Abstract
The aim of this study was to investigate the possible role of the interaction of different potassium channels in dog ventricular muscle, by applying the conventional microelectrode and whole cell patch-clamp techniques at 37°C.
Complete block of IKr by 1 μM dofetilide lengthened action potential duration (APD) by 45.6±3.6% at 0.2 Hz (n=13). Chromanol 293B applied alone at 10 μM (a concentration which selectively blocks IKs) did not markedly lengthen APD (<7%), but when repolarization had already been prolonged by complete IKr block with 1 μM dofetilide, inhibition of IKs with 10 μM chromanol 293B substantially delayed repolarization by 38.5±8.2% at 0.2 Hz (n=6).
BaCl2, at a concentration of 10 μM which blocks IKl without affecting other currents, lengthened APD by 33.0±3.1% (n=11), but when IKr was blocked with 1 μM dofetilide, 10 μM BaCl2 produced a more excessive rate dependent lengthening in APD, frequently (in three out of seven preparations) initiating early afterdepolarizations.
These findings indicate that if only one type of potassium channels is inhibited in dog ventricular muscle, excessive APD lengthening is not likely to occur. Dog ventricular myocytes seem to repolarize with a strong safety margin (‘repolarization reserve'). However, when this normal ‘repolarization reserve' is attenuated, otherwise minimal or moderate potassium current inhibition can result in excessive and potentially proarrhythmic prolongation of the ventricular APD. Therefore, application of drugs which are able to block more than one type of potassium channel is probably more hazardous than the use of a specific inhibitor of one given sort of potassium channel, and when simultaneous blockade of several kinds of potassium channel may be presumed, a detailed study is needed to define the determinants of ‘repolarization reserve'.
Keywords: Potassium channels, action potential duration, repolarization reserve, proarrhythmia, dofetilide, chromanol 293B, BaCl2
Introduction
Lengthening of cardiac action potential duration (APD) is an important mode of action of antiarrhythmic drugs (Singh & Vaughan Williams, 1970). Several non-cardiac drugs also prolong repolarization in the ventricular muscle and Purkinje fibres (Pinney et al., 1995; Gintant et al., 2001) by inhibition of one or more potassium currents (Antzelevitch et al., 1996; Rampe & Murawsky, 1997; Ducic et al., 1997; Drici & Barhanin, 2000). This latter phenomenon is an undesirable effect since it is known that prolongation of cardiac APD, in certain situations, may lead to proarrhythmic complication (torsade de pointes ventricular tachycardia), thereby increasing drug induced overall mortality.
Ventricular repolarization is governed by a fine balance of inward currents, such as the fast sodium (INa) and the L-type calcium (ICa) currents, and outward currents, such as the transient outward (Ito), rapid delayed rectifier (IKr), slow delayed rectifier (IKs) and inward rectifier (IKl) potassium currents. Under normal conditions impairment or block of one type of outward potassium channels can not be expected to cause excessive and potentially dangerous APD lengthening, since the other potassium currents may provide sufficient repolarizing capacity, which can be considered as a ‘repolarization reserve'. However, in situations where the density of one or more types of potassium channel is decreased by inheritance or remodelling (Roden et al., 1996; Tomaselli & Marban, 1999) inhibition of other potassium channels may lead to unexpectedly augmented APD prolongation, resulting in proarrhythmic reactions. In genetic channelophathies certain potassium channels, which normally contribute to repolarization, can attenuate the capability of the heart to repolarize.
Recently, we have found that selective IKs block only minimally lengthens repolarization in normal dog ventricular muscle, but when the ‘repolarization reserve' is attenuated by E-4031 and veratrine (Varró et al., 2000) IKs block substantially delays repolarization. It was therefore of interest to further investigate the possible role of the ‘repolarization reserve' and the interaction of different potassium channels in cardiac repolarization in dog ventricular muscle.
Methods
All experiments were carried out in compliance with the Guide for the Care and Use of Laboratory Animals (U.S.A. NIH publication No 85-23, revised 1985). The protocols were approved by the Review Board of the Committee on Animal Research of the University of Szeged (54/1999 OEj).
Conventional microelectrode technique
Adult mongrel dogs (8–14 kg) of either sex were used. Following anaesthesia (sodium pentobarbitone, 30 mg kg−1 administered intravenously), the heart of each animal was rapidly removed through right lateral thoracotomy. The hearts were immediately rinsed in oxygenated Tyrode's solution containing (in mM): NaCl, 115; KCl, 4; CaCl2, 1.8; MgCl2, 1; NaHCO3, 20; and glucose, 11. The pH of this solution was 7.40–7.45 when gassed with 95% O2 and 5% CO2 at 37°C. Tip of the papillary muscles obtained from the right ventricle were individually mounted in a tissue chamber (volume≈50 ml). Each ventricular preparation was initially stimulated (HSE (Hugo Sachs Elektronik) stimulator type 215/II, March-Hugstetten, Germany) at a basic cycle length of 1000 ms (frequency=1 Hz), using 2 ms rectangular constant voltage pulses isolated from ground and delivered across bipolar platinum electrodes in contact with the preparation. Each preparation was allowed at least 1 h to equilibrate while they were continuously superfused with Tyrode's solution. Temperature of the superfusate was kept constant at 37°C. Transmembrane potentials were recorded using conventional microelectrode techniques. Microelectrodes filled with 3M KCl and having tip resistances of 5–20 MOhm were connected to the input of a high impedance electrometer (HSE microelectrode amplifier type 309), which was connected to ground. The first derivative of transmembrane potentials was electronically obtained by an HSE differentiator (type 309). The voltage outputs from all amplifiers were displayed on a dual beam memory oscilloscope (Tektronix 2230 100 MHz digital storage oscilloscope, Beaverton, OR, U.S.A.).
The maximum diastolic potential, action potential amplitude and APD measured at 50 and 90% repolarization (APD50-90) were obtained using a software developed in our department (HSE-APES) on an IBM 386 microprocessor based personal computer connected to the digital output of the oscilloscope. After control measurements the preparations were superfused for 60 min with Tyrode's solution containing the compound under study, and then the electrophysiological measurements were resumed. Chromanol 293B (gift from Aventis Pharma, Frankfurt, Germany) and dofetilide (Institute for Drug Research, Budapest, Hungary) were dissolved in 100% DMSO to make 1 mM and 10 mM stock solutions. The stock solutions were further diluted in the tissue bath to obtain the desired final drug concentrations.
Whole cell configuration of the patch-clamp technique
Ventricular myocytes were enzymatically dissociated from hearts of mongrel dogs of either sex weighing 10–20 kg following anaesthesia (sodium pentobarbitone, 30 mg kg−1 i.v.) as described earlier in detail (Varró et al., 2000).
One drop of cell suspension was placed within a transparent recording chamber mounted on the stage of an inverted microscope (TMS, Nikon, Tokyo, Japan), and individual myocytes were allowed to settle and adhere to the chamber bottom for at least 5 min before superfusion was initiated. Only rod shaped cells with clear cross striations were used. HEPES buffered Tyrode's solution served as the normal superfusate. This solution contained (mM): NaCl 144, NaH2PO4 0.33, KCl 4.0, CaCl2 1.8, MgCl2 0.53, Glucose 5.5, and HEPES 5.0 at pH of 7.4.
Patch-clamp micropipettes were fabricated from borosilicate glass capillaries (Clark, Reading, U.K.) using a P-97 Flaming/Brown micropipette puller (Sutter Co, Novato, CA, U.S.A.). These electrodes had resistances between 1.5 and 2.5 MΩ when filled with pipette solution containing (in mM): K-aspartate 100, KCl 45, ATP 3, MgCl2 1, EGTA 10 and HEPES 5. The pH of this solution was adjusted to 7.2 by KOH. Cell capacitance (214.4±26.2 pF, n=50) was measured by applying a 10 mV hyperpolarizing pulse from −10 mV. The holding potential was −90 mV. The capacity was measured by integration of the capacitive transient divided by the amplitude of the voltage step (10 mV). Measuring K+ currents, nisoldipine (1 μM) (gift from Bayer AG, Leverkusen, Germany) was added to the external solution to eliminate inward L-type Ca2+ current (ICa). The rapid IKr and slow IKs components of the delayed rectifier potassium current were separated by using the selective IKr blocker E-4031 (1 μM, Institute for Drug Research, Budapest, Hungary) or the IKs blocker L-735,821 (100 nM, a gift from Merck-Sharpe & Dohme, West-Point, PA, U.S.A.). Membrane currents were recorded with Axopatch-1D and 200B patch-clamp amplifiers (Axon Instruments, Union City, CA, U.S.A.) using the whole-cell configuration of the patch-clamp technique. After establishing a high (1–10 Gohm) resistance seal by gentle suction, the cell membrane beneath the tip of the electrode was disrupted by suction or by application of 1.5 V electrical pulses for 1–5 ms. The series resistance was typically 4–8 MΩ before compensation (50–80%, depending on the voltage protocols). Experiments where the series resistance was high, or substantially increased during measurement, were discarded. Membrane currents were digitized using a 333 kHz analogue-to-digital converter (Digidata 1200, Axon Instruments) under software control (pClamp 6.0 and 7.0 Axon Instruments). Analyses were performed using Axon (pClamp 6.0) software after low-pass filtering at 1 kHz. All patch-clamp data were collected at 37°C.
Statistical analyses
Results were compared using Student's t-tests for paired and unpaired data. Differences were considered significant when P<0.05. Data are expressed as mean±s.e.mean.
Results
Effect of IKs and combined IKs and IKr inhibition on the APD
Chromanol 293B is considered as an effective and relatively selective blocker of IKs (Busch et al., 1996). In our experiments we used 10 μM chromanol 293B since at higher concentrations the compound was reported also to block Ito (Bosch et al., 1998; Sun et al., 2001) or possibly other current(s). In order to establish the selectivity of chromanol 293B on IKs we have investigated the possible effects of chromanol on various transmembrane potassium currents. In all experiments L-type calcium current (ICa) was fully blocked by addition of 1 μM nisoldipine.
IKl was measured by applying 36 s ramp pulses from −120 mV to +60 mV from the holding potential of −90 mV at the pulse frequency of 0.02 Hz. As Figure 1A shows the current traces, which represent IKl, were overlapping and therefore indistinguishable from each other indicating that 10 μM chromanol 293B did not affect IKl. The transient outward current (Ito) was elicited by 300 ms test voltage pulses from −20 to 50 mV from the holding potential of −90 mV and with 0.33 Hz pulse frequency. The amplitude of the current was determined as the difference of the peak current at the start of the pulse and the current level measured at the end of the pulse. Figure 1B and Table 1 show that 10 μM chromanol 293B did not affect significantly Ito. The rapid delayed rectifier current (IKr) was measured by 0.5–1 s test voltage pulses from −10 to 50 mV with pulse frequency of 0.05 Hz. The holding potential in these experiments was −40 mV. The deactivating so-called tail current was measured as IKr after returning the voltage from the test potential to −40 mV. The amplitude of the IKr tail current was determined as the difference between the peak tail current and the holding current level at −40 mV. Figure 1C and Table 1 show that chromanol 293B, even at the high 100 μM concentration, exerted only minimal effect on IKr. In these experiments IKs was blocked by 100 nM L-735,821.
Figure 1.
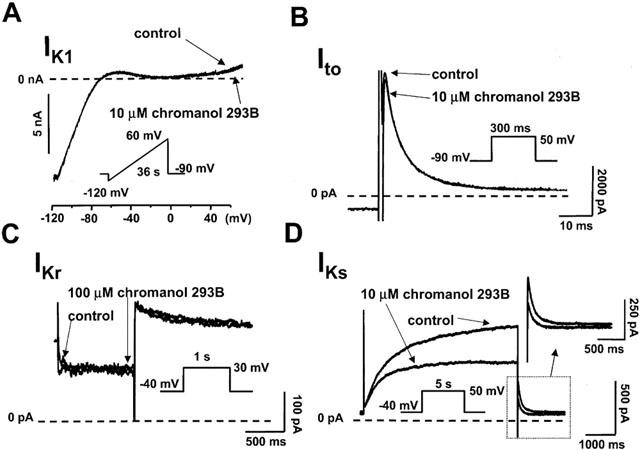
Effect of chromanol 293B on the inward rectifier (IKl), transient outward (Ito), rapid delayed rectifier (IKr) and slow delayed rectifier (IKs) potassium currents in dog ventricular myocytes. When measuring IKr, L-735,821 (0.1 μM) was used to completely block IKs, and E-4031 (2 μM) was applied to block IKr when measuring IKs. The applied voltage protocols are shown in the insets and explained in the text in more detail. In all experiments ICa was fully blocked by addition of 1 μM nisoldipine. The dotted lines represent the zero current levels.
Table 1.
Effect of chromanol 293B and BaCl2 on various potassium currents in dog ventricular myocytes
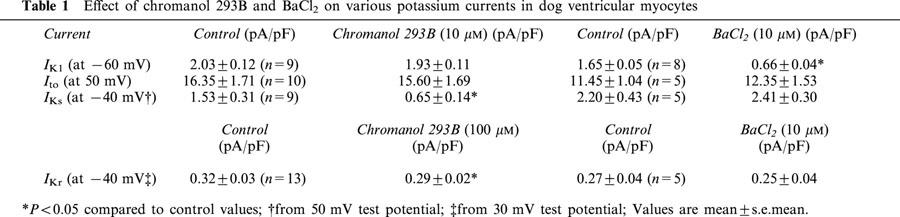
The slow delayed rectifier (IKs) was measured by 5 s test voltage pulses from −20 to +50 mV with the pulse frequency of 0.1 Hz. The holding potential in these experiments was −40 mV. IKr was completely blocked by 2 μM E-4031. The deactivating IKs tail current was measured after returning the voltage from +50 mV to −40 mV. The amplitude of the IKs tail current was determined as the difference between the peak tail current and the holding current level at −40 mV. Figure 1D and Table 1 show that 10 μM chromanol 293B caused a more than 50% inhibition of IKs.
These experiments suggested that chromanol 293B at 10 μM can be applied as a tool inhibiting IKs rather specifically.
Figure 2 shows that 10 μM chromanol 293B alone lengthened APD only to a small extent (<7%) in dog right ventricular papillary muscle at stimulation cycle lengths ranging from 300 to 5000 ms. However, when the same concentration of chromanol 293B was added to preparations in which IKr was previously blocked by the specific IKr blocker dofetilide (1 μM), it induced a marked and significant further prolongation of the APD (Figure 3), suggesting a strong additive effect of the two compounds.
Figure 2.
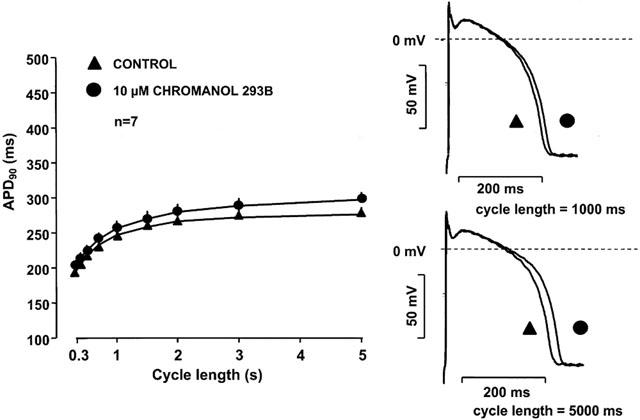
Frequency dependent effect of IKs block (chromanol 293B) on the action potential duration in dog right ventricular papillary muscle. Note that the error bars are often smaller than the corresponding symbols.
Figure 3.
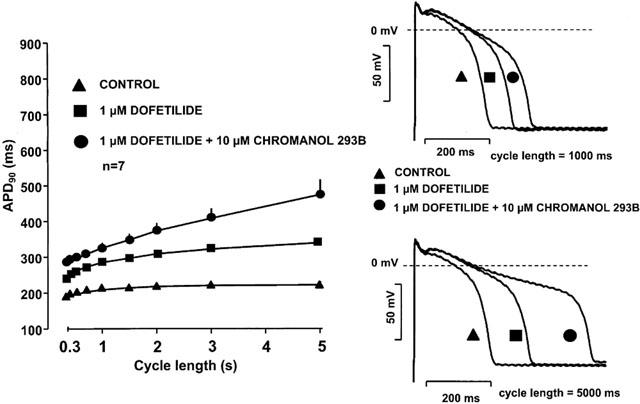
Frequency dependent effect of IKr (dofetilide) and combined IKr (dofetilide) and IKs (chromanol 293B) block on the action potential duration in dog right ventricular papillary muscle. Note that the error bars are often smaller than the corresponding symbols, and the drug combination augmented the reverse rate dependent APD prolongation.
Effect of IKl and combined IKl and IKr inhibition on the APD
It was reported that a low concentration of BaCl2 inhibited IKl in cardiac myocytes (Liu et al., 2001). IKl and Ito were measured as previously described. Since BaCl2 markedly reduced IKl, which appeared as a voltage independent background current when measuring IKr and IKs, the following protocols were applied to determine the effect of BaCl2 on IKr and IKs. First the previously described voltage protocols were used in control condition. Then 10 μM BaCl2 was added, and the voltage protocols were repeated. To distinguish between the effect of BaCl2 on IKl and IKr/IKs, 2 μM E-4031 or 100 nM L-735,821 were added to the tissue bath and measurements were repeated again. Subtracting current traces after application of the specific blockers for IKr or IKs in the presence of BaCl2 resulted in E-4031 or L-735,821 sensitive currents, which can be considered as IKr or IKs, respectively. These E-4031 and L-735,821 sensitive currents, in the presence of BaCl2, were compared to control IKr and IKs measurement to determine the possible effect of 10 μM BaCl2 on IKr and IKs. As Figures 4B,C,D and Table 1 show, 10 μM BaCl2 did not significantly affect Ito, IKr and IKs. Figure 4A and Table 1 indicate, however, that 10 μM BaCl2 inhibited IKl very effectively (>70%) suggesting that at this concentration BaCl2 can be used as a rather selective tool to inhibit IKl.
Figure 4.
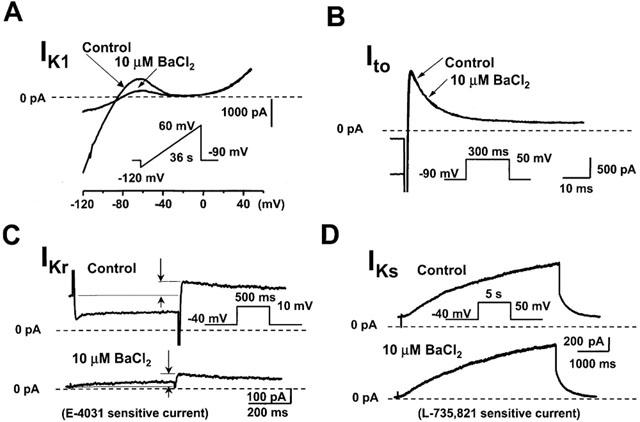
Effect of BaCl2 on the inward rectifier (IKl), transient outward (Ito), rapid delayed rectifier (IKr) and slow delayed rectifier (IKr) potassium currents in dog ventricular myocytes. For measuring the effect of BaCl2 on IKr the following protocol was used; (1) current recording under control conditions; (2) current recording in the presence of 10 μM BaCl2; (3) current recording after application of 2 μM E-4031 (in the presence of 10 μM BaCl2). Subtracting (3) from (2) gave the E-4031 sensitive current (i.e. IKr) in the presence of 10 μM BaCl2. For measuring the effect of BaCl2 on IKs a similar protocol was used but 0.1 μM L-731,821 was applied for determining IKs. The applied voltage protocols are shown in the insets, and explained in the text in more detail. In all experiments ICa was fully blocked by addition of 1 μM nisoldipine. The dotted lines represent the zero current levels.
Figure 5 shows that 10 μM BaCl2 alone lengthened APD in a reverse rate dependent manner inducing APD prolongation of 9.4±2.1% at cycle length of 300 ms (n=11, P<0.05) and 33.0±3.3% at cycle length of 5000 ms (n=11, P<0.05). When the same concentration of BaCl2 was applied but to preparations in which IKr was previously blocked by 1 μM dofetilide, BaCl2 induced a more excessive reverse-rate dependent further prolongation of APD (Figure 6). This excessive APD lengthening at long cycle lengths frequently (in three out of seven experiments) resulted in early afterdepolarizations (EADs) as shown in Figure 7).
Figure 5.
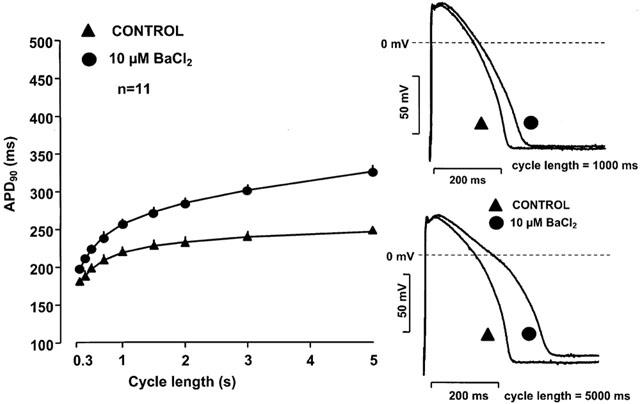
Frequency dependent effect of IKl block (BaCl2) on the action potential duration in dog right ventricular papillary muscle. Note that the error bars are often smaller than the corresponding symbols.
Figure 6.
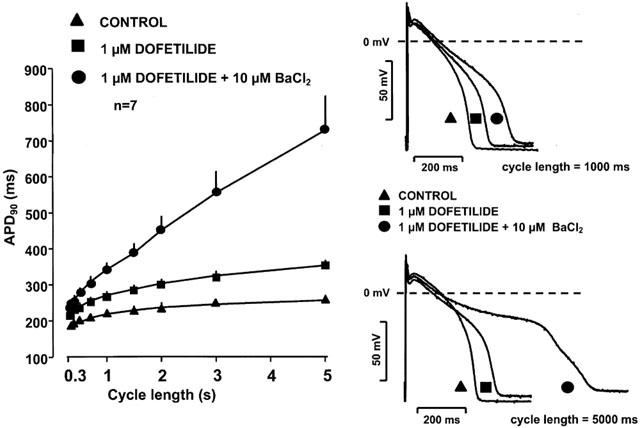
Frequency dependent effect of IKr (dofetilide) and combined IKr (dofetilide) and IKl (BaCl2) block on the action potential duration in dog right ventricular papillary muscle. Note that the error bars are often smaller than the corresponding symbols, and the drug combination augmented the reverse rate dependent APD prolongation.
Figure 7.
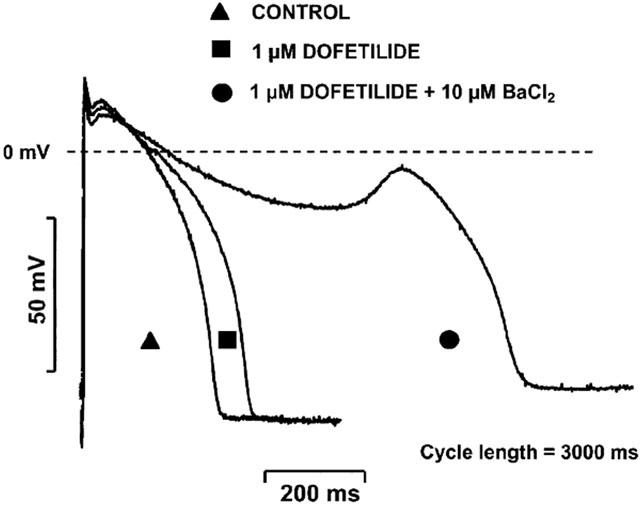
Early afterdepolarization (EAD) evoked by the combined IKr (dofetilide) and IKl (BaCl2) block in dog right ventricular papillary muscle.
Discussion
The most important finding of this study is that IKs or IKl inhibition resulted in an augmented reverse rate dependent repolarization lengthening in the presence of IKr block.
In our experiments 10 μM chromanol 293B and 10 μM BaCl2 were applied in order to partially but selectively inhibit IKs and IKl, respectively. To apply these two agents at higher concentrations in which they would cause complete block of IKs and IKl was hampered by their known capability to inhibit various other potassium channels (Bosch et al., 1998; Weerapura et al., 2000; Sun et al., 2001). However, at the concentration of 10 μM, as our Figures demonstrate (Figures 1 and 4), they can be regarded as fairly selective blockers of IKs or IKl, respectively. In spite of this, it can not be entirely ruled out that chromanol 293B and BaCl2 at the applied concentration, can affect other, so far uninvestigated, transmembrane cardiac ion channels or electrogenic transport mechanisms. Further studies would be worthwhile to rule out or establish such possibilities.
Our results are in good agreement with the results of previous studies. Our recent findings indicate that chromanol 293B alone only slightly lengthens APD in dog papillary muscle, but when APD is lengthened by previous superfusion of E-4031 and veratrine chromanol 293B resulted in a marked prolongation of APD (Varró et al., 2000) which can be explained by the kinetic properties of IKs. Accordingly, at longer APD IKs has more time to be activated and thereby it can more importantly contribute to repolarization. This led us to conclude that IKs could provide an important means of limiting excessive APD lengthening when action potentials are prolonged beyond normal by other mechanisms. In the present work we extended this previous observation, suggesting now that any kind of additive potassium channel block would greatly limit the capability of the ventricular muscle to repolarize.
Vos et al. (1995) developed an experimental torsade de pointes arrhythmia model in dogs, in which they found that after complete chronic atrioventricular block downregulation of IKs and IKr can be observed (Volders et al., 1999). These animals showed longer QTc interval and monophasic APD, and had augmented susceptibility to develop torsade de pointes ventricular tachycardia after administration of certain Class III antiarrhythmic drugs (Verduyn et al., 1997). It is also known that various potassium channels are downregulated during heart failure (Näbauer & Kääb, 1998), resulting in longer APD (Kääb et al., 1996) and in increased risk of proarrhythmia.
In some forms of inherited long QT syndrome, like LQT1 and LQT2 (Roden et al., 1996; Priori et al., 2001), loss of functional potassium channel proteins or possible decrease of their density does not always result in marked or manifest QTc lengthening (Swan et al., 1998; Priori et al., 1998). These patients, however, are susceptible to drugs, which can further depress the function of different types of potassium channels, and in such patients this can cause excessive and unexpected APD lengthening, often inducing torsade de pointes tachycardia. These observations strongly argue for the role of the so-called ‘repolarization reserve' i.e. that different potassium channels may compensate each other to secure the repolarization process (Roden, 1998).
Similar to our results, very recently, Burashnikov & Antzelevitch (2002) reported that attenuation of the repolarization reserve by combining IKr and IKs block can result in excessive APD lengthening and development of EADs.
The present experiments may have some important therapeutical and practical implications. Based on these results it seems obvious that in situations where the ‘repolarization reserve' is decreased, otherwise minor loss of the function of the potassium channels may lead to excessive repolarization lengthening and under certain conditions to development of torsade de pointes arrhythmia. Therefore, patients admitted to hospital should be carefully tested how they respond to Class III antiarrhythmic drugs, and only those patients should receive long-term out of hospital treatment who respond to these drugs with QTc lengthening within the expected range. Similar conclusion can be drawn also from DIAMOND trial (Brendorp et al., 2001) as well, since dofetilide treatment proved to be neutral concerning overall mortality, but in a subgroup when mortality was related to QTc lengthening it was found that in patients in whom QTc lengthening was less, the dofetilide induced mortality figures tended to be favourable, but in patients where the QTc lengthening was above average, after dofetilide treatment, the mortality ratio was somewhat increased.
Another possible implication of our study is that we should re-evaluate our safety pharmacology concepts related to drug induced QTc lengthening. Most of the routine safety pharmacology investigations are carried out in channel expression systems or in in vitro and in vivo tests performed in normal preparations where the ‘repolarization reserve' is intact, frequently even at fast heart rates. In these situations the possible proarrhythmic danger of both cardiac and noncardiac investigational compounds is often underestimated, since the drugs may not cause significant APD lengthening under normal conditions, but in patients where the ‘repolarization reserve' is impaired, the risk for excessive APD prolongation and consequently for torsade de pointes arrhythmia is greatly enhanced. Therefore, the preclinical safety pharmacology screenings should be extended to preparations in which ‘repolarization reserve' is attenuated.
In summary we can conclude that: (a) In the dog ventricular muscle when only one type of potassium channels is inhibited, excessive APD lengthening is not likely to occur. This is probably due to the capability of the various potassium channels to substitute each other. Dog ventricular myocytes seem to repolarize with a strong safety margin (‘repolarization reserve'). (b) When the normal ‘repolarization reserve' is attenuated (due to drugs, remodelling or genetic disorders), the otherwise minimal or moderate potassium current inhibition can result in excessive and potentially proarrhythmic prolongation of the ventricular action potential duration. Also, application of drugs which are able to block more than one type of potassium channels is probably more hazardous than the use of specific inhibitors of a given potassium channel. (c) We should re-evaluate our safety pharmacology concept related to possible QT lengthening effect of drugs to apply tests in preparations where the ‘repolarization reserve' is impaired instead of using preparations where this reserve is normal.
Acknowledgments
This work was supported by grants from the Hungarian National Research Foundation (OTKA T-032558, T-035018 and T-037520), Hungarian Ministry of Health (ETT 532/2000, 536/2000 and T-144/2001), Hungarian Ministry of Education (FKFP 0064/2001), National Research and Development Programmes (NKFP 1A/0011/2002), the Hungarian Academy of Sciences and by János Bolyai Research Scholarship (For L. Virág and N. Iost). Chromanol 293B was a gift from AVENTIS Pharma (Frankfurt, Germany) and L-735,821 was a gift from Merck-Sharpe & Dohme (West Point, PA, U.S.A.).
Abbreviations
- APD
action potential duration
- APD50 and APD90
action potential durations at 50% and 90% of repolarization
- CL
cycle length
- EAD
early afterdepolarization
- ICa
L-type calcium current
- IKl
inward rectifier potassium current
- IKr
rapid component of the delayed rectifier potassium current
- IKs
slow component of the delayed rectifier potassium current
- INa
inward fast sodium current
- Ito
transient outward current
- LQT
long QT syndrome
References
- ANTZELEVITCH C., SUN Z.Q., ZHANG Z.Q., YAN G.X. Cellular and ionic mechanisms underlying erythromycin-induced long QT intervals and torsade de pointes. J. Am. Coll. Cardiol. 1996;28:1836–1848. doi: 10.1016/S0735-1097(96)00377-4. [DOI] [PubMed] [Google Scholar]
- BOSCH R.F., GASPO R., BUSCH A.E., LANG H.J., NATTEL S. Effects of the chromanol 293B, a selective blocker of the slow component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc. Res. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- BRENDORP B., ELMING H., JUN L., KOBER L., MALIK M., JENSEN G.B., TORP-PEDERSEN C. The DIAMOND Study Group. QTc interval as a guide to select those patients with congestive heart failure and reduced left ventricular systolic function who will benefit from antiarrhythmic treatment with dofetilide. Circulation. 2001;103:1422–1427. doi: 10.1161/01.cir.103.10.1422. [DOI] [PubMed] [Google Scholar]
- BURASHNIKOV A., ANTZELEVITCH C. Prominent IKs in epicardium and endocardium contributes to development of transmural dispersion of repolarization but protects against development of early afterdepolarizations. J. Cardiovasc. Electrophysiol. 2002;13:172–177. doi: 10.1046/j.1540-8167.2002.00172.x. [DOI] [PubMed] [Google Scholar]
- BUSCH A.E., SUESSBRICH H., WALDEGGER S., SAILER E., GREGER R., LANG H., GIBSON K.J., MAYLIE J.G. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflügers Archiv-Eur. J. Physiol. 1996;432:1094–1096. doi: 10.1007/s004240050240. [DOI] [PubMed] [Google Scholar]
- DRICI M.D., BARHANIN J. Cardiac K+ channels and drug-acquired long QT syndrome. Therapie. 2000;55:185–193. [PubMed] [Google Scholar]
- DUCIC I., KO C.M., SHUBA Y., MORAD M. Comparative effects of loratidine and terfenadine on cardiac K+ channels. J. Cardiovasc. Pharmacol. 1997;30:42–54. doi: 10.1097/00005344-199707000-00007. [DOI] [PubMed] [Google Scholar]
- GINTANT G.A., LIMBERIS J.T., MCDERMOTT J.S., WEGNER C.D., COX B.F. The canine Purkinje fiber: an in vitro model system for acquired long QT syndrome and drug-induced arrhythmogenesis. J. Cardiovasc. Pharmacol. 2001;37:607–618. doi: 10.1097/00005344-200105000-00012. [DOI] [PubMed] [Google Scholar]
- KÄÄB S., NUSS H.B., CHIAMVIMONVART N., O'ROURKE B., KASS D.A., MARBAN E., TOMASELLI G.F. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ. Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- LIU G.X., DERST C., SCHLICHTHÖRL G., HEINEN S., SEEBOHM G., BRÜGGEMANN A., KUMMER W., VEH R.W., DAUT J., PREISIG-MÜLLER R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J. Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NÄBAUER M., KÄÄB S. Potassium channel down-regulation in heart failure. Cardiovasc. Res. 1998;37:324–334. doi: 10.1016/s0008-6363(97)00274-5. [DOI] [PubMed] [Google Scholar]
- PINNEY S.P., KOLLER B.S., FRANZ M.R., WOOSLEY R.L. Terfenadine increases the QT interval in isolated guinea pig heart. J. Cardiovasc. Pharmacol. 1995;25:30–40. doi: 10.1097/00005344-199501000-00006. [DOI] [PubMed] [Google Scholar]
- PRIORI S.G., BLOISE R., CROTTI L. The long QT syndrome. Europace. 2001;3:16–27. doi: 10.1053/eupc.2000.0141. [DOI] [PubMed] [Google Scholar]
- PRIORI S.G., NAPOLITANO C., BLOISE R., SCHWARTZ P.J.Low penetrance in the long QT syndrome: the importance of molecular diagnosis Eur. Heart J. 199819424Abstract suppl [Google Scholar]
- RAMPE D., MURAWSKY M.K. Blockade of the human cardiac K+ channel Kv1.5 by the antibiotic erythromycin. Naunyn-Schmiedebergs Arch. Pharmacol. 1997;355:743–750. doi: 10.1007/pl00005008. [DOI] [PubMed] [Google Scholar]
- RODEN D.M. Taking the “idio” out of “idiosyncratic”: Predicting torsades de pointes. PACE. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- RODEN D.M., LAZZARA R., ROSEN M., SCHWARTZ P.J., TOWBIN J., VINCENT G.M., for the SADS Foundation Task Force on LQTS Multiple mechanisms in the long-QT syndrome current knowledge, gaps, and future directions. Circulation. 1996;94:1996–2012. doi: 10.1161/01.cir.94.8.1996. [DOI] [PubMed] [Google Scholar]
- SINGH B.N., VAUGHAN WILLIAMS E.M. A third class of anti-arrhythmic action. Effects on atrial and ventricular intracellular potentials, and other pharmacological actions on cardiac muscle, of MJ 1999 and AH 3474. Br. J. Pharmacol. 1970;39:675–687. doi: 10.1111/j.1476-5381.1970.tb09893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN Z.Q., THOMAS G.P., ANTZELEVITCH C. Chromanol 293B inhibits slowly activating delayed rectifier and transient outward currents in canine left ventricular myocytes. J. Cardiovasc. Electrophysiol. 2001;12:472–478. doi: 10.1046/j.1540-8167.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- SWAN H., SAARINEN K., KONTULA K., TOIVONEN L., VIITASALO M. Evaluation of QT interval duration and dispersion and proposed clinical criteria in diagnosis of long QT syndrome in patients with a genetically uniform type of LQT1. J. Am. Coll. Cardiol. 1998;32:486–491. doi: 10.1016/s0735-1097(98)00248-4. [DOI] [PubMed] [Google Scholar]
- TOMASELLI G.F., MARBAN E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc. Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- VARRÓ A., BALÁTI B., IOST N., TAKÁCS J., VIRÁG L., LATHROP D.A., LENGYEL C., TÁLOSI L., PAPP J.G.Y. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J. Physiol. 2000;523:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERDUYN S.C., VOS M.A., VAN DER ZANDE J., VAN DER HULST F.F., WELLENS H.J. Role of interventricular dispersion of repolarization in acquired torsade-de-pointes arrhythmias: reversal by magnesium. Cardiovasc. Res. 1997;34:453–463. doi: 10.1016/s0008-6363(97)00067-9. [DOI] [PubMed] [Google Scholar]
- VOLDERS P.G.A., SIPIDO K.R., VOS M.A., SPÄTJENS R.L.H.M.G., LEUNISSEN J.D.M., CARMELIET E., WELLENS H.J.J. Downregulation of delayed rectifier K+ currents in dog with chronic complete atrioventricular block and acquired torsades de pointes. Circulation. 1999;100:2455–2461. doi: 10.1161/01.cir.100.24.2455. [DOI] [PubMed] [Google Scholar]
- VOS M.A., VERDUYN S.C., GORGELS A.P.M., LIPCSEI G.C., WELLENS H.J.J. Reproducible induction of early afterdepolarizations and torsade de pointes arrhythmias by d-sotalol and pacing in dogs with chronic atrioventricular block. Circulation. 1995;91:846–872. doi: 10.1161/01.cir.91.3.864. [DOI] [PubMed] [Google Scholar]
- WEERAPURA M., NATTEL S., COURTEMANCHE M., DOERN D., ETHIER N., HÉBERT T.E. State-dependent barium block of wild-type and inactivation-deficient HERG channels in Xenopus oocytes. J. Physiol. 2000;526:265–278. doi: 10.1111/j.1469-7793.2000.t01-1-00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


