Abstract
The cyclic peptide human urotensin II (U-II) has been recently recognized as the endogenous ligand of an orphan GPCR, subsequently named the UT receptor. No synthetic ligands are available for investigating this novel peptide-receptor system. A novel UT receptor ligand, [Orn8]U-II, was synthesized and evaluated in calcium functional assays performed on HEK293 cells expressing the recombinant rat and human UT receptor and in the rat aorta bioassay. [Orn8]U-II behaves as a full agonist (pEC50≈8) at both human and rat UT receptors in the FlipR calcium assay eliciting similar maximal effects as the natural ligand U-II. On the contrary, in the rat aorta bioassay, [Orn8]U-II behaves as a competitive and selective antagonist (pA2=6.56) showing however a small but consistent residual agonist activity. It is therefore proposed that [Orn8]U-II is a partial agonist at UT receptors.
Keywords: [Orn8]urotensin II, recombinant and native UT receptors, calcium assay, rat aorta bioassay
Introduction
Urotensin II (U-II) is a cyclic peptide that was isolated from the urophysis of the teleost fish (Pearson et al., 1980). The gene coding for the human peptide was cloned (Coulouarn et al., 1998) and U-II was demonstrated to selectively bind and activate a G-protein coupled receptor (Ames et al., 1999; Nothacker et al., 1999), subsequently named the UT receptor (Douglas & Ohlstein, 2000b). This receptor is mainly expressed in the heart, vascular smooth muscle, and central nervous system (spinal cord and cerebellum) (Douglas & Ohlstein, 2000a). The vascular activity of U-II is the most studied action of this peptide. Several groups reported contractile effects of U-II in some vascular smooth muscle preparations with huge regional, species, and interindividual variations (Camarda et al., 2002; Douglas et al., 2000c; Maguire et al., 2000). In the few vessels sensitive to U-II, the peptide always displayed very high potency but low maximal effects. Worthy of mention is the recent demonstration of vasoconstrictive properties of U-II in humans in vivo (Bohm & Pernow, 2002). Altogether, the biological actions mediated by the U-II / UT receptor system are poorly known due to the lack of selective receptor ligands (particularly antagonists) and knockout or transgenic animal models (Douglas & Ohlstein, 2000a).
In the frame of a structure-activity study on U-II, we identified [Orn8]U-II and we report herein on the in vitro pharmacological profile of this novel UT receptor ligand.
Methods
Experiments were performed on HEK293 cells stably expressing the recombinant human (hUT) or rat (rUT) UT receptor and in the rat isolated aorta. Details about hUT receptor cloning and generation of stable cell lines expressing the hUT protein have been previously reported (Flohr et al., 2002). The rat UT receptor coding sequence flanked by a 5′ EcoRI and 3′ NotI site was amplified via PCR from rat kidney cDNA and cloned into the mammalian pEAK8 expression vector. Correctness of the construct was verified by dideoxy sequencing in both directions. The pEAK8 construct was used for generation of cell lines stably expressing the rUT receptor.
Saturation studies performed in our laboratories indicated that the binding of [125I]Tyr9-U-II was characterized by a KD of 1.66 nM and a Bmax of 1100 fmol mg protein−1 for the hUT receptor, and a KD of 1.23 nM and Bmax of 3700 fmol mg protein−1 for rUT receptor membranes.
Functional experiments were performed on HEK293-hUT and HEK293-rUT cells by measuring [Ca2+]i levels with the fluorometric imaging plate reader FlipR™, (Molecular Devices, Sunnyvale CA, U.S.A.) and 4 μM of the fluorescent calcium indicator Fluo-4 as described previously in detail (Flohr et al., 2002). Fluorescence data were generated in duplicate.
Bioassay experiments were performed as previously described (Camarda et al., 2002). Briefly, thoracic aorta were taken from male Sprague–Dawley rats (200–250 g) decapitated under ether anaesthesia. The tissues were suspended in organ baths containing oxygenated Krebs solution at 37°C and at pH 7.4, stretched to a resting tension of 1 g and allowed to equilibrate for 1 h. Contractions were measured with isometric transducers (GRASS FT03) and recorded by a multichannel polygraph (LINSEIS L2005). Cumulative concentration response curves to U-II, noradrenaline, angiotensin-II and endothelin-1 were performed in the absence and in the presence of [Orn8]U-II. A few experiments were also performed in the electrically stimulated guinea-pig ileum where the effects of [Orn8]U-II were tested against the inhibitory effect of somatostatin.
Concentration response curve data were analysed by non-linear curve fitting using Graph Pad Prism version 3.02; the equation used was as follows:
where y=effect; bottom=baseline; top=maximal effect; EC50=concentration of an agonist that produces 50% of the maximal effect; x=log of agonist concentration.
The peptides used in this study were synthesized and purified in house at the Department of Pharmaceutical Sciences of the University of Ferrara. All other substances and reagents were from Sigma (St. Louis, MO, U.S.A.) or Tocris (Bristol, U.K.).
The data are expressed as means±s.e.mean of n experiments. Data were statistically analysed using the Student's t-test for unpaired data or one way ANOVA followed by Dunnett test for multiple comparison. All experimental procedures complied with the standards of the European Communities Council directives (86/609/EEC).
Results
U-II increased intracellular calcium levels in HEK293 hUT and rUT cells with similar high potencies (pEC50 8.51±0.18 and 8.54±0.14, respectively) and maximal effects (Figure 1), and evoked concentration dependent contractions (pEC50 8.17±0.18) in the rat isolated aorta (Figure 2, top panel). These results are in line with several published reports (Ames et al., 1999; Camarda et al., 2002; Douglas et al., 2000c; Nothacker et al., 1999). The synthetic analogue, [Orn8]U-II, behaved as a full agonist in the calcium assay in HEK293 hUT and rUT cells, inducing maximal effects similar to those of U-II. The potency of the analogue at both the hUT and rUT receptor (pEC50 7.93±0.16 and 8.06±0.22, respectively) was, however, 3 fold lower than that of U-II. Quite different results have been obtained in the rat aorta bioassay, where [Orn8]U-II produced negligible contractile effects at 0.1 and 1 μM, and caused at 10 μM a stable contraction of 0.12±0.04 g (corresponding to about 25% of the maximal effect of U-II). In this range of concentrations, however, [Orn8]U-II produced a concentration dependent rightward shift of the concentration response curve to U-II, without significantly modifying the maximal effects induced by the natural ligand (Figure 2, top panel). Although the analysis of these data is biased by the residual agonist activity of [Orn8]U-II, Schild plot is compatible with a competitive type of antagonism (the slope of the regression line is not significantly different from unity) and a pA2 value of 6.56±0.15 was calculated (Figure 2, bottom panel). Ten μM [Orn8]U-II did not modify (either in terms of potency or of maximal effects) the concentration response curves to noradrenaline, angiotensin II and endothelin-1 in the rat aorta or that to somatostatin in the electrically stimulated guinea-pig ileum (data not shown).
Figure 1.
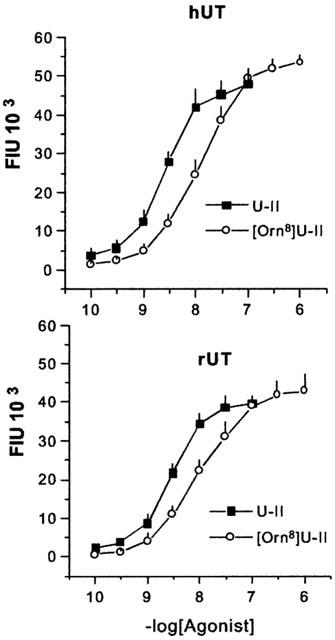
Concentration-response curves to human urotensin II (U-II) and [Orn8]U-II in HEK293 cells expressing human (hUT, top panel) or the rat (rUT, bottom panel) UT receptor. Intracellular calcium levels were expressed as fluorescence intensity units (FIU) × 103. Points indicate the means and vertical bars the s.e.mean of at least three experiments performed in duplicate.
Figure 2.
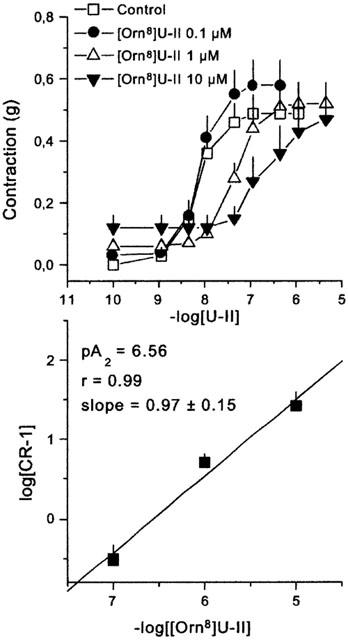
Concentration-response curves to human urotensin II (U-II) obtained in the absence (control) and in presence of increasing concentrations (0.1, 1, and 10 μM) of [Orn8]U-II. The bottom panel shows the corresponding Schild plot. Points indicate the means and vertical bars the s.e.mean of 6–8 separate experiments.
Discussion
The natural peptide U-II selectively activates the UT receptor modulating cardiovascular functions (Douglas & Ohlstein, 2000a). Selective UT receptor antagonists are required for investigating the (patho)physiological relevance of this novel peptide-receptor system. Here we described the identification and in vitro characterization of [Orn8]U-II, a novel peptide ligand for the UT receptor. The chemical modification (Lys8→(Orn) used for generating this peptide was suggested by previous structure-activity studies demonstrating that the Lys8 residue in U-II sequence is the most important for biological activity (Flohr et al., 2002). In the calcium functional assay [Orn8]U-II behaves as a full agonist, inducing similar maximal effects as U-II. In contrast, different results were obtained in the rat aorta bioassay where the compound mainly behaved as a competitive antagonist showing however at the highest concentration tested (10 μM) a small (≈25%) but consistent residual agonist activity. The discrepancy between the results obtained in the cell assay vs tissue bioassay can be interpreted assuming that [Orn8]U-II is actually a partial agonist (low efficacy agonist) whose final behaviour (agonist vs antagonist) strongly depends on the preparation under study. In preparations like our HEK293 cells that express a very high number of receptors (Bmax 1–4 pmol mg protein−1) the efficiency of the stimulus-response coupling is probably very high and the maximal effects can be elicited even by low efficacy agonists. On the contrary, in preparations like the rat aorta that express a low number of receptors (Bmax 1–20 fmol mg protein−1 (Ames et al., 1999)), the efficiency of the stimulus-response coupling is so low that low efficacy agonists behave mainly as competitive antagonists. For further details on the importance of stimulus-response coupling on the estimation of ligand efficacy see Kenakin (2002). Such a preparation-dependent pharmacological behaviour displayed by [Orn8]U-II at UT receptors is not unique, since other examples have been reported in the GPCR field as for instance the nociceptin/orphanin FQ receptor ligand [F/G]N/OFQ(1-13)NH2 (Calo et al., 2000), the kinin B2 receptor ligand FR 190997 (Rizzi et al., 1999), or the β1 adrenoceptor ligand prenalterol (Kenakin & Beek, 1984). Based on these considerations we propose to classify the novel UT receptor ligand [Orn8]U-II as a partial agonist. However, further investigations on the pharmacological behaviour of [Orn8]U-II, especially at mammalian UT receptors from different species (i.e. mouse, monkey), are mandatory. Despite limitations related to the residual agonist activity of [Orn8]U-II, we expect that this molecule can be useful for at least two purposes: (1) as a template for future SAR studies aimed to reduce the residual agonistic activity thus leading to the identification of pure antagonists, (2) as a selective UT receptor antagonist to be used in pharmacological studies performed in vitro on tissues and organs expressing native receptors or in vivo in intact animals. In these preparations, the number of UT sites is generally very low and [Orn8]U-II may mainly behave as a receptor antagonist.
Acknowledgments
This work was supported by the 60% grant to D Regoli from the University of Ferrara and by the G00C437 grant to A Rizzi from the Consiglio Nazionale delle Ricerche.
Abbreviations
- hUT
human urotensin II receptor
- rUT
rat urotensin II receptor
- U-II
Urotensin II
References
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- BOHM F., PERNOW J. Urotensin II evokes potent vasoconstriction in humans in vivo. Br. J. Pharmacol. 2002;135:25–27. doi: 10.1038/sj.bjp.0704448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALO G., GUERRINI R., RIZZI A., SALVADORI S., REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMARDA V., RIZZI A., CALO' G., GENDRON G., PERRON S.I., KOSTENIS E., ZAMBONI P., MASCOLI F., REGOLI D. Effects of human urotensin II in isolated vessels of various species; comparison with other vasoactive agents. Naunyn Schmiedeberg's Arch. Pharmacol. 2002;365:141–149. doi: 10.1007/s00210-001-0503-0. [DOI] [PubMed] [Google Scholar]
- COULOUARN Y., LIHRMANN I., JEGOU S., ANOUAR Y., TOSTIVINT H., BEAUVILLAIN J.C., CONLON J.M., BERN H.A., VAUDRY H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H. Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease. Trends Cardiovasc. Med. 2000a;10:229–237. doi: 10.1016/s1050-1738(00)00069-4. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H.Urotensin receptors The IUPHAR compendium of receptor characterization and classification. 2000bLondon: IUPHAR Media; 365–372.ed. Girdlestone, D. pp [Google Scholar]
- DOUGLAS S.A., SULPIZIO A.C., PIERCY V., SARAU H.M., AMES R.S., AIYAR N.V., OHLSTEIN E.H., WILLETTE R.N. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br. J. Pharmacol. 2000c;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOHR S., KURZ M., KOSTENIS E., BRKOVICH A., FOURNIER A., KLABUNDE T. Identification of non-peptide urotensin II receptor antagonists by virtual screening based on a pharmacophore model derived from SAR and NMR studies on urotensin II. J. Med. Chem. 2002;45:1799–1805. doi: 10.1021/jm0111043. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Drug efficacy at g protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P., BEEK D. Relative efficacy of prenalterol and pirbuterol for beta-1 adrenoceptors: measurement of agonist affinity by alteration of receptor number. J. Pharmacol. Exp. Ther. 1984;229:340–345. [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Orphan-receptor ligand human urotensin II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br. J. Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOTHACKER H.P., WANG Z., MCNEILL A.M., SAITO Y., MERTEN S., O'DOWD B., DUCKLES S.P., CIVELLI O. Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nat. Cell. Biol. 1999;1:383–385. doi: 10.1038/14081. [DOI] [PubMed] [Google Scholar]
- PEARSON D., SHIVELY J.E., CLARK B.R., GESCHWIND BARKLEY M., II, NISHIOKA R.S., BERN H.A. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc. Natl. Acad. Sci. U.S.A. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIZZI A., RIZZI C., AMADESI S., CALO G., VARANI K., INAMURA N., REGOLI D. Pharmacological characterisation of the first non-peptide bradykinin B2 receptor agonist FR 190997: an in vitro study on human, rabbit and pig vascular B2 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1999;360:361–367. doi: 10.1007/s002109900087. [DOI] [PubMed] [Google Scholar]


