Abstract
Human urotensin-II (hU-II), a cyclic undecapeptide, is amongst the most potent mammalian vasoconstrictors identified, suggesting that hU-II and its G-protein-coupled receptor (UT) may regulate cardiovascular homeostasis. Such a hypothesis would benefit greatly from the development of selective UT antagonists.
Although the somatostatin (SST) antagonist SB-710411 (Cpa-c[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide) is purported to block U-II-induced contractions in rat isolated aorta, little is known about its specific pharmacological properties.
SB-710411 (10 μM) inhibited hU-II-induced contraction in rat isolated aorta causing a significant, parallel shift in the agonist concentration-response curve (pKb 6.28±0.11; n=8) with no suppression of the Emax. In contrast, SB-710411 did not alter the contractile actions of angiotensin-II, phenylephrine, or KCl. Paradoxically, however, SB-710411 potentiated the contractile response to endothelin-1 (pEC50 8.02±0.16 and 8.54±0.11, P<0.01; n=8). Rather than being specific toSB-710411, this phenomenon appears to be related to somatostatin receptor affinity and not intrinsic activity since the SST agonist somatostatin-14 and antagonist cyclo-somatostatin also potentiated endothelin-1-induced contraction.
SB-710411 (10 μM) did not inhibit carbachol, sodium nitroprusside, IBMX, isoprenaline, and levcromakalim-induced reversal of tone established with noradrenaline. In contrast, however, SB-710411 significantly inhibited the reversal of tone established with endothelin-1 using the same vasorelaxants.
In summary, although SB-710411 inhibits the vasoconstrictor actions of hU-II in a competitive, surmountable manner, it also possesses additional pharmacological actions. Thus, whilst the present study is amongst the first to detail the properties of a functional U-II receptor antagonist, the data suggest caution be used when assessing data generated utilizing this moiety and other SST analogues.
Keywords: Urotensin-II, GPR14, UT receptor, somatostatin, endothelin-1, vasoconstriction, vasorelaxation, sodium nitroprusside, carbachol, SB-710411
Introduction
Human urotensin-II (hU-II), a cyclic undecapeptide, is the cognate ligand for the human G-protein-coupled receptor, UT (formerly termed GPR14/SENR; see Douglas & Ohlstein, 2000a,b). Based on the observation that hU-II and UT are both highly expressed within the mammalian cardiovasculature, it was proposed that they play a role in the (patho)physiological regulation of cardiovascular homeostasis (Douglas & Ohlstein, 2000a,b). hU-II has been characterized as the most potent, or amongst the most potent, mammalian vasoconstrictor identified (1–2 orders of magnitude more potent than endothelin-1) in a diverse range of blood vessels including those from rat, rabbit, dog, pig, monkey, (Douglas et al., 2000; Saetrum Opgaard et al., 2000; Camarda et al., 2002) and man (Maguire et al., 2000; Russell et al., 2001; Paysant et al., 2001; Camarda et al., 2002).
Further evidence for a role in the regulation of mammalian cardiohaemodynamic function came with the observation that systemic administration of hU-II in the anaesthetized monkey increased total peripheral resistance leading to a catastrophic decrease in cardiac contractility (Ames et al., 1999). Indeed, not only was hU-II a vascular smooth muscle spasmogen, it was also shown to be a positive inotrope in human isolated right atrial trabeculae (Russell et al., 2001). In accord, recent studies in man have reported that intradermal hU-II causes vasoconstriction in vivo (attenuated skin microcirculatory flow; Leslie et al., 2000) and that hU-II infused into the brachial artery induces a dose-dependent reduction in forearm blood flow (venous occlusion plethysmography; Bohm & Pernow, 2002). In addition to regulating smooth muscle and cardiac contractility, hU-II may also influence vascular remodelling, stimulating vascular smooth muscle proliferation (Sauzeau et al., 2001; Watanabe et al., 2001) and cardiomyocyte hypertrophy (Tzanidis et al., 2001; Zou et al., 2001) in vitro. As such, the ability of U-II/UT to regulate the contractility and growth properties of cardiac and peripheral vascular tissue suggests a putative role in the aetiology of cardiovascular disorders e.g. hypertension, heart failure etc. However, a definitive role(s) for U-II/UT in the pathophysiology of such disorders would be greatly assisted by the development of selective hU-II receptor antagonists.
To this end, it is of interest to note that, recently, a preliminary report by Coy et al. (2000) described the identification of a novel peptidic somatostatin (SST) ligand, herein referred to as SB-710411, with moderate affinity for U-II receptors. This peptidic moiety was reported to possess the ability to inhibit U-II-induced contraction in rat isolated thoracic aorta. However, little detail was provided regarding the specific pharmacological properties of this peptidic molecule. As such, the present study has extended these preliminary studies by investigating the selectivity and nature of antagonism of SB-710411 using a rat aorta functional assay.
An initial account of some of the data described in this manuscript was presented to the Mid-Atlantic Pharmacology Society (Behm et al., 2001).
Methods
General preparation of isolated thoracic aorta
All procedures were performed in accredited facilities in accordance with institutional guidelines (Animal Care and Use Committee, GlaxoSmithKline) and the Guide for the Care and Use of Laboratory Animals (DHSS no. NIH 85-23). Proximal descending thoracic aortae were isolated from adult male Sprague–Dawley rats (400 g) anaesthetized with 5% isoflurane in O2 as described previously (Douglas et al., 2000). Vessel rings, approximately 2–3 mm in length, were suspended in 10 ml organ baths containing Krebs of the following composition (mM): NaCl 112.0; KCl 4.7; KH2PO4 1.2; MgSO4 1.2; CaCl2 2.5; NaHCO3 25.0; dextrose 11.0. Krebs was maintained at 37±1°C and aerated with 95%O2:5%CO2 (pH 7.4). Changes in isometric force were measured under optimal resting tension (1 g) using FT03 force-displacement transducers (Grass Instruments, Quincy, MA, U.S.A.) coupled to Model 7D polygraphs. Following a 60 min equilibration period, the vessels were treated with standard concentrations of KCl (60 mM) and noradrenaline (10 μM). Subsequent agonist-induced responses were normalized to these responses.
Utilization of vessels for constriction studies
Vessels were denuded of endothelium by rubbing with a pair of fine forceps (functional loss was confirmed using 10 μM carbachol). Indomethacin (10 μM, 0.1% ethanol, v v−1) was added to the Krebs solution to inhibit eicosanoid generation. Tissues were pretreated with either vehicle (0.1% DMSO), 10 μM SB-710411 or 10 μM cyclo-somatostatin for 30 min prior to the generation of spasmogen-induced cumulative concentration-response curves (the vehicle used for somatostatin-14-treated vessels was H2O). Vasoconstrictor agents studied included hU-II and angiotensin-II (0.01 nM–1 μM), phenylephrine (0.01 nM–3 μM), endothelin-1 (0.01–300 nM) and KCl (5–90 mM). Aortic rings were used to generate concentration-response curves to a single agonist only.
Utilization of vessels for relaxation studies
Relaxation studies were performed in endothelium intact vessels in the absence of indomethacin. After a 30 min pretreatment with antagonist, tissues were contracted with an EC80 concentration of either endothelin-1 (30 nM) or noradrenaline (100 nM) and responses were expressed as percent reversal of the original tone established in the tissue by either endothelin-1 or noradrenaline. Vasorelaxant-induced cumulative concentration-response curves were generated to the following agents: carbachol (0.01–100 μM), sodium nitroprusside (0.01 nM–300 μM), isoprenaline and levcromakalim (0.1–3 μM) and IBMX (0.01 nM–100 μM).
Statistical and data analysis
All values are expressed as mean±s.e.mean and n represents the total number of animals from which the vessels were isolated. Statistical comparisons were made using a paired, two-tailed t-test and differences were considered significant when P<0.05.
Concentration-response curves were fitted to a logistic equation as previously described (Douglas et al., 1995):
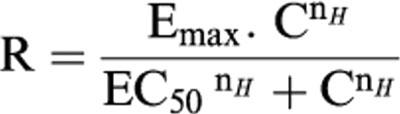 |
where R is the contraction or relaxation response; C, the concentration of vasoactive agent; EC50, the concentration of agonist required to produce a half maximal response; nH, the Hill coefficient and Emax, the maximal contraction or relaxation response.
Drugs and reagents
hU-II and SB-710411 (Cpa-c[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide; Cpa, 4-chlorophenylalanine and Pal, 3-pyridylalanine; Coy et al., 2000) were synthesized by California Peptide Research Inc. (Napa, CA, U.S.A.). American Peptide Company Inc. (Sunnyvale, CA, U.S.A.) and Bachem Inc. (Torrance, CA, U.S.A.) synthesized endothelin-1 and cyclo-somatostatin (Cyclo[-7-aminoheptanoyl-Phe-D-Trp-Lys-Thr(Bzl)] acetate), respectively. Angiotensin-II, carbachol, indomethacin, IBMX, isoprenaline, noradrenaline, phenylephrine, sodium nitroprusside and somatostatin-14 were from Sigma (St. Louis, MO, U.S.A.). Levcromakalim was from Tocris Cookson Inc. (Ellisville, MO, U.S.A.). All other reagents used were of analytical grade.
Results
SB-710411 antagonizes hU-II-induced vasoconstriction in the rat isolated aorta
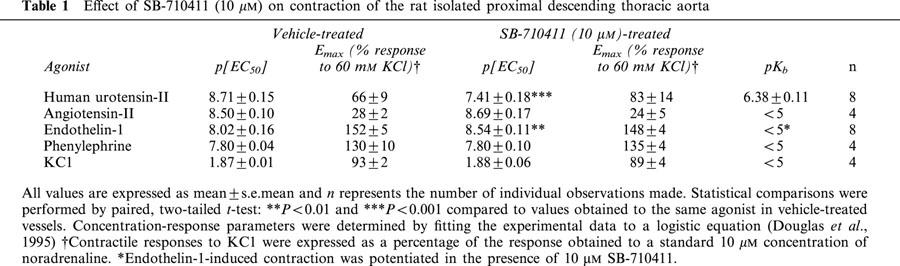
SB-710411 inhibited hU-II induced contraction of the rat isolated thoracic aorta in a surmountable manner (Figure 1, Table 1). Exposure to 10 μM SB-710411 induced a significant (P<0.001) 20-fold, parallel, (nHs approximated to unity), rightward shift in the concentration-response curve to hU-II without causing a significant suppression of the Emax, suggesting a competitive type of antagonism.
Figure 1.
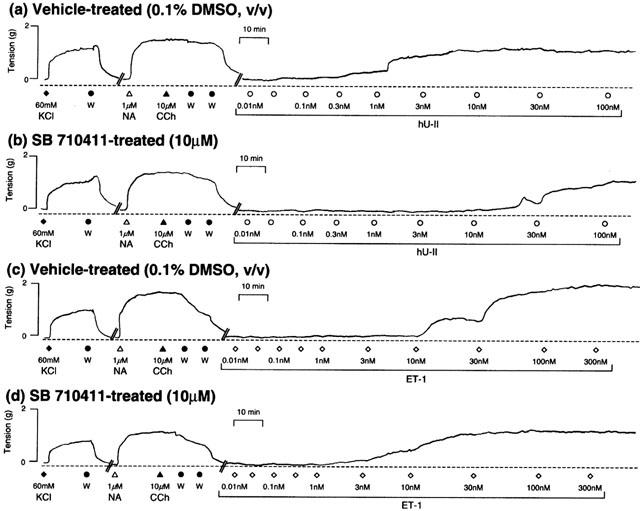
Representative experimental traces illustrating that relative to vehicle-treated vessels (a and c), SB-710411 (10 μM; b and d) inhibits or augments the concentration-dependent contractile actions of hU-II or endothelin-1 (ET-1) in rat isolated aorta, respectively. All experiments were performed in endothelium-denuded vessels in the presence of 10 μM indomethacin. In order to normalize the contractile responses to hU-II and ET-1, vessels were precontracted with 60 mM KCl. Following washing (W), endothelial cell integrity was evaluated by assessing the ability of 10 μM carbachol (CCh) to reverse tone established in the isolated aorta with 1 μM noradrenaline (NA). The vertical and horizontal scale bars represent tension (2 g) and time (10 min), respectively.
Table 1.
Effect of SB-710411 (10 μM) on contraction of the rat isolated proximal descending thoracic aorta
SB-710411 augments endothelin-1-induced vasoconstriction in the rat isolated aorta
The selectivity of the antagonism against urotensin-II was demonstrated by the observation that, relative to vehicle-treated vessels, the presence of 10 μM SB-710411 did not attenuate the contractile potency or efficacy of angiotensin-II, KCl or phenylephrine (Table 1). Paradoxically, however, 10 μM SB-710411 caused a significant (P<0.01), parallel 3-fold leftward shift in the concentration-response curve to endothelin-1 (i.e. an enhancement of endothelin-1 contractile potency with no concomitant alteration in Emax or nH; Figures 1 and 2, Table 1).
Figure 2.
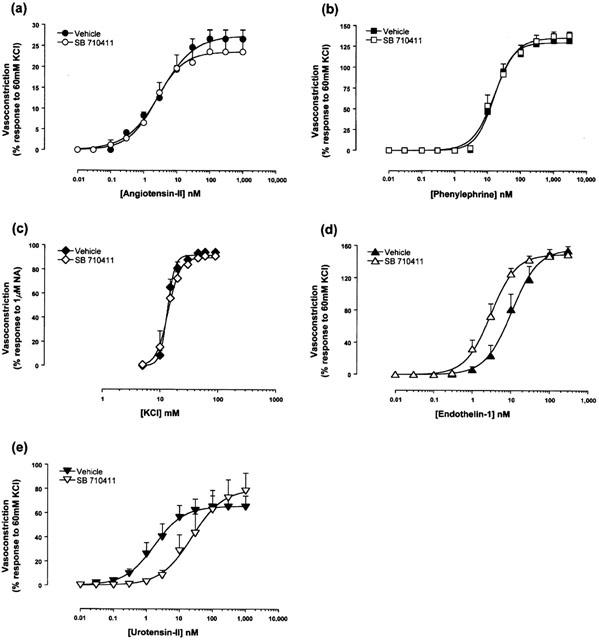
SB-710411 (10 μM) does not inhibit (a) angiotensin-II, (b) phenylephrine, or (c) KCl induced contraction in the rat isolated aorta. Paradoxically, (d) SB-710411 potentiates endothelin-1-induced vasoconstriction. In contrast, however, (e) SB-710411 inhibits hU-II-induced contraction causing a parallel shift in the concentration-response curve to the agonist with no suppression of the maximum contractile response. All experiments were performed in endothelium-denuded vessels in the presence of 10 μM indomethacin. Values are mean and vertical bars represent the s.e.mean. Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995).
Somatostatin-14 and cyclo-somatostatin also potentiate endothelin-1-induced vasoconstriction in the rat isolated aorta
Since the somatostatin antagonist SB-710411 potentiated the potency of the contractile response to endothelin-1, the effects of the somatostatin agonist somatostatin-14 and the somatostatin antagonist cyclo-somatostatin were determined. Compared to vehicle-treated vessels, both somatostatin-14 and cyclo-somatostatin potentiated ET-1-induced contraction (pEC50 of 7.54±0.25 and 8.54±0.30; 8.38±0.16 and 9.25±0.14) with no suppression of the maximal contractile response (Emax of 125±8 and 122±4% response to 60 mM KCl; 133±4 and 123±2% response to 60 mM KCl; n=8 and 4, respectively).
SB-710411 does not inhibit vasorelaxation in rat isolated aorta preconstricted with noradrenaline
SB-710411 (10 μM) did not inhibit the relaxant actions of a range of vasodilators, namely, carbachol, IBMX, isoprenaline, levcromakalim, and sodium nitroprusside when vessels were preconstricted with noradrenaline (Figure 3, Table 2).
Figure 3.
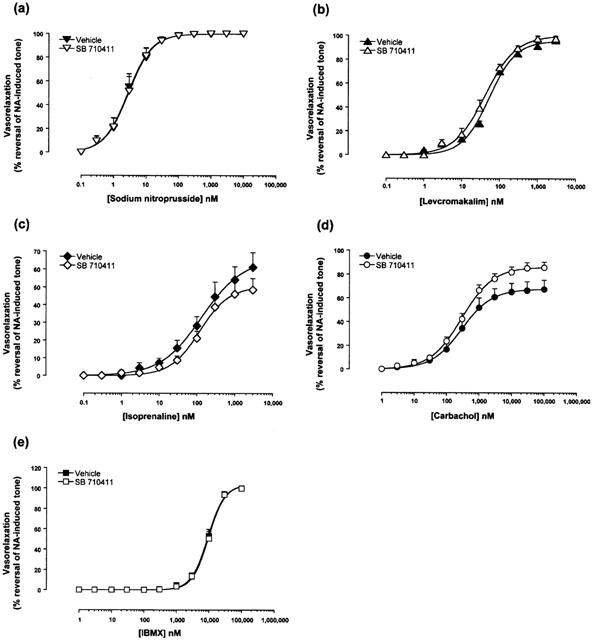
SB-710411 (10 μM) does not inhibit reversal of tone establish with noradrenaline. The Figure illustrates concentration-dependent relaxation response curves to (a) carbachol, (b) IBMX, (c) isoprenaline, (d) levcromakalim and (e) sodium nitroprusside in vehicle- and drug-treated rat isolated aortae following precontraction with 100 nM noradrenaline (NA). Responses are expressed as percent reversal of the original tone established in the tissue by NA. All experiments were performed in endothelium-intact vessels. Values are mean and vertical bars represent the s.e.mean. Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995).
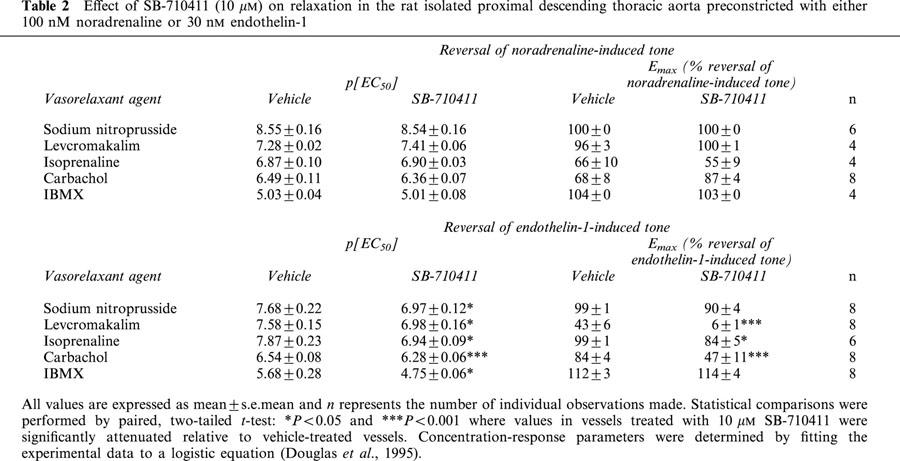
Table 2.
Effect of SB-710411 (10 μM) on relaxation in the rat isolated proximal descending thoracic aorta preconstricted with either 100 nM noradrenaline or 30 nM endothelin-1
SB-710411 attenuates relaxation in rat isolated aorta preconstricted with endothelin-1
In contrast to the observations made where tone was established in isolated aortae with noradrenaline, ‘non-selective' inhibition of vasorelaxation was observed in tissues preconstricted with endothelin-1. SB-710411 (10 μM) significantly inhibited carbachol- (2-fold reduction in relaxant potency, 44% suppression of Rmax; P<0.001), IBMX- (9-fold reduction in potency, P<0.05), isoprenaline- (4-fold reduction in potency, 86% suppression of Emax; P<0.05 and 0.001, respectively), levcromakalim- (9-fold reduction in relaxant potency, 15% suppression of Emax; P<0.05) and sodium nitroprusside- (5-fold reduction in relaxant potency, P<0.05) induced reversal of tone established with 30 nM endothelin-1 (Figures 4 and 5, Table 2).
Figure 4.
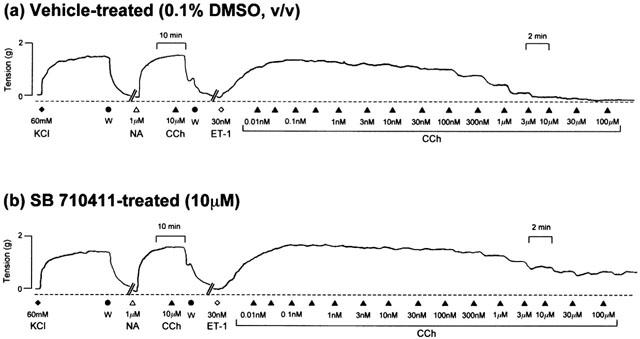
Representative experimental traces illustrating that relative to vehicle-treated vessels (a), SB-710411 (10 μM; b) inhibits the carbachol-induced reversal of tone in rat isolated aorta precontracted with 30 nM endothelin-1 (ET-1). All experiments were performed in endothelium-intact vessels. Vessels were precontracted with 60 mM KCl. Following washing (W), endothelial cell integrity was evaluated by adding 1 μM noradrenaline (NA) and observing the response to 10 μM carbachol (CCh). The vertical and horizontal scale bars represent tension (2 g) and time (10 min), respectively.
Figure 5.
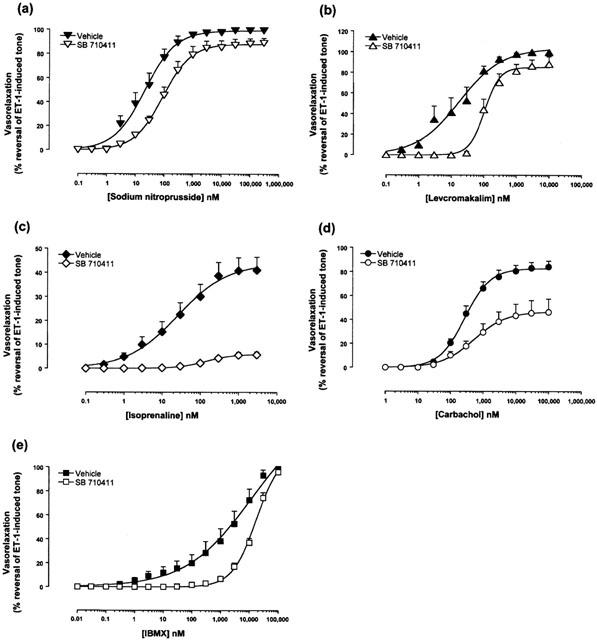
Relative to vehicle-treated vessels, 10 μM SB-710411 inhibits (a) sodium nitroprusside, (b) isoprenaline, (c) levcromakalim, (d) carbachol and (e) IBMX-induced reversal of tone established with endothelin-1 (ET-1). Responses are expressed as per cent reversal of the original tone established in the tissue by 30 nM ET-1. All experiments were performed in endothelium-intact vessels. Values are mean and vertical bars represent the s.e.mean. Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995).
Discussion
hU-II, the most potent, or amongst the most potent, mammalian vasoconstrictor identified to date, may play a role(s) in regulating cardiovascular homeostasis. The development of a selective hU-II antagonist may assist in determining this role(s).
Due to sequence similarities between hU-II and somatostatin, Coy et al. (2000) screened several somatostatin analogues in a rat aortic contraction assay and demonstrated that Cpa-c[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide, herein referred to as SB-710411, inhibited hU-II-induced contraction. In accord with this preliminary study, the present manuscript confirms that SB-710411 inhibits hU-II-induced contraction in the rat isolated thoracic aorta in a surmountable manner with a parallel shift in the concentration-response curves (nH ∼1) and no suppression of the Emax, suggesting competitive antagonism. SB-710411 inhibited hU-II-induced contraction with a pKb of 6.38±0.11 in the present study, a value which is consistant with the IC50 (100 nM) and Kd (240 nM) values reported by Coy et al. (2000).
In order to determine the selectivity of SB-710411, the ability of this putative UT receptor antagonist to alter various spasmogen-induced contractions was assessed. Relative to vehicle-treated vessels, the contractile potency and efficacy of angiotensin-II, phenylephrine, and KCl was unaltered in the presence of 10 μM SB-710411. Paradoxically, however, 10 μM SB-710411 potentiated the contractile response to endothelin-1. The underlying mechanism for this phenomenon remains undetermined. However, whilst such data suggest that SB-710411 interacts directly with endothelin receptor(s) present in the rat aorta, it has been determined that SB-710411 lacks significant affinity for rat ETA and ETB receptors (10 μM SB-710411 produced less than 10% inhibition of [125I]endothelin-1 binding in rat A-10 aortic smooth muscle cell [ETA] and cerebellar [ETB] membranes; data not shown). Also, it is of interest to note that both Herold et al. (2001) and Behm et al. (2001) demonstrated that SB-710411 had moderate affinity for members of the somatostatin receptor family e.g. SB-710411 displayed a higher affinity for the cloned human somatostatin SSTR3 receptor in competition binding studies (pKi=7.57±0.20; n=3), and was only slightly less potent than somatostatin-14 (pKi=7.91±0.14; n=7).
To further examine the finding that SB-710411 potentiates endothelin-1-induced contraction in the rat isolated aorta, the effects of other somatostatin analogues on endothelin-1-induced contraction were also studied. SB-710411 is a somatostatin receptor antagonist since it reverses somatostatin-14-induced inhibition of cAMP production in HEK293 cells expressing both SSTR2 and SSTR5 (data not shown). Therefore, one may hypothesize that other somatostatin receptor antagonists may also potentiate the contractile potency of endothelin-1 in the rat isolated aorta. In accord, the present study demonstrates that the somatostatin receptor antagonist cyclo-somatostatin (Long et al., 1992) caused a significant (P<0.01), parallel 7-fold leftward shift in the concentration-response curve to endothelin-1 (i.e. an enhancement of endothelin-1 contractile potency without altering Emax or nH). Interestingly, however, the somatostatin receptor agonist somatostatin-14 also potentiated the response to endothelin-1 in a similar fashion (P<0.01; parallel 7-fold leftward shift in the concentration-response curve to endothelin-1). Therefore, these data suggest that the affinity and not the intrinsic activity at the somatostatin receptor of somatostatin analogues contribute to the potentiation of endothelin-1-induced contraction since this effect is seen with both somatostatin receptor agonists and antagonists. In accord with these data, Huang et al. (2002) demonstrated that the somatostatin receptor agonists somatostatin-14 and octreotide significantly enhanced endothelin-1-induced vasoconstriction in portal hypertensive rats. Further, Wiest et al. (2001) witnessed a similar phenomenon in the rat superior mesenteric arterial vascular bed (although, in contrast to the observations made in the current manuscript where somatostatin analogues selectively potentiated endothelin-1 induced contraction, the contractile actions of several pharmacologically distinct protein kinase C-dependent vasoconstrictors were also augmented e.g. α1-adrenergic). As such, it would be of interest in the future to assess the ability of other putative peptidic urotensin-II antagonists derived from SST (PRL-2882 (4Fpa-c[D-Cys-3Pal-D-Trp-Lys-Val-Cys]-Nal-NH2), PRL-2903 (4Fpa-c[D-Cys-Pal-D-Trp-Lys-Tle-Cys-Nal-NH2), and PRL-2915 (4Cpa-c[D-Cys-Pal-D-Trp-Lys-Tle-Cys-Nal-NH2]; Rossowski et al., 2002) to potentiate endothelin-1-induced contraction.
As such, the rat aorta functional assay data suggest SB-710411 is not merely a UT receptor antagonist. This complex pharmacological profile is amplified further by findings of Herold et al. (2001) that demonstrate SB-710411 is a competitive antagonist at rat UT receptors stably transfected in HEK293 cells, inhibiting hU-II-induced inositol phosphate (IP) formation with a pKb of 6.50±0.05. However, in HEK293 cells expressing human or monkey UT receptor, IP formation induced by either SB-710411 or hU-II were similar, suggesting SB-710411 is an efficacious agonist at the primate UT receptors.
In order to further investigate the specificity of the proposed hU-II antagonist, vessels were precontracted with either noradrenaline or endothelin-1 and relaxed with either the nitrate vasodilator sodium nitroprusside, the β-adrenoceptor agonist isoprenaline, the potassium channel activator levcromakalim, the muscarinic receptor agonist carbachol or the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX). Relative to vehicle-treated vessels, the presence of 10 μM SB-710411 does not alter relaxant-induced reversal of tone established with noradrenaline. The antagonist does not appear to alter general vasorelaxant mechanisms in the vascular smooth muscle.
Since SB-710411 potentiated the contractile response of endothelin-1, the effects of SB-710411 on relaxation in vessels precontracted with this particular ligand were determined. SB-710411 (10 μM) inhibited the relaxant effects of isoprenaline, levcromakalim, carbachol, sodium nitroprusside and IBMX. However, these inhibitory effects may be due to SB-710411-induced potentiation of the ET-1 contractile response i.e. in comparison to vehicle-treated vessels, the contractile effects of 30 nM ET-1 are 3-fold stronger in the presence of SB-710411 and therefore a higher concentration of vasorelaxant would be necessary to produce the same response. No matter the cause, the ‘non-selective' inhibition of vasorelaxation in vessels precontracted with endothelin-1 provides further evidence that SB-710411-induced antagonism is not selective to the UT receptor.
Thus, the present study is the first to explore the pharmacological properties of the putative peptidic UT receptor antagonist SB-710411 (Cpa-c[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide). In summary, the pharmacological properties of SB-710411 exceed simple inhibition of the UT receptor binding/function since it also potentiates endothelin-1-induced contraction in the rat isolated aorta. Therefore, caution must be taken when interpreting data generated with this moiety as a tool compound for selective UT receptor function.
Acknowledgments
The authors would like to thank N. Aiyar, Z. Ao, W. Crowell, J. Disa, B. Guida, G. McCafferty, D. Naselsky, C. Sauermelch, and A. Sulpizio for their assistance and comments during the preparation of this manuscript.
Abbreviations
- Cpa
4-chlorophenylalanine
- DMSO
dimethylsulphoxide
- IBMX
isobutyl methylxanthine
- Pal
3-pyridylalanine
- (h)U-II
(human)urotensin-II
- UT
urotensin-II receptor
References
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W.P., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- BEHM D.J., NASELSKY D.P., HEROLD C.L., AIYAR N.V., KNIGHT S.D., DHANAK D., DOUGLAS S.A. Pharmacological characterization of a novel peptidic urotensin-II receptor antagonist. Pharmacologist. 2001;43:190. [Google Scholar]
- BOHM F., PERNOW J. Urotensin II evokes potent vasoconstriction in humans in vivo. Br. J. Pharmacol. 2002;135:25–27. doi: 10.1038/sj.bjp.0704448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMARDA V., RIZZI A., CALO G., GENDRON G., PERRON S.I., KOSTENIS E., ZAMBONI P., MASCOLI F., REGOLI D. Effects of human urotensin II in isolated vessels of various species; comparison with other vasoactive agents. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;365:141–149. doi: 10.1007/s00210-001-0503-0. [DOI] [PubMed] [Google Scholar]
- COY D.H., ROSSOWSKI W.J., CHENG B.L., HOCART S.J., TAYLOR J.E. Novel urotensin-II (UII) antagonists point to multiple receptor involvement in UII bioactivity. Reg. Pep. 2000;94:48. [Google Scholar]
- DOUGLAS S.A., BECK G.R., ELLIOTT J.D., OHLSTEIN E.H. Pharmacological evidence for the presence of three distinct functional endothelin receptor subtypes in the rabbit lateral saphenous vein. Br. J. Pharmacol. 1995;114:1529–1540. doi: 10.1111/j.1476-5381.1995.tb14936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H. Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease. Trends Cardiovasc. Med. 2000a;10:229–237. doi: 10.1016/s1050-1738(00)00069-4. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H.Urotensin Receptors The IUPHAR Compendium of Receptor Characterization and Classification 2000bLondon: IUPHAR Media; 365–372.ed. Girdlestone, D. pp [Google Scholar]
- DOUGLAS S.A., SULPIZIO A.C., PIERCY V., SARAU H.M., AMES R.S., AIYAR N.V., OHLSTEIN E.H., WILLETTE R.N. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br. J. Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEROLD C.L., BEHM D.J., AIYAR N.V., NASELSKY D.P., DOUGLAS S.A. The somatostatin derivative, Cpa-c-[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide, is an agonist at the human UT receptor but not the rat UT receptor in transfected HEK293 cells. Pharmacologist. 2001;43:189. [Google Scholar]
- HUANG H., LEE F., CHAN C., CHANG F., WANG S., LIN H., HOU M., CHEN C., TAI C., LAI I., LEE S. Effects of somatostatin and octreotide on portal-systemic collaterals in portal hypertensive rats. J. Hepatol. 2002;36:163–168. doi: 10.1016/s0168-8278(01)00267-7. [DOI] [PubMed] [Google Scholar]
- LESLIE S.J., DENVIR M., WEBB D.J. Human urotensin-II causes vasoconstriction in the human skin microcirculation. Circulation. 2000;102:II–113. [Google Scholar]
- LONG J.B., RIGAMONTI D.D., DOSAKA K., KRAIMER J.M., MARTINEZ-ARIZALA A. Somatostatin causes vasoconstriction, reduces blood flow and increases vascular permeability in the rat central nervous system. Br. J. Pharmacol. 1992;260:1425–1432. [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Orphan-receptor ligand human urotensin-II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br. J. Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYSANT J., RUPIN A., SIMONET S., FABIANI J.N., VERBEUREN T.J. Comparison of the contractile responses of human coronary bypass grafts and monkey arteries to human urotensin-II. Fundam. Clin. Pharmacol. 2001;15:227–231. doi: 10.1046/j.1472-8206.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- ROSSOWSKI W.J., CHENG B.L., TAYLOR J.E., DATTA R., COY D.H. Human urotensin II-induced aorta ring contractions are mediated by protein kinase C, tyrosine kinases and Rho-kinase: inhibition by somatostatin receptor antagonists. Eur. J. Pharmacol. 2002;438:159–170. doi: 10.1016/s0014-2999(02)01341-9. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., MOLENAAR P., O'BRIEN D.M. Cardiostimulant effects of urotensin-II in human heart in vitro. Br. J. Pharmacol. 2001;132:5–9. doi: 10.1038/sj.bjp.0703811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAETRUM OPGAARD O., NOTHACKER H., EHLERT F.J., KRAUSE D.N. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur. J. Pharmacol. 2000;406:265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE MELLIONNEC E., BERTOGLIO J., SCALBERT E., PACAUD P., LOIRAND G. Human urotensin II-induced contraction and arterial smooth muscle proliferation are mediated by RhoA and Rho-kinase. Circ. Res. 2001;88:1102–1104. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- TZANIDIS A., HANNAN R., THOMAS W., ONAN D., AUTELITANO D., SEE F., KRUM H. Increased expression of urotensin II and its receptor in an experimental model of left ventricular myocardial infarction in the rat: implications for cardiac remodeling. European Heart Journal. 2001;22:141. [Google Scholar]
- WATANABE T., PAKALA R., KATAGIRI T., BENEDICT C.R. Synergistic effect of urotensin II with mildly oxidized LDL on DNA synthesis in vascular smooth muscle cells. Circulation. 2001;104:16–18. doi: 10.1161/hc2601.092848. [DOI] [PubMed] [Google Scholar]
- WIEST R., TSAI M.H., GROSZMANN R.J. Octreotide potentiates PKC-dependent vasoconstrictors in portal-hypertensive and control rats. Gastroenterology. 2001;120:975–983. doi: 10.1053/gast.2001.22529. [DOI] [PubMed] [Google Scholar]
- ZOU Y., NAGAI R., YAMAZAKI T. Urotensin-II induces hypertrophic responses in cultured cardiomyocytes from neonatal rats. FEBS Lett. 2001;508:57–60. doi: 10.1016/s0014-5793(01)03015-0. [DOI] [PubMed] [Google Scholar]


