Abstract
The aim of the present study was to determine the relative contribution of prostanoids, nitric oxide and K+ channels in the bradykinin-induced relaxation of bovine pulmonary supernumerary arteries.
In endothelium-intact, but not denuded rings, bradykinin produced a concentration-dependent relaxation (pEC50, 9.6±0.1), which was unaffected by the cyclo-oxygenase inhibitor indomethacin. The nitric oxide scavenger hydroxocobalamin (200 μM, pEC50, 8.5±0.2) and the nitric oxide synthase inhibitor L-NAME (100 μM, pEC50, 8.9±0.1) and the combination of L-NAME and hydroxocobalamin (pEC50, 8.1±0.2) produced rightward shifts in the bradykinin concentration response curve.
The guanylyl cyclase inhibitor ODQ (10 μM, pEC50, 9.6±0.4) did not affect the response to bradykinin.
Elevating the extracellular [K+] to 30 mM did not affect the response to bradykinin but abolished the response when ODQ or L-NAME was present.
The K+ channel blocker apamin (100 nM), combined with charybdotoxin (100 nM), produced a small reduction in the maximum response to bradykinin but they abolished the response to bradykinin when ODQ, L-NAME or hydroxocobalamin were present. Apamin (100 nM) combined with iberiotoxin (100 nM) also reduced the response to bradykinin in the presence of hydroxocobalamin or L-NAME.
The concentration response curve for sodium nitroprusside-induced relaxation was abolished by ODQ (10 μM) and shifted to the right by apamin and charybdotoxin.
These studies suggest that in bovine pulmonary supernumerary arteries bradykinin can stimulate the formation of nitric oxide and activate an EDHF-like mechanism and that either of these pathways alone can mediate the bradykinin-induced relaxation. In addition nitric oxide, acting through guanylyl cyclase, can activate an apamin/charbydotoxin-sensitive K+ channel in this tissue.
Keywords: Endothelium-derived hyperpolarizing factor, nitric oxide, K+ channels, guanylyl cyclase, endothelium
Introduction
Pulmonary supernumerary arteries are small muscular arteries that arise from their parent conventional artery at 90° (Elliott & Reid, 1965; Shaw et al., 1999). Since they account for a substantial part of the total cross-sectional area of the pulmonary vasculature (Elliott & Reid, 1965) they are likely to be important in influencing pulmonary vascular resistance. Vascular endothelium is believed to play an important role in maintaining low pulmonary vascular resistance by releasing various relaxing factors. These EDRFs include nitric oxide (Furchgott & Zawadzski, 1980; Ignarro et al., 1987; Palmer et al., 1987; Moncada et al., 1988) prostanoids, predominantly prostacyclin (PGI2) (Moncada & Vane, 1979) and a hyperpolarizing factor (EDHF; Taylor & Weston, 1988; Garland et al., 1995; Feletou & Vanhoutte, 1999). The contribution of these factors to endothelium-dependent relaxation appears to vary depending on the species and the anatomical region from which the blood vessels are derived (Nagao et al., 1992; Kato et al., 1997). In general it is thought that nitric oxide plays a greater role in endothelium-dependent relaxation of large arteries (Nagao et al., 1992; Cohen et al., 1997), whereas EDHF is considered to play a more important role in the relaxation of smaller arteries and arterioles (Nagao et al., 1992; Garland et al., 1995; Shimokawa et al., 1996). In contrast the involvement of prostanoids and in particular PGI2 in endothelium-dependent relaxation remains unclear.
The exact identity of EDHF remains elusive, fuelling the argument over whether it is a diffusable substance (Edwards et al., 1998; Fisslthaler et al., 1999; Campbell et al., 1996; Randall et al., 1996) or involves intracellular activation of myoendothelial gap junctions to facilitate charge conductance (Kuhberger et al., 1994; Chaytor et al., 1998; Dora et al., 1999; Edwards et al., 1999). Several reports indicate that the hyperpolarizing action of EDHF involves the activation of K+ channels which are sensitive to the combination of charybdotoxin, which inhibits BKCa, and apamin, which blocks SKCa (Nelson & Quayle, 1995; Cook & Quast, 1990).
Thus, this study aimed to determine the relative involvement of prostanoids, nitric oxide and the guanylyl cyclase pathway as well as K+ channels in bradykinin-induced relaxation of bovine supernumerary arteries.
Methods
Tissue preparation
Bovine lungs were obtained from a local abattoir within 30 min of slaughter. Ring segments of supernumerary artery (external diameter 0.5–1 mm) were dissected from the lung and freed of surrounding connective tissue. Care was taken not to damage the luminal surface of the preparation. In some experiments, artery rings had their endothelium removed by gently abrading the luminal surface with forceps. The vessels were suspended between two stainless steel wire hooks in 10 ml Linton vessel chambers containing Krebs/Henseleit physiological salt solution (PSS) of the following composition (mM): NaCl (119), KCl (4.7), NaHCO3 (24.8), MgSO4 (1.2), KH2PO4 (1.2), CaCl (2.5), glucose (11.1). Tissues were maintained at 37°C under a tension of 1.5 g, and gassed with a mixture of 95% O2/5% CO2. Solutions of high K+ PSS (30 mM) were made by equimolar replacement of NaCl with KCl. Changes in isometric tension were measured by force-displacement transducer (Grass Instruments, FT03). The output from the transducer was amplified and displayed on a Lectromed MTR8P chart recorder.
Experimental protocols
Following an equilibration period of 60 min the artery rings were constricted with a sub-maximal concentration of the thromboxane-A2 mimetic U46619 (0.3 μM). The endothelium-dependent vasodilator bradykinin (100 nM) was then used to indicate that the endothelium was intact. This was verified at the beginning of each experiment. Tissues displaying less than 70% relaxation to bradykinin were discarded. All tissues were washed with PSS over a period of 30 min then pre-treated with the angiotensin converting enzyme inhibitor captopril (10 μM) for 30 min to prevent bradykinin degradation.
Studies examining the effect of indomethacin, L-NAME, hydroxocobalamin and ODQ on bradykinin-induced relaxation
To assess the relative contribution of prostanoids, nitric oxide and the guanylyl cyclase pathway to endothelin-dependent relaxation induced by bradykinin in bovine pulmonary supernumerary arteries, tissues were either (a) untreated (control) or treated with (b) the cyclo-oxygenase inhibitor indomethacin (10 μM), (c) the nitric oxide synthase inhibitor L-NAME (100 μM), (d) the combination of indomethacin plus L-NAME, (e) the combination of indomethacin plus the nitric oxide scavenger hydroxycobalamin (200 μM; Danser et al., 2000). A further goal was to determine whether responses mediated by nitric oxide were dependent on guanylyl cyclase. For this, indomethacin-treated tissues were preincubated with (a) the soluble guanylyl cyclase inhibitor ODQ (10 μM; Schrammel et al., 1996), (b) the combination of indomethacin with hydroxocobalamin and ODQ. After 30 min incubation all artery rings were contracted with U46619 (0.3 μM). Upon reaching a steady level of contraction bradykinin was added to the organ baths cumulatively in half-log increments from 100 pM–1 μM to construct a concentration-response curve for relaxation to bradykinin.
Studies examining the effect of high extracellular K+ and K+ channel blockers on bradykinin-induced relaxation
A further set of experiments were designed to examine the involvement of, and to characterize the K+ channels involved in mediating the bradykinin-induced relaxation. (a) Using indomethacin-treated tissues, in the absence and presence of L-NAME (100 μM) or ODQ (10 μM), concentration response curves to bradykinin were constructed in normal or high (30 mM) extracellular K+. (b) Indomethacin-treated issues in the absence and presence of L-NAME (100 μM), hydroxocobalamin (200 μM) or ODQ (10 μM) were further treated with either apamin (100 nM), an inhibitor of SKCa (Nelson & Quayle, 1995), charybdotoxin (ChTX, 100 nM), an inhibitor of BKCa, KV and intermediate-conductance calcium-sensitive K+ channels (IKCa) (Nelson & Quayle, 1995; Chandy & Gutman, 1995; Kaczorowski et al., 1996) or iberiotoxin (IbTX, 100 nM), a selective inhibitor of BKCa (Galvoz et al., 1990). The combination of apamin (100 nM) and ChTX (100 nM), or apamin (100 nM) and IbTX (100 nM) were also examined.
Statistics
Relaxations were expressed as per cent decrease in the U46619-induced contraction. Data are expressed as means±s.e.mean. Mean sensitivity (pEC50 values), maximum relaxation (Rmax) and their standard errors (s.e.) were then calculated for each response curve and expressed as the negative log molar concentration of bradykinin required to elicit 50% of the maximum relaxation (−log EC50). n is the number of preparations (each from different animals) used. The significance between mean pEC50 and Rmax values was calculated by Student's t-test. Multiple comparisons were conducted using Bonferroni Post-test. All differences were considered as statistically significant when P<0.05.
Chemicals
Bradykinin (acetate salt), captopril ([2S]-1-(3-Mercapto-2-methylpropionyl)-L-proline, Nω-nitro-L-arginine methyl ester HCl (L-NAME), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), sodium (nitroferricyanide) nitroprusside, indomethacin (1-[p-chlorobenzoyl]-5-methoxy-2-methylindole-3-acetic acid) (dissolved in absolute ethanol), hydroxocobalamin (acetate salt) (dissolved in distilled water at 90°C for 5 min of day of experiment), were purchased from Sigma Chemical Company. 9,11-dideoxy-11α(9α-epoxymethano-prostaglandinF2α) (U46619, Affiniti Research Products Limited) was dissolved in absolute ethanol and stored at −20°C for up to 6 months as 1 mM stocks. Apamin was purchased from Bachem and was dissolved in 50 mM acetic acid. Charybdotoxin and iberiotoxin were purchased from Calbiochem. Unless otherwise stated compounds were dissolved in distilled water. Fresh dilutions of stocks were made up in PSS at the beginning of each experiment.
Results
Effect of bradykinin on preconstricted endothelium-intact and endothelium-denuded supernumerary arteries
In endothelium-intact but not denuded supernumerary artery rings contracted with U46619 (0.3 μM), bradykinin (100 pM–1 μM) evoked concentration-dependent relaxations (pEC50, 9.6±0.1, Rmax 100.5±4.1%, n=25).
Effect of indomethacin on bradykinin-induced relaxation of supernumerary arteries
Indomethacin (10 μM) had no effect on the concentration-response curve to bradykinin (pEC50, 9.6±0.2, Rmax 109.3±14.1, n=7) and did not affect the concentration response curve for bradykinin in the presence of L-NAME data not shown). Although this suggests that prostanoids are not involved in bradykinin-mediated relaxation all subsequent experiments included indomethacin to ensure that cyclo-oxygenase-derived products were excluded.
Effect of L-NAME, hydroxocobalamin and ODQ on bradykinin-induced relaxation of supernumerary arteries
(pEC50 values, Rmax values and significance are given in Table 1). L-NAME (100 μM) produced a small but significant reduction in the tissue sensitivity to bradykinin. Hydroxocobalamin (200 μM) produced a significant greater reduction in the tissue sensitivity to bradykinin than L-NAME. The reduction in sensitivity produced by L-NAME and hydroxocobalamin was similar to hydroxocobalamin alone. However, the sensitivity to bradykinin was unaffected by the guanylyl cyclase inhibitor ODQ (10 μM) and in the presence of ODQ, hydroxocobalamin did not reduce the tissue sensitivity to bradykinin (Table 1).
Table 1.
Sensitivity (pE50) and maximum relaxation (Rmax) to bradykinin in U46619 (0.3 μM)-pre-constricted bovine pulmonary supernumerary arteries in the absence and presence of the treatment indicated
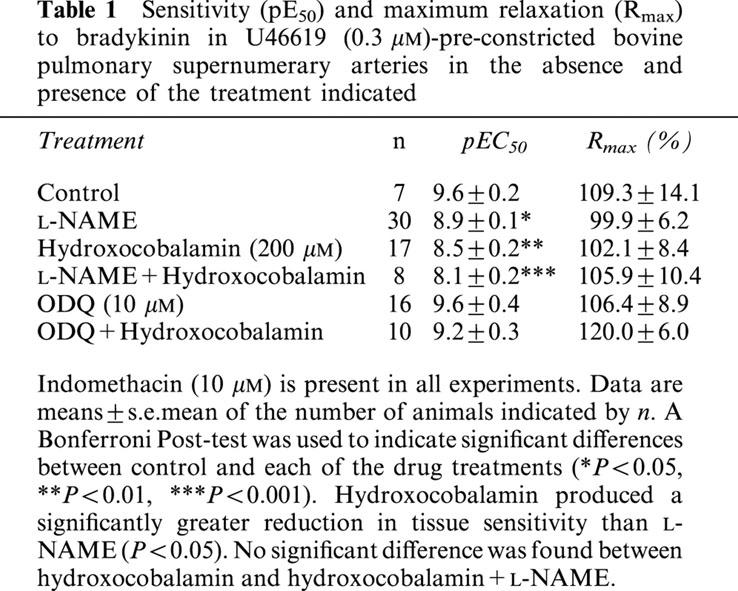
Effect of high extracellular [K+] on bradykinin-induced relaxation of supernumerary arteries in the absence and presence of L-NAME or ODQ
The bradykinin concentration response curve was unchanged when the extracellular [K+] was elevated to 30 mM. The combination of either L-NAME (100 μM) or ODQ (10 μM) and elevated [K+] completely abolished the response to bradykinin (Figure 1).
Figure 1.
Concentration response curve for bradykinin-induced relaxation of U46619-(0.3 μM)-preconstricted bovine supernumerary arteries in normal Krebs-PSS and in Krebs-PSS adjusted to 30 mM K+ in the absence (a, n=6) and presence of 100 μM L-NAME (b, n=6) or 10 μM ODQ (c, n=6). Data are expressed as mean±s.e.mean. The number of experiments (n) is shown in parenthesis.
Effect of K+ channel blockers on bradykinin-induced relaxation of supernumerary arteries in the absence and presence of L-NAME, hydroxocobalamin or ODQ
Neither apamin (100 nM), IbTX (100 nM) nor ChTX (100 nM) alone affected the bradykinin-induced relaxation in the absence or presence of L-NAME (100 μM), hydroxocobalamin (200 μM) or ODQ (10 μM) (data not shown).
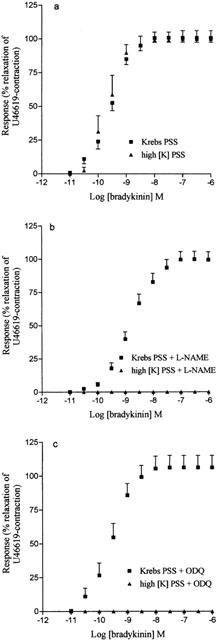
The combination of apamin (100 nM) and ChTX (100 nM) produced a small reduction in the maximum relaxation to bradykinin (Figure 2a) but completely abolished the relaxation when L-NAME (Figure 2b), hydroxocobalamin (Figure 2c) or ODQ (Figure 2d) were present.
Figure 2.
Concentration response curve to bradykinin in (a) control tissues (n=5) and in tissues treated with (b) L-NAME (100 μM, n=7) (c) hydroxocobalamin (200 μM, n=6) and (e) ODQ (10 μM, n=5) in the absence and presence of apamin (100 nM) and ChTX (100 nM). Data are expressed as mean±s.e.mean. The number of experiments (n) is shown in parenthesis.
Similarly the combination of apamin and iberiotoxin had no effect on the bradykinin concentration response curve but reduced the maximum relaxation by 57±12% and 46±15% when L-NAME and hydroxocobalamin were present respectively (data not shown).
Effect of ODQ and the K+ channel blockers apamin and ChTX on sodium nitroprusside-induced relaxation of supernumerary arteries
Sodium nitroprusside (0.1 nM–50 μM) induced a concentration-dependent relaxation (pEC50, 7.6±0.5; Rmax 87.9±7.7, n=8) in the absence but not in the presence of ODQ (10 μM). In the presence of apamin and ChTX (both 100 nM) the concentration response curve for sodium nitroprusside induced relaxation was shifted to the right (Figure 3).
Figure 3.
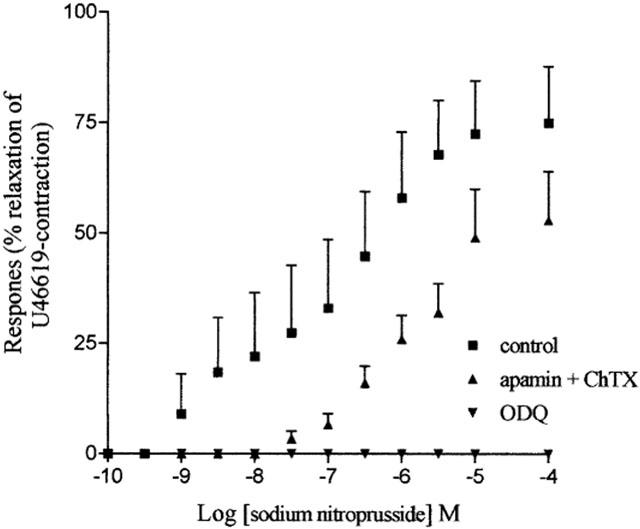
Concentration response curve for sodium nitroprusside-induced relaxation of U46619-(0.3 μM)-preconstricted supernumerary arteries in the absence (n=5) and presence of ODQ (10 μM, n=5) or apamin and ChTX (both 100 nM, n=5). The data is expressed as mean±s.e.mean. The number of experiments (n) is shown in parenthesis.
Discussion
The main finding of the present study is that in bovine pulmonary supernumerary arteries bradykinin induces endothelium-dependent relaxation by stimulating the nitric oxide/guanylyl cyclase pathway and by activating an EDHF-like pathway. Since blocking either pathway on its own only has a marginal effect on the bradykinin-relaxation, this suggests that either the nitric oxide- or EDHF-pathway alone can independently mediate the bradykinin-induced relaxation.
The involvement of prostanoids in the vasorelaxant responses to bradykinin in supernumerary arteries
Because indomethacin did not alter the bradykinin concentration response curve and because the concentration response curve for bradykinin in the presence of indomethacin- and L-NAME was not significantly different from the response in the presence of L-NAME alone, this suggests that prostanoids do not have an obvious role in bradykinin-induced relaxation of supernumerary arteries. However to be absolutely sure of excluding cyclo-oxygenase-derived vasoactive metabolites we included indomethacin in all subsequent studies.
Role of nitric oxide and guanylyl cyclase in the bradykinin-induced relaxation
Since removal of nitric oxide by L-NAME or hydroxocobalamin reduced the tissue sensitivity to bradykinin, this may suggest that bradykinin-induced nitric oxide formation is responsible for the greater tissue sensitivity to bradykinin seen in the absence of L-NAME or hydroxocobalamin. The nitric oxide scavenger hydroxocobalamin produced a greater reduction in the tissue sensitivity than L-NAME. The combination of the two produced no greater reduction in sensitivity than hydroxocobalamin alone. The reason for this difference between L-NAME and hydroxocobalamin is unclear. It has been suggested that L-NAME at the concentrations used here may not completely block NOS (Cohen et al., 1997). The possibility that bradykinin may release a pool of stored nitric oxide, resistant to the action of L-NAME, has also been suggested (Danser et al., 2000). Equally however, hydroxocobalamin may have effects other than scavenging nitric oxide, which could account for its greater effect. Since the bradykinin-mediated relaxation that remained in the presence of L-NAME/hydroxocobalamin was sensitive to high extracelluar [K+] and K+ channel blockers apamin and charybdotoxon, this suggests, albeit indirectly, that the L-NAME/hydroxocobalamin-resistant relaxation was mediated by an EDHF.
Since the relaxant response to bradykinin in the presence of ODQ is sensitive to apamin and charybdotoxin and to high [K+], this would suggest that this response to bradykinin is also mediated by an EDHF when guanylyl cyclase is blocked. However, unlike L-NAME and hydroxocobalamin, no reduction in the tissue sensitivity to bradykinin was seen with ODQ.
It would appear, therefore, that when nitric oxide is removed the bradykinin-induced relaxation is mediated by an EDHF but the tissue sensitivity to bradykinin is reduced. Yet, when guanylyl cyclase is inhibited with ODQ the bradykinin-induced relaxation is clearly mediated by an EDHF but the tissue sensitivity to bradykinin is not reduced. There is general acceptance that nitric oxide induces smooth muscle relaxation by activating a soluble guanylyl cyclase and cGMP-dependent protein kinase in the smooth muscle cell (Ignarro, 1990) and because ODQ is reported to inhibit the nitric oxide-stimulated activity of guanylyl cyclase without affecting its basal activity (Schrammel et al., 1996) the effect of ODQ and removal of nitric oxide should be similar. The fact that nitric oxide has been shown to directly activate calcium-sensitive K+ channels in several preparations, independently of the guanylyl cyclase/cGMP pathway, raises the possibility that the bradykinin-induced relaxation in the presence of ODQ could be mediated by K+ channels activated by nitric oxide as well as an EDHF. The fact that in these arteries the tissue sensitivity to sodium nitroprusside was reduced in the presence of apamin and ChTX supports the possibility that calcium-sensitive K+ channels may mediate part of the relaxation to (exogenous) nitric oxide. Clearly however, the major pathway for nitric oxide-mediated relaxation is via the activation of a guanylyl cyclase since relaxation to sodium nitroprusside was abolished by blockade of guanylyl cyclase. While a direct action of nitric oxide on calcium sensitive K+ channels in supernumerary arteries is possible, it seems unlikely that this mechanism is involved in the relaxant response to bradykinin in the presence of ODQ because the additional removal of nitric oxide with hydroxocobalamin did not significantly alter the bradykinin concentration response curve in the presence of ODQ. This study suggests therefore, that blockade of guanylyl cyclase has an additional effect over removal of nitric oxide, which results in an increase in the tissue sensitivity to the EDHF.
The K+ channels involved in the EDHF-mediated relaxation to bradykinin in supernumerary arteries
Apamin, which has been reported to selectively block SKCa (Cook & Quast, 1990; Nelson & Quayle, 1995) did not affect the EDHF component of the bradykinin-induced relaxation, suggesting that SKCa alone do not mediate this relaxant response. This is in agreement with other findings (Jiang et al., 2000; Ohlmann et al., 1997; Petersson et al., 1997). Yet, in other arterial preparations, apamin has been reported to partially, if not fully, inhibit the EDHF-mediated response (Murphy & Brayden, 1995; Adeagbo & Triggle, 1993; Yamakawa et al., 1997; Drummond et al., 2000). ChTX, an inhibitor of BKCa, IKCa and also certain types of KV (Chandy & Gutman, 1995; Cook & Quast, 1990; Nelson & Quayle, 1995) and IbTX, which is reported to be a selective inhibitor of BKCa in arterial smooth muscle (Nelson & Quayle, 1995; Chandy & Gutman, 1995), did not affect the EDHF-mediated response to bradykinin. These results are in agreement with similar experiments in other vascular preparations where ChTX alone (Doughty et al., 1999; Chataigneau et al., 1998), or IbTX alone (Petersson et al., 1997; Urakami-Harasawa et al., 1997; Chataigneau et al., 1998) did not inhibit the EDHF-mediated response.
In common with a number of other vascular tissues (Petersson et al., 1997; Zygmunt & Högestatt, 1996; Chataigneau et al., 1998; Edwards et al., 1998) the combination of the K+ channel blockers apamin and ChTX was required to fully inhibit the EDHF-mediated response in bovine pulmonary supernumerary arteries. This particular combination of K+ channel inhibitors appears to have a synergistic action. There are a number of possible explanations for this observation, it is possible that EDHF might activate two distinct calcium-sensitive K+ channels to cause relaxation; an apamin-sensitive (SKCa) channel and one (or more) of the channels that are sensitive to ChTX. The fact that blockade by apamin or ChTX alone is ineffective may suggest that the channels can fully compensate for one another if one is blocked. ChTX can block BKCa, IKCa, and some types of KV. Further support for the involvement of BKCa comes from the observation that IbTX, which is reported to be more selective for BKCa than ChTX (Galvoz et al., 1990), also substantially inhibited the EDHF-mediated response when combined with apamin.
The present results implicate the involvement, of at least, SKCa and BKCa channels in the bradykinin-induced EDHF-mediated response in bovine pulmonary supernumerary arteries. However, the identity of EDHF and the location of the calcium-sensitive K+ channels in these arteries remain to be established.
Role of K+ channel in the nitric oxide mediated relaxation
The observation that relaxation induced by sodium nitroprusside is sensitive to apamin and ChTX suggests that in these vessels (exogenous) nitric oxide mediates relaxation, in part, by activating apamin/ChTX-sensitive K+ channels. Although direct activation of Ca2+-sensitive K+ channels by nitric oxide has been reported (Archer et al., 1994; Bolotina et al., 1994; Mistry & Garland, 1998) the fact that the response was abolished by ODQ suggests that the K+ channel activation by sodium nitroprusside is dependent on guanylyl cyclase.
Nitric oxide or EDHF
The fact that the bradykinin-induced vasorelaxant response was abolished only by blocking the nitric oxide/guanylyl cyclase pathway together with the EDHF-like pathway but was only marginally affected by blockade of each individual pathway suggests that either both pathways are activated by bradykinin and that each is largely capable of mediating the relaxant response, or that inhibition of one pathway is rapidly compensated by activation/upregulation of the other system. Whether one or both pathways are normally activated by bradykinin remains to be clearly demonstrated. There is some evidence, however, that the nitric oxide/cGMP inhibits some aspects of bradykinin type II receptor transduction (Miyamoto et al., 1997) in the endothelial cell and it is also reported to inhibit the formation/action of EDHF (Olmos et al., 1995; Bauersachs et al., 1996; McCulloch et al., 1997). Thus it is suggested that in the absence of nitric oxide production, the nitric oxide-independent component(s) of vasorelaxation can be up-regulated and can compensate for a reduction in the nitric oxide system (Kilpatrick & Cocks, 1994; Drummond & Cocks, 1996; Kemp et al., 1995).
In conclusion, the results of the present study suggest that in bovine isolated supernumerary arteries with an intact endothelium, bradykinin has the capacity to induce relaxation by both a nitric oxide and EDHF-like mechanism, which is sensitive to high extracellular K+ and the SKCa inhibitor apamin combined with the BKCa inhibitors ChTX or IbTX. This study also suggests that nitric oxide, acting through guanylyl cyclase, can activate an apamin/ChTX-sensitive K+ channel in this tissue.
Abbreviations
- BKCa
big conductance calcium-sensitive K+ channel
- ChTX
charybdotoxin
- EDHF
endothelium-derived hyperpolarizing factor
- IbTX
iberiotoxin
- IKCa
intermediate-conductance calcium-sensitive K+ channel
- KV
voltage-dependent K+ channel
- L-NAME
Nω-nitro-L-arginine methyl ester
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PSS
physiological saline solution
- PGI2
prostacyclin
- Rmax
maximum relaxation
- SKCa
small-conductance calcium-sensitive K+ channel
References
- ADEAGBO A.S.O., TRIGGLE C.R. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J. Cardiovasc. Pharmacol. 1993;21:423–429. [PubMed] [Google Scholar]
- ARCHER S.L., HUANG J.M.C., HAMPL V., NELSON D.P., SCHULTZ P.J., WEIR E.K. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin sensitive K+ channel by cGMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUERSACHS J., POPP R., HECKER M., SAUER E., FLEMING I., BUSSE R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- CAMPBELL W.B., GEBREMEDHIN D., PRATT P.F., HERDER D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- CHANDY I.G., GUTMAN G.A.Voltage-gated potassium channel genes Ligand- and Voltage-Gated Ion Channels. 1995Boca Raton: CRC Press; 2–71.ed. North, R.A. pp [Google Scholar]
- CHATAIGNEAU T., FELETOU M., DUHAULT J., VANHOUTTE P.M. Epoxyeicotrienoic acids, potassium channel blockers and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br. J. Pharmacol. 1998;78:574–580. doi: 10.1038/sj.bjp.0701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J. Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN R.A., PLANE F., NAJIBI S., JUK I., MALINSKI T., GARLAND C.J. Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit coronary artery. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4193–4198. doi: 10.1073/pnas.94.8.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK N.S., QUAST U.Potassium channel pharmacology Potassium channels: structure, classification, function and therapeutic potential. 1990New York: Halsted Press; 181–325.ed. Cook, N.S. pp [Google Scholar]
- DANSER A.H.J., TOM B., DE VRIES R., SAXENA P.R. L-NAME-resistant bradykinin-induced relaxation in porcine coronary arteries is NO-dependent: effects of ACE inhibition. Br. J. Pharmacol. 2000;131:195–202. doi: 10.1038/sj.bjp.0703555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORA K.A., MARTIN P.E.M., CHAYTOR A.T., EVANS W.H., GARLAND C.J., GRIFFITH T.M. Role of heterocellular gap junctional communication in endothelium-dependent smooth muscle hyperpolarisation: inhibition by a connexin-mimetic peptide. Biochem. Biophys. Res. Commun. 1999;254:27–31. doi: 10.1006/bbrc.1998.9877. [DOI] [PubMed] [Google Scholar]
- DOUGHTY J.M., PLANE F., LANGTON P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am. J. Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- DRUMMOND G.R., COCKS T.M. Evidence for mediation by endothelium-derived hyperpolarizing factor of relaxation to bradykinin in the bovine isolated coronary artery independently of voltage-operated Ca2+ channels. Br. J. Pharmacol. 1996;117:1035–1040. doi: 10.1111/j.1476-5381.1996.tb16693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUMMOND G.R., SELEMIDIS S., COCKS T.M. Apamin-sensitive, non-nitric oxide (NO) endothelium-dependent relaxations to bradykinin in the bovine isolated coronary artery: no role for cytochrome P450 and K+ Br. J. Pharmacol. 2000;129:811–819. doi: 10.1038/sj.bjp.0703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., FELETOU M., GARDENER M.J., THOLLON C., VANHOUTTE P.M., WESTON A.H. Role of gap junctions in the responses to EDHF in rat and guinea-pig small arteries. Br. J. Pharmacol. 1999;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT F.M., REID L.M. Some new facts about the pulmonary artery and its branching pattern. Clin. Radiol. 1965;16:193–198. doi: 10.1016/s0009-9260(65)80042-3. [DOI] [PubMed] [Google Scholar]
- FELETOU M., VANHOUTTE P.M. The Alternative: EDHF. J. Mol. Cell. Cardiol. 1999;31:15–22. doi: 10.1006/jmcc.1998.0840. [DOI] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZSKI J.V. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;286:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GALVOZ A., GIMINEZ-GALLEGO G., REUBEN J.P., ROY-CONTANCIN L., FEIGENBAUM P., KACZOROWSKI G.J.F., GARCIA M.L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J. Biol. Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- GARLAND C.J., PLANE F., KEMP B.K., COCKS T.A. Endothelium-derived hyperpolarizing factor. Trends Pharmacol. Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUGA G.M., WOOD K.S., BYRNS R.E. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGNARRO L.J. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- JIANG F., GUANG G., RAND M.J. Mechanisms of nitric oxide-independent relaxations induced by carbachol and acetylcholine in rat isolated renal arteries. Br. J. Pharmacol. 2000;130:1191–1200. doi: 10.1038/sj.bjp.0703408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KACZOROWSKI G.J., KNAUS H.G., LEONARD R.J., MCMANUS O.B., GARCIA M.L. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J. Bioenerg. Biomembr. 1996;28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- KATO M., SHIODE N., YAMAGATA T., MATSUURA H., KAJIYAMA G. Bradykinin induced dilatation of human epicardial and resistance coronary arteries in vivo: effect of inhibition of nitric oxide synthesis. Heart. 1997;78:493–498. doi: 10.1136/hrt.78.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP B.K., SMOLICH J.J., RITCHIE B.C., COCKS T.M. Endothelium-dependent relaxations in sheep pulmonary arteries and veins: resistance to block by NG-nitro-L-arginine in pulmonary hypertension. Br. J. Pharmacol. 1995;116:2457–2467. doi: 10.1111/j.1476-5381.1995.tb15096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILPATRICK E.V., COCKS T.M. Evidence for differential roles of nitric oxide (NO) and hyperpolarization in endothelium-dependent relaxation of pig isolated coronary artery. Br. J. Pharmacol. 1994;112:557–565. doi: 10.1111/j.1476-5381.1994.tb13110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUHBERGER E., GROSCHNER K., KUKOVETZ W.R., BRUNNER F. The role of myoendothelial cell contact in non-nitric oxide-, non prostanoid-mediated endothelium-dependent relaxation of porcine coronary artery. Br. J. Pharmacol. 1994;113:1289–1294. doi: 10.1111/j.1476-5381.1994.tb17138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCULLOCH A.I., BOTTRILL F.E., RANDALL M.D., HILEY C.R. Characterization and modulation of EDHF-mediated relaxations in the rat isolated superior mesenteric arterial bed. Br. J. Pharmacol. 1997;120:1431–1438. doi: 10.1038/sj.bjp.0701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISTRY D.K., GARLAND C.J. NO-induced activation of large-conductance Ca2+-dependent K+ channels (BK(Ca)) in smooth muscle cells isolated from the rat mesenteric artery. Br. J. Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAMOTO A., LAUFS U., PARDO C., LIAO J.K. Modulation of bradykinin receptor ligand binding affinity and its coupled G-proteins by nitric oxide. J. Biol. Chem. 1997;272:19601–19608. doi: 10.1074/jbc.272.31.19601. [DOI] [PubMed] [Google Scholar]
- MONCADA S., VANE J.R. Pharmacology and endogenous roles of prostaglandin endoperoxydes, thromboxane A2 and prostacyclin. Pharmacol. Rev. 1979;30:293–331. [PubMed] [Google Scholar]
- MONCADA S., RADOMSKI M.W., PALMER R.M.J. Endothelium-derived factor. Identification as Nitric Oxide and role in the control of vascular tone and platelet function. Biochem. Pharmacol. 1988;37:2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- MURPHY M.E., BRAYDEN J.E. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J. Physiol. 1995;498:723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAO T., ILLIANO S., VANHOUTTE P.M. Heterogenous distribution of endothelium-dependent relaxations resistant to NG-nitro-L-arginine in rats. Am. J. Physiol. 1992;263:H1090–H1094. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- OHLMANN P., MARTINEZ M.C., SCHNEIDER F., STOCLET J.C., ANDRIANSITOHAINA R. Characterization of endothelium-derived relaxing factors released by bradykinin in human resistance arteries. Br. J. Pharmacol. 1997;121:657–664. doi: 10.1038/sj.bjp.0701169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLMOS L., MOMBOULI J.-V., ILLIANO S., VANHOUTTE P.M. cGMP mediates the desensitisation to bradykinin in isolated canine coronary arteries. Am. J. Physiol. 1995;268:H865–H870. doi: 10.1152/ajpheart.1995.268.2.H865. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., FERRIDGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PETERSSON J., ZYGMUNT P.M., HOGESTATT E.D. Characterization of the potassium channels involved in EDHF-mediated relaxation in cerebral arteries. Br. J. Pharmacol. 1997;120:1344–1350. doi: 10.1038/sj.bjp.0701032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P.H., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- SCHRAMMEL A., BEHRENDS S., SCHMIDT K., KOESLING D., MAYER B. Characterization of 1H (1,2,4-) oxadiazolo (4,3-a)quinoxalin-1-one as a heme-site inhibitor of NO-sensitive guanylyl cyclase. Mol. Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- SHAW A.M., BUNTON D., FISHER A., DALY C., MCGRATH J.C., MACDONALD A. V-shaped cushion at the origin of bovine pulmonary supernumerary arteries: structure and putative function. J. Appl. Physiol. 1999;87:2348–2356. doi: 10.1152/jappl.1999.87.6.2348. [DOI] [PubMed] [Google Scholar]
- SHIMOKAWA H., YASUTAKE H., FUJII K., OWADA M.K., NAKAIKE R., FUKUMOTO Y., TAKAYANAGI T., NAGAO T., EGASHIRA K., FUJISHIMA M., TAKESHITA A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J. Cardiovasc. Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- TAYLOR S.G., WESTON A.H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol. Sci. 1988;9:272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- URAKAMI-HARASAWA L., SHIMOKAWA H., NAKASHIMA M., EGASHIRA K., TAKESHITA A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J. Clin. Invest. 1997;100:2793–2799. doi: 10.1172/JCI119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAKAWA N., OHHASHI M., WAGA S., ITOH T. Role of endothelium in regulation of smooth muscle membrane potential and tone in the rabbit middle cerebral artery. Br. J. Pharmacol. 1997;121:1315–1322. doi: 10.1038/sj.bjp.0701285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HÖGESTATT E.D. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br. J. Pharmacol. 1996;117:1600–1606. doi: 10.1111/j.1476-5381.1996.tb15327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


