Abstract
We investigated the profound involvement of mast cell chymase, an alternative angiotensin II-generating enzyme, in angiogenesis using a specific chymase inhibitor. We also studied the functional profiles of this novel inhibitor in basic fibroblast growth factor (bFGF)-induced angiogenesis.
In this study, angiogenesis was induced by daily injections of bFGF (0.3 μg site−1 day−1), angiotensin I (2 nmol site−1 day−1) or angiotensin II (2 nmol site−1 day−1) into sponges implanted to male hamsters subcutaneously for 7 days. Angiogenesis in the granulation tissue surrounding sponges was evaluated by measuring the haemoglobin (Hb) content and local blood flow as the parameters for angiogenesis.
A chymase inhibitor, BCEAB (4-[1-{[bis-(4-methyl-phenyl)-methyl]-carbamoyl}-3-(2-ethoxy-benzyl)-4-oxo-azetidine-2-yloxy]-benzoic acid), was simultaneously administered into the implanted sponges (2 or 5 nmol site−1 day−1, for 7 days) treated with bFGF and strongly suppressed the haemoglobin contents in sponge granulomas. In the studies using a laser doppler perfusion imager, BCEAB (5 nmol site−1 day−1) also attenuated the bFGF-induced increase of local blood flow around the implanted sponge granuloma.
In bFGF-induced angiogenesis, chymase activity in sponge granulomas was substantially increased. It was also confirmed that the chymase activity increased by bFGF was significantly and dose-dependently inhibited by BCEAB (2, 5 nmol site−1 day−1).
BCEAB inhibited the Hb contents and the expression of vascular endothelial growth factor (VEGF) mRNA induced by angiotensin I but not by angiotensin II.
These results suggest that the significance of chymase in bFGF-induced angiogenesis was confirmed, and a novel inhibitor, BCEAB, strongly suppresses the bFGF-induced angiogenesis through the chymase-angiotensin II-VEGF dependent pathway.
Keywords: Chymase, angiotensin, angiogenesis, inhibitor, VEGF, bFGF
Introduction
A disabled regulation of angiogenesis is strongly implicated in the pathogenesis of numerous diseases including chronic inflammatory diseases, diabetic retinopathy and tumour growth (Folkman, 1971; Michaelson, 1948; Wise, 1956; Peacock et al., 1992; Colville-Nash & Scott, 1992). Angiogenesis is a multistep event and is regulated by various factors such as basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), or epidermal growth factor (EGF) positively or negatively (Fan & Brem, 1992; Folkman & Shing, 1992).
Angiotensin II also enhances neovascularization in several animal models and increases blood flow in ischaemia-induced angiogenesis (Fernandez et al., 1985; Le Noble et al., 1991; Emanueli et al., 2002; Sasaki et al., 2002; Tamarat et al., 2002). From the results of experiments in a mouse sponge model and in a laser-induced macular degeneration model using angiotensin II receptor antagonists, it has been suggested that the effects of angiotensin II in angiogenesis are mediated by the angiotensin type I (AT1) receptor (Walsh et al., 1997; Hikichi et al., 2001).
Angiotensin converting enzyme (ACE) has been believed to predominate in the production of angiotensin II. An alternative pathway for generating angiotensin II, i.e. the chymase-dependent angiotensin II-generating system, has been identified (Okunishi et al., 1984). Chymase is a chymotrypsin-like serine protease originally found as a mast cell protease (Langunoff & Benditt, 1963). The enzymatic character of chymase has been identified in some species, it cleaves the C-terminal of peptides just after aromatic amino acids such as Phe, Tyr and Trp. There are species differences in the cleavage of angiotensin I (Okunishi et al., 1993). In humans, monkeys, dogs and hamsters, chymase generates angiotensin II from angiotensin I by cleaving the Phe8-His9 bond, but, in rats, mice and rabbits, chymase cleaves Tyr4-Ile5 of angiotensin I to inactive fragments. Recently, many pathophysiological possibilities for chymase through the formation of angiotensin II have been reported such as vascular proliferation in dog grafted vein (Takai et al., 2000) and remodelling after myocardial infarction (Jin et al., 2001; 2002). We have demonstrated that chymase acts as a pro-angiogenic factor because the exogenous injections of chymase gene or purified chymase into implanted sponges strongly facilitate angiogenesis in a hamster sponge implant model (Muramatsu et al., 2000a). The source of this chymase is mainly mast cells and it is a major mediator in mast cell-associated angiogenesis (Muramatsu et al., 2000b).
In the previous report (Muramatsu et al., 2000a), we have also demonstrated the involvement of endogenous chymase in bFGF-induced angiogenesis using chymostatin, limabean trypsin inhibitor (LBTI) and soybean trypsin inhibitor (SBTI). The inhibitions of chymase activities are common among these inhibitors. These inhibitors are able to inhibit chymase activity, while it was a problem that each inhibitor has wide effects against other serine proteases such as elastase, tryptase and trypsin.
In this study, we investigated the precise significance of endogenous chymase in bFGF-induced angiogenesis using a specific chymase inhibitor, BCEAB and also studied the functional profiles of it on inhibition of angiogenesis.
Methods
Hamster sponge model
Angiogenesis model was produced by the method described previously (Muramatsu et al., 2000a). Briefly, polyurethane sponge discs (5-mm thick, 1.3 cm in diameter) were implanted in the subcutaneous air pouch in the dorsum of individual male Syrian hamsters (6 weeks old) purchased from Japan SLC (Shizuoka, Japan), under light anaesthesia with pentobarbital (50 mg kg−1, i.p.). Angiogenesis was induced by the injection of bFGF (0.3 μg site−1 day−1), angiotensin I (2 nmol site−1 day−1) or angiotensin II (2 nmol site−1 day−1) into the implanted sponges for 7 days. One hundred microlitres of saline were injected into the sponges as a control. A chymase inhibitor, BCEAB (2 or 5 nmol site−1 day−1) was administered into the sponge simultaneously with the inducer. At the end of the experimental period, the animal was sacrificed, and the granuloma tissues were excised immediately, together with the enclosed sponge implants. The obtained tissues were divided into halves, one half for measurement of haemoglobin contents and the other half for measurement of chymase activity, detection or analysis of VEGF mRNA expression.
Measurement of haemoglobin content
We measured the haemoglobin contents of the implant samples according to the method of Majima et al. (1997) as a parameter for angiogenesis. Briefly, the sample granuloma tissues were weighed and homogenized with a Polytrone homogenizer in distilled water (4 ml g−1 wet weight). After centrifugation at 5000×g for 20 min, the haemoglobin concentration in the supernatant was determined by means of haemoglobin assay kit (Hemoglobin B-test WAKO).
Measurement of local blood flow in sponge granuloma
The local blood flow in sponge granuloma tissues was measured by a laser doppler perfusion imager (PIM II, Lisca Developments Co., Linköping, Sweden). After the experimental period, the hamsters were anaesthetized with pentobarbital sodium (50 mg kg−1, i.p.) and were put into the prone position, keeping a precise distance to the laser. Defined areas on the whole back or the site around the sponge granuloma tissues were scanned at 2176 or 2500 pixels, respectively, and local blood flow was detected. The obtained data were visualized with colour-coded images and were quantified by the mean value for blood flow in defined areas.
Measurement of chymase activity
To measure chymase activity, each sample was homogenized in 20 mM phosphate buffer (pH 7.4) containing 2 M KCl and 0.1% Nonidet P-40. After centrifugation at 15,000 r.p.m. for 30 min, the obtained supernatant was used for the measurement of chymase activity. Aliquots of the tissue extracts were incubated for 1 h at 37°C with 770 μM angiotensin I in 48.5 mM borax-borate buffer (pH 8.5) containing 8 mM dipyridyl, 770 μM diisopropyl phosphorofluoridate, and 5 mM ethylenediaminetetraacetic acid as inhibitors of ACE and angiotensinases. The reactions were terminated by addition of 15% cold trichloroacetic acid and the mixtures were centrifuged at 15,000 r.p.m. for 5 min. The concentration of His-Leu, an enzymatic cleavage product of angiotensin I, in the supernatant was determined using 10% o-phthaldialdehyde. One unit of chymase activity was defined as the amount of enzyme that cleaved 1 μmol His-Leu min−1.
Analysis of VEGF mRNA by reverse-transcription polymerase chain reaction (RT–PCR)
Total RNA was extracted from the excised sponge granuloma tissues, which were immediately frozen with liquid nitrogen according to the acid guanidium-phenol-chloroform (AGPC) method. First-strand cDNA was synthesized by the reverse transcription reaction using oligo (dT)12-18 primer and reverse transcriptase (SuperScript II). As PCR primers, 5′-ggaccctggctttactgctg-3′ (sense) and 5′-gtgattttctggctttgttc-3′ (antisense) for hamster VEGF and 5′-ccaaggccaaccgcgagaagatgac-3′ (sense) and 5′-agggtacatggtggtgccgccagac-3′ (antisense) for β-actin cDNA were used for the amplifications. The amplifications for VEGF and β-actin were performed at 25 cycles of 94°C for 30 s 50°C for 30 s, and 68°C for 30 s. PCR products were separated on a 2% agarose gel.
Drugs
All chemicals were reagent grade. Recombinant human bFGF was purchased from Genzyme/Techne (Minneapolis, USA). Angiotensin I and angiotensin II were purchased from Peptide Institue Inc. (Osaka, Japan). Hemoglobin B-test®, diisopropyl phosphorofluoridate and o-phthaldialdehyde were purchased from Wako Pure Chemical Industries (Osaka, Japan). Taq DNA polymerase and reverse transcriptase (SuperScript II) were purchased from Life Technologies (Rockville, USA). Oligo (dT)12-18 primer was purchased from Invitrogen (Groningen, Netherlands). Primers for VEGF and β-actin were ordered from Funakoshi (Tokyo, Japan). BCEAB (4-[1-{[bis-(4-methyl-phenyl)-methyl]-carbamoyl}-3-(2-ethoxy-benzyl)-4-oxo-azetidine-2-yloxy]-benzoic acid) was a kind gift from Shionogi Co. (Osaka, Japan).
Statistics
Results are given as mean±standard error of the mean (s.e.mean). Differences among groups were examined for statistical significance using one-way ANOVA. P values of lower than 0.05 indicated significant differences.
Results
Inhibition of bFGF-induced angiogenesis by a chymase inhibitor
In the specimens treated with bFGF alone, granuloma tissues rich with newly formed vessels were observed (Figure 1a). On the other hand, BCEAB strongly suppressed the microvessel formation and granulation. As shown in Figure 1b, significant decreases of haemoglobin contents due to BCEAB of 2 and 5 nmol site−1 were observed and this inhibition was dose-dependent.
Figure 1.
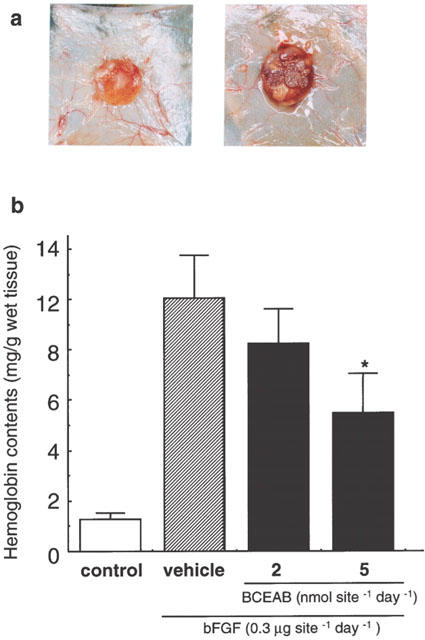
Inhibition of bFGF-induced angiogenesis by BCEAB in hamster sponge model. Angiogenesis was induced by the administration of bFGF (0.3 μg site−1 day−1) for 7 days and a chymase inhibitor, BCEAB, was also injected into implanted sponges (2 or 5 nmol site−1 day−1). (a) Typical photographs of a sponge treated with bFGF alone (right) and a sponge treated with bFGF and BCEAB in the concentration of 5 nmol site−1 (left). (b) Haemoglobin contents in sponge granuloma tissues were significantly suppressed by BCEAB in a dose-dependent manner. Data are the means±s.e.mean of five hamsters. *P<0.05 versus the bFGF-treated group.
To confirm that the haemoglobin contents in sponges are not stagnated blood, the local blood flow in sponge granuloma tissues was measured by the laser doppler perfusion imager. Figure 2a,b show the intensity of local blood flows in the whole back of the hamster. The area circled with the dotted line indicating the site of the sponge implant exhibited a strong signal with bFGF treatment. This signal was clearly reduced by simultaneous administration of BCEAB (Figure 2c, d). In quantitative analysis, BCEAB (5 nmol site−1 day−1) also inhibited the local blood flow induced by bFGF (Figure 3).
Figure 2.
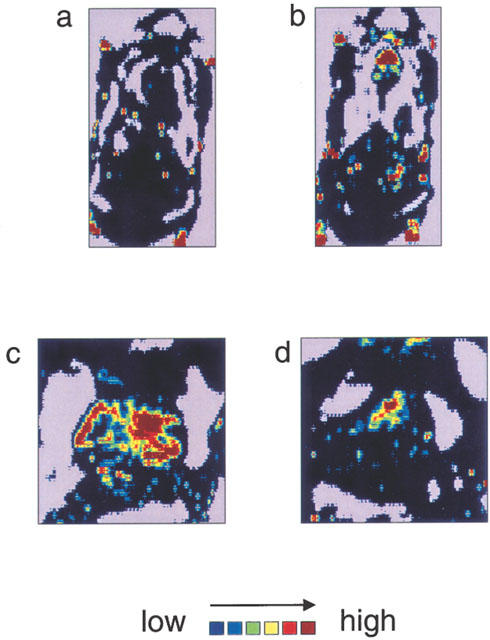
Increase in blood flow in sponge granulomas induced by bFGF treatment and inhibitory effect of BCEAB on this blood-flow increase. On day 7 after the sponge implantation, the local blood flow around the sponge granulomas was measured by the laser doppler perfusion imager. The scanned data are shown by the colour-coded image, which indicates blood flow in the whole back of hamsters treated with saline (a) or bFGF (0.3 μg site−1 day−1) (b). The areas circled with dotted lines indicate the sites of sponge implantation. Panels (c) and (d) show the local blood flow around the sponge implantations, which were treated with bFGF alone (c) or bFGF plus BCEAB (5 nmol site−1 day−1) (d).
Figure 3.
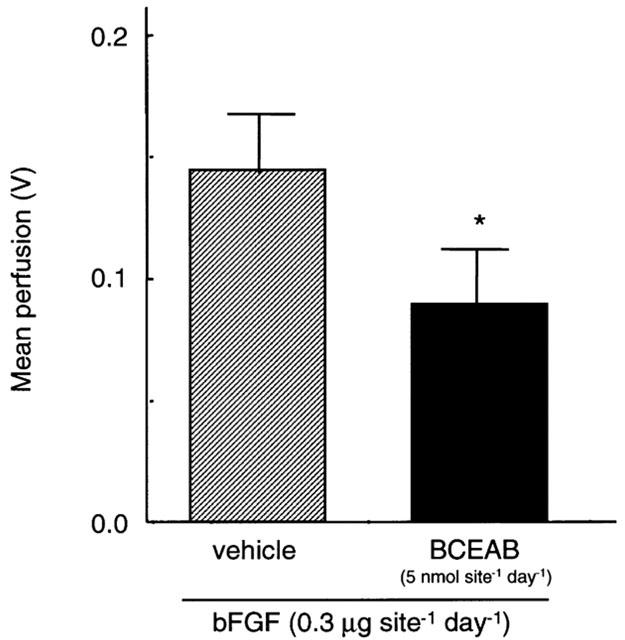
Quantitative analysis of local blood flows in sponge granuloma tissues. On day 7 after the sponge implantation, the local blood flow around the sponge granuloma was quantified by the laser doppler perfusion imager and is shown as the mean value for the defined area. Data are presented as means±s.e.mean of four hamsters. *P<0.05 versus the bFGF-treated group.
Chymase activity in sponge implants
On day 7 after the continuous administration of bFGF with or without BCEAB, the granuloma tissues were excised and the chymase activities in the extracts obtained from granulomas were assessed. The administration of bFGF strongly promotes chymase activity in sponge granulomas in comparison to saline-treated implants. The increase of chymase activity with bFGF treatment was completely suppressed by BCEAB of 5 nmol site−1 (Figure 4).
Figure 4.
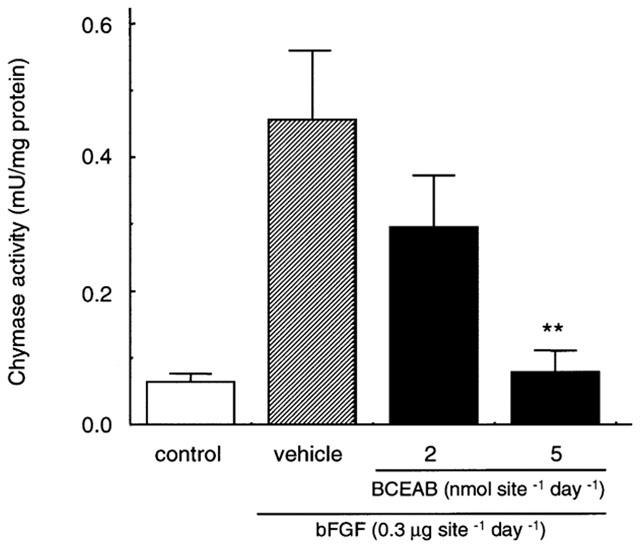
Effects of BCEAB on chymase activity in sponge granuloma tissues. The sponges with or without BCEAB treatment (2, 5 nmol site−1 day−1) were excised and an extract buffer was obtained from each sponge. The chymase activity in the buffered extract was determined as the His-Leu releasing activity from angiotensin I. Data are presented as means±s.e.mean of five hamsters. **P<0.01 versus the bFGF-treated group.
VEGF mRNA expression induced by angiotensin II
We examined whether the inhibitory effect of angiogenesis by BCEAB was mediated via the angiotensin II/VEGF dependent pathway. Daily administrations of angiotensin II (2 nmol site−1 day−1) into implanted sponges for 7 days induced angiogenesis and 2 nmol site−1 of angiotensin I, which is the inactive precursor of angiotensin II, also induced angiogenesis to an almost equal extent (Figure 5a). As shown in Figure 5a, BCEAB significantly blocked the angiogenesis induced by angiotensin I but not by angiotensin II.
Figure 5.
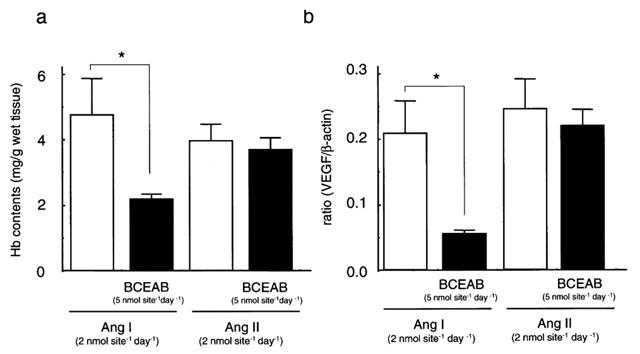
Angiogenesis and VEGF mRNA expression in angiogenesis induced by angiotensin I or angiotensin II. After the administration of angiotensin I (2 nmol site−1 day−1) or angiotensin II (2 nmol site−1 day−1) for 7 days with or without BCEAB (5 nmol site−1 day−1), haemoglobin contents and VEGF mRNA expression were determined in the sponge granulomas. Haemoglobin contents in sponge granuloma tissue (a). The ratio of VEGF mRNA expression against β-actin (b). Results are shown as means±s.e.mean of five sponge granulomas. **P<0.01 versus each vehicle-treated group.
The expression of VEGF mRNA was detectable in the sponge granuloma stimulated with angiotensin I or angiotensin II. As shown in Figure 5b, BCEAB suppressed the expression of VEGF mRNA stimulated with angiotensin I but not with angiotensin II.
Discussion
Chymase is a serine protease contained in granules of mast cells and several of its pathophysiological roles based on the production of angiotensin II have been reported (Takai et al., 2000; Jin et al., 2001; 2002). Previously, we demonstrated that hamster chymase is angiogenic when injected exogenously as genes or purified proteins into implanted sponges (Muramatsu et al., 2000a) and we also confirmed that the origin of chymase was the mast cells that accumulated in the sponge granulomas (Muramatsu et al., 2000b). We have also investigated the significance of endogenous chymase in angiogenesis induced by bFGF using several protease inhibitors such as chymostatin, LBTI and SBTI (Muramatsu et al., 2000a). Because of the absence of a specific chymase inhibitor, we previously demonstrated our hypothesis by using a multiple number of inhibitors. These inhibitors are able to inhibit chymase, but also have inhibitory effects against other serine proteases. It was problematic that chymostatin also inhibits elastase and cathepsin G, and that LBTI inhibits tryptase and trypsin. To clarify the precise role of endogenous chymase in angiogenesis, a specific chymase inhibitor was sought.
BCEAB inhibited purified hamster chymase activity with an IC50 value of 5.4 nM but it did not suppress ACE, elastase and tryptase activities (Takai et al., 2001), and it weakly inhibits trypsin activity (IC50=6400 nM). Tryptase is reported as an angiogenic factor (Blair et al., 1997). Elastase, which is mainly present in neutrophils, generates an anti-angiogenic factor, angiostatin (O'reilly et al., 1994). As BCEAB did not inhibit the activities of these other proteases involved in angiogenesis, by using it, we could observe an unambiguous effect of endogenous chymase in bFGF-induced angiogenesis. We also investigated the anti-angiogenic profile of a novel chymase inhibitor, BCEAB, in bFGF-induced angiogenesis.
To investigate the significance of endogenous chymase in pathophysiological angiogenesis, the typical and potent angiogenic factor, bFGF, was used as an inducer in this model. It was reported that bFGF is released from various cancer cells (Relf et al., 1997) and it is a chemoattractant of mast cells (Gruber et al., 1995). By treatment with bFGF, implanted sponges grew into granulation tissues rich in fibrous matrix and CD31-positive microvessels (data not shown). BCEAB strongly reduced the Hb content induced by bFGF in a dose-dependent manner and its maximum inhibition was about 60% (Figure 1b). Furthermore, we also detected the blood flow in the sponge granuloma. The continuous administration of bFGF caused an apparent increase of blood flow in the sponge granuloma, but BCEAB (5 nmol site−1 day−1) suppressed it (Figures 2 and 3). Since BCEAB is an inhibitor highly selective for chymase rather than other proteases such as elastase, tryptase and trypsin, this result indicated that endogenous chymase is obviously involved in angiogenesis induced by bFGF. As the administration of BCEAB in vivo apparently inhibited chymase activity in the sponge granuloma, the suppression of angiogenesis by BCEAB may be mediated through the inhibition of chymase activity (Figure 4). BCEAB strongly suppressed bFGF-induced angiogenesis, but did not completely inhibit it. Basic FGF itself powerfully facilitates angiogenesis by acting on endothelial cells, and it also synergistically accelerates angiogenesis together with various other factors. It was reported that bFGF increased the expression of the KDR receptor for VEGF (Hata et al., 1999) and a VEGF receptor antagonist suppressed bFGF-induced angiogenesis (Tille et al., 2001). In addition, bFGF also promotes the expression of matrix metalloproteinase-1 (MMP-1), which is important when endothelial cells invade the extracellular matrix (Okamura et al., 1991). Thus, the reason why BCEAB did not inhibit angiogenesis completely might be that the effects of bFGF itself or downstream factors partly contributed to the bFGF-induced angiogenesis.
It has been reported that angiotensin II facilitates angiogenesis via the augmentation of VEGF mRNA expression via the angiotensin II type I receptor in several types of cells in vitro (Chua et al., 1998; Otani et al., 2000; Pupilli et al., 1999). To confirm the inhibitory characteristics of BCEAB in this model, the expression of VEGF mRNA in angiotensin-induced angiogenesis was examined. BCEAB blocked the angiogenesis and VEGF mRNA expression in sponge granulomas induced by angiotensin I but not by angiotensin II (Figure 5a). These results show that the suppression of angiogenesis by BCEAB is mediated via the inhibition of chymase activity that converts angiotensin I to angiotensin II, and the reduction of VEGF mRNA expression as a result of the attenuation of angiotensin II stimuli.
In this model, we hypothesize that chymase derived from mast cells, which were accumulated as a result of bFGF stimuli, generates angiotensin II. Angiotensin II facilitates the up-regulation of VEGF but also the synthesis and expression of bFGF (Itoh et al., 1993; Peifley & Winkles, 1998). Basic FGF induced by angiotensin II could further accelerate the accumulation of mast cells and angiotensin II generation by chymase. Thus the significance of angiotensin II in angiogenesis is strongly suggested.
We primarily examined the angiotensin-VEGF dependent pathway for the angiogenic effect of chymase in this study. Interestingly, chymase cleaves not only angiotensin I but also various substrates associated with angiogenesis such as interleukin (IL)-β (Mizutani et al., 1991), latent transforming growth factor (TGF)-β (Taipale et al., 1995; Lindstedt et al., 2001) and pro-gelatinase B (Fang et al., 1997). Thus, the possibility that chymase promotes angiogenesis synergistically through other pathways by the activation of these substrates, is also suggested. This hypothesis is supported by the result that TCV-116, an angiotensin II type 1 receptor antagonist, did not completely inhibit the angiogenesis induced by purified chymase (Muramatsu et al., 2000a).
In the present study, we demonstrated the significant role of endogenous chymase in angiogenesis induced by bFGF by using a specific chymase inhibitor and this effect was mediated via the chymase-angiotensin-VEGF-dependent pathway. Moreover, several possibilities for treatment with chymase inhibitors in angiogenesis-associated diseases such as cancer, diabetic retinopathy, rheumatoid arthritis and so on, are suggested. Further studies are required to clarify the actual roles of chymase in angiogenesis other than through the angiotensin II-VEGF-dependent pathway.
Acknowledgments
We thank Shionogi Co. for kindly supplying BCEAB. This study was supported in part by grants from the Pharmacological Research Foundation and the Hoansha Foundation.
Abbreviations
- ACE
angiotensin converting enzyme
- bFGF
basic fibroblast growth factor
- Hb
haemoglobin
- VEGF
vascular endothelial growth factor
References
- BLAIR R.J., MENG H., MARCHESE M.J., REN S., SCHWARTZ L.B., TONNESEN M.G., GRUBER B.L. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J. Clin. Invest. 1997;99:2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUA C.C., HAMDY R.C., CHUA B. Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochim. Biophys. Acta. 1998;1401:187–194. doi: 10.1016/s0167-4889(97)00129-8. [DOI] [PubMed] [Google Scholar]
- COLVILLE-NASH P.R., SCOTT D.L. Angiogenesis and rheumatoid arthritis: pathogenic and therapeutic implication. Ann. Rheum. Dis. 1992;51:919–925. doi: 10.1136/ard.51.7.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMANUELI C., SALIS M.B., STACCA T., PINNA A., GASPA L., MADDEDDU P. Angiotensin AT(1) receptor signaling modulates reparative angiogenesis induced by limb ischaemia. Br. J. Pharmacol. 2002;135:87–92. doi: 10.1038/sj.bjp.0704461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAN T.P.D., BREM S.The search for anti-cancer drugs Cancer Biology and Medicine Series 1992Dordrecht: Kluwer Academic Publishing; 185–229.ed. Waring, M.J. & Ponder, B.A.J. pp [Google Scholar]
- FANG K.C., RAYMOND W.W., BLOUNT J.L., CAUGHEY G.H. Dog mast cell alpha-chymase activates progelatinase B by cleaving the Phe88-Gln89 and Phe91-Glu92 bonds of the catalytic domain. J. Biol. Chem. 1997;272:25628–25635. doi: 10.1074/jbc.272.41.25628. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ L.A., TWICKLER J., MEAD A. Neovascularization produced by angiotensin II. J. Lab. Clin. Med. 1985;105:141–145. [PubMed] [Google Scholar]
- FOLKMAN J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- FOLKMAN J., SHING Y. Angiogenesis. J. Biol. Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- GRUBER B.L., MARCHESE M.J., KEW R. Angiogenic factors stimulate mast-cell migration. Blood. 1995;86:2488–2493. [PubMed] [Google Scholar]
- HATA Y., ROOK S., AIELLO L.P. Basic fibroblast growth factor induces expression of VEGF receptor KDR through a protein kinase C and p44/p42 mitogen-activated protein kinase-dependent pathway. Diabetes. 1999;48:1145–1155. doi: 10.2337/diabetes.48.5.1145. [DOI] [PubMed] [Google Scholar]
- HIKICHI T., MORI F., TAKAMIYA A., SASAKI M., HORIKAWA Y., TAKEDA M., YOSHIDA A. Inhibitory effects of losartan on laser-induced choroidal neovascularization. Am. J. Ophthalmol. 2001;132:587–589. doi: 10.1016/s0002-9394(01)01139-4. [DOI] [PubMed] [Google Scholar]
- ITOH H., MUKOYAMA M., PRATT R.E., GIBBONS G.H., DZAU V.J. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J. Clin. Invest. 1993;9:2268–2274. doi: 10.1172/JCI116454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN D., TAKAI S., YAMADA M., SAKAGUCHI M., YAO Y., MIYAZAKI M. Possible roles of cardiac chymase after myocardial infarction in hamster hearts. Jpn. J. Pharmacol. 2001;86:203–214. doi: 10.1254/jjp.86.203. [DOI] [PubMed] [Google Scholar]
- JIN D., TAKAI S., YAMADA M., SAKAGUCHI M., MIYAZAKI M. Beneficial effects of cardiac chymase inhibition during the acute phase of myocardial infarction. Life Science. 2002;71:437–446. doi: 10.1016/s0024-3205(02)01689-2. [DOI] [PubMed] [Google Scholar]
- LANGUNOFF D., BENDITT E.P. Proteolytic enzymes of mast cells. Ann. N.Y. Acad. Sci. 1963;103:185–198. doi: 10.1111/j.1749-6632.1963.tb53698.x. [DOI] [PubMed] [Google Scholar]
- LE NOBLE F.A.C., HEKKING J.W.M., VAN STRAATEN H.W.M., SLAAF D.W., BOUDIER H.A.J.S. Angiotensin II stimulates angiogenesis in the chorio-allantoic membrane of the chick embryo. Eur. J. Pharmacol. 1991;195:305–306. doi: 10.1016/0014-2999(91)90552-2. [DOI] [PubMed] [Google Scholar]
- LINDSTEDT K.A., WANG Y., SHIOTA N., SAARINEN J., HYYTIAINEN M., KOKKONEN J.O., KESKI-OJA J., KOVANEN P.T. Activation of paracrine TGF-β1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001;15:1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- MAJIMA M., ISONO M., IKEDA Y., HAYASHI I., HATANAKA K., HARADA Y., KATSUMATA O., YAMASHINA S., KATORI M. Significant roles of inducible cyclooxygenase (COX)-2in angiogenesis in rat sponge implants. Jpn. J. Pharmacol. 1997;75:105–114. doi: 10.1254/jjp.75.105. [DOI] [PubMed] [Google Scholar]
- MICHAELSON I.C. The mode of development of the vascular system of the retina, with some observations on its significance for certain retinal diseases. Trans. Ophthalmol. U.K. 1948;68:137–180. [Google Scholar]
- MIZUTANI H., SCHECHTER N., LAZARUS G., BLACK R.A., KUPPER T.S. Rapid and specific conversion of precursor interleukin 1β (IL-1β) to an active IL-1 species by human mast cell chymase. J. Exp. Med. 1991;174:821–825. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAMATSU M., KATADA J., HAYASHI I., MAJIMA M. Chymase as a proangiogenic factor; a possible involvement of chymase-angiotensin-dependent pathway in the hamster sponge angiogenesis model. J. Biol. Chem. 2000a;275:5545–5552. doi: 10.1074/jbc.275.8.5545. [DOI] [PubMed] [Google Scholar]
- MURAMATSU M., KATADA J., HATTORI M., HAYASHI I., MAJIMA M. Chymase mediates mast cell-induced angiogenesis in hamster sponge granulomas. Eur. J. Pharmacol. 2000b;402:181–191. doi: 10.1016/s0014-2999(00)00350-2. [DOI] [PubMed] [Google Scholar]
- OKAMURA K., SATO Y., MATSUDA T., HAMANAKA R., ONO M., KOHNO K., KUWANO M.. Endogenous basic fibroblast growth factor-dependent induction of collagenase and interleukin-6 in tumor necrosis factor treated human microvascular endothelial cells. J. Biol. Chem. 1991;266:19162–19165. [PubMed] [Google Scholar]
- OKUNISHI H., OKA Y., SHIOTA N., KAWAMOTO T., SONG K., MIYAZAKI M. Marked species-difference in the vascular angiotensin II-forming pathways: humans versus rodents. Jpn. J. Pharmacol. 1993;62:207–210. doi: 10.1254/jjp.62.207. [DOI] [PubMed] [Google Scholar]
- OKUNISHI H., MIYAZAKI M., TODA N. Evidence for a putatively new angiotensin II-generating enzyme in the vascular wall. J. Hypertens. 1984;2:277–284. [PubMed] [Google Scholar]
- O'REILLY M.S., HOLMGREN L., SHING Y., CHEN C., ROSENTHAL R.A., MOSES M., LANE W.S., CAO Y., SAGE E.H., FOLKMAN J. Angiostatis: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- OTANI A., TAKAGI H., OH H., SUZUMA K., MATSUMURA M., IKEDA E., HONDA Y. Angiotensin II-stimulated vascular endothelial growth factor expression in bovine retinal pericyte. Invest. Ophthalmol. Vis. Sci. 2000;41:1192–1199. [PubMed] [Google Scholar]
- PEACOCK D.J., BANQUERIGO M.L., BRAHN E. Angiogenesis inhibition suppresses collagen arthritis. J. Exp. Med. 1992;175:1135–1138. doi: 10.1084/jem.175.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEIFLEY K.A., WINKLES J.A. Angiotensin II and endothelin-1 increase fibroblasts growth factor-2 mRNA expression in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1998;242:202–208. doi: 10.1006/bbrc.1997.7940. [DOI] [PubMed] [Google Scholar]
- PUPILLI C., LASAGNI L., ROMAGNANI P., BELLINI F., MANNELLI M., MISCIGLIA N., MAVILLIA C., VELLEI U., VILLARI D., SERIO M. Angiotensin II stimulates the synthesis and secretion of vascular permeability factor/vascular endothelial growth factor in human mesangial cells. J. Am. Soc. Nephrol. 1999;10:245–255. doi: 10.1681/ASN.V102245. [DOI] [PubMed] [Google Scholar]
- RELF M., LEJEUNE S., SCOTT P.A., FOX S., SMITH K., LEEK R., MOGHADDAM A., WHITEHOUSE R., BICKNELL R., HARRIS A.L. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, transforming growth factor β-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- SASAKI K., MUROHARA T., IKEDA H., SUGAYA T., SHIMADA T., SHINTANI S., IMAIZUMI T. Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J. Clin. Invest. 2002;109:603–611. doi: 10.1172/JCI13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAIPALE J., LOHI J., SAARINE J., KOVANEN P.T., KESKI-OJA J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-β from the extracellular matrix of cultured human epithelial and endothelial cells. J. Biol. Chem. 1995;270:4689–4696. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- TAKAI S., YUDA A., JIN D., NISHIMOTO M., SAKAGUCHI M., SASAKI S., MIYAZAKI M. Inhibition of chymase reduces vascular proliferation in dog grafted veins. FEBS Lett. 2000;467:141–144. doi: 10.1016/s0014-5793(00)01125-x. [DOI] [PubMed] [Google Scholar]
- TAKAI S., JIN D., SAKAGUCHI M., KIRIMURA K., MIYAZAKI M. An orally active chymase inhibitor, BCEAB, suppresses heart chymase activity in the hamster. Jpn. J. Pharmacol. 2001;86:124–126. doi: 10.1254/jjp.86.124. [DOI] [PubMed] [Google Scholar]
- TAMARAT R., SILVESTRE J.S., KUBIS N., BENESSIANO J., DURIEZ M., DEGASPARO M., HENRION D., LEVY B.I. Endothelial nitric oxide synthase lies downstream from angiotensin II-induced angiogenesis in ischemic hindlimb. Hypertension. 2002;39:830–835. doi: 10.1161/hy0302.104671. [DOI] [PubMed] [Google Scholar]
- TILLE J.C., WOOD J., MANDRIOTA S.J., SCHNELL C., FERRARI S., MESTAN J., ZHU Z., WITTE K., PEPPER M.S. Vascular endothelial growth factor (VEGF) receptor-2 antagonists inhibit VEGF- and basic fibroblast growth factor-induced angiogenesis in vivo and in vitro. J. Pharmacol. Exp. Ther. 2001;299:1073. [PubMed] [Google Scholar]
- WALSH D.A., HU D.E., WHARTON J., CATRAVAS J.D., BLAKE D.R., FAN T.P. Sequential development of angiotensin receptors and angiotensin I converting enzyme during angiogenesis in the rat subcutaneous sponge granuloma. Br. J. Pharmacol. 1997;120:1302–1311. doi: 10.1038/sj.bjp.0701062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISE G.N. Retinal neovascularization. Trans. Am. Ophthalmol. Soc. 1956;54:729–826. [PMC free article] [PubMed] [Google Scholar]


