 50 and 100 μM) dose-dependently antagonized the vasoconstriction caused by TNS (from 0.5 to 64 Hz) and by NE (from 0.01 to 10 μM). The vasorelaxant activity of NCX 4016 (100 μM) in NE-precontracted arteries was concomitant with a marked increase of tissue cyclic GMP (4.9 fold, P<0.001) and was significantly antagonized by the inhibitors of soluble guanylate cyclase, methylene blue and 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one.
The effect of NCX 4016 was endothelium NO-independent since, in preparations perfused with NG-monomethyl-L-arginine (10 μM), this compound prevented the rise in basal perfusion pressure and reversed the accentuation of vasoconstrictor responses caused by NO synthase inhibition.
Aspirin-moiety released by NCX 4016 inhibited the 6-keto-PGF1α formation without interfering with the vasorelaxant activity of NCX 4016, while aspirin (100 μM) was devoid of any activity against vasoconstriction induced by both TNS and NE in perfused rat tail artery.
NCX 4016 moderated adrenergic vasoconstriction in perfused rat tail arteries by a direct donation of NO without involving the relaxant factors such as PGI2 and NO from endothelial cells.
The results obtained with NCX 4016 in perfused rat tail artery bears some therapeutical potential in conditions associated with vascular smooth muscle hyperreactivity to adrenergic stimulation.
Keywords: NCX 4016, adrenergic vasoconstriction, nitric oxide, cyclic GMP, perfused rat tail artery
Introduction
It is well established that the endothelium plays a pivotal role in the control of vascular smooth muscle tone primarily by production and release of the nitric oxide (NO) (Ignarro et al., 1987; Palmer et al., 1987; 1988; Sakuma et al., 1988) and in some circumstances by prostacyclin (PGI2) (Berti et al., 1993; 1994; Rossoni et al., 1996). The metabolic pathway of NO generation has been recognized in various cells, where it provides a signal transduction leading to soluble guanylate cyclase stimulation, intracellular guanosine 3′:5′-cyclic monophosphate (cyclic GMP) accumulation and vasodilation (Ignarro, 1990; Moncada et al., 1991). In vitro studies have demonstrated that NO is also involved in non-adrenergic non-cholinergic neurogenic relaxant responses in blood vessels (Ahlner et al., 1991; Toda et al., 1991) and the presence of endothelium decreases neurogenic vasoconstriction (Tesfamarian et al., 1987). Bucher et al. (1992) analysing the role of L-arginine/NO pathway and cyclic GMP in the rat tail artery suggested that either NO or an NO-like substance of endothelial origin modulated electrical field stimulation-induced noradrenaline release and vasoconstriction of this resistance vessel.
All this information prompted us to investigate whether a nitroderivative of aspirin, NCX 4016 (2-acetoxy-benzoate 2-[1-nitroxy-methyl]-phenyl ester), by releasing the NO-moiety (Lechi et al., 1996; Wallace et al., 1999; Carini et al., 2001), might affect the reactivity of the perfused rat tail artery and control the vasospasm elicited by adrenergic receptors activation. Previous experiments have already shown that, in contrast to aspirin, NCX 4016, by NO donation, exerts a significant cardioprotection in the rabbit (Rossoni et al., 2000; 2002) and reduces the infarct size caused by ischaemia-reperfusion in the anaesthetized rat (Rossoni et al., 2001) and pig (Wainwright et al., 2002). Moreover, a recent study by Muscarà et al. (2001) indicates that NCX 4016 lessens the increase in systemic blood pressure induced in the rat by chronic administration of the NO synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME), added to drinking water. These authors suggest that this property of NCX 4016 is not due simply to a direct vasodilatory action of the NO released but possibly to interference with the effects of endogenous vasoconstrictors.
Experiments on perfused rat tail artery preparations will provide new insight on the mode of action of NCX 4016, particularly on the domain of sympathetic nervous system activation in a resistance vessel. The activity of a nitro-phenyl ester of salicylic acid (NCX 4023), a compound that does not inhibit prostaglandin synthesis, will also be investigated.
Methods
Animals
Male Wistar rats (300±20 g of body weight) obtained from Charles River Italia (Calco, LC, Italy) were used for all experiments. All experimental procedures were approved by the Animal Care Committee of the University of Milan, Italy, and the investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996).
Tail artery preparation
Rats were killed by decapitation, and a proximal segment of tail artery (3.5–4 cm) was quickly dissected out and placed in oxygenated (95% O2+5% CO2) Krebs Henseleit solution (KHS; 37°C; pH 7.4) of the following composition (mM): NaCl 118, KCl 4.8, KH2PO4 1.2, CaCl2 1.6, MgSO4 1.2, NaHCO3 25, glucose 11.5, ascorbic acid 0.3 and EDTA 0.03.
The vascular tissues were cannulated (20 gauge-needle) using the procedures described by Berti et al. (1971) and Spokas & Folco (1984). The tail arteries were bathed (10 ml) extraluminally with gassed KHS at 37°C, maintained in a vertical position with a small weight (0.5 g) on the distal end, and perfused intraluminally through the needle at a constant flow rate of 5 ml min−1 with gassed KHS via a Minipuls-3 peristaltic pump (Gilson, Villiers Le Bel, France). The experiments started after a 30- to 45-min equilibration period. Changes in perfusion pressure, which represent changes in vascular resistance, were monitored with a Bentley pressure transducer (model 800, U. Basile, Comerio, VA, Italy), and the resulting electrical signals were recorded by a two-channel polygraph (model Gemini, U. Basile).
Vasoconstrictor responses
Vasoconstriction of perfused tail arteries was induced by transmural nerve stimulation (TNS) or by the addition of norepinephrine (NE) to the perfusion stream.
TNS was delivered with a Grass S-88 stimulator (Grass Instruments Co., Quincy, MA, U.S.A.) through platinum electrodes (40 mm long, 0.5 mm diameter; U. Danuso, Bresso, MI, Italy) placed 5 mm away from the vascular tissue. Stimulation parameters were 60 V (supramaximal voltage), 1 ms pulse duration, with various frequencies (from 0.5 to 64 Hz; 2 fold increments in frequencies) or with single frequency at 2 or 8 Hz to better evaluate TNS-responses potentiation or reduction, respectively. Vasoconstriction of perfused tail arteries to TNS were conducted using 5-s train duration at 3-min intervals. No reduction in responses was observed when the time interval between responses was 3 min. Vasoconstriction of perfused tail artery preparations was also elicited by bolus injections (5 min apart) of increasing concentrations of NE (from 0.01 to 10 μM). In some experiments, to precontract the vascular segment, NE was perfused at the concentration of 30 nM. Vascular responses to TNS or NE were expressed as a percentage of the maximal vasoconstriction to 120 mM KCl in individual experiments.
NCX 4016-relationship to adrenergic vasoconstriction
Two sets of five different groups of tail arteries were perfused with vehicle, NCX 4016 (25, 50 and 100 μM) and aspirin (100 μM) for 15 min before subjecting the preparations to TNS at the various frequencies (from 0.5 to 64 Hz) or to a challenge with NE at different concentrations (from 0.01 to 10 μM), respectively. The perfusion with the above compounds was continued throughout the experiment.
To investigate the relevance of vascular endothelium cyclo-oxygenase activity inhibition, several groups of different tail artery preparations subjected to 8 Hz TNS were treated with vehicle, NCX 4016 (from 12.5 to 100 μM), aspirin (100 μM), NCX 4023 (from 12.5 to 100 μM) and salicylic acid (100 μM). In these experiments, the above four drugs were all perfused for 15 min during 8 Hz TNS. At the end of this time, the organ bath was cleared out and the bathing solution (10 ml) was stored at −70°C for 6-keto-PGF1α determination which was performed by means of a specific enzyme immunoassay kit according to the method described by Pradelles et al. (1985). The release of 6-keto-PGF1α was expressed as picograms per ml per mg of wet tissue (pg ml−1 mg w.t.−1). Furthermore, in the same tail arteries the activity of the above compounds against neurogenic vasoconstriction was evaluated and, for NCX 4016 and NCX 4023, the inhibitory effect was expressed as a concentration able to reduce the response induced by 8 Hz TNS by 50% (IC50).
NCX 4016-relationship to cyclic GMP formation
To study the mechanism of action of NCX 4016, the vasorelaxant activity of this compound in NE-precontracted tail arteries and the concomitant increment of tissue cyclic GMP content were evaluated.
Five different groups of perfused tail artery preparations were contracted with NE (30 nM) and when the increase of perfusion pressure reached the plateau (about 5 min), NCX 4016 (100 μM), NCX 4023 (100 μM), sodium nitroprusside (SNP, 1 μM) or acetylcholine (ACh, 10 μM) were perfused for 1 min. Within this time these four compounds caused the maximal vasorelaxation. The tail arteries were then rapidly removed (10 s) from the organ bath, immediately frozen in liquid nitrogen and stored at −70°C for cyclic GMP determination following the procedure described by Bossaller et al. (1987). The tissue cyclic GMP content was assayed in duplicate by using a commercially available enzyme immunoassay kit (cross-reactivity with adenosine 3′ : 5′-cyclic monophosphate is less than 0.0001%). Baseline cyclic GMP was defined as the tissue cyclic GMP content at the maximal effect of NE. Results were expressed as picomoles of cyclic GMP per mg of protein (pmol mg−1 protein).
To investigate the involvement of soluble guanylate cyclase activation, two well-known inhibitors of the above enzyme system, methylene blue (MB) or 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (Garthwaite et al., 1995; Moro et al., 1996) were used in another series of experiments. For these studies, six different groups of perfused tail artery were precontracted with NE (30 nM) and the vasorelaxant effects of bolus injection of graded concentrations (from 3.1 to 200 μM) of both NCX 4016 and NCX 4023 were recorded in absence (vehicle) and in presence of MB (1 μM) or ODQ (1 μM). These two guanylate cyclase inhibitors were perfused for 15 min before NE and the treatment was maintained throughout the experiment.
NCX 4016-relationship to NO synthase activity
The influence of NO synthase inhibition with NG-monomethyl-L-arginine (L-NMMA, 10 μM) given for 14 min to different groups of perfused tail artery preparations on both basal perfusion pressure and its changes due to low frequency of TNS (2 Hz) was evaluated. Similar experiments were performed in a separate group of perfused tail arteries by bolus injections (5 min apart) of low concentration of NE (0.1 μM). In all these experiments, NCX 4016 (25, 50 and 100 μM), aspirin (100 μM), L-arginine (100 μM) and D-arginine (100 μM) were perfused through the arteries for 15 min before L-NMMA and perfusion with these compounds continued until the end of L-NMMA treatment.
Statistical analysis
Data are presented as mean±s.e.mean and n reflects the number of rats from which the tail arteries were obtained. In some figures the error bars fall within the symbol size. Concentration-effect curves were fitted using the computer program Origin 3.5 (Microcal Software Inc., Northampton, MA, U.S.A.). Experimental groups were compared by ANOVA and, when appropriate, with Bonferroni's test for multiple comparisons. Differences were considered statistically significant at P<0.05.
Drugs
The following drugs were used: NCX 4016 and NCX 4023 (NicOx S.A., Valbonne-Sophia Antipolis, France); aspirin, salicylic acid, acetylcholine chloride, norepinephrine bitartrate, sodium nitroprusside, methylene blue, NG-monomethyl-L-arginine, 1H - [1,2,4] Oxadiazolo [4,3-a] quinoxalin-1-one (ODQ) and dimethylsulphoxide (DMSO) (Sigma Chem. Co., St. Louis, MO, U.S.A.); kits for guanosine 3′ : 5′-cyclic monophosphate and 6-keto-prostaglandin F1α determinations (Amersham Italia, MI, Italy). NCX 4016, NCX 4023, aspirin and ODQ were first dissolved in 100% DMSO to produce a concentrated stock solution (10 mM) from which final bath dilutions were made. The DMSO (vehicle) concentration did not elicit any effects per se on the parameters tested. Norepinephrine was dissolved in Krebs Henseleit solution with 70 μM ascorbic acid added as an antioxidant. All the drugs were prepared daily.
Results
NCX 4016-relationship to adrenergic vasoconstriction
When the perfused rat tail artery preparations were subjected to TNS (from 0.5 to 64 Hz), a frequency-dependent rise in perfusion pressure was observed (Figure 1). Tetrodotoxin (1 μM) or phentolamine (1 μM) fully antagonized TNS-induced vasoconstriction indicating adrenergic nerve activation in the vascular segment (data not shown). NCX 4016 (25, 50 and 100 μM) perfused for 15 min did not change the basal perfusion pressure (22±2 mmHg) of rat tail arteries, but significantly reduced the vasoconstriction due to TNS at the established frequencies (Figure 1). Similar results were obtained by challenging the vascular preparations with NE (from 0.01 to 10 μM). In this instance NCX 4016 dose-dependently inhibited the rise of perfusion pressure due to NE (Figure 2). The activity of NCX 4016 was rather prompt in onset, attaining the peak effect in 10–12 min. The duration of this activity against both TNS or NE-induced vasoconstriction was proportional to the concentration of NCX 4016 used being at 100 μM around approximately 60 min (data not shown). Aspirin (100 μM) did not affect the resting perfusion pressure nor did it change the responses to TNS or NE (Figures 1 and 2).
Figure 1.
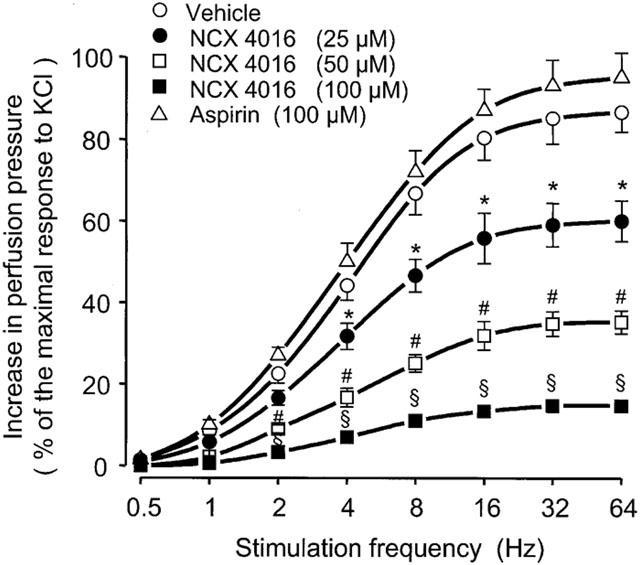
Effects of vehicle, NCX 4016 and aspirin on transmural nerve stimulation (TNS; from 0.5 to 64 Hz)-induced vasoconstriction in perfused rat tail arteries. Treatment with drugs started 15 min before TNS and was maintained throughout the experiment. Values are means±s.e.mean, n=7 different arteries per group. *P<0.05, #P<0.01, §P<0.001 compared to the corresponding TNS-frequency in vehicle-treated preparations.
Figure 2.
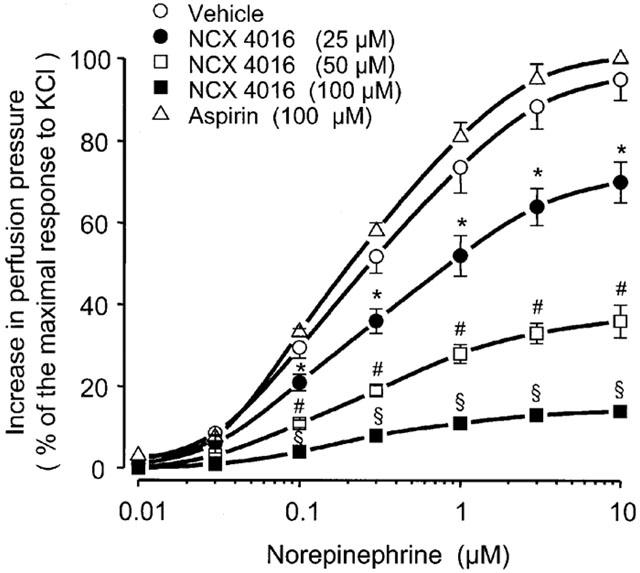
Effects of vehicle, NCX 4016 and aspirin on norepinephrine (NE; bolus injection from 0.01 to 10 μM)-induced vasoconstriction in perfused rat tail arteries. Treatment with drugs started 15 min before NE and was maintained throughout the experiment. Values are means±s.e.mean, n=7 different arteries per group. *P<0.05, #P<0.01, §P<0.001 compared to the corresponding NE-concentration in vehicle-treated preparations.
Figure 3 shows the inhibitory activity of NCX 4016, NCX 4023 (the nitro-phenyl ester of salicylic acid), aspirin and salicylic acid on both neurogenic vasoconstriction and 6-keto-PGF1α release in the same perfused tail artery preparation. NCX 4016 (IC50=27.2 μM; conf. lim. 23.3–31.6 μM) was 1.6 fold more potent (P<0.05) than NCX 4023 (IC50=44.0 μM; conf. lim. 35.6–52.4 μM) in antagonizing the increase of perfusion pressure due to 8 Hz TNS whereas neither aspirin nor salicylic acid changed the reactivity of the arteries to neurogenic stimulation (Figure 3). In these same experiments, the amount of 6-keto-PGF1α found in the organ bath at the end of 15 min 8 Hz TNS increased 4.8 fold as compared to that measured in tail arteries not subjected to 8 Hz TNS (8.5±1 pg ml−1 mg w.t.−1). NCX 4016 dose-dependently reduced the release of 6-keto-PGF1α (IC50=24.8 μM; conf. lim. 20.6–29.3 μM) whereas neither salicylic acid (100 μM) nor NCX 4023 (100 μM) affected the generation of 6-keto-PGF1α during 8 Hz TNS. Aspirin (100 μM) fully abolished the amount release of this lipidic material (Figure 3).
Figure 3.
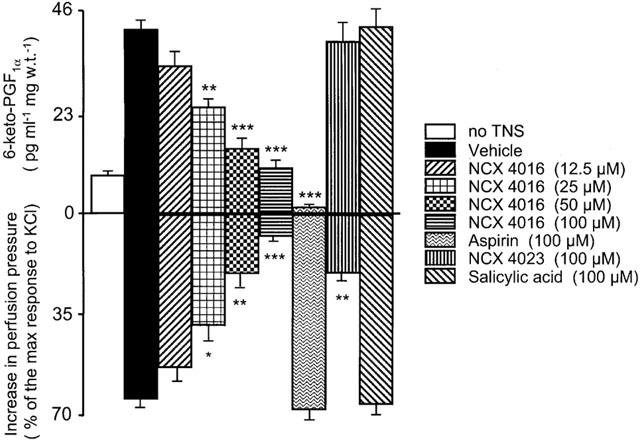
Effects of vehicle, NCX 4016, NCX 4023, aspirin and salicylic acid on the release of 6-keto-PGF1α (upper panel) from perfused rat tail arteries submitted to 8 Hz transmural nerve stimulation (TNS) (lower panel). Drugs were perfused through the arteries for 15 min and the related perfusates were collected at the end of 15 min for 6-keto-PGF1α determination. Values are means±s.e.mean, n=8 different arteries per group. 8 Hz TNS-elicited 6-keto-PGF1α formation was significantly inhibited by NCX 4016 and aspirin (*P<0.05, **P<0.01, ***P<0.001 compared to vehicle-treated preparations).
NCX 4016-relationship to cyclic GMP formation
The vasorelaxant activity of bolus injection of NCX 4016 (100 μM), NCX 4023 (100 μM), SNP (1 μM) and ACh (10 μM) through NE-precontracted perfused rat tail artery preparations was accompanied by an important increase of vascular tissue cyclic GMP (Figure 4). In particular, the activity of NCX 4016 (96±3% reduction of NE-induced contraction; P<0.001) was associated to a marked accumulation of cyclic GMP in the vasculature (4.9 fold; P<0.001) as compared to that found in vehicle-treated arteries (0.37±0.04 pmol mg−1 protein) at the maximal effect of NE (Figure 4).
Figure 4.
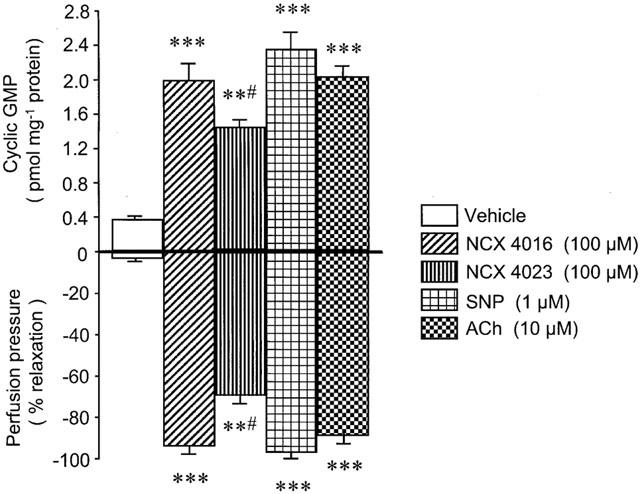
Cyclic GMP-tissue accumulation (upper panel) during maximal (1 min) vasorelaxant response (lower panel) induced by bolus injection of vehicle, NCX 4016, NCX 4023, sodium nitroprusside (SNP) and acetylcholine (ACh) in perfused rat tail arteries precontracted with norepinephrine (30 nM). Values are means±s.e.mean, n=8 different arteries per group. **P<0.01 and **P<0.001 compared to vehicle-treated preparations. #P<0.05 compared to NCX 4016, SNP and ACh-treated preparations.
When the soluble guanylate cyclase was impaired with MB (1 μM) or ODQ (1 μM) in perfused rat tail artery preparations the ability to reverse NE-induced vasoconstriction of both NCX 4016 and NCX 4023, even at the higher concentrations, was remarkably reduced (Figure 5).
Figure 5.
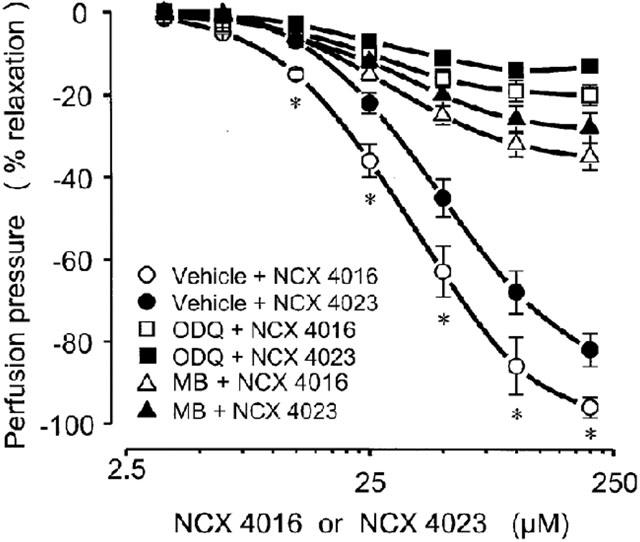
Vasorelaxant effects of bolus injection of graded concentrations of NCX 4016 and NCX 4023 (from 3.1 to 200 μM) in absence (vehicle) or in presence of soluble guanylate cyclase inhibitors, methylene blue (MB; 1 μM) or 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 1 μM), in perfused rat tail arteries precontracted with norepinephrine (30 nM). Vehicle, MB and ODQ were perfused through the arteries for 15 min before the dose-response curves with NCX 4016 or NCX 4023 and these treatments were maintained throughout the experiment. Values are means±s.e.mean, n=8 different arteries per group. *P<0.05 only compared to vehicle+NCX 4023-treated preparations. Both MB and ODQ significantly decreased (P<0.01) the corresponding vasodilator effect in vehicle+NCX 4016 and vehicle+NCX 4023-treated preparations.
NCX 4016-relationship to NO synthase activity
When the rat tail arteries were perfused for 14 min with L-NMMA (10 μM), the resistance of the vessel progressively increased being 31±2% (P<0.001 vs vehicle) of the maximal response to KCl (158±11 mmHg) at the end of L-NMMA perfusion (Figure 6). This effect of L-NMMA was almost completely prevented by L-arginine (100 μM) but not by D-arginine (100 μM). Moreover, at variance with aspirin (100 μM), NCX 4016 (25, 50 and 100 μM) dose dependently attenuated the rise in perfusion pressure of tail artery preparations (Figure 6).
Figure 6.
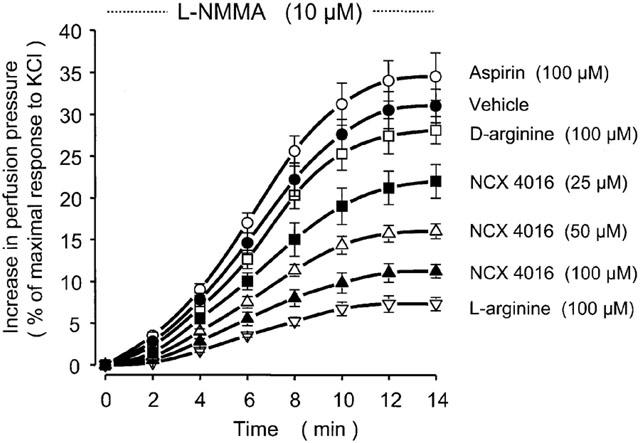
Effects of vehicle, NCX 4016, aspirin, L-arginine and D-arginine on perfusion pressure of perfused rat tail arteries during 14 min-treatment with NG-monomethyl-L-arginine (L-NMMA). Drugs were given through the arteries for 15 min before L-NMMA and this treatment was maintained until the end of L-NMMA administration. Values are means±s.e.mean, n=7 different arteries per group. Considering the area under the curves: P<0.05 for NCX 4016 (25 μM), P<0.01 for NCX 4016 (50 μM), P<0.001 for NCX 4016 (100 μM) and L-arginine (100 μM) all compared to vehicle-treated preparations.
The blockade of NO generation with L-NMMA in perfused rat tail artery resulted in a remarkably increased sensitivity of the preparation to 2 Hz TNS (Figures 7 and 8). In fact, in vehicle-treated arteries, changes in perfusion pressure caused by 2 Hz TNS in presence of L-NMMA was 2.8 fold (P<0.01) greater than that obtained with 2 Hz TNS in absence of L-NMMA (22.4±2.3% of the maximal response to KCl) (Figure 7). NCX 4016 (100 μM), perfused through the artery before and during L-NMMA treatment, abolished the increased sensitivity of the vascular tissue to 2 Hz TNS. In this instance, the increase in perfusion pressure caused by 2 Hz TNS in presence of L-NMMA was only 14.5±3% of the maximal response to KCl (Figure 7). Two representative traces of the experiments performed are depicted in Figure 8. Furthermore, similar results were obtained in other separate experiments using bolus injection (5 min apart) of low concentration (0.1 μM) of NE. In this case, the enhanced reactivity (3.1 fold; P<0.01) of the perfused rat tail artery preparations to NE in presence of L-NMMA was completely abolished by NCX 4016 (100 μM) (data not shown). L-arginine (100 μM), but not D-arginine (100 μM), was effective in reducing the increased sensitivity of the vascular segments to 2 Hz TNS. Aspirin (100 μM) was devoid of any activity in preventing the hyperreactivity of the perfused vessels to 2 Hz TNS caused by NO synthase inhibition (Figure 7).
Figure 7.
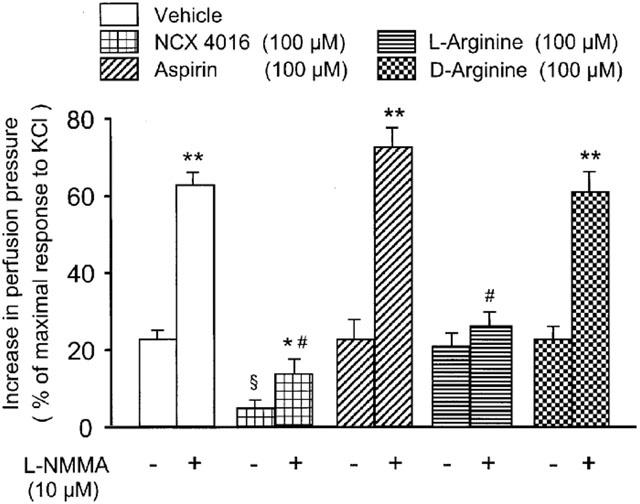
Effects of vehicle, NCX 4016, aspirin, L-arginine and D-arginine in absence (−) or in presence (+) of NG-monomethyl-L-arginine (L-NMMA) on the perfusion pressure of perfused rat tail artery preparations subjected to 2 Hz transmural nerve stimulation (TNS). Drugs were perfused for 15 min before L-NMMA and for another period of 14 min in presence of L-NMMA. Values are means±s.e.mean, n=7 different arteries per group. §P<0.01 compared to 2 Hz TNS-response in vehicle-treated preparations without L-NMMA, *P<0.05 and **P<0.01 compared to the corresponding 2 Hz TNS-response without L-NMMA, #P<0.01 compared to 2 Hz TNS-response in vehicle-treated preparations plus L-NMMA.
Figure 8.
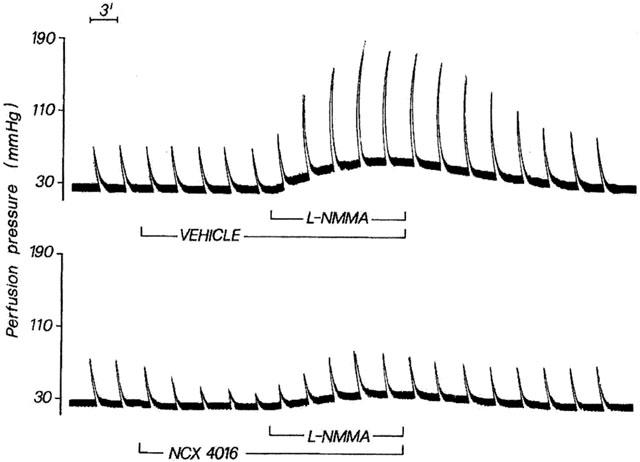
Two original traces related to perfused rat tail arteries subjected to 2 Hz transmural nerve stimulation and perfused for 14 min with L-NMMA (10 μM) in presence of vehicle or NCX 4016 (100 μM). Vehicle or NCX 4016 were perfused for 15 min before L-NMMA and for another period of 14 min in presence of L-NMMA.
Discussion
The results reported here clearly demonstrate that the NO-releasing derivative of aspirin, NCX 4016, reduces vasoconstriction due to TNS at various frequencies and prevents the effect of different concentrations of NE in perfused rat tail artery preparations with intact endothelium.
The dominant effects observed with NCX 4016 in these vascular preparations appear to be mediated by NO-donation. This important point has already been addressed in vitro (Wallace et al., 1995) in human platelets by measuring NO generation by chemiluminescence and in vivo (Takeuchi et al., 1998) in pylorus-ligated rats where NCX 4016 administration resulted in elevated levels of NO both in gastric contents and plasma. Furthermore, nitrosylhaemoglobin, a marker of NO-release, has been detected by electron spin resonance spectroscopy in venous blood of rats treated orally with NCX 4016 (Carini et al., 2001). The hypothesis of NO-donation is also supported by the fact that the vasorelaxant action of NCX 4016 (100 μM) in NE-precontracted tail arteries is accompanied by a remarkable increase (4.9-fold; P<0.001) in tissue levels of cyclic GMP and is substantially antagonized by inhibition of guanylate cyclase activity with MB or ODQ. The latter is a potent inhibitor of NO-stimulated soluble guanylate cyclase activity, without actions on particulate guanylate cyclase or adenylate cyclase (Garthwaite et al., 1995). Therefore, it is reasonable to speculate that NCX 4016 activity in tail artery preparations may involve a direct and specific increase in cyclic GMP in vascular smooth muscle cells.
Cyclic GMP is considered to be an important regulator of smooth muscle function, and the action of NO-containing vasodilators seems to be mediated by cyclic GMP. Although the mechanism of action of this cyclic nucleotide is unknown, studies in several laboratories have demonstrated that cyclic GMP lowers intracellular Ca2+ in agonist-treated and depolarized smooth muscles (Kaur et al., 1998; Lincoln et al., 2001; Brophy et al., 2002). On this ground, Cornwell & Lincoln (1989), working in primary rat aortic cells, reported that cyclic GMP-dependent protein kinase appears to be the mediator of the reduction in Ca2+ levels upon elevation of the intracellular cyclic GMP. An interference of NCX 4016 via NO-donation with the prejunctional mechanisms regulating NE release is unlikely, since it has already been demonstrated (Bucher et al., 1992) that during TNS of rat tail artery preparations the concentration of NE found in perfusates is unaltered by compound modifying NO-production. Besides that, and according to these authors, the lack of prejunctional effects of both soluble and membrane-associated guanylate cyclase activators indicates that the endogenous cyclic GMP production, if present in sympathetic nerves, may not be involved in the regulation of NE release. However, since in the present experiments NE-concentration in perfusates was not measured, a modulation of perivascular adrenergic nerve activity by cyclic GMP cannot be completely ruled out.
The results obtained with L-NMMA-treated rat tail artery preparations clearly support the concept that the activity of NCX 4016 does not depend on NO released by the vascular endothelial cells. NCX 4016, likely by a direct donation of NO, which overcomes the inhibition of NO synthase activity, prevents the rise in perfusion pressure of vascular segments and abolishes the increased sensitivity of vascular smooth muscles to neurogenic stimulation and NE.
Another point of interest emerging from the present study is the low significance of the aspirin-moiety on the overall activity of NCX 4016. This compound maintains its activity against vasoconstriction in spite of inhibition of 6-keto-PGF1α generation, a lipidic material which is known in other in vitro models of vascular preparations to take part with NO in the modulation of vascular tonus and vasoconstriction (Bessenge, 1992; Berti et al., 1993). The results obtained with the NO releaser derivative of salicylic acid, NCX 4023, further support the above observation. This compound does not interfere with 6-keto-PGF1α formation and appears to counteract adrenergic vasoconstriction via cyclic GMP accumulation in vascular tissue with a potency close to that of NCX 4016. It is likely that slight differences in potencies among NCX 4023 and NCX 4016 in antagonizing adrenergic vasoconstriction my be ascribed to differences in NO-releasing properties of the two compounds. It is also worth pointing out that PGI2, which is known to be produced in the rat tail artery via α1-adrenergic receptors stimulation by NE (Golub et al., 1985a, b), does not appear to play a significant modulatory role during vasoconstriction in normal tail artery preparations. Although, 6-keto-PGF1α production is abolished by aspirin the basal tonus of the vascular segment did not increase nor were the vasoconstrictor responses potentiated. This would imply that the subluminal release of PGI2 from endothelial cells toward adjacent vascular smooth muscles is slight compared with NO release. Therefore when eicosanoid formation is blocked, the abluminal release of NO from endothelial cells is sufficient to maintain the vascular tonus within the normal range. It is likely that cyclo-oxygenase becomes more important and PGI2 production may substitute NO only when generation of this mediator from endothelial cells is lost.
The present experiments, however, demonstrate that this does not appear to be the case. In fact, in tail arteries perfused with L-NMMA, the concomitant inhibition of prostaglandin formation did not result in a further increase in basal perfusion pressure, nor did it induce hyperreactivity of the vascular segments to adrenergic stimulation, hyperreactivity already enhanced by NO synthase inhibition.
The fact that NCX 4016 antagonizes adrenergic vasoconstriction via cyclic GMP-mediated mechanism has recently been reported by Muscarà et al. (2001) in phenylephrine-precontracted aortic rings harvested from L-NAME-treated and control rats. In this study, the delivery of NO by NCX 4016 partially compensated the endothelial dysfunction caused by chronic administration of L-NAME and the observed decrease of vascular smooth muscle contraction to NE may have played a contributory role in the explanation of the antihypertensive effect of NCX 4016 in these rats.
Considering that NO insufficiency limits NO-mediated signal transduction of normal or protective physiological process, replacement or augmentation of endogenous NO by NCX 4016, may provide the foundation for a broad field of pharmacotherapeutics in cardiovascular medicine. This is particularly true for this NO-releasing derivative of aspirin, a compound endowed with many beneficial properties like a potent antithrombotic action (Lechi et al., 1996), restenosis control after arterial injury (Napoli et al., 2001) and gastrointestinal safety in those conditions of increased sensitivity of gastric damage (Tashima et al., 2000; Kato et al., 2001).
Finally, the ability of NCX 4016 to attenuate vascular reactivity to adrenergic stimulation, even when the endothelial-dependent relaxant function is impaired, may bear therapeutic potential for the treatment of ongoing process related to those conditions of the cardiovascular systems complicated by sustained catecholamines production.
Abbreviations
- ACh
acetylcholine
- cyclic GMP
guanosine 3′ : 5′-cyclic monophosphate
- DMSO
dimethyl sulphoxide
- 6-keto-PGF1α
6-keto-prostaglandin F1α
- KHS
krebs Henseleit solution
- L-NAME
NG-nitro-L-arginine methyl ester
- L-NMMA
NG-monomethyl-L-arginine
- MB
methylene blue
- NE
norepinephrine
- NCX 4016
2-acetoxy-benzoate 2-[1-nitroxy-methyl]-phenyl ester
- NCX 4023
2-hydroxybenzoic acid 3-(nitrooximethyl)-phenyl ester
- NO
nitric oxide
- ODQ
1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one
- PGI2
prostacyclin
- SNP
sodium nitroprusside
- TNS
transmural nerve stimulation
References
- AHLNER J., LISEGREN M.E., GRUNDSTRÖM N., AXELSSON K.L. Role of nitric oxide and cyclic GMP as mediators of endothelium-independent neurogenic relaxation in bovine mesenteric artery. Circ. Res. 1991;68:756–762. doi: 10.1161/01.res.68.3.756. [DOI] [PubMed] [Google Scholar]
- BERTI F., BERNAREGGI V., MANDELLI V. Contraction and relaxation of in vitro perfused rat caudal artery: a possible role for 3′,5′-AMP. Arch. Int. Pharmacodyn. Ther. 1971;192:247–254. [PubMed] [Google Scholar]
- BERTI F., ROSSONI G., DELLA BELLA D., VILLA L.M., BUSCHI A., TRENTO F., BERTI M., TONDO C. Nitric oxide and prostacyclin influence coronary vasomotor tone in perfused rabbit heart and modulate endothelin-1 activity. J. Cardiovasc. Pharmacol. 1993;22:321–326. doi: 10.1097/00005344-199308000-00023. [DOI] [PubMed] [Google Scholar]
- BERTI F., ROSSONI G., FERRO L., TRENTO F., VILLA L.M., POMPILIO G., SALA A. Defibrotide, via prostacyclin release, antagonizes endothelin-1 induced vasospasm in rabbit and human coronary artery. Int. Med. 1994;2:49–56. [Google Scholar]
- BESSENGE E.Endothelial regulation of coronary tone Endothelial regulation of vascular tone 1992New York: Marcel Dekker Inc; 225–264.ed. Ryan, U.S. & Rubanyi, G.M. pp [Google Scholar]
- BOSSALLER C., HABIB G.B., YAMAMOTO H., WILLIAMS C., WELLS S., HENRY P.D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5′-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J. Clin. Invest. 1987;79:170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROPHY C.M., WOODDRUM D.A., POLLOCK J., DICKINSON M., KOMALAVILAS P., CORNWELL T.L., LINCOLN T.M. CGMP-dependent protein kinase expression restores contractile function in cultured vascular smooth muscle cells. J. Vasc. Res. 2002;39:95–103. doi: 10.1159/000057758. [DOI] [PubMed] [Google Scholar]
- BUCHER B., OUEDRAOGO S., TSCHÖPL M., PAYA D., STOCLET J.C. Role of the L-arginine-NO pathway and cyclic GMP in electrical field-induced noradrenaline release and vasoconstriction in the rat tail artery. Br. J. Pharmacol. 1992;107:976–982. doi: 10.1111/j.1476-5381.1992.tb13394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARINI M., ALDINI G., STEFANI R., FACINO R.M. Nitrosylhemoglobin, an unequivocal index of nitric oxide release from nitroaspirin: in vitro and in vivo studies in the rat by electron spin resonance (ESR) spectroscopy. J. Pharm. Biomed. Anal. 2001;26:509–518. doi: 10.1016/s0731-7085(01)00478-2. [DOI] [PubMed] [Google Scholar]
- CORNWELL T.L., LINCOLN T.M. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. J. Biol. Chem. 1989;264:1146–1155. [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GOLUB M.S., BERGER M.E., POWELL M. Adrenergic stimulation of prostacyclin production in the rat tail artery. I. Response to agonist and antagonist. Prostaglandins Leukot. Med. 1985a;20:299–311. doi: 10.1016/0262-1746(85)90152-0. [DOI] [PubMed] [Google Scholar]
- GOLUB M.S., BERGER M.E., CARNAZZOLA A.E. Adrenergic stimulation of prostacyclin production in the rat tail artery. II. The role of calcium. Prostaglandins Leukot. Med. 1985b;20:313–324. doi: 10.1016/0262-1746(85)90153-2. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BYRNS R.E., BUGA G.M., WOOD K.S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ. Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- KATO S., SUZUKI K., UKAWA H., KOMOIKE Y., TAKEUCHI K. Low gastric toxicity of nitric oxide-releasing aspirin, NCX-4016, in rats with cirrhosis and arthritis. Dig. Dis. Sci. 2001;46:1690–1699. doi: 10.1023/a:1010601520497. [DOI] [PubMed] [Google Scholar]
- KAUR K., YAO J., PAN X., MATTHEWS C., HASSID A. NO decreases phosphorylation of focal adhesion proteins via reduction of Ca in rat aortic smooth muscle cells. Am. J. Physiol. 1998;274:H1613–H1619. doi: 10.1152/ajpheart.1998.274.5.H1613. [DOI] [PubMed] [Google Scholar]
- LECHI C., ANDRIOLI G., GAINO S., TOMMASOLI R., ZULIANI V., ORTOLANI R., DEGAN M., BENONI G., BELLAVITE P., LECHI A., MINUZ P. The antiplatelet effects of a new nitroderivative of acetylsalicylic acid: an in vitro study of inhibition on the early phase of platelet activation and on TXA2 production. Thromb. Haemost. 1996;76:791–798. [PubMed] [Google Scholar]
- LINCOLN T.M., DEY N., SELLAK H. Invited review: cyclic GMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J. Appl. Physiol. 2001;91:1421–1430. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MORO M.A., RUSSELL R.J., CELLEK S., LIZASOAIN I., SU Y., DARLEY-USMAR V.M., RADOMSKI M.W., MONCADA S. Cyclic GMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 1996;82:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCARÀ M.N., LOVREN F., MCKNIGHT W., DICAY M., DEL SOLDATO P., TRIGGLE C.R., WALLACE J.L. Vasorelaxant effects of nitric oxide-releasing aspirin derivative in normotensive and hypertensive rats. Br. J. Pharmacol. 2001;133:1314–1322. doi: 10.1038/sj.bjp.0704209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAPOLI C., CIRINO G., DEL SOLDATO P., SORRENTINO R., SICA V., CONDORELLI M., PINTO A., IGNARRO L.J. Effect of nitric oxide-releasing aspirin versus aspirin on restenosis in hypercholesterolemic mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2860–2864. doi: 10.1073/pnas.041602898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.M.J., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., ASHTON D.S., MONCADA S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- PRADELLES P., GRASSI J., MACLOUF J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioimmunoassy. Anal. Chem. 1985;57:1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- ROSSONI G., SALA A., BUCCELLATI C., MACLOUF J., FOLCO G.C., BERTI F. Vasoconstriction to polymorphonuclear leukocytes in the isolated, perfused rabbit heart: inhibition by prostacyclin mimetics. J. Cardiovasc. Pharmacol. 1996;27:680–685. doi: 10.1097/00005344-199605000-00010. [DOI] [PubMed] [Google Scholar]
- ROSSONI G., BERTI M., DE GENNARO COLONNA V., BERNAREGGI M., DEL SOLDATO P., BERTI F. Myocardial protection by the nitroderivative of aspirin, NCX 4016: in vitro and in vivo experiments in the rabbit. Ital. Heart. J. 2000;1:146–155. [PubMed] [Google Scholar]
- ROSSONI G., MANFREDI B., DE GENNARO COLONNA V., BERNAREGGI M., BERTI F. The nitroderivative of aspirin, NCX 4016, reduces infarct size caused by myocardial ischemia-reperfusion in the anaesthetized rat. J. Pharmacol. Exp. Ther. 2001;297:380–387. [PubMed] [Google Scholar]
- ROSSONI G., MUSCARA M.N., CIRINO G., WALLACE J.L. Inhibition of cyclo-oxygenase-2 exacerbates ischemia-induced acute myocardial dysfunction in the rabbit. Br. J. Pharmacol. 2002;135:1540–1546. doi: 10.1038/sj.bjp.0704585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKUMA I., STUEHR D.J., GROSS S.S., NATHAN C., LEVI R. Identification of arginine as a precursor of endothelium-derived relaxing factor (EDRF) Proc. Natl. Acad. Sci. U.S.A. 1988;85:8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOKAS E.G., FOLCO G.C. Intima-related vasodilatation of the perfused rat caudal artery. Eur. J. Pharmacol. 1984;100:211–217. doi: 10.1016/0014-2999(84)90225-5. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI K., UKAWA H., KONAKA A., KITAMURA M., SUGAWA Y. Effect of nitric oxide-releasing aspirin derivative on gastric functional and ulcerogenic responses in rats: comparison with plain aspirin. J. Pharmacol. Exp. Ther. 1998;286:115–121. [PubMed] [Google Scholar]
- TASHIMA K., FUJITA A., UMEDA M., TAKEUCHI K. Lack of gastric toxicity of nitric oxide-releasing aspirin, NCX 4016, in the stomach of diabetic rats. Life Sci. 2000;67:1639–1652. doi: 10.1016/s0024-3205(00)00746-3. [DOI] [PubMed] [Google Scholar]
- TESFAMARIAN B., WEISBROD R.M., COHEN R.A. Endothelium inhibits responses of rabbit carotid artery to adrenergic nerve stimulation. Am. J. Physiol. 1987;253:H792–H798. doi: 10.1152/ajpheart.1987.253.4.H792. [DOI] [PubMed] [Google Scholar]
- TODA N., YOSHIDA K., OKAMURA T. Analysis of the potentiating action of NG-nitro-L-arginine on the contraction of the dog temporal artery elicited by transmural stimulation of noradrenergic nerves. Naunyn.-Schmiedebergs. Arch. Pharmacol. 1991;343:221–224. doi: 10.1007/BF00168614. [DOI] [PubMed] [Google Scholar]
- WAINWRIGHT C.L., MILLER A.M., WORK L.M., DEL SOLDATO P. NCX 4016 (NO-aspirin) reduces myocardial infarct size and suppresses arrhythmias following myocardial ischaemia/reperfusion in pigs. Br. J. Pharmacol. 2002;135:1882–1888. doi: 10.1038/sj.bjp.0704646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., DEL SOLDATO P., BAYDOUN A.R., CIRINO G. Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing aspirin derivative. J. Clin. Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE J.L., MUSCARA M.N., MCKNIGHT W., DICAY M., DEL SOLDATO P., CIRINO G. In vivo antithrombotic effects of a nitric oxide-releasing aspirin derivative, NCX 4016. Thromb. Res. 1999;93:43–50. [Google Scholar]


