Abstract
Toluene is a representative example of a class of industrial solvents that are voluntarily inhaled as drugs of abuse. Previous data from this lab and others has shown that toluene modulates the function of N-methyl-D-aspartate (NMDA), γ-aminobutyric acid (GABA) and glycine receptors at concentrations that do not affect non-NMDA receptors.
We utilized two-electrode voltage-clamp and whole cell patch-clamp techniques to assess the effects of toluene on neuronal nicotinic acetylcholine receptors expressed in oocytes and cultured hippocampal neurons. Toluene (50 μM to 10 mM) produced a reversible, concentration-dependent inhibition of acetylcholine-induced current in Xenopus oocytes expressing various nicotinic receptor subtypes. The α4β2 and α3β2 subunit combinations were significantly more sensitive to toluene inhibition than the α4β4, α3β4 and α7 receptors.
Receptors composed of α4 and β2(V253F) subunits showed α4β4-like toluene sensitivity while those containing α4 and β4(F255V) subunits showed α4β2-like sensitivity.
In hippocampal neurons, toluene (50 μM to 10 mM) dose-dependently inhibited ACh-mediated responses with an IC50 of 1.1 mM.
Taken together, these results suggest that nicotinic receptors, like NMDA receptors, show a subunit-dependent sensitivity to toluene and may represent an important site of action for some of the neurobehavioural effects of toluene.
Keywords: Toluene, Xenopus oocyte, hippocampal neuron, nicotinic acetylcholine receptor
Introduction
Volatile solvents including toluene and related compounds represent one of the largest classes of abused inhalants. These solvents are found in many household and commercial products including gasoline, paint thinner, glues and other adhesives. The voluntary inhalation of these substances (‘huffing' or ‘bagging') is a world-wide drug problem and is especially prevalent among adolescent males. It is among the most common form of substance abuse in countries such as Japan, Mexico, and Colombia and inhalant abuse in the US and Canada is rising. About 6% of children in the United States have tried inhalants by the time they reach fourth grade and 15–20% of junior-high and high school students report using abused inhalants at least once (NIDA, 1988). Rates of inhalant abuse are even higher in native American populations.
Behavioural studies have shown that toluene causes psychomotor impairment (Bowen & Balster, 1996), has anti-convulsant and anti-anxiety effects (Wood et al., 1984) and shows ethanol and barbiturate-like effects in drug discrimination tests (Rees et al., 1987a, b). Based on these findings, it has been hypothesized that abused inhalants like toluene may share sites and mechanisms of action with other better characterized CNS depressants.
Recent studies examining the effects of volatile solvents on native and recombinant ion channels support this hypothesis. For example, toluene and other alkylbenzenes dose-dependently inhibited the function of NMDA but not non-NMDA receptors expressed in Xenopus oocytes (Cruz et al., 1998; 2000). The NR1/2B receptor subtype was more sensitive to toluene inhibition than either NR1/2A or NR1/2C with IC50 values for toluene of (in mM) 0.17, 1.4 and 2.1, respectively (Cruz et al., 1998). Toluene potentiated the effects of GABA and glycine on oocytes injected with GABAA or glycine receptor subunits and shifted the agonist dose response curves for these receptors left-ward similar to that seen for barbiturates and benzodiazepines (Beckstead et al., 2000). These results suggest that toluene and other abused solvents may target ligand-gated ion channels that are also sensitive to ethanol and other CNS depressants.
One such class of receptors is the family of nicotinic acetylcholine receptors (nAChRs) that are widely distributed throughout the central and peripheral nervous systems. Unlike nAChRs located on skeletal muscle that contain four different subunits, neuronal nAChRs are composed of α and β subunits that are distinct from their muscle-type counterparts. These receptors can be classified as either heteromeric (α/β) or homomeric (α) and two major brain subtypes are the α4/β2 and the α7 receptors. Homomeric α7 receptors are calcium permeable and blocked by α-bungarotoxin while heteromeric nAChRs show little calcium permeability and are insensitive to α-bungarotoxin. Nicotinic receptors have been implicated in regulating various aspects of synaptic neurotransmission and may be involved in cognition and memory (Albuquerque et al., 1997; Colquhoun & Patrick, 1997).
Although nAChRs are best known as targets for nicotine delivered from tobacco smoking, a variety of CNS depressants including volatile anaesthetics and alcohols have also been shown to modulate nicotinic receptor function. For example, heteromeric neuronal nAChRs containing the β2 subunit are sensitive to general anaesthetics such as halothane (Violet et al., 1997; Yamakura et al., 2000). In addition, alcohols produce bi-phasic effects on native and recombinant heteromeric neuronal nAChRs with short-chain alcohols such as ethanol producing potentiation and long-chain alcohols causing inhibition (Coverton & Connolly, 1997; Cardoso et al., 1999; Aistrup et al., 1999; Godden et al., 2001). Based on the effects of volatile anaesthetics and alcohols on neuronal nicotinic nAChRs, we hypothesized that toluene would also affect the function of these channels. The results show that nAChRs are inhibited by toluene in a subunit-dependent manner. Some of this work has been presented in preliminary form (Bale et al., 2000).
Methods
Synthesis of mRNA
cDNA clones of the rat nAChRs (α4, α3, β2, α7 provided by Dr J. Patrick, β4 provided by Dr MW Nowak, β2(V253F), β4(F255V) provided by Dr RA Harris) were linearized downstream from the coding sequence with restriction enzymes, and purified by phenol/chloroform extraction. The extracts were precipitated in ethanol and resuspended in diethyl pyrocarbonate-treated water. The mMessage machine kit (Ambion, Austin, TX, USA) was used to synthesize mRNA from the linearized transcripts.
Preparation of oocytes
Oocytes were prepared as previously described by Cruz et al. (1998). Briefly, adult Xenopus laevis female frogs were purchased from Xenopus I (Ann Arbor, MI, USA) and were housed in a de-chlorinated water compartment and fed twice weekly with beef liver. Frogs were anaesthetized in a 0.25% MS-222 (Sigma-Aldrich, St. Louis, MO, USA) solution and oocytes were surgically removed and placed in OR-2 buffer (in mM: NaCl 82, KCl 2, HEPES 5, MgCl2 1, pH 7.3). Oocytes were treated with collagenase (1 mg ml−1) in OR-2 buffer for 1 h to remove the follicular membrane just prior to mRNA injection.
mRNA injections
Oocytes (stage V and VI) were injected with 1–30 ng (with a total volume of 41.4 nl per oocyte) each of various nAChR subunit mRNAs (1 : 1 ratio unless otherwise noted) and maintained in 0.5×L-15 Leibovitz media (pH 7.4) supplemented with 10 mg l−1 penicillin G, 10 mg l−1 streptomycin and 15.5 mg l−1 gentamycin. Oocytes were incubated at 18°C for up to 7 days before recording.
Neuronal cell culture
Hippocampal cell cultures were prepared as previously described by Smothers et al. (1997). Briefly, hippocampi were excised from 18-day-old Sprague-Dawley rat foetuses. The tissue was trypsinized in 0.125% trypsin for 3 min and triturated with a narrow-bore pipette. The resulting dissociated cells were distributed onto sterile poly-L-lysine-coated sterile 35 mm culture dishes. The plating media for these cells consisted of Eagle's minimal essential medium (MEM) supplemented with 10% foetal calf serum, 10% heat-inactivated horse-serum and 2 mM L-glutamine. After 24 h of incubation at 37°C (95% CO2/5% O2), the plating media was replaced with MEM supplemented with 5% heat-inactivated horse-serum and 2 mM L-glutamine (feeding media). One week after neuronal plating 0.13 mg ml−1 of 5-fluoro-2′-deoxyuridine (FdU) and 0.3 mg ml−1 uridine were added to the feeding media to control astrocyte overgrowth. Cultures were incubated at 37°C (95% CO2/5% O2) and the feeding media was changed at least once a week. Neurons were recorded from after 4–6 weeks in culture.
Preparation of drug solutions
Drug solutions for Xenopus oocyte studies were prepared using a barium-containing extracellular solution (in mM: NaCl 115, KCl 2.5, HEPES (4-2-(hydroxyethyl)-1-piperazine-ethanesulfonic acid) 10, BaCl2 1.8 at a pH of 7.2). Barium was used as the divalent cation to prevent activation of any endogenous calcium-dependent chloride currents. Atropine (10 μM) was added to block endogenous muscarinic receptors expressed by the oocyte. In the whole cell patch clamp studies, drugs were prepared in a recording solution (in mM: NaCl 135, KCl 5.4, CaCl2 1.8, HEPES 10, glucose 10, pH 7.4). The solution was adjusted to 325 mOsm with sucrose. Tetrodotoxin (TTX; 100 nM) was added to prevent spontaneous activity and atropine was included to block muscarinic receptor responses. In both studies, solutions containing 3 mM toluene or less were made by dissolving toluene directly in buffer and were prepared fresh daily. Solutions containing greater than 6 mM toluene were made by mixing toluene and the vehicle, emulphor at a 1 : 1 ratio and diluting this suspension to the desired concentration with the extracellular buffer (Cruz et al., 1998).
Two-electrode voltage-clamp recordings
Oocytes were placed in a 200 μl recording chamber and continually perfused with extracellular solution at a flow rate of approximately 8–10 ml min−1. Microelectrodes filled with 3 M KCl (0.5–3.0 MΩ) were used to voltage clamp oocytes at a membrane potential of −80 mV using a GeneClamp 500 amplifier (Axon Instruments Inc., Foster City, CA, USA). The nAChRs were stimulated by applying a solution containing ACh (either 1, 1, 5, 30 or 150 μM for α4β2, α4β4, α3β2, α3β4 and α7 respectively). Oocytes were pretreated for 30 s in extracellular solution containing toluene before agonist plus toluene was applied. Following the toluene exposure, oocytes were perfused with extracellular solution for a period of 5–10 min before a second control ACh stimulation was performed. For the current-voltage(I-V) studies, current amplitudes (generated from agonist) were measured in the presence and absence of 1 or 3 mM toluene as membrane voltage was stepped from −120 to +80 mV in 400 ms intervals. Currents generated from oocytes were filtered at 10 Hz, digitized with a 16-bit analogue-to-digital interface (Instrutech, Port Washington, NY, USA) and recorded on a Macintosh computer running under the IGOR-Pro graphics platform (Wavemetrics, Lake Oswego, OR, USA).
Whole cell patch-clamp recordings
Neurons were continually perfused with extracellular recording solution as described above. Patch pipettes (4–6 MΩ) were filled with an internal solution (in mM: N-methyl glucamine 100, CsCl 40, MgCl2 2, ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) 10, ATP 2 and HEPES 10, pH 7.4, osmolarity adjusted to 325 mOsm with sucrose). The cell membrane potential was clamped at −50 mV using an Axon 200B amplifier (Axon Instruments Inc., Foster City, CA, USA). Control responses were obtained by rapidly (<10 ms) switching the extracellular solution to one containing 3 mM ACh using a SF-77A stepper (Warner Instruments, Hamden, CT, USA). Toluene was applied for 1 min before stimulating the neuron with solution containing ACh and toluene. ACh was re-applied after a 3–5 min washout of the toluene-containing solution to obtain a second control response. Currents from neurons were filtered at 5 kHz, digitized using an Instrutech analogue-to-digital interface, and collected on a Macintosh G3 computer with the Igor Pro software program (Wavemetrics).
Materials
Collagenase, tricaine methanesulphonate (MS-222), acetylcholine chloride, atropine and other reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA). TTX was purchased from RBI (Natwick, MA, USA). HPLC grade toluene was purchased from Aldrich (Milwaukee, WI, USA). Alkamuls EL-620 (emulphor) was obtained from Rhone-Poulenc (Princeton, NJ, USA). The mMessage machine kit was purchased from Ambion (Austin, TX, USA)
Data and statistical analysis
Current amplitudes were analysed using Axograph version 4.0 software (Axon Inc. Software, Foster City, CA, USA). The response obtained in the presence of toluene was divided by the average control response to give the per cent inhibition of receptor response. Dose response curves were generated using Prism 2.0a (GraphPad Software Inc., San Diego, CA, USA). IC50 values and 95% confidence limits were calculated using least squares linear regression analysis, followed by calculation of the confidence limits (Bliss, 1967). IC50 values for toluene between nAChRs were considered significant only if there was not an overlap of the 95% confidence limits.
Results
Acetylcholine induced large and reproducible inward currents in oocytes expressing various nicotinic receptor subunits. As previously reported, toluene itself had no effects on resting membrane currents at concentrations below 20 mM (Cruz et al., 1998). However, toluene significantly inhibited ACh-induced responses from all the heteromeric nAChRs tested (Figure 1). The concentration of ACh used in the toluene experiments was based on the approximate EC50 value reported for each subunit combination (Colquhoun & Patrick, 1997; Yamakura et al., 2000). Toluene inhibition was rapid in onset and was fully reversible upon washout of the toluene-containing solution. At 1 mM, toluene inhibited α4β4 receptors by 30% and α3β4 receptors by 40%. This inhibition increased to approximately 80% for the α4β2 and α3β2 receptors. Atropine (10 μM) was typically used to block endogenous muscarinic receptor activity in the oocyte. Atropine has been shown to cause subunit-dependent alterations in nicotinic acetylcholine receptors (Zwart & Vijverberg, 1997). To test whether atropine affected the degree of toluene inhibition, toluene-mediated inhibition (1 mM) of α4β2 and α4β4 nAChRs was assessed in the presence of 0.1 μM atropine or 10 μM atropine. In the presence of 0.1 μM atropine, toluene inhibited α4β4 and α4β2 receptors by 27% (±10.08) and 77.32% (±3.10) respectively (n=5). These values did not significantly differ from those obtained in the presence of 10 μM atropine (α4β4, 29.70±6.37%; α4β2, 74.04±2.79%).
Figure 1.
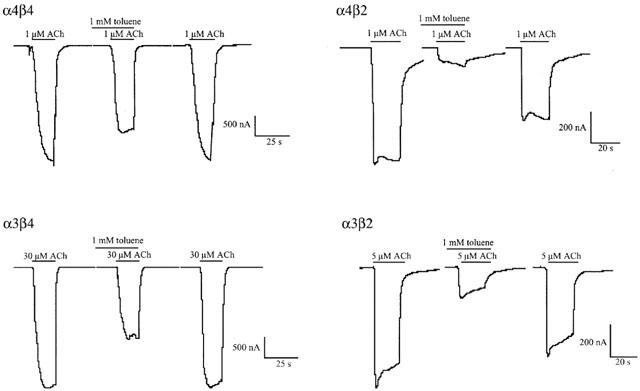
Effects of toluene on α4β2, α3β2, α3β4 and α4β4 nAChRs expressed in Xenopus oocytes. Representative traces are shown for each receptor subtype in response to ACh (1–30 μM) with and without toluene. Each set of the three traces comes from a separate oocyte voltage clamped at −80 mV.
Figure 2 shows the dose-response curves for toluene inhibition of α4β2, α3β2, α4β4, and α3β4 receptors. Inhibition was calculated as a percentage of the averaged control value for each oocyte tested and was determined using current amplitudes measured at the end of agonist application when currents were stable. Calculated IC50 values (95% confidence limits in parentheses) for toluene inhibition are listed in Table 1. The rank order of toluene sensitivity was α4β2>α3β2>α3β4>α4β4.
Figure 2.
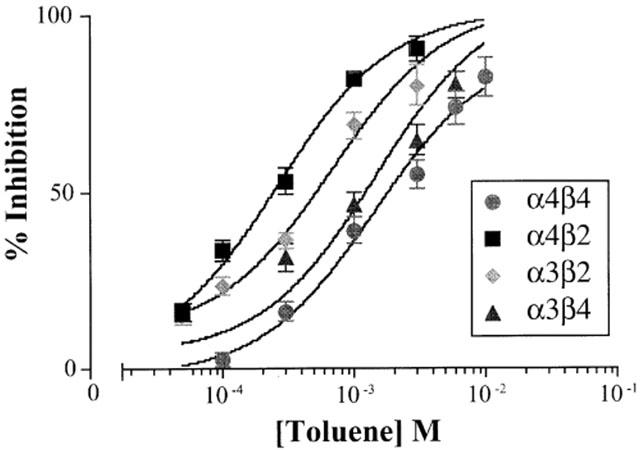
Concentration-response curves for toluene-inhibition of ACh-induced currents in oocytes expressing different nAChR subtypes. Each point represents the per cent inhibition of the steady state response by toluene and is the mean (±s.e.mean) from 5–12 oocytes from at least two separate frogs. The IC50 values for the nAChRs are listed in Table 1.
Table 1.
The mean and 95% confidence limits for toluene IC50 values for the nAChRs tested in this study
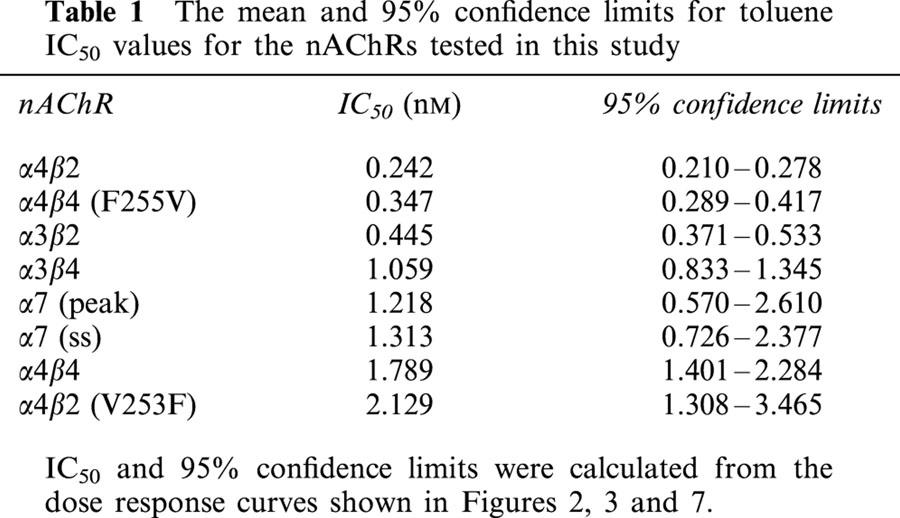
Figure 3a shows representative traces from a Xenopus oocyte expressing α7 nAChRs during stimulation with 150 μM ACh in the absence and presence of 300 μM toluene. Toluene inhibited ACh-induced currents by approximately 30%. The α7 receptor response recovered fully following washout of the toluene solution. Figure 3b shows the concentration response curves for toluene inhibition of peak and steady state α7 currents. As shown in Table 1, the IC50 values for toluene inhibition of peak and steady state α7 currents were not different.
Figure 3.
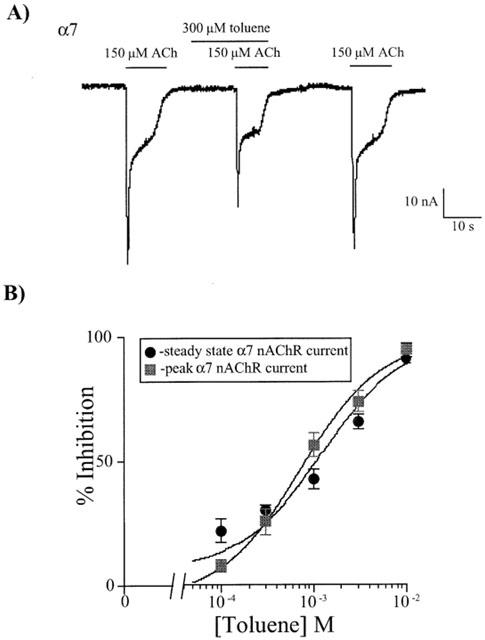
Inhibitory effects of toluene on the α7 nAChR. (A) Representative traces from an oocyte expressing α7 receptors. The lines above each current represent the length of time that the oocyte was perfused with either 150 μM ACh or 300 μM toluene. At this concentration, ACh-mediated responses were inhibited by 20% in comparison to control. (B) Concentration response curves were generated for the inhibition of the α7 receptor at both the peak and steady state portions of the current. Each point represents the per cent inhibition of the response by toluene and is the mean (±s.e.mean) from 5–8 oocytes from at least two separate frogs. IC50 values for the inhibition at both these portions are listed in Table 1 and do not differ significantly.
Figure 4 shows the effects of the holding potential on toluene's inhibition of heteromeric (α4β2, α3β2) and homomeric (α7) receptors. Data were expressed as a ratio of the leak-subtracted current obtained in the presence and absence of 1 mM toluene at each voltage tested. Slight differences in the magnitude of toluene inhibition at the different holding potentials were observed for α4β2, α3β2 and α7 receptors although these were not statistically significant (ANOVA with repeated measures, P>0.05). There was a small reduction in the toluene inhibition of α3β2 receptors at −120 mV, but this was not significantly different from the values obtained at the other holding potentials. Overall, these data suggest that the degree of toluene inhibition of recombinant nicotinic acetylcholine receptors is similar over a physiological range of membrane holding potentials.
Figure 4.
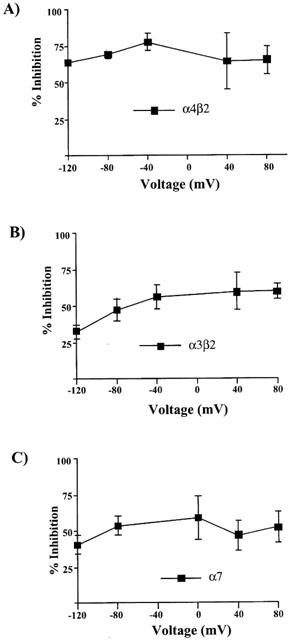
Effects of toluene on current-voltage (I-V) relationships of nAChRs expressed in Xenopus oocytes. Toluene inhibition for α4β2 (A), α3β2 (B), and α7 (C) nAChRs was calculated at each voltage (n=4–5 oocytes) and plotted. None of the values were significant with respect to each other (one-way ANOVA).
Additional studies were conducted with all receptor subtypes to test whether toluene is a competitive antagonist at the ACh-binding site. In these studies, the effects of toluene (1 or 3 mM) were tested in the presence of increasing concentrations of acetylcholine. If toluene competes with acetylcholine at its binding site, the degree of inhibition should decrease at higher agonist concentrations. As shown in Figure 5, increasing ACh up to 100 times the EC50 for each subunit combination did not significantly affect toluene's inhibition of ACh-induced currents.
Figure 5.
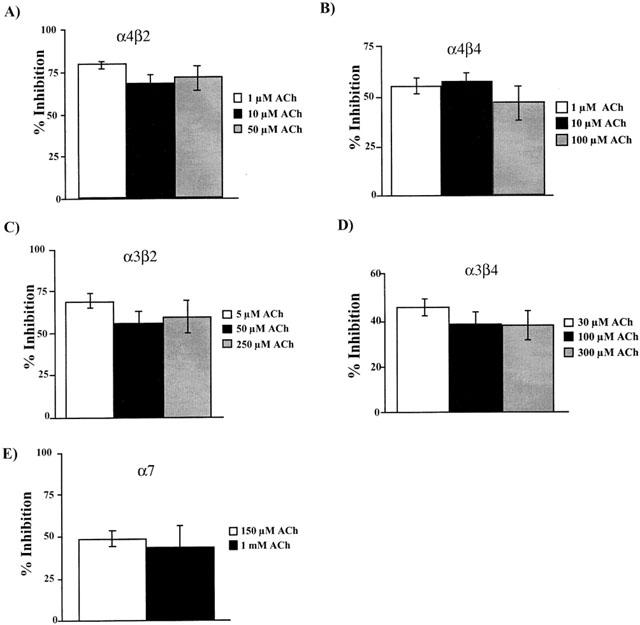
Per cent inhibition of nAChRs by toluene in the presence of increasing ACh concentrations. Oocytes expressing the indicated nAChR were exposed to 1 mM (α4β2, α3β2, α3β4, α7) or 3 mM (α4β4) toluene at different ACh concentrations. Values represent the per cent inhibition of the corresponding control current by toluene and are the mean (±s.e.mean) from 5–8 oocytes from at least two separate frogs.
Toluene's differential inhibition of heteromeric nAChRs was similar to that previously reported for certain general anaesthetics, including nitrous oxide and isoflurane. As with toluene, these compounds produced greater inhibition of β2 subunit containing receptors as compared to those containing the β4 subunit (Yamakura et al., 2000). A single amino acid in the TM2 domain of the β subunit was found to be a critical determinant of this effect. Expression of mutant β2 (V253F) subunits, resulted in receptors with anaesthetic sensitivities similar to that of β4-containing receptors. The corresponding mutation in the β4 subunit (F255V), increased the anaesthetic sensitivity of these receptors to that of β2-containing receptors. In the next series of experiments in the present study, toluene sensitivity of these mutant receptors was determined.
Figure 6a shows representative traces from an oocyte expressing α4β4 (F255V) receptors. The EC50 of ACh for this receptor was higher than its wild-type counterpart and therefore a higher ACh concentration (10 μM) was used. ACh-induced currents from these receptors were inhibited by 80% in the presence of 1 mM toluene, similar to the per cent inhibition observed with the α4β2 nAChR. Toluene (1 mM) inhibited α4β2(V253F) receptors by approximately 25%, similar to that observed for α4β4 receptors (Figure 6b). Figure 7 shows the concentration-response curves for toluene inhibition of α4β2(V253F) and α4β4(F255V) receptors. Dose response curves for wild-type receptors are shown for comparison and are taken from Figure 2.
Figure 6.
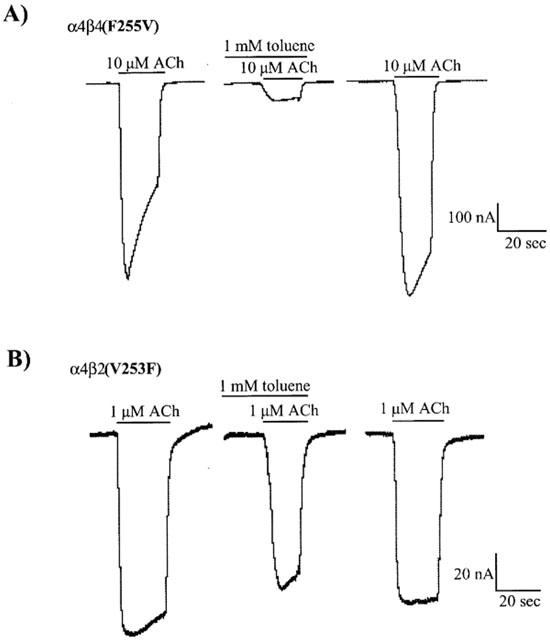
Effects of toluene on α4β2(V253F) and α4β4(F255V) nAChRs expressed in Xenopus oocytes. Representative traces are shown for each receptor subtype in response to ACh (1 or 10 μM) with and without 1 mM toluene. Each set of the three traces comes from a separate oocyte voltage-clamped at −80 mV.
Figure 7.
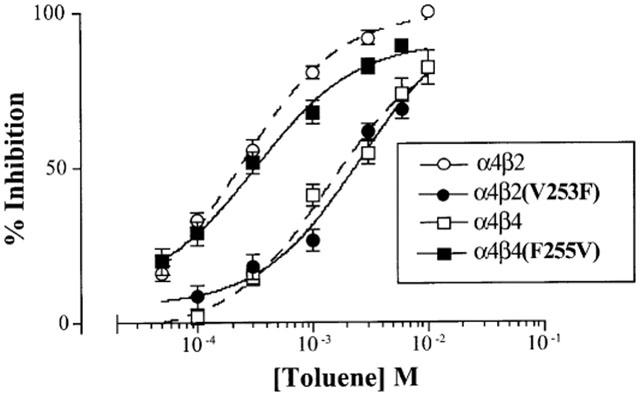
Concentration-response curves for toluene-inhibition of ACh-induced currents in oocytes expressing mutant nAChR subtypes. Each point on the curve represents the per cent inhibition of the steady state response by toluene and is the mean (±s.e.mean) from 5–7 oocytes from at least two separate frogs. Concentration-response curves for the corresponding wild type nAChR (α4β2 and α4β4) receptors are displayed as dashed lines and are taken from Figure 2. The IC50 values for the mutant nAChRs are listed in Table 1.
Finally, the effect of toluene on native nAChRs expressed in brain neurons was determined. In the majority of hippocampal neurons tested, ACh elicited rapidly desensitizing currents consistent with the expression of α7 nicotinic receptors (Jones & Yakel, 1997). Non-desensitizing currents indicative of heteromeric receptors were occasionally seen but were too few to obtain a full dose-response relationship. Toluene itself did not alter resting membrane currents in hippocampal neurons but significantly inhibited ACh-induced currents (Figure 8a). This inhibition was fully reversible upon washout. Due to the highly desensitizing nature of these currents, toluene inhibition was measured at the peak of the ACh response. As shown in Figure 8b, toluene dose-dependently inhibited ACh-induced currents in hippocampal neurons with an estimated IC50 value of 1100 μM (650–1900 μM).
Figure 8.
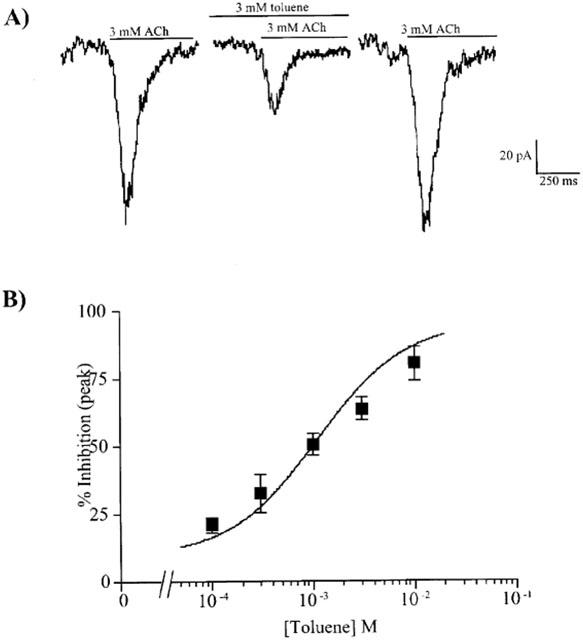
Toluene inhibition of ACh-mediated currents in cultured hippocampal neurons. (A) A representative set of currents generated from one neuron. Traces represent currents induced by ACh (3 mM) in the absence and presence of toluene (3 mM) from a neuron voltage-clamped at −50 mV. The line above each current indicates the specified treatment (3 mM ACh or 3 mM ACh/3 mM toluene). (B) A dose response curve for the toluene-inhibition of ACh-induced currents in hippocampal neurons is shown. Each point represents the per cent inhibition of control currents by toluene and is the mean (±s.e.mean) from 5–9 neurons. The IC50 value of toluene for the ACh response was 1.1 mM (0.65–1.9 mM).
Discussion
The major finding of this work is that toluene inhibits recombinant and native neuronal nAChRs in a concentration-dependent and reversible manner. Toluene caused inhibition of nAChRs at concentrations that did not significantly alter the resting membrane conductance of oocytes or hippocampal neurons. These results suggest that the effect of toluene on nAChRs was not due to a non-specific disruption of membrane integrity. Additionally, toluene's inhibition of ACh-mediated currents was not significantly altered by the holding potential used and was unaffected by increasing agonist concentrations.
Heteromeric nAChRs containing the β2 subunit were more sensitive to toluene inhibition than those containing β4 subunits or those generated by the α7 subunit. For example, α4β2 receptors were approximately 8 fold more sensitive to toluene than α4β4 receptors. A similar, although less marked effect was observed between the α3β2 and α3β4 receptors. The sensitivity of the α4β2 receptors to toluene was reduced when the α4 subunit was co-expressed with a mutant β2(V253F) subunit while α4β4 sensitivity was increased by the complementary β4(F255V) mutation. These mutations have been previously shown to modulate the sensitivity of neuronal nAChRs to nitrous oxide, pentobarbital and hexanol, but not ketamine (Yamakura et al., 2000). Thus, Valine-253 in the second transmembrane domain of the β2 subunit may represent an important site of action for an array of centrally active compounds. However, although the β2-V253 site appears to regulate the anaesthetic/toluene sensitivity of heteromeric nicotinic receptors, it seems unlikely that it may be an actual binding site for these compounds since occupation of this site with a thiol derivative, in a cysteine-substituted mutant, did not alter the anaesthetic sensitivity of the receptor (Yamakura et al., 2000).
The concentration-dependent inhibition of recombinant nAChRs by toluene in oocytes was also observed in hippocampal neurons grown in culture. The IC50 value for toluene in neurons was similar to the IC50 value obtained for the α7 receptors expressed in oocytes. Previous data has shown that these rapidly desensitizing currents are blocked by α7-subunit specific antagonists (α-bungarotoxin and methyllycaconitine; Jones & Yakel, 1997; Alkondon et al., 1998), and α-bungarotoxin binding sites in the rat contain only α7 subunits (Chen & Patrick, 1997). These results suggest that the responses measured in the present study were mediated mainly via α7 receptors.
Concentrations of toluene that inhibited neuronal nicotinic receptors in the present study cause acute behavioural effects in man and animals. For example, blood levels measured in toluene-intoxicated humans from one study were between 150–200 μM (Hobara et al., 2000). Brain levels of toluene are highly correlated with blood levels and are generally 2–3 times higher. Brain toluene concentrations obtained during autopsy in humans after accidental exposure range from approximately 100–900 μM (Ameno et al., 1992; Winek & Collom, 1971; Takeichi et al., 1986).
Several studies have measured brain and blood concentrations of toluene after exposure of experimental animals to toluene vapor. In one study, Benignus et al. (1981) found brain levels of toluene of approximately 200 μM after a two hour exposure to 575 p.p.m. toluene. Those authors did not comment on whether this level of exposure produced any significant behavioural effects. In fact, there are few studies that have directly correlated brain levels of toluene with vapour concentrations that produce behavioural effects. In a recent study with mice, Warren et al. (2000) showed that locomotor activity increased at brain concentrations of trichloroethane between 500 and 1500 μM and decreased at higher concentrations. Trichloroethane has been shown to produce effects on NMDA and GABAA receptors that are generally similar to those produced by toluene (Cruz et al., 2000; Beckstead et al., 2000).
Overall, these data suggest that brain concentrations of toluene and other abused solvents between 50–1000 μM represent a relevant range with respect to the use of these compounds as drugs of abuse. It is not as clear, however what specific behavioural effects result directly from toluene's inhibition of nicotinic receptors.
Toluene causes anaesthesia (Tegeris & Balster, 1994), memory impairment (Evans & Balster, 1991) and has anti-anxiety and anti-convulsant effects (Wood et al., 1984) similar to that observed for other CNS depressants. Recent data has suggested that inhibition of heteromeric nicotinic receptors may contribute to the sedative effects produced by volatile anaesthetics such as isoflurane and nitrous oxide (Flood et al., 1997; Yamakura et al., 2000). In addition, more selective nAChR antagonists such as mecamylamine and dihydro-β-erythrodine (DHβE) interfere with cognition in animals (Levin & Simon, 1998) but do not show anti-convulsant properties as do NMDA antagonists and GABAA agonists. These results suggest that toluene's inhibition of neuronal nicotinic receptors may contribute to the sedative and cognitive impairments observed during toluene intoxication.
In summary, we have shown that toluene inhibits nAChR function when expressed in oocytes and in hippocampal neurons. This inhibition is concentration-dependent and reversible upon washout. Based on these findings, the nAChR family of ligand-gated ion channels may represent an important target for the actions of toluene and other abused solvents.
Acknowledgments
The authors would like to acknowledge Tana Blevins for her technical assistance. They would also like to thank Dr J. Patrick (Baylor College of Medicine, Houston, TX, USA), Dr M.W. Nowak (Medical University of South Carolina, Charleston, SC, USA), and Dr R.A. Harris (University of Texas, Austin, TX, USA) for kindly providing the nAChR cDNA clones that were used in this study. This research was supported by National Institute of Health grants AA09986, KO2-AA00238 to J.J. Woodward and T32-DA07027 to A.S. Bale.
Abbreviations
- ACh
acetylcholine
- EGTA
Ethylene Glycol-bis(β-Aminoethyl Ether)-N,N,N′,N′-Tetraacetic Acid
- FdU
5-fluoro-2′-deoxyuridine
- GABA
γ-aminobutyric acid
- HEPES
4-2-(Hydroxyethyl)-1-Piperazine-ethanesulfonic acid
- MEM
minimal essential media
- MS-222
tricaine methanesulphonate
- nAChR
nicotinic acetylcholine receptor
- NMDA
N-methyl-D-aspartate
- TTX
Tetrodotoxin
References
- AISTRUP G.L., MARZALEC W., NARAHASHI T. Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Mol. Pharmacol. 1999;55:39–49. doi: 10.1124/mol.55.1.39. [DOI] [PubMed] [Google Scholar]
- ALBUQUERQUE E.X., ALKONDON M., PEREIRA E.F.R., CASTRO N.G., SCHRATTENHOLZ A., BARBOSA C.T.F., BONFANTE-CARBARCAS R., ARACAVA Y., EISENBERG H.M., MAELICKE A. Properties of neuronal nicotinic acetylcholine receptors: Pharmacological characterization and modulation of synaptic function. J. Pharmacol. Exp. Ther. 1997;280:1117–1136. [PubMed] [Google Scholar]
- ALKONDON M., PEREIRA E.F.R., ALBUQUERQUE E.X. Alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- AMENO K., KIRIU T., FUKE C., AMENO S., SHINOHARA T., IJIRI I. Regional brain distribution of toluene in rats and in a human autopsy. Arch. Toxicol. 1992;66:153–156. doi: 10.1007/BF02342512. [DOI] [PubMed] [Google Scholar]
- BALE A.S., SMOTHERS C.T., BLEVINS T., WOODWARD J.J. Comparison of the mechanisms and sites of action of toluene and alcohol. Alcohol. Clin. Exp. Res. 2000;24:84A. [Google Scholar]
- BECKSTEAD M.J., WEINER J.L., EGER E.I., II, GONG D.H., MIHIC S.J. Glycine and gamma-aminobutyric acid (A) receptor function is enhanced by inhaled drugs of abuse. Mol. Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- BENIGNUS V.A., MULLER K.E., BARTON C.N., BITTIKOFER J.A. Toluene levels in blood and brain of rats during and after respiratory exposure. Toxicol. App. Pharmacol. 1981;61:326–334. doi: 10.1016/0041-008x(81)90353-7. [DOI] [PubMed] [Google Scholar]
- BLISS C.I. Statistics in Biology. New York: McGraw-Hill; 1967. [Google Scholar]
- BOWEN S.E., BALSTER R.L. Effects of inhaled 1,1,1-trichloroethane on locomotor activity in mice. Neurotoxicol. Teratol. 1996;18:77–81. doi: 10.1016/0892-0362(95)02024-1. [DOI] [PubMed] [Google Scholar]
- CARDOSO R.A., BROZOWSKI S.J., CHAVEZ-NORIEGA L.E., HARPOLD M., VALENZUELA C.F., HARRIS R.A. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- CHEN D., PATRICK J.W. The alpha-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the alpha7 subunit. J. Biol. Chem. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN L.M., PATRICK J.W. Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Adv. Pharmacol. 1997;39:191–220. doi: 10.1016/s1054-3589(08)60072-1. [DOI] [PubMed] [Google Scholar]
- COVERTON P.J.O., CONNOLLY J.G. Differential modulation of rat neuronal nicotinic receptor subtypes by acute application of ethanol. Br. J. Pharmacol. 1997;122:1661–1668. doi: 10.1038/sj.bjp.0701568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUZ S.L., BALSTER R.L., WOODWARD J.J. Effects of volatile solvents on recombinant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 2000;131:1303–1308. doi: 10.1038/sj.bjp.0703666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUZ S.L., MIRSHAHI T., THOMAS B., BALSTER R.L., WOODWARD J.J. Effects of abused solvent toluene on recombinant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1998;286:334–340. [PubMed] [Google Scholar]
- EVANS E.B., BALSTER R.L. CNS depressant effects of volatile organic solvents. Neurosci. Biobehav. Rev. 1991;15:233–241. doi: 10.1016/s0149-7634(05)80003-x. [DOI] [PubMed] [Google Scholar]
- FLOOD P., RAMIREZ-LATORRE J., ROLE L. α4β2 neuronal nicotinic acetylcholine receptors in the central nervous system are inhibited by isoflurane and propofol, but α7-type are nicotinic acetylcholine receptors are unaffected. Anesthesiology. 1997;86:859–865. doi: 10.1097/00000542-199704000-00016. [DOI] [PubMed] [Google Scholar]
- GODDEN E.L., HARRIS R.A., DUNWIDDIE T.V. Correlation between molecular volume and effects of n-alcohols on human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 2001;296:716–722. [PubMed] [Google Scholar]
- HOBARA T., OKUDA M., GOTOH M., OKI K., SEGAWA H., KUNITSUGU I. Estimation of the lethal toluene concentration from the accidental death of painting workers. Ind. Health. 2000;38:228–231. doi: 10.2486/indhealth.38.228. [DOI] [PubMed] [Google Scholar]
- JONES S., YAKEL J.L. Functional nicotinic ACh receptors on the interneurones in the rat hippocampus. J. Physiol. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN E.D., SIMON B.B. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- NATIONAL INSTITUTE ON DRUG ABUSE (NIDA) Rockville, MD: The Institute; 1988. 1985 National Household Survey on Drug Abuse, Division of Epidemiology and Statistical Analysis. [Google Scholar]
- REES D.C., KNISELY J.S., BREEN T.J., BALSTER R.L. Toluene, halothane, 1,1,1-trichloroethane and oxazepam produce ethanol-like discriminative stimulus effects in mice. J. Pharmacol. Exp. Ther. 1987a;243:931–937. [PubMed] [Google Scholar]
- REES D.C., KNISELY J.S., JORDAN S., BALSTER R.L. Discriminative stimulus properties of toluene in the mouse. Toxicol. Appl. Pharmacol. 1987b;88:97–104. doi: 10.1016/0041-008x(87)90273-0. [DOI] [PubMed] [Google Scholar]
- SMOTHERS C.T., MROTEK J.J., LOVINGER D.M. Chronic ethanol exposure leads to a selective enhancement of N-methyl-D-aspartate receptor function in cultured hippocampal neurons. J. Pharmacol. Exp. Ther. 1997;283:1214–1222. [PubMed] [Google Scholar]
- TAKEICHI S., YAMADA T., SHIKATA I. Acute toluene poisoning during painting. Forensic Sci. Int. 1986;32:109–115. doi: 10.1016/0379-0738(86)90146-5. [DOI] [PubMed] [Google Scholar]
- TEGERIS J.S., BALSTER R.L. A comparison of the acute behavioral effects of alkylbenzenes using a functional observational battery in mice. Fundam. Appl. Toxicol. 1994;22:240–250. doi: 10.1006/faat.1994.1028. [DOI] [PubMed] [Google Scholar]
- VIOLET J.M., DOWNIE D.L., NAKISA R.C., LIEB W.R., FRANKS N.P. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology. 1997;86:760–762. doi: 10.1097/00000542-199704000-00017. [DOI] [PubMed] [Google Scholar]
- WARREN D.A., BOWEN S.E., JENNINGS W.B., DALLAS C.E., BALSTER R.L. Biphasic effects of 1,1,1-trichloroethane on the locomotor activity of mice: relationship to blood and brain solvent concentrations. Toxicol. Sci. 2000;56:365–373. doi: 10.1093/toxsci/56.2.365. [DOI] [PubMed] [Google Scholar]
- WINEK C.L., COLLOM W.D. Benzene and toluene fatalities. J. Occup. Med. 1971;13:259–261. [PubMed] [Google Scholar]
- WOOD R.W., COLEMAN J.B., SCHULER R., COX C. Anticonvulsant and antipunishment effects of toluene. J. Pharmacol. Exp. Ther. 1984;230:407–412. [PubMed] [Google Scholar]
- YAMAKURA T., BORGHESE C., HARRIS R.A. A transmembrane site determines sensitivity of neuronal nicotinic acetylcholine receptors to general anesthetics. J. Biol. Chem. 2000;275:40879–40886. doi: 10.1074/jbc.M005771200. [DOI] [PubMed] [Google Scholar]
- ZWART R., VIJVERBERG H.P. Potentiation and inhibition of neuronal nicotinic receptors by atropine: competitive and noncompetitive effects. Mol. Pharmacol. 1997;52:886–895. doi: 10.1124/mol.52.5.886. [DOI] [PubMed] [Google Scholar]


