Abstract
Relaxin (RLX) is a multifunctional hormone best known for its role in pregnancy and parturition, that has been also shown to influence coronary perfusion and mast cell activation through the generation of endogenous nitric oxide (NO). In this study we report on the effects of RLX on the biochemical and mechanical changes of ex vivo perfused hearts isolated from ovalbumin-sensitized guinea-pigs induced by challenge with the specific antigen. The possible involvement of NO in the RLX action has been also investigated.
A 30-min perfusion with RLX (30 ng ml−1) before ovalbumin challenge fully abated the positive chronotropic and inotropic effects evoked by anaphylactic reaction to the antigen. RLX also blunted the short-term coronary constriction following to antigen challenge. Conversely, perfusion with chemically inactivated RLX had no effect.
The release of histamine in the perfusate and the accumulation of calcium in heart tissue induced by antigen challenge were significantly decreased by RLX, while the amounts of nitrites in the perfusate were significantly increased, as were NO synthase activity and expression and cGMP levels in heart tissue.
These findings indicate that RLX has a protective effect in cardiac anaphylaxis which involves an up-regulation of the NO biosynthetic pathway.
Keywords: Relaxin, nitric oxide, cardiac anaphylaxis, histamine, cyclic GMP, intracellular calcium
Introduction
Relaxin (RLX) is a peptide hormone of the insulin superfamily, best known for its effects on the female reproductive organs during pregnancy (Bani, 1997). In recent years, evidence is accumulating that RLX has a major influence on the cardiovascular system (Bigazzi et al., 2001). In particular, RLX has been shown to increase the expression of the inducible NO synthase isoform (iNOS) in vascular smooth muscle cells and coronary endothelial cells in vitro, thereby increasing endogenous NO generation (Bani et al., 1998a; Failli et al., 2001). Through this mechanism, relaxin exerts a widespread vasodilatation, that may account for its effectiveness in increasing coronary flow (Bani Sacchi et al., 1995) and in counteracting ischemia-reperfusion-induced cardiac injury in experimental models of myocardial infarction in vivo and ex vivo in rats and guinea-pigs (Masini et al., 1997; Bani et al., 1998b). Of note, in these animal species, the RLX-induced increase in coronary flow is 100 to 1000 fold higher than acetylcholine or sodium nitroprusside respectively (Bani Sacchi et al., 1995). Moreover, atrial cardiocytes have been shown to produce and secrete RLX (Taylor & Clark, 1994), which in turn has specific receptors in the heart (Hsu et al., 2002) and raises levels in blood of patients with heart failure (Dschietzig et al., 2001). These findings suggest that the heart may be a physiological target for this hormone.
RLX, besides its direct effects on the vascular components, may also act on perivascular mast cells, which are physiological regulators of blood-tissue exchanges and which play a major pathogenic role in allergic inflammation (Taylor & Metcalfe, 2001). In fact, RLX has been shown to inhibit histamine release and granule exocytosis by isolated mast cells activated by immunologic and non-immunologic stimuli, acting through the stimulation of intrinsic NO generation (Masini et al., 1994a). This property of RLX may have important physiopathological consequences, as indicated by previous findings that this hormone counteracts asthma-like reaction and bronchial hyperresponsiveness induced by antigen inhalation in sensitized guinea-pigs (Bani et al., 1997). NO itself may afford protection against allergic activation of mast cells and consequent inflammatory tissue damage, as the NO donors sodium nitroprusside and glyceryl trinitrate have been found to down-regulate the release of histamine from tissues of sensitized guinea-pig upon immunologic and non-immunologic stimuli (Masini et al., 1994b). In this context, cardiac anaphylaxis due to systemic antigen dissemination is characterized by functional and biochemical changes of the heart that are mainly attributed to the release of pro-inflammatory and vasoactive mediators from cardiac mast cells (Capurro & Levi, 1975). A reliable model of cardiac anaphylaxis ex vivo can be reproduced using perfused hearts isolated from sensitized guinea-pigs and subjected to challenge with the specific antigen (Giotti et al., 1966). In the present study, we used this model to investigate whether RLX could counteract cardiac anaphylaxis and, if so, whether a NO-driven mechanism is involved.
Methods
Animals
Nineteen male adult guinea-pigs, Dunkin-Hartley strain, were used. They were purchased from a commercial dealer (Rodentia, Bergamo, Italy) and quarantined for 7 days at 22–24°C under a 12-h light, 12-h dark cycle before use. Standard laboratory chow (Rodentia), fresh vegetables and water were available ad libitum. The experimental protocol was designed in compliance with the recommendations of the European Economic Community (86/609/CEE) for the care and use of laboratory animals and was approved by the animal care committee of the University of Florence, Italy. At the end of the treatments, the animals weighed 350–400 g.
Induction of cardiac anaphylaxis
The guinea-pigs were sensitized by two intraperitoneal injections, 1 ml each, of ovalbumin 1% in PBS given on two consecutive days (Feigen et al., 1960). The animals were killed by decapitation 15–30 days after sensitization, and the hearts were quickly removed and mounted in a Langendorff perfusion apparatus through a cannula inserted into the aorta. Hearts were perfused with Tyrode solution at 37°C at a constant pressure of 30 mmHg, corresponding to 40 cm water at the instrument's pressure regulator, and gassed with a mixture of 97% O2–3% CO2 giving a final pH of 7.45. Composition of the perfusion fluid was as follows (mM): Na+ 149.3, K+ 2.7, Ca2+ 1.8, Mg2+ 1.05, Cl− 145.4, HCO3− 11.9, H2PO4− 0.4 and (+)-glucose 5.6 (Dieterich & Löffelholz, 1977). Hearts were allowed to equilibrate for 1 h before further treatments.
Heart rate and contraction were recorded by a force displacement transducer connected to a clip hooked to the heart apex under an initial load of 2 g and coupled to a thermic pen recorder (MARB, Florence, Italy). The onset and type of arrhythmias were monitored by a bipolar surface electrocardiograph. Coronary perfusates were collected over intervals of 5 min in graduated tubes to determine coronary flow rates and kept frozen at −20°C until needed.
Cardiac anaphylaxis was elicited by injection of 0.1 ml of 1% solution of albumin in Tyrode solution into the aortic cannula. Coronary perfusates were collected every 5 min for 30 min before and after the ovalbumin challenge. In some hearts (n=8) RLX was added to the perfusion fluid at the concentration of 30 ng ml−1 30 min before antigen challenge. This RLX concentration was chosen because it has been proven effective in preventing cardiac injury due to ischemia and reperfusion in isolated guinea-pig hearts (Masini et al., 1997). Hearts not treated with RLX were used as controls (n=8). As specificity controls, some hearts (n=3) were perfused with inactivated porcine RLX (iRLX) at the dose of 30 ng ml−1, 30 min before antigen challenge. RLX was inactivated according to the method of Büllesbach & Schwabe (1988). Briefly, 1 mg porcine RLX was dissolved in 0.1 M borate buffer, pH 8.9, with a 10 fold molar excess of 1,2 cyclohexanedione (Sigma Chemical Co., St. Louis, MO, U.S.A.), which specifically binds to the arginines of the receptor-binding domain of the RLX B chain thus preventing the modified hormone from binding to the RLX receptor. Excess reagent was removed by dialysis against distilled water. Successful inactivation was assessed by inability of iRLX to increase the coronary flow in isolated and perfused guinea-pig hearts, at variance with authentic RLX. At the end of the experiments, samples of cardiac tissue were collected to determine the content of calcium, histamine and cGMP, as well as to evaluate mast cell degranulation and nitric oxide synthase activity.
Determination of histamine content in the perfusates and in the heart
Histamine content in the hearts and the perfusates was measured fluorimetrically according to the method of Kremzer & Wilson (1961), as described in detail by Masini et al. (1985). Briefly, o-phthaldialdehyde 0.1% in methanol was added directly to the perfusates after alkalinization with 0.5 ml of NaOH 0.5 N. The reaction was stopped after 5 min by adding 0.2 ml of H3PO4 2.5 N. The same procedure was used for the tissue samples after extraction with alkaline n-butanol, separation of the organic phase with n-heptane and recovery of histamine in the aqueous phase with 0.1 M HCl. The authenticity of the extracted histamine was checked through the fluorescence spectra. Histamine in the samples was determined by fluorimetric measurement using an excitation wave length of 365 nm and an emission wave length of 455 nm (Shöre et al., 1959). The values of histamine release were expressed as the percentage of total ‘initial' histamine, i.e. the sum of histamine appearing in the perfusates and that remained in the heart (Mongar & Schild, 1952). Because of fluctuations in the release of cardiac histamine (Giotti et al., 1966), the effect of RLX on the release of histamine was assessed by comparing histamine release in the presence of the hormone with matched controls. At the concentrations present in the perfusates, RLX did not interfere with the fluorimetric assay of histamine.
Computer-assisted densitometry of cardiac mast cells
Tissue samples from the hearts were fixed by immersion in isotonic formaldehyde-acetic acid (IFAA), dehydrated in graded ethanol and embedded in paraffin wax. Sections 5 μm thick were cut and stained with Astra blue which selectively binds heparin contained in mast cell granules. Light transmittance across mast cells, which is inversely related to their granule content, was evaluated by a computer-assisted method, as described previously (Masini et al., 1994a). The mast cells were viewed by a CCTV television camera (Sony, Tokyo, Japan) applied to a Reichert-Jung Microstar IV light microscope (Cambridge Instruments Inc, Buffalo, NY, U.S.A.) with a ×100 oil immersion objective, and interfaced with a personal computer through a Matrox Marvel G400-TV card (Matrox Graphics, Dorval, Canada). The card allows for the light transmitted across the microscopic slide to be determined within a range of 256 gray levels, which are comprised between 0 (black level) and 255 (white level). The card also allows for a digitized image of mast cells to be reproduced on the basis of the values estimated. Measurements of transmittance were carried out using the Scion Image Beta 4.0.2 image analysis program (Scion Corp., Frederick, MD, U.S.A.). The transmittance of 100 different mast cells, 10 from each animal of the different groups, was analysed and the mean transmittance value (±s.e.mean) was then calculated.
Nitrite analysis
Release of NO2−, stable end-products of NO metabolism, in the heart perfusates was measured spectrophotometrically by the Griess reaction. Half of each perfusate (about 15–20 ml) was lyophilized separately and resuspended in 4 ml of water. The samples were centrifuged and each supernatant was allowed to react with Griess reagent (1% sulfanilamide and 0.1% N-[1-naphtyl]ethylendiamine in 5% phosphoric acid) to form a stable chromophore which absorbed at 465 nm wave length. The optical density was measured with a Bio-Rad 550 micro plate reader. Nitrite concentrations in the supernatants were calculated by comparison with standard concentrations of NaNO2 dissolved in Tyrode solution. Results were expressed as nmol of NO2− ml−1 (Salvemini et al., 1992).
Western blot analysis of iNOS
Cardiac samples were homogenized in 1 ml of lysis buffer of the following composition: NaCl 0.9%, TRIZMA-HCl 1 M, pH 7.6, Triton X100 0.1%, PMSF 0.05 M, leupeptin 0.36 mg ml−1. Samples were kept on ice for 15 min and then centrifuged at 4°C for 10 min at 9000×g. In the supernatant, protein concentration was determined by BCA protein assay (Pierce, Rockford, IL, U.S.A.). The samples, each containing 40 μg of total proteins, were electrophoresed by SDS–PAGE (200 V, 1 h) using a denaturing 8% polyacrilamide gel with proper molecular weight markers (Biolabs, Beverly, MA, U.S.A.) and blotted (150 V, 1 h) into nitrocellulose membranes (Amersham Biosciences, Milan, Italy). After thorough washings in phosphate-buffered saline (PBS), pH 7.4, added with 0.1% Tween 20 (T-PBS), the membranes were treated with albumin 5% in T-PBS to block aspecific binding sites and incubated with rabbit polyclonal antibodies against iNOS (Chemicon, Temecula, CA, U.S.A.), diluted 1 : 1000 in T-PBS at 4°C for 2 h under stirring. After three washes with T-TBS, immune reaction was visualized by a chemiluminescence kit using anti-rabbit polyclonal antibodies (Pierce) following closely the manufacturer's instructions.
Determination of nitric oxide synthase (NOS) activity
Fragments of cardiac tissues were homogenized at −4°C in buffer containing 0.32 M sucrose, 20 mM HEPES (pH 7.2), 0.5 M EDTA, and 1 mM dithiothreitol. NO synthase activity was carried out by the [3H]-arginine conversion assay, according to Mollace et al. (1993), with minor modifications. Part of the tissue homogenates was used for determination of total NOS activity. The samples (340 μl) were added with 60 μl of a medium composed by: NADPH+ 13.2 mM, CaCl2 3 mM, calmodulin (free base, Sigma) 10 μg ml−1, L-arginine 200 mM, and [3H]L-arginine 32 μCi ml−1. After 60 min of incubation at 37°C, the mixture was loaded on 1 ml Dowex AG50WX-8/Na+ form column (Sigma) and eluted by 1 ml HEPES buffer followed by 5 ml distilled water. The [3H]-citrulline obtained from enzyme activity was measured with a β-counter (Packard, Zürich, Switzerland) and the ratio between [3H]-citrulline (nmol) and mg of proteins, the latter evaluated by the Bradford reagent, was taken as NOS activity. Another part of the tissue homogenate was used to determine the activity of Ca2+/calmodulin independent NOS. In this latter case, the samples were treated as above, except for the use of a Ca2+/calmodulin-free medium added with EDTA (6.6 mM) and the calmodulin inhibitor trifluperazine (660 μM, Sigma). The activity of Ca2+/calmodulin-dependent NOS was determined from the difference between the values of total NOS activity and the values of Ca2+/calmodulin independent NOS activity.
Evaluation of cGMP
Because NOS is known to up-regulate cGMP levels through its product NO (Ignarro, 1991), we looked for possible changes in cGMP content in hearts from the different experimental groups. Cardiac tissue samples were homogenized in the presence of 3′-isobutyl-1-methylxanthine (IBMX, 50 μM) to inhibit phosphodiesterase activity. The levels of cGMP were measured in the aqueous phase of 5% TCA extracts of the homogenates, as described previously (Bani et al., 1998a). The values are expressed as fmol of cGMP per mg of protein. The protein concentrations were determined by the Bradford reagent.
Calcium content
Total Ca2+ content was determined in heart tissue fragments by atomic absorption spectrometry, as previously described (Masini et al., 1997). Briefly, after washing with a Ca2+-free buffered solution, 30 mg of tissue were cut from each fragment, dried and digested overnight at 80°C with 65% HNO3. The samples were dried at 45°C under nitrogen, resuspended in 50 μl of 32% HCl and added with 5 ml of an aqueous solution of lanthanum chloride (LaCl3.7H2O) to provide a final concentration of 1% lanthanum (weight/volume). Lanthanum was used to remove Ca2+-binding ions which could interfere with the measurements. Upon centrifugation to remove particulate residues, the amounts of Ca2+ in the supernatants were read in an atomic absorption spectrophotometer (Perkin-Elmer 303, Überlingen, Germany) at 422 nm wave length, which is characteristic for calcium. The experimental values were determined by comparison with a standard curve at graded concentrations of CaCl2 and were expressed as ng of Ca2+ mg−1 of tissue (dry weight).
Statistic analysis
Data are expressed as mean±s.e.mean. Statistical analysis was performed by Kruskal Wallis test. Calculations were carried out using a GraphPad Prism 2.0 statistical program (GraphPad Software, San Diego, CA, U.S.A.). P<0.05 was considered significant.
Results
Mechanical activity and coronary flow
Ovalbumin challenge of untreated control hearts from sensitized guinea-pigs resulted in a typical anaphylactic crisis, characterized by arrhythmias, sinusal tachycardia and increase in the strength of contraction (Figure 1). These mechanical abnormalities were accompanied by a fast, short-lasting decrease in the coronary flow followed by a sustained flow increase (Figure 2). In the hearts pretreated with RLX, no significant changes were observed in either beat rate or contraction strength upon antigen challenge (Figure 1). Moreover, no short-lasting decrease in coronary flow took place, and the subsequent flow increase was significantly higher than in the untreated control hearts (Figure 2). In the hearts pretreated with iRLX, antigen challenge caused the same positive chronotropic and inotropic effects and changes in the coronary flow as in the untreated control hearts (Figures 1, 2).
Figure 1.
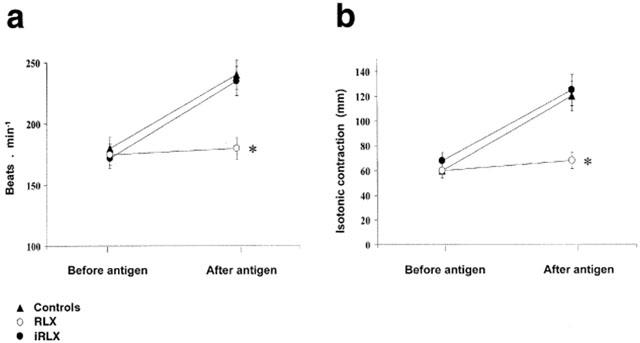
Heart rate (a) and strength of contraction (b) evaluated before and 30 min after antigen challenge of hearts from sensitized guinea-pigs (10 μg ovalbumin). Authentic RLX prevents the chronotropic and inotropic effects of antigen administration observed in the untreated control hearts. Conversely, iRLX is ineffective. Data are the mean±s.e.mean of six experiments. *P<0.05 vs control and P<0.05 RLX-treated vs iRLX-treated hearts.
Figure 2.
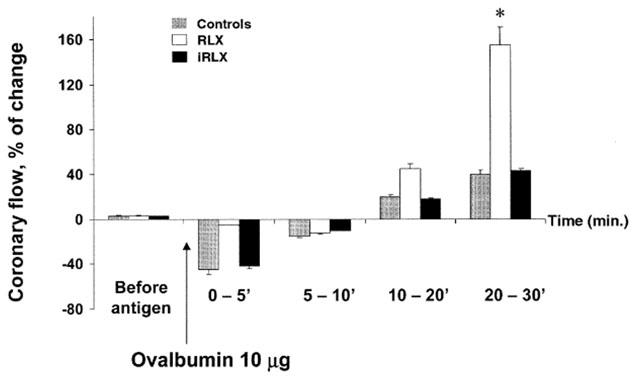
Coronary flow in hearts from sensitised guinea-pigs challenged with the antigen (10 μg ovalbumin). Compared with the untreated control hearts, RLX pretreatment prevents the quick, short-lasting decrease in the coronary flow evoked by antigen administration and promotes a subsequent, sustained flow increase. Conversely, iRLX is ineffective. Data are the mean±s.e.mean of eight experiments. *P<0.05 vs control and P<0.05 RLX-treated vs iRLX-treated hearts.
Histamine release and mast cell degranulation
The overall histamine release evaluated 30 min after antigen challenge showed that, in the untreated control hearts, about 58% of the endogenous histamine was released. The amount of histamine appearing in the perfusates showed a peak (353.7±22 ng ml−1) within the first 5 min from antigen challenge, followed by a significant decline (98.7±6.2 ng ml−1) at 30 min (Figure 3a). The amount of histamine released by hearts pretreated with RLX (30 ng ml−1) was significantly lower than that of the controls, especially in the first 5 min (from 353.7±22 to 110.6±7.2 ng ml−1) (Figure 3a). On the contrary, in the hearts pretreated with iRLX, the amount of histamine released after exposure to antigen was not different from that of the controls (Figure 3a). The amount of histamine retained at the end of perfusion in the untreated control hearts subjected to antigen challenge was more than 3 fold lower (476 ng g−1 wet weight) than that of hearts pretreated with RLX before antigen challenge (1376 ng g−1 wet weight). Conversely, in the hearts pretreated with iRLX, tissue histamine content was similar to controls (586 ng g−1 wet weight) (Figure 3b). Consistently, the light transmittance across mast cells, which is inversely related to their granule content, was high in the untreated control hearts and decreased markedly in the RLX-pretreated hearts. Once again, the hearts pretreated with iRLX before antigen challenge showed high transmittance levels, similar to those of the untreated control hearts (Figure 3c).
Figure 3.
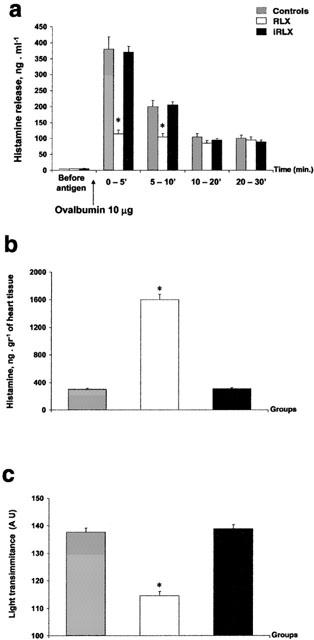
Histamine in hearts from sensitized guinea-pigs challenged with the antigen (10 μg ovalbumin). (a) Histamine release in the perfusates of untreated control hearts, hearts pretreated with RLX, and hearts pretreated with iRLX. Authentic RLX but not iRLX cause a marked reduction of antigen-stimulated increase of histamine in the perfusates. (b) Histamine retained in the hearts upon antigen challenge. Compared with the untreated control hearts, the hearts pretreated with RLX contain significantly higher amounts of histamine. The same does not occur upon iRLX pretreatment. (c) Light transmittance across Astra blue-stained cardiac mast cells. Compared with the untreated control hearts, the RLX-pretreated hearts show a significant decrease in light transmittance, indicating a higher amount of intracellular secretion granules. Conversely, iRLX is ineffective. Data are expressed as mean±s.e.mean of eight experiments. *P<0.05 vs control and P<0.05 RLX-treated vs iRLX-treated hearts.
NO2− release and NOS expression and activity
Ovalbumin challenge of untreated control hearts caused a long-lasting release of NO2−. The amount of NO2− in the perfusates showed a peak (1.1±0.3 nmol ml−1) within the first 10 min after antigen challenge, followed by a significant decline (0.41±0.02 nmol ml−1) at 30 min. The release of NO2− in the perfusates was significantly increased in the hearts pretreated with authentic RLX, particularly at 10 min from antigen challenge (1.23±0.4 nmol ml−1), but not in those treated with iRLX (Figure 4). The increase in NO2− release after pretreatment with RLX was accompanied by an increase in the expression of iNOS protein, as shown by Western blot analysis (Figure 5a). As expected, no change of iNOS protein expression was found in the hearts treated with iRLX as compared with the untreated controls. Moreover, the [3H]-arginine conversion assay showed that, as compared with the untreated control hearts, the hearts pretreated with RLX underwent a significant increase in both Ca2+-dependent (from 189.7±13.2 to 402.4±22.7 nmol mg−1 of proteins) and, even more, Ca2+-independent (from 56.3±4.2 to 224.3±12.7 nmol mg−1 of proteins) NOS activity (Figure 5b).
Figure 4.
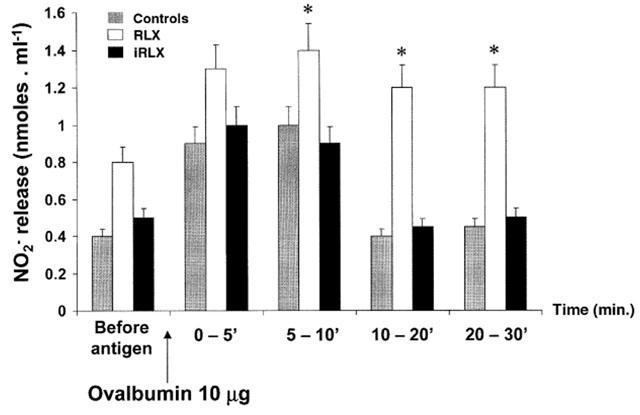
NO2− release in the perfusates of hearts from sensitized guinea-pigs challenged with the antigen (10 μg ovalbumin). Compared with the untreated control hearts, RLX pretreatment causes a prompt and sustained increase in NO2− amounts, whereas iRLX is ineffective. Data are expressed as mean±s.e.mean of six experiments. *P<0.05 vs control and P<0.05 RLX-treated vs iRLX-treated hearts.
Figure 5.
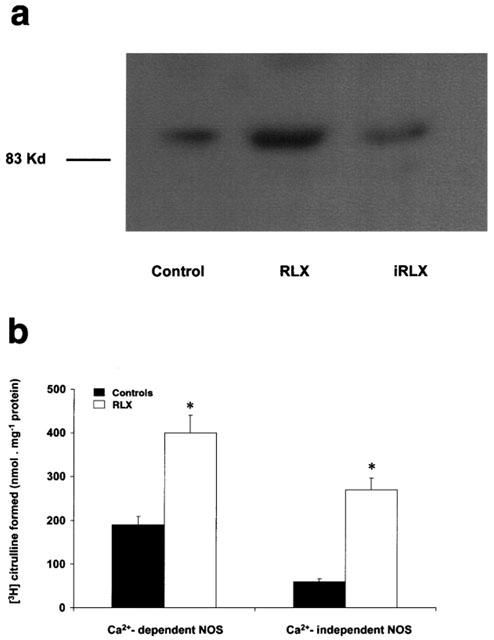
Expression and activity of iNOS by hearts from sensitized guinea-pigs challenged with the antigen (10 μg ovalbumin). (a) Representative Western blot analysis showing an increase in iNOS protein expression upon RLX, whereas iRLX is ineffective. (b) [3H]-arginine conversion assay showing an increase in both Ca2+-dependent and Ca2+-independent NOS activity in the RLX-treated hearts compared with the untreated controls. Data are expressed as mean±s.e.mean of six experiments. *P<0.05 vs controls.
Intracellular cGMP and calcium levels
In the hearts pretreated with RLX a significant increase in cGMP levels took place as compared with the untreated control hearts. On the other hand, pretreatment with iRLX had no effect (Figure 6a). It is known that cGMP inhibits intracellular Ca2+ fluxes in cardiac tissue in vitro and in vivo (Tohse et al., 1995; Liu et al., 2001). In our experiments, treatment of the hearts with RLX, but not with iRLX, before antigen challenge caused a significant reduction of calcium content in myocardial tissue as compared with the untreated control hearts (Figure 6b).
Figure 6.
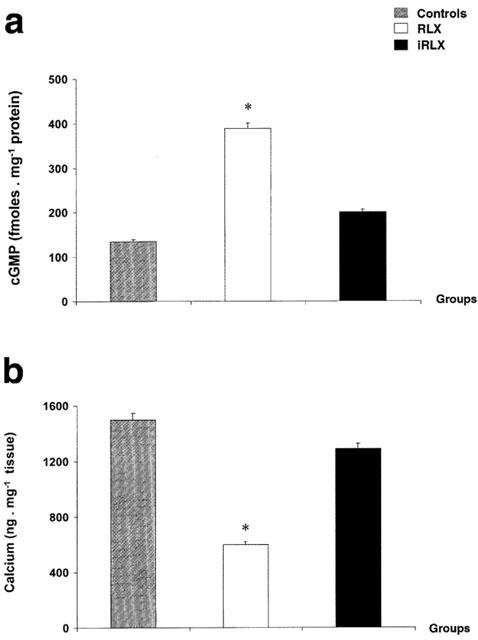
Tissue levels of cGMP (a) and Ca2+ (b) in hearts from sensitized guinea-pigs challenged with the antigen (10 μg ovalbumin). Compared with the untreated control hearts, those pretreated with RLX show higher levels of cGMP and lower levels of Ca2+. Pretreatment with iRLX gave values not significantly different from those of the controls. Data are expressed as mean±s.e.mean of eight experiments. *P<0.05 vs control and P<0.05 RLX-treated vs iRLX-treated hearts.
Discussion
The present study indicates that ex vivo perfusion of hearts isolated from ovalbumin-sensitized guinea-pigs with the hormone RLX affords a marked protection against anaphylactic reaction induced by challenge with the specific antigen. The protective effect of RLX, which is exerted at low, nanomolar concentrations (30 ng ml−1) and for short exposure times (30 min), results in a significant reduction of histamine release from resident mast cells, which are the main repository of cardiac histamine, and is accompanied by a marked increase in coronary flow that counteracts the transient flow reduction upon antigen challenge typical of the untreated control hearts. The increase in coronary flow by RLX, a typical property of this hormone (Bani Sacchi et al., 1995; Masini et al., 1997), may substantially concur to cardiac protection for it could promptly remove pre-stored and newly-synthesized inflammatory mediators released from activated mast cells (Capurro & Levi, 1975). As a consequence of this protection, RLX has been shown to reduce the cardiac tissue levels of Ca2+, whose accumulation into myocardial cells is deemed a reliable biochemical marker of inflammatory cell injury (Zimmerman & Hulsman, 1966; Duncan et al., 1992).
Inhibition of cardiac mast cells appears to play a crucial role in the anti-anaphylactic action of RLX. This is an expected finding, since RLX had been previously demonstrated to hinder immunologic activation of mast cells (Masini et al., 1994a). The beneficial effect of RLX on mechanical heart dysfunction observed in the present study is in keeping with mast cell inhibition, endogenous histamine being a potent arrhythmogenic, chronotropic and inotropic substance to the heart (Wolff & Levi, 1986). In the present study, the protection afforded by RLX against cardiac anaphylaxis clearly emerges as a specific property of this hormone since it could not be reproduced by replacing authentic RLX with iRLX, which is similar to RLX except for its inability to bind to RLX receptors. Specificity of the cardiac actions of RLX is also indicated by the recent discovery of specific RLX receptors in the heart (Hsu et al., 2002).
The molecular mechanism of the anti-anaphylactic action of RLX appears to involve an up-regulation of the NO biosynthetic pathway. Upon perfusion with RLX, the release of NO2− – the stable end-products of NO metabolism – in the perfusates raised and remained high until the end of the experimental period. Concurrently, RLX treatment caused a clear-cut increase in the expression of iNOS protein and in the activity of NOS, both the Ca2+-dependent and the Ca2+-independent isoforms, in cardiac tissue. Indirect evidence for a role of NO in the action of RLX also comes from the finding that this hormone increases the tissue levels of cGMP, which is crucial in intracellular signalling of NO (Ignarro, 1991). In turn, cGMP may contribute to the observed increase in coronary flow via vasorelaxation, as reported previously in studies on vascular smooth muscle cells (Ignarro, 1991; Dominiczak & Bohr, 1992), as well as to the reduction of cardiac tissue levels of Ca2+ (Tohse et al., 1995; Liu et al., 2001).
From the present data, it is not possible to identify which cellular targets in the heart are responsible for NOS up-regulation and increased NO generation in response to RLX. Based on the current literature, it is conceivable that several cell types of the heart may be involved. These types likely include coronary endothelial cells and vascular smooth muscle cells, which have been shown to respond to RLX by increasing NO production and iNOS expression (Bani et al., 1998a; Failli et al., 2001), as well as mast cells themselves, which can also produce NO upon stimulation with RLX (Masini et al., 1994a). Nitrergic innervation to the heart could also be a target for RLX, thus contributing additional NO. Of note, RLX has been found to up-regulate the nitrergic activity of the inhibitory neural component of mouse gastric strips in vitro (unpublished observation). The readily diffusible NO, by paracrine and autocrine pathways, may provide a major contribution to the anti-anaphylactic effects of RLX since it can down-regulate the release of histamine from cardiac mast cells and the vascular tone of coronary vessels by increasing cGMP and by decreasing intracellular Ca2+, which is critical for mast cell granule exocytosis and vascular smooth muscle contraction. RLX is both secreted and bound specifically by cardiocytes (Taylor & Clark, 1994; Hsu et al., 2002). Hence, RLX could act as an autocrine regulator of cardiac function. Since RLX can up-regulate iNOS expression and activity and NO production by the heart, it is likely that it could be involved in the defensive cellular adaptive phenomenon whereby brief ischemic stimuli render the heart resistant to subsequent similar stress, known as ischaemic preconditioning, in which NO has been shown to play a major role (Dawn & Bolli, 2002).
The present study extends our knowledge on the beneficial action of RLX in experimental allergic diseases and fits well with previous reports that RLX, administered systemically to sensitized guinea-pigs, counteracts bronchial hyperresponsiveness and inflammatory lung injury induced by antigen inhalation (Bani et al., 1997). Taken together with the reported ability of RLX to shift cultured T cells from a Th2 to a Th1 pattern of cytokine secretion (Piccinni et al., 1999), the above findings suggest that RLX could represent an endogenous defence mechanism against anaphylactic reactions due to either immunologic or non-immunologic causes. It is tempting to speculate that RLX, which attains the highest plasma levels during pregnancy (O'byrne & Steinetz, 1976; Bell et al., 1987), could play a role in protecting the mother against the risk of developing anaphylaxis in response to foetal soluble antigens, as may occur in amniotic fluid embolism (Benson et al., 2001). Going a step further, it cannot be ruled out that RLX itself or RLX-derived drugs could be developed in the future as a new therapeutic approach to allergic diseases.
Acknowledgments
The authors are grateful to Prof. O.D. Sherwood, from the Department of Molecular and Integrative Physiology, University of Illinois at Urbana Champaign, Urbana, U.S.A., for having provided purified porcine relaxin as a gift, and to Prof. Christoph Thiemermann, William Harvey Research Institute, London, for valuable suggestions.
Abbreviations
- cGMP
cyclic guanosine 3′,5′monophosphate
- EDTA
ethylene-diammino-tetracetic acid
- eNOS
endothelial nitric oxide synthase
- HEPES
2-[4-(2-hydroxyethyl)-1-piperazynyl]ethanesulphonic acid
- IBMX
3′-isobutyl-1-methylxanthine
- iNOS
inducible nitric oxide synthase
- iRLX
inactivated relaxin
- N2
nitrogen
- NO
nitric oxide
- OPT
ortophthalaldehyde
- RLX
relaxin
- TBS
Tris-buffered saline
- TCA
trichloroacteic acid
References
- BANI D. Relaxin: a pleiotropic hormone. Gen. Pharmacol. 1997;28:13–22. doi: 10.1016/s0306-3623(96)00171-1. [DOI] [PubMed] [Google Scholar]
- BANI D., BALLATI L., MASINI E., BIGAZZI M., BANI SACCHI T. Relaxin counteracts asthma-like reaction induced by inhaled antigen in sensitized guinea-pigs. Endocrinology. 1997;138:1909–1915. doi: 10.1210/endo.138.5.5147. [DOI] [PubMed] [Google Scholar]
- BANI D., FAILLI P., BELLO M.G., THIEMERMANN C., BANI SACCHI T., BIGAZZI M., MASINI E. Relaxin activates the L-arginine-nitric oxide pathway in vascular smooth muscle cells in culture. Hypertension. 1998a;31:1240–1247. doi: 10.1161/01.hyp.31.6.1240. [DOI] [PubMed] [Google Scholar]
- BANI D., MASINI E., BELLO M.G., BIGAZZI M., BANI SACCHI T. Relaxin protects against myocardial injury caused by ischemia and reperfusion in rat heart. Am. J. Pathol. 1998b;152:1367–1376. [PMC free article] [PubMed] [Google Scholar]
- BANI SACCHI T., BIGAZZI M., BANI D., MANNAIONI P.F., MASINI E. Relaxin-induced increased coronary flow through stimulation of nitric oxide production. Br. J. Pharmacol. 1995;116:1589–1594. doi: 10.1111/j.1476-5381.1995.tb16377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL R.J., EDDIE L.W., LESTER A.R., WOOD E.C., JOHNSTON P.D., NIALL H.D. Relaxin in human pregnancy serum measured with an homologous radioimmunoassay. Obstet. Gynecol. 1987;69:585–589. [PubMed] [Google Scholar]
- BENSON M.D., KOBAYASHI H., SILVER R.K., OI H., GREENBERGER P.A., TERAO T. Immunologic studies in presumed amniotic fluid embolism. Obstet. Gynecol. 2001;97:510–514. doi: 10.1016/s0029-7844(00)01213-8. [DOI] [PubMed] [Google Scholar]
- BIGAZZI M., BANI D., BANI SACCHI T. Relaxin: a possible future preventative therapy for cardiovascular disease in postmenopausal women and men. Climacteric. 2001;4:137–143. [PubMed] [Google Scholar]
- BÜLLESBACH E.E., SCHWABE C. On the receptor binding sites of relaxins. Int. J. Pept. Protein Res. 1988;32:361–367. doi: 10.1111/j.1399-3011.1988.tb01271.x. [DOI] [PubMed] [Google Scholar]
- CAPURRO N., LEVI R. The heart is a target organ in systemic allergic reactions: comparison of cardiac anaphylaxix in vivo and in vitro. Circ. Res. 1975;36:520–528. doi: 10.1161/01.res.36.4.520. [DOI] [PubMed] [Google Scholar]
- DAWN B., BOLLI R. Role of nitric oxide in myocardial preconditioning. Ann. N.Y. Acad. Sci. 2002;962:18–41. doi: 10.1111/j.1749-6632.2002.tb04053.x. [DOI] [PubMed] [Google Scholar]
- DIETERICH H.A., LÖFFELHOLZ K. Effect of coronary perfusion rate on the hydrolysis of exogenous and endogenous acetylcholine in the isolated heart. Naunyn-Schmied. Arch. Exp. Path. Pharmak. 1977;296:143–148. doi: 10.1007/BF00508466. [DOI] [PubMed] [Google Scholar]
- DOMINICZAK A.F., BOHR D.F. Mechanisms of vasorelaxation. Cardiovasc. Drug Rev. 1992;10:243–258. [Google Scholar]
- DUNCAN E., ONODERA T., ASHRAF M. Production of hydroxyl radicals and their disassociation from myocardial cell injury during calcium paradox. Free Radical Biol. Med. 1992;12:11–18. doi: 10.1016/0891-5849(92)90053-j. [DOI] [PubMed] [Google Scholar]
- DSCHIETZIG T., RICHTER C., BARTSCH C., LAULE M., ARMBRUSTER F.P., BAUMANN G., STANGL K. The pregnancy hormone relaxin is a player in human heart failure. FASEB J. 2001;15:2187–2195. doi: 10.1096/fj.01-0070com. [DOI] [PubMed] [Google Scholar]
- FAILLI P., NISTRI S., QUATTRONE S., MAZZETTI L., BIGAZZI M., BANI SACCHI, T., BANI D.Relaxin up-regulates inducible nitric oxide synthase expression and nitric oxide generation in rat coronary endothelial cells FASEB J. 2001200216252–254.(Dec 14, 2001) 10.1096/fj.01-0569fje Summary [DOI] [PubMed] [Google Scholar]
- FEIGEN G.A., VAUGHANWILLIAMS E.M., PETERSON J.K., NILSEN C.B. Histamine release and intracellular potentials during anaphylaxis in the isolated heart. Circ. Res. 1960;8:713–723. doi: 10.1161/01.res.8.4.713. [DOI] [PubMed] [Google Scholar]
- GIOTTI A., GUIDOTTI A., MANNAIONI P.F., ZILLETTI L. The influences of adrenotropic drugs and noradrenaline on the histamine release in cardiac anaphylaxis in vitro. J. Physiol. 1966;184:924–941. doi: 10.1113/jphysiol.1966.sp007957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU S.Y., NAKABAYASHI K., NISHI S., KUMAGAI J., KUDO M., SHERWOOD O.D., HSUEH A.J. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- IGNARRO L. Signal transduction mechanisms involving nitric oxide. Biochem. Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- KREMZER L.T., WILSON I.B. A procedure for the determination of histamine. Biochim. Biophys. Acta. 1961;50:364–367. doi: 10.1016/0006-3002(61)90339-0. [DOI] [PubMed] [Google Scholar]
- LIU H., SONG D., LEE S.S. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G68–G74. doi: 10.1152/ajpgi.2001.280.1.G68. [DOI] [PubMed] [Google Scholar]
- MASINI E., BANI D., BELLO M.G., BIGAZZI M., MANNAIONI P.F., BANI SACCHI T. Relaxin counteracts myocardial damage induced by ischemia-reperfusion in isolated guinea-pig hearts: evidence for an involvement of nitric oxide. Endocrinology. 1997;138:4713–4720. doi: 10.1210/endo.138.11.5520. [DOI] [PubMed] [Google Scholar]
- MASINI E., BANI D., BIGAZZI M., MANNAIONI P.F., BANI SACCHI T. Effects of relaxin on mast cells. In vitro and in vivo studies in rats and guinea-pigs. J. Clin. Invest. 1994a;94:1974–1980. doi: 10.1172/JCI117549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASINI E., PISTELLI A., GAMBASSI F., DI BELLO M.G., MANNAIONI P.F.The role of nitric oxide in anaphylactic reaction of isolated guinea-pig hearts and mast cells Nitric Oxide: Brain and Immune System 1994bLondon: Portland Press; 277–287.ed. Moncada, S., Nisticò G. & Higgs, A.E. pp [Google Scholar]
- MASINI E., PLANCHENAULT J., PEZZIARDI F., GAUTIER P., GAGNOL J.P. Histamine-releasing properties of Polysorbate 80 in vitro and in vivo: correlation with its hypotensive action in the dog. Agents Actions. 1985;16:470–477. doi: 10.1007/BF01983649. [DOI] [PubMed] [Google Scholar]
- MOLLACE V., COLASANTI M., RODINO P., MASSOUD R., LAURO G.M., NISTICÒ G. Cytokine-induced nitric oxide generation by cultured astrocytoma cells involves Ca++-calmodulin-independent NO-synthase. Biochem. Biophys. Res. Commun. 1993;191:327–334. doi: 10.1006/bbrc.1993.1221. [DOI] [PubMed] [Google Scholar]
- MONGAR J.L., SCHILD H.O. A comparison of the effects of anaphylactic shock and chemical histamine releasers. J. Physiol. 1952;118:461–478. doi: 10.1113/jphysiol.1952.sp004808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'BYRNE E.M., STEINETZ B.G. Radioimmunoassay of relaxin in sera of various species using an antiserum to porcine relaxation. Proc. Soc. Exp. Biol. Med. 1976;152:272–276. doi: 10.3181/00379727-152-39377. [DOI] [PubMed] [Google Scholar]
- PICCINNI M.P., BANI D., BELONI L., MANUELLI C., MAVILIA C., VOCIONI F., BIGAZZI M., BANI SACCHI T., ROMAGNANI S., MAGGI E. Relaxin favors the development of Th1-like T cells. Eur. J. Immunol. 1999;29:2241–2247. doi: 10.1002/(SICI)1521-4141(199907)29:07<2241::AID-IMMU2241>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MOLLACE V., PISTELLI A., ÅNGGÄRD E., VANE J. Metabolism of glyceryl trinitrate to nitric oxide by endothelial cells and smooth muscle cells and its induction by Escherichia coli lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. 1992;89:982–986. doi: 10.1073/pnas.89.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHÖRE P.A., BURKHALTER A., COHN V.A. A method for the fluorimetric assay of histamine in tissues. J. Pharmacol. Exp. Ther. 1959;127:182–186. [PubMed] [Google Scholar]
- TAYLOR M.J., CLARK C.L. Evidence for a novel source of relaxin: atrial cardiocytes. J. Endocrinol. 1994;143:R5–R8. doi: 10.1677/joe.0.143r005. [DOI] [PubMed] [Google Scholar]
- TAYLOR M.L., METCALFE D.D. Mast cells in allergy and host defense. Allergy Asthma Proc. 2001;22:115–119. doi: 10.2500/108854101778148764. [DOI] [PubMed] [Google Scholar]
- TOHSE N., NAKAYA H., TAKEDA Y., KANNO M. Cyclic GMP-mediated inhibition of L-type Ca2+ channel activity by human natriuretic peptide in rabbit heart cells. Br. J. Pharmacol. 1995;114:1076–1082. doi: 10.1111/j.1476-5381.1995.tb13316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF A.A., LEVI R. Histamine and cardiac arrhythmias. Circ. Res. 1986;58:1–16. doi: 10.1161/01.res.58.1.1. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN A.N.E., HULSMAN W.C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966;211:646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]


