Abstract
Fructose-1,6-diphosphate (FDP), a glycolytic metabolite, is reported to ameliorate inflammation and inhibit the nitric oxide production in murine macrophages stimulated with endotoxin. It is also reported that FDP has cytoprotective effects against hypoxia or ischaemia/reperfusion injury in brain and heart. However, underlying mechanisms of its various biological activities are not completely understood.
In this study, we examined the effects of FDP on UVB-induced prostaglandin production in HaCaT keratinocytes.
Ultraviolet B (UVB, 280–320 nm) irradiation (30 mJ cm−2) increased prostaglandin E2(PGE2) production, which was significantly decreased by FDP in a concentration dependent manner. NS-398, a cyclo-oxygenase-2 (COX-2) selective inhibitor completely inhibited UVB-induced PGE2 production showing that COX-2 activity is responsible for the increase in PGE2 production under our experimental conditions.
UVB irradiation increased total COX activity and COX-2 mRNA in HaCaT keratinocytes, which were significantly blocked by FDP in a concentration dependent manner.
N-acetylcysteine (NAC) significantly attenuated UVB-induced PGE2 production, COX activity and COX-2 mRNA expression indicating oxidative components might contribute to these events.
FDP reduced UVB-induced increase in cellular reactive oxygen species (ROS) level although it did not show direct radical scavenging effect in the experiment using 1,1-diphenyl-2picrylhydrazil (DPPH). FDP preserved the cellular antioxidant capacity including catalase activity and GSH content after irradiation.
Our data obtained hitherto suggest that FDP may have a protective role in UVB-injured keratinocyte by attenuating PGE2 production and COX-2 expression, which are possibly through blocking intracellular ROS accumulation.
Keywords: FDP, HaCaT keratinocytes, UVB irradiation, cyclo-oxygenase-2, prostaglandin E2, reactive oxygen intermediates
Introduction
Ultraviolet (UV) irradiation induces cutaneous inflammation, characterized by erythema and oedema. UVB (280–320 nm) exposure increases the production of a variety of inflammatory mediators such as prostaglandins and leukotrienes (Greaves & Sondergaard, 1970; Hruza & Pentland, 1993). UVB-induced formation of proinflammatory eicosanoids has been suggested to be primarily due to enhanced activity and synthesis of phospholipase A2 (Chen et al., 1996; Gresham et al., 1996; Kang-Rotondo et al., 1993). However, a recent study showed that enhanced cyclo-oxygenase-2 (COX-2) expression may also have an important role(s) in the increased prostaglandin synthesis and inflammatory reaction after UV irradiation (Isoherranen et al., 1999; Soriani et al., 1999). These reports parallel previous studies in which cyclo-oxygenase inhibitors suppressed UV-induced erythema (Farr & Diffey, 1986; Ibbotson et al., 1996; Wilgus et al., 2000). It has been suggested that the upregulation of COX-2 by UV radiation may contribute to photocarcinogenesis in the same way that COX-2 has been shown to contribute to colon cancer (Athar et al., 2001; Pentland et al., 1999). These reports suggest that COX-2 could be an effective target for the regulation of UV-induced skin disorders.
Fructose-1,6-diphosphate (FDP), a glycolytic metabolite, is reported to have cytoprotective effects against ischaemia and postischaemic reperfusion injury of brain and heart, presumably by augmenting anaerobic carbohydrate metabolism (Farias et al., 1990; Sola et al., 1996; Takeuchi et al., 1998). It has been also shown to mitigate the adverse effect of endotoxin by regulating the generation of nitric oxide (Edde et al., 1998). In addition, it has been demonstrated that FDP completely inhibits generation of oxygen free radicals by stimulated neutrophils (Sun et al., 1990). It is well documented that UVB increases oxidative stress in irradiated tissue and oxidant components play an important role in the signalling events leading to gene activation after UV irradiation (Tyrrell, 1996). Although mechanisms by which UVB upregulates COX-2 are not clearly defined, UVB-induced formation of reactive oxygen intermediates has been suggested to be involved (Isoherranen et al., 1999; Soriani et al., 1999). Based on these premises, we examined whether FDP could attenuate UVB-induced generation of reactive oxygen species and COX-2 expression in HaCaT keratinocytes.
Methods
Cell cultures
The experiments were performed with human skin keratinocyte cell line HaCaT which was kindly gifted from Dr Fusenig of the German Cancer Research Center (DKFZ). These keratinocytes were derived from normal skin of a male patient and spontaneously immortalized (Boukamp et al., 1988). They are aneuploid and form normal epidermis when transplanted to nude mice. Cells were grown in DMEM medium (Gibco, Grand Island, NY, USA) containing 10% fetal calf serum, antibiotics (penicillin, 100 U ml−1; streptomycin, 100 μg ml−1) and antimycotic (amphotericin B 0.25 μg ml−1). For experiments, cells were maintained in DMEM supplemented with 1% fetal calf serum (FCS) for 18 h. FDP (1–20 mM), NS-398 (1 μM) and N-acetylcysteine (NAC, 20 mM) were added immediately after UV-irradiation.
In vitro ultraviolet B irradiation
UVB radiation was provided by a bank of Sankyo Denki G15T8E, a fluorescent bulb emitting 270∼320 nm wave with a peak at 313 nm. Minimal erythema dose varies between 30–80 mJ, depending on the skin type and light source. Thus, UVB was delivered with a dose of 30 mJ cm−2 and the irradiance was monitored with an IL1700 radiometer (International Light Inc., Newburyport, MA, U.S.A.). Before UVB exposure the cells were washed twice in phosphate-buffered saline (PBS), and the cells were covered with PBS during UV irradiation. After the UBV exposure, fresh culture medium was added and the cells and media were harvested at the indicated time points for further experiments.
Determination of PGE2 and COX activity
Spent media was removed at the indicated time and the accumulated levels of PGE2 in the media were determined using enzyme immunoassay kit from Cayman chemical (Ann Arbor, MI, U.S.A.). To determine the COX activity, cells were harvested at the indicated time after irradiation, and rinsed with phosphate saline (PBS, 0.01 M, pH 7.4) prior to addition of fresh media containing arachidonic acid (10 μM). After incubation for 10 min at 37°C, media was removed and subjected to enzyme immunoassay for the measurement of PGE2. Release of PGE2 from exogenous arachidonic acid was taken as an index of COX activity (Fu et al., 1990).
Northern blot analysis
Total cellular RNA was isolated from cell cultures using the easy-BLUETM RNA isolation kit (iNtRON, Seoul, Korea) and subjected to Northern analysis. Twenty micrograms of total RNA were fractionated on 1.2% agarose/formaldehyde gels in 1× MOPS and transferred to nylon membranes (HybondTM, Amersham, Arlington Heights, IL, U.S.A.) and hybridized to a 32P-labelled cDNA probe. The membranes were exposed to phosphor imaging plates and the amounts of COX-2 mRNAs were determined by a phosphor imaging plate scanner (Fujifilm, Tokyo, Japan, BS-5000). Human cDNA probe for COX-2 was a kind gift from Dr D.H. Hwang of Pennington Biomedical Research Center, Louisiana State University, U.S.A. 32P-labelled cDNA probe for human COX-2 was prepared by random primed synthesis.
ROS determination
Cells grown on a glass-bottom dish were loaded with 5 μM 2,7′-dichlorofluorescein diacetate (DCFH-DA; Molecular Probes, Eugene, OR, U.S.A.) in PBS. Cultures were incubated for 30 min at 37°C, washed three times with PBS, and the fluorescence signal of DCFH (Ex=490 nm; Em=510 nm), the oxidation product of DCFH-DA by free radicals, was analysed on the stage of a Nikon Diaphot inverted microscope equipped with a 100 W Xenon lamp. To minimize background signal caused by direct oxidation of DCFH-DA by illumination at 490 nm, intracellular levels of ROS were analysed within 3 s after illumination using a Quanticell 700 system (Applied Imaging).
Glutathione(GSH) measurement
Total glutathione (GSH plus GSSG) was determined by photometric determination of 5-thio-2-nitrobenzoate, formed from 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) at a 405 nm, according to Akerboom & Sies (1981). The assay mixture was 18 mM Tris-HCl buffer (pH 7.4) containing 0.47 mg ml−1 BSA, 0.007% Tween 20, 0.7 mM glucose 6-phosphate, 21.5 mM NADP, 1.4 U ml−1 yeast glucose 6-phosphate dehydrogenase, 0.61 mM DTNB, 0.26 U ml−1 glutathione reductase and 25 μl cell lysates.
Determination of catalase and superoxide dismutase (SOD) activities
Catalase activity was measured according to a modified method of Beer et al. (Baudhuin et al., 1964). The assay mixture contained 0.01 M phosphate buffer, pH 7.0 and 0.015 M hydrogen peroxide in a final volume of 1 ml and 50 μg protein of cell lysates. Changes in the optical density at 240 nm were spectrophotometrically monitored. Catalase activity was expressed as units mg−1 protein. One unit of enzyme activity was defined as the amount of the enzyme which decreases 1 μmol of the hydrogen peroxide per min under defined conditions. SOD activity was measured using the xanthine/xanthine oxidase and cytochrome c reduction assay described by Mccord & Fridovich (1969). The assay mixture contained 5 μmol xanthine in 0.001 N NaOH, 2 μmol cytochrome c in 50 mM phosphate buffer, pH 7.8 and 0.1 mM EDTA, 0.2 U xanthine oxidase in 0.1 mM EDTA and 20 μl of cell lysates. SOD activity was expressed as units mg−1 protein. One unit of enzyme activity was defined as the amount of the enzyme which reduces 50% of cytochrome c reduction by superoxide induced by xanthine/xanthine oxidase system under defined conditions.
Statistical analysis
Statistical analysis was performed with Student's t-test. A P value of 0.05 was selected as the limit of statistical significance.
Results
FDP attenuates UVB-induced PGE2 production and COX activity
HaCaT keratinocytes exposed to UVB irradiation dose of 30 mJ cm−2 showed a significant induction of PGE2 production in a time dependent manner up to 24 h (the accumulated levels of PGE2 were 40.72±1.48, 60.86±1.19, 255.4±3.38, 324.4±5.35 and 378.7±16.74 ng mg protein−1 at 0, 3, 6, 12 and 24 h after irradiation, respectively). Thus, levels of PGE2 and COX-2 activity were measured 24 h after irradiation in the following experiments. FDP reduced the accumulated levels of PGE2 in the media in a concentration dependent manner when added to the culture media immediately after irradiation (Figure 1). UVB-induced PGE2 production was also significantly inhibited by NS-398 (1 μM), a COX-2 selective inhibitor, and NAC (20 mM), an antioxidant. NS-398 was dissolved in ethanol. Final concentration of ethanol in media was 0.1%(v v−1). Vehicle alone did not affect UV-induced PGE2 production (20.85±1.28 and 19.91±2.24 ng ml−1 for control and vehicle, respectively). Total cellular COX activity was significantly increased when measured 24 h post irradiation. This increase in UVB-induced COX activity was significantly attenuated by FDP in a concentration dependent manner (Figure 2). NAC also mitigated the induction of total cellular COX activity by UVB irradiation.
Figure 1.
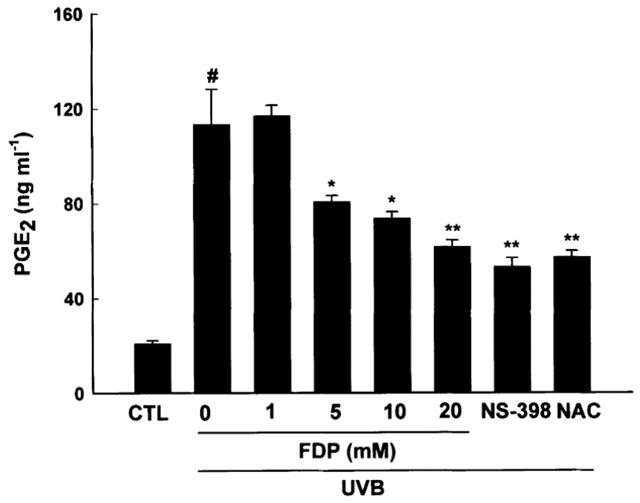
FDP attenuates UVB-induces PGE2 production. HaCaT keratinocytes that had been pretreated with aspirin (100 μM) for 30 min were exposed to UV irradiation (30 mJ cm−2). After the UVB exposure, cells were incubated with FDP, NS-398 (1 μM) or NAC (20 mM) in fresh media containing 10% FCS for 24 h and the accumulated levels of PGE2 in the media were determined. The results are expressed as mean±s.e.mean in three different experiments. UVB-irradiation significantly increased PGE2 production compared with unirradiated control (#P<0.01). A significant difference in PGE2 concentrations relative to UVB control is indicated with *P<0.05 or **P<0.01.
Figure 2.
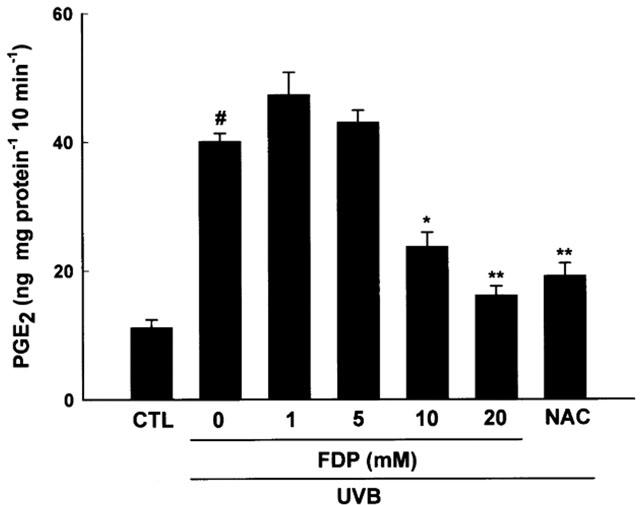
FDP attenuates UVB-induced COX activity. HaCaT keratinocytes that had been pretreated with aspirin (100 μM for 30 min were exposed to UV irradiation (30 mJ cm−2). After the UVB exposure, cells were incubated with FDP or NAC (20 mM) in fresh culture media containing 10% FCS for 24 h. COX activity was determined as described in Methods. The results are expressed as mean±s.e.mean in three different experiments. UVB-irradiation significantly increased COX activity compared with unirradiated control (#P<0.01). A significant difference in COX activity relative to UVB control is indicated with *P<0.05 or **P<0.01.
FDP attenuates UVB-induced COX-2 expression
A single UVB exposure (30 mJ cm−2) induced COX-2 gene expression in HaCaT cells. The maximal induction of COX-2 mRNA was observed between 3 and 6 h post irradiation (data not shown). UVB irradiation stimulated the expression of COX-2 mRNA to 3.5 fold of the control level in 3 h. This result is consistent with a previous report (Isoherranen et al., 1999). The increase in COX-2 expression stimulated by UVB was markedly reduced by FDP to 1.7 (20 mM) and 2.1 fold (10 mM), respectively (Figure 3). The antioxidant NAC (20 mM) suppressed UVB-induced COX-2 mRNA expression significantly (1.6 fold of control level).
Figure 3.
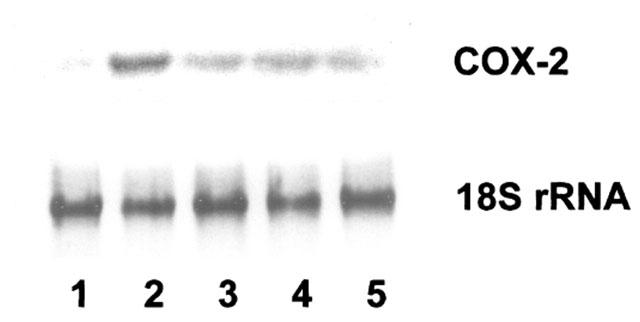
FDP attenuates UVB-induced COX-2 gene expression. HaCaT keratinocytes that had been pretreated with aspirin (100 μM) for 30 min were exposed to UV irradiation (30 mJ cm−2). After the UVB exposure, cells were incubated with FDP or NAC (20 mM) in fresh culture media containing 10% FCS. Total RNA was extracted 3 h later, and 20 μg of total RNA was hybridized with COX-2 cDNA probe. Lane 1: unirradiated control; Lane 2: UVB control; Lane 3: UVB+FDP (20 mM); Lane 4: UVB+FDP (10 mM); Lane 5: UVB+NAC (20 mM).
FDP attenuates accumulation of ROS and preserves cellular total glutathione level and catalase activity after UVB irradiation
In order to explore the relevant cellular events that may be involved in the regulation of COX-2 by FDP, intracellular levels of reactive oxygen species (ROS) were analysed using DCFH-DA, a redox sensitive dye. The time course for the levels of ROS in HaCaT keratinocytes afer UVB irradiation showed that the increase of cellular ROS level was observed from 1 h post irradiation and maximum level was reached in 3 h (Figure 4a). UVB-induced ROS accumulation was attenuated to control level by FDP (10 mM) at all time points of measurement (Figure 4a). The increase in [ROS]i was significantly reduced in the presence of FDP in a concentration dependent manner measured at 3 h after irradiation (Figure 4b). FDP did not show direct radical scavenging effect in the experiment using 1,1-diphenyl-2-picrylhydrazil (DPPH) (data not shown). On the other hand, FDP preserved the cellular antioxidant capacity such as catalase and glutathione which were significantly reduced after UVB irradiation (Figure 5). UVB-irradiation reduced catalase activity to 86±4% of control, which was reversed by FDP. Cellular glutathione content was also reduced to 83±7% of control after irradiation, and FDP restored it to 101±6%. The baseline levels of catalase and glutathione were significantly increased in HaCaT cells treated with FDP (125±4%, P<0.05 vs control; 138±11%, P<0.05 vs control, respectively). FDP failed to alter the cellular levels of superoxide dismutase (SOD) whether or not cells were irradiated.
Figure 4.
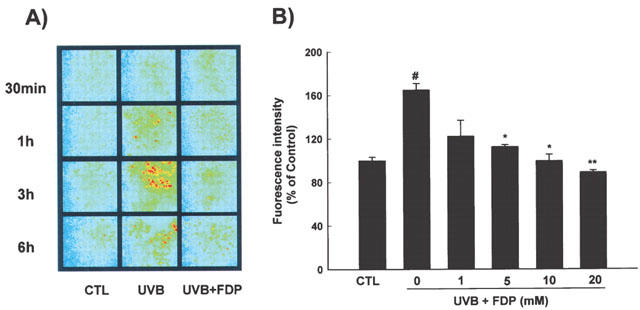
FDP attenuates the intracellular levels of UVB-induced reactive oxygen species (ROS). (A) Maximum level of cellular ROS was reached in 3 h after UVB-irradiation. UVB-induced ROS accumulation was reduced to control level by FDP (10 mM). (B) FDP attenuates the cellular accumulation of ROS measured at 3 h after irradiation. The results are expressed as mean±s.e.mean in four different experiments. UVB-irradiation significantly increased ROS production compared with unirradiated control (#P<0.01). A significant difference relative to UVB control is indicated with *P<0.05 or **P<0.01.
Figure 5.
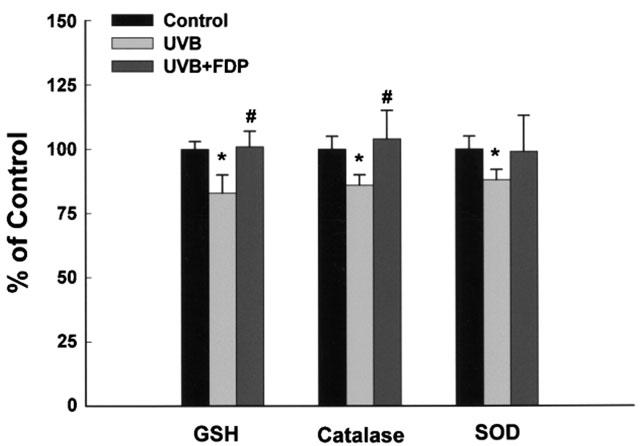
FDP preserves the cellular antioxidant capacity. HaCaT keratinocytes that had been exposed to UV irradiation (30 mJ cm−2) were incubated with F1,6DP (10 mM) in fresh culture media containing 10% FCS for 24 h. The results are expressed as mean±s.e.mean in four different experiments. UVB-irradiation significantly decreased the total glutathione level and activities of catalase and SOD compared with unirradiated control (*P<0.05). A significant difference relative to UVB control is indicated with #P<0.05.
Discussion
The UV-induced erythema leading to vasodilation and oedema correlates with the activation of inflammatory mechanisms including prostaglandin pathways. Previous studies have suggested that the enhanced COX-2 expression may be an important component in UVB-induced prostaglandin production and inflammatory reaction (Isoherranen et al., 1999; Wilgus et al., 2000). In the present study, we also observed that a physiological dose of UVB irradiation caused a significant induction of COX-2 expression in HaCaT keratinocytes. A single UVB exposure significantly enhanced COX activity and, thereby, the increase of prostaglandin synthesis. Together with these results, the significant inhibition of prostaglandin production by NS-398, a COX-2 selective inhibitor, further supports the role of COX-2 in UVB-induced prostaglandin synthesis.
The present study showed that FDP inhibited the induction of total cellular COX activity and abolished COX-2 expression in HaCaT keratinocytes exposed to UVB. FDP has been reported to ameliorate inflammation. Hepatitis-like injury in rats was prevented by repeated dosings of FDP (350 mg kg−1, i.p. ×3) at 24 h after galactosamine injection (Markov et al., 1991). FDP (0.5–2 g kg−1, i.p. and p.o.) suppressed the paw oedema of rats at 1–5 h after local carageenan injection (Planas et al., 1993). FDP (100 mg kg−1, i.p.) enhanced the suppression of ischaemic and histamine oedema by dexamethasone in oxyradicals and NO-dependent manner (Oyanagui, 1998). These effects of FDP have been explained by the maintenance of ATP level to stabilize cell membrane or to support ATP-dependent glucocorticoid receptor recycling or by the action to inhibit the generation of oxygen free radicals and attachment to the blood vessels of neutrophils. However, its anti-inflammatory effects in connection with COX-2 expression and prostaglandin synthesis have not been studied. Thus, our results add another possible mechanism to the anti-inflammatory action of FDP.
Increased COX-2 expression is also known to be linked to tumorigenesis. COX-2 expression is upregulated in colorectal and gastric carcinomas (Rao et al., 1995; Dubois et al., 1998) and overexpression of COX-2 produces a carcinogenic phenotype in rat intestinal epithelial cells (Thujii & Dubois, 1995). Furthermore, knockout mutation of COX-2 gene reduces the risk for intestinal polyposis in mice (Oshima et al., 1996). Recently it was also shown that the number of UVB-induced murine skin tumors is reduced by the selective COX-2 inhibitor, celecoxib (Pentland et al., 1999). Therefore, continually increased COX-2 expression after UV exposure may have an important role in UV-induced carcinogenesis. In this regard, it would be worth examining whether FDP could reverseUVB-induced skin tumorigenesis.
Recent studies have suggested that reactive oxygen intermediates contribute to the signalling events leading to gene expression after UV irradiation (Garmyn & Degreef, 1997; Tyrrell, 1996). In our study, it was observed that NAC reduced the prostaglandin production and the induction of the total cellular COX activity by UVB irradiation. NAC also mitigated the induction of UVB-induced COX-2 mRNA in HaCaT keratinocytes after UV-exposure. These results coincide with the previous reports that oxidant components play an important role in COX-2 induction after UV irradiation (Isoherranen et al., 1999; Soriani et al., 1999). In this context, intracellular ROS levels were analysed to explore the relevant cellular events that may be involved in the regulation of COX-2 by FDP. It was reported that FDP completely inhibited oxyradical generation by stimulated canine and human neutrophils although the precise mechanism was not known (Sun et al., 1990). In this study it was observed that FDP significantly reduced the generation of ROS after UVB irradiation and this was not due to direct radical scavenging activity. It has been suggested that FDP could be a substrate for or stimulator of anaerobic ATP production (Bickler & Buck, 1996). It was also shown that FDP might upregulate the pentose phosphate pathway (PPP), possibly by inhibiting phosphofructokinase (Kelleher et al., 1995; 1996; Espanol et al., 1999). It is well documented that the pentose phosphate pathway plays an important role in the cellular redox regulation, by providing NADPH which is the principal intracellular reductant and a critical modulator of the redox potential in all cell types (Ben-Yoseph et al., 1996). NADPH is used for the regeneration of glutathione and also required for the formation of active catalase tetramers (Salvemini et al., 1999). Our data showed that FDP preserved cellular catalase activity and glutathione levels (Figure 5) and increased the activity of glucose-6-phosphate dehydrogenase, the rate limiting enzyme of PPP, in keratinocytes exposed to UVB (data not shown). Thus, it is highly plausible that FDP reduces ROS generation via activation of PPP.
FDP is reported to have cytoprotective effects against ischaemia and postischaemic reperfusion injury in various tissues, presumably by augmenting anaerobic carbohydrate metabolism (Farias et al., 1990; Sola et al., 1996; Takeuchi et al., 1998). In this study, it was also observed that FDP could block the LDH release from HaCaT keratinocytes exposed to UVB (data not shown). Because keratinocytes are major epidermal cells injured directly by UV light, it would be of interest to test the potential application of FDP as a protective agent against UVB-induced skin damage. Together with its anti-inflammatory effect, this possibility is under investigation in vivo.
There is no information on endogenous level of FDP in skin. It is reported that the calculated total FDP concentrations in aerobic and ischaemic hearts are 90 and 250 μM, respectively (Hardin et al., 2001). Thus, on the assumption that skin and heart contain the same levels of FDP, the levels being used exogenously in this study is more than 10 times of endogenous level. Because FDP is a highly charged molecule at physiological pH, it is generally not expected to readily cross the cell membrane. However, it was reported that metabolism of exogenous fructose was undetectable or if any, only about 10% of that found with FDP in vascular smooth muscle and heart, providing evidence that FDP enters cells rather than being converted to fructose (Hardin & Roberts, 1995; Tavazzi et al., 1992). It has been demonstrated that FDP is capable of crossing artificial lipid bilayers in a concentration dependent manner (Ehringer et al., 2000). Furthermore, a recent report showed FDP could be transported into the cell via a dicarboxylate transport system (Hardin et al., 2001). These results strongly suggest that FDP has an intracellular site of action. Whether or not this speculation is true, our data add the possible usefulness for UVB-induced skin disorder to the list of the beneficial effects of FDP such as the prevention of endotoxic shock (Markov et al., 1981) and ischaemic injury in a wide variety of tissues including brain (Sola et al., 1996), heart (Takeuchi et al., 1998, kidney (Didlake et al., 1989) and intestine (Sun et al., 1990) and amelioration of traumatic (Markov et al., 1983) and inflammatory disease (Planas et al., 1993; Oyanagui, 1998).
In summary, our data hitherto obtained show that FDP could attenuate UVB-induced COX-2 expression, thereby reducing the prostaglandin production in HaCaT keratinocytes and this could be ascribed, at least in part, to the diminution of intracellular oxidative stress although the nature of its effects on generation of reactive oxygen species remains to be elaborated.
Acknowledgments
This work was supported by Korea Research Foundation Grant (KRF-99-800-20010387).
Abbreviations
- COX
cyclo-oxygenase
- DCFH-DA
2,7′-dichlorofluorescein diacetate
- DMEM
Dulbecco's minimum essential medium
- DPPH
1,1-diphenyl-2-picrylhydrazil
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- EDTA
ethylenediamine tetraacetate
- FCS
fetal calf serum
- FDP
fructose-1,6-diphosphate
- GSH
glutathione
- NAC
N-acetylcysteine
- NADP
nicotineamide disphosphate
- PBS
phosphate-buffered saline
- PGE2
prostaglandin E2
- PPP
pentose phosphate pathway
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- UVB
ultraviolet B
References
- AKERBOOM T.P.M., SIES H.Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples Methods in Enzymology 77: Detoxication and Drug Metabolism: Conjugation and Related systems 1981New York: Academic Press; 373–382.ed. Jakoby W.B. [DOI] [PubMed] [Google Scholar]
- ATHAR M., AN K.P., MOREL K.D., KIM A.L., ASZTERBAUM M., LONGLEY J., EPSTEIN E.H., JR., BICKERS D.R. Ultraviolet B (UVB)-induced COX-2 expression in murine skin: an immunohistochemical study. Biochem. Biophys. Res. Commun. 2001;280:1042–1047. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- BAUDHUIN P., BEAUFAY H., RAHMAN-LI Y., SELLINGER O.Z., WATTIAUX R., JACQUES P., DE DUVE C. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat liver tissue. Biochem. J. 1964;92:179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEN-YOSEPH O., BOXER P., ROSS B. Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. J. Neurochem. 1996;66:2329–2337. doi: 10.1046/j.1471-4159.1996.66062329.x. [DOI] [PubMed] [Google Scholar]
- BICKLER P.E., BUCK L.T. Effects of fructose-1,6-bisphosphate on glutamate release and ATP loss from rat brain slices during hypoxia. J. Neurochem. 1996;67:1463–1468. doi: 10.1046/j.1471-4159.1996.67041463.x. [DOI] [PubMed] [Google Scholar]
- BOUKAMP P., PETRUSSEVSKA R.T., BREITKREUTZ D., HORNUNG J., MARKHAM A., FUSENIG N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN X., GRESHAM A., MORRISON A., PENTLAND A.P. Oxidative stress mediates synthesis of cytosolic phospholipase A2 after UVB injury. Biochim. Biophys. Acta. 1996;1299:23–33. doi: 10.1016/0005-2760(95)00166-2. [DOI] [PubMed] [Google Scholar]
- DIDLAKE R.H., KIRCHNER K.A., LEWIN J., BOWER J.D., MARKOV A.K. Attenuation of ischemic renal injury with fructose-1,6-diphosphate. J. Surg. Res. 1989;47:220–226. doi: 10.1016/0022-4804(89)90111-x. [DOI] [PubMed] [Google Scholar]
- DUBOIS R.N., ABRAMSON S.B., CROFFORD L., GUPTA R.A., SIMON L.S., VAN DE PUTTE L.B., LIPSKY P.E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- EDDE L., ZHOU Z., EATON J.W., SHERMAN M.P. Induction of nitric oxide synthase in macrophages: inhibition by fructose-1,6-diphosphate. Biochem. Biophys. Res. Commun. 1998;243:683–687. doi: 10.1006/bbrc.1998.8163. [DOI] [PubMed] [Google Scholar]
- EHRINGER W.D., NIU W., CHIANG B., WANG O.L., GORDON L., CHIEN S. Membrane permeability of fructose-1,6-diphosphate in lipid vesicles and endothelial cells. Mol. Cell. Biochem. 2000;210:35–45. doi: 10.1023/a:1007059214754. [DOI] [PubMed] [Google Scholar]
- ESPANOL M.T., LITT L., HASEGAWA K., CHANG L., MACDONALD J.M., GREGORY G., JAMES T.L., CHAN P.H. Fructose-1,6-bisphosphate preserves adenosine triphosphate but not intracellular pH during hypoxia in respiring neonatal rat brain slices. Anesthesiology. 1999;88:461–472. doi: 10.1097/00000542-199802000-00025. [DOI] [PubMed] [Google Scholar]
- FARIAS L.A., SMITH E.E., MARKOV A.K. Prevention of ischemic-hypoxic brain injury and death in rabbits with fructose-1,6-diphosphate. Stroke. 1990;21:606–613. doi: 10.1161/01.str.21.4.606. [DOI] [PubMed] [Google Scholar]
- FARR P.M., DIFFEY B.L. A quantitative study of the effect of topical indomethacin on cutaneous erythema induced by UVB and UVC radiation. Br. J. Dermatol. 1986;115:453–466. doi: 10.1111/j.1365-2133.1986.tb06240.x. [DOI] [PubMed] [Google Scholar]
- FU J.Y., MASFERRER J.L., SEIBERT K., RAZ A., NEEDLEMAN P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J. Biol. Chem. 1990;265:16737–16740. [PubMed] [Google Scholar]
- GARMYN M., DEGREEF H. Suppression of UVB-induced c-fos and c-jun expression in human keratinocytes by N-acetylcysteine. J. Photochem. Photobiol. B. 1997;37:125–130. doi: 10.1016/s1011-1344(96)07340-x. [DOI] [PubMed] [Google Scholar]
- GREAVES M.W., SONDERGAARD J. Pharmacological agents released in ultraviolet inflammation studied by continuous skin perfusion. J. Invest. Dermatol. 1970;54:365–367. doi: 10.1111/1523-1747.ep12259058. [DOI] [PubMed] [Google Scholar]
- GRESHAM A., MASFERRER J., CHEN X., LEAL-KHOURI S., PENTLAND A.P. Increased synthesis of high molecular weight cPLA2 mediates early UV-induced PGE2 in human skin. Am. J. Physiol. 1996;270:C1037–C1050. doi: 10.1152/ajpcell.1996.270.4.C1037. [DOI] [PubMed] [Google Scholar]
- HARDIN C.D., LAZZARINO G., TAAZZI B., DI PIERRO D., ROBERTS T.M., GIARDINA B., ROVETTO M.J. Myocardial metabolism of exogenous FDP is consistent with transport by a dicarboxylate transporter. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2654–H2660. doi: 10.1152/ajpheart.2001.281.6.H2654. [DOI] [PubMed] [Google Scholar]
- HARDIN C.D., ROBERTS T.M. Compartmentation of glucose and fructose 1,6-bisphosphate metabolism in vascular smooth muscle. Biochemistry. 1995;34:1323–1331. doi: 10.1021/bi00004a027. [DOI] [PubMed] [Google Scholar]
- HRUZA L., PENTLAND A.P. Mechanisms of UV-induced inflammation. J. Invest. Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- IBBOTSON S.H., DIFFEY B.L., FARR P.M. The effect of topical indomethacin on ultraviolet-radiation-induced erythema. Br. J. Dermatol. 1996;135:523–527. [PubMed] [Google Scholar]
- ISOHERRANEN K., PUNNONEN K., JANSEN C., UOTILA P. Ultraviolet irradiation induces cyclooxygenase-2 expression in keratinocytes. Br. J. Dermatol. 1999;140:1017–1022. doi: 10.1046/j.1365-2133.1999.02897.x. [DOI] [PubMed] [Google Scholar]
- KANG-ROTONDO C.H., MILLER C.C., MORRISON A.R., PENTLAND A.P. Enhanced keratinocyte prostaglandin synthesis after UV injury is due to increased phospholipase activity. Am. J. Physiol. 1993;264:C396–C401. doi: 10.1152/ajpcell.1993.264.2.C396. [DOI] [PubMed] [Google Scholar]
- KELLEHER J.A., CHAN P.H., CHAN T.Y.Y., GREGORY G.A. Energy metabolism in hypoxic astrocytes: protective mechanism of fructose-1,6-bisphosphate. Neurochem. Res. 1995;20:785–792. doi: 10.1007/BF00969690. [DOI] [PubMed] [Google Scholar]
- KELLEHER J.A., CHAN T.Y.Y., CHAN P.H., GREGORY G.A. Protection of astrocytes by fructose 1,6-bisphosphate and citrate ameliorates neuronal injury under hypoxic conditions. Brain Res. 1996;726:167–173. [PubMed] [Google Scholar]
- MARKOV A.K., FARIAS L.A., BENNETT W.S., SUBRAMONY C., MIHAS A.A. Prevention of galactosamine-induced hepatotoxicity in rats with fructose-1,6-diphosphate. Pharmacology. 1991;43:310–317. doi: 10.1159/000138861. [DOI] [PubMed] [Google Scholar]
- MARKOV A.K., OGLETHORPE N.C., GRILLIS M., NEELY W.A., HELLEMS H.K. Therapeutic action of fructose-1,6-diphosphate in traumatic shock. World J. Surg. 1983;7:430–436. doi: 10.1007/BF01658096. [DOI] [PubMed] [Google Scholar]
- MARKOV A.K., TURNER M.D., OGLETHORPE N.C., NEELY W.A., HELLEMS H.K. Fructose-1,6-diphosphate: an agent for treatment of experimental endotoxin shock. Surgery. 1981;90:482–488. [PubMed] [Google Scholar]
- MCCORD J., FRIDOVICH I. Superoxide dismutase; An enzymatic function for erythrocuprein. J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- OSHIMA M., DINCHUK J.E., KARGMAN S.L., OSHIMA H., HANCOCK B., KWONG E., TRZASKOS J.M., EVANS J.F., TAKETO M.M. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- OYANAGUI Y. Fructose-1,6-diphosphate enhanced oxyradicals and nitric oxide-dependent suppressions by dexamethasone of ischemic and histamine paw edema of mice. Life Sci. 1998;62:241–249. doi: 10.1016/s0024-3205(98)00073-3. [DOI] [PubMed] [Google Scholar]
- PENTLAND A.P., SCHOGGINS J.W., SCOTT G.A., KAHN K.N.M., HAN R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- PLANAS M.E., SANCHEZ S., GONGALEZ P., RODRIGUES DE OLIVEIRA J., BARTRONS R. Protective effect of fructose 1,6-bisphosphate against carrageenan-induced inflammation. Eur. J. Pharmacol. 1993;237:251–255. doi: 10.1016/0014-2999(93)90276-n. [DOI] [PubMed] [Google Scholar]
- RAO C.V., RIVENSON A., SIMI B., ZANG E., KELLOFF G., STEELE V., REDDY B.S. Chemoprevention of colon carcinogenesis by sulindac, a non-steroidal anti-inflammatory agent. Cancer Res. 1995;55:1464–1472. [PubMed] [Google Scholar]
- SALVEMINI F., FRANZE A., IERVOLINO A., FILOSA S., SALZANO S., URSINI M.V. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J. Biol. Chem. 1999;274:2750–2757. doi: 10.1074/jbc.274.5.2750. [DOI] [PubMed] [Google Scholar]
- SOLA A., BERRIOS M., SHELDON R.A., FERREIRO D.M., GREGORY G.A. Fructose 1,6-bisphosphate after hypoxic ischemic injury is protective to the neonatal rat brain. Brain Res. 1996;742:294–299. doi: 10.1016/s0006-8993(96)00984-5. [DOI] [PubMed] [Google Scholar]
- SORIANI M., LUSCHER P., TYRRELL R.M. Direct and indirect modulation of ornithine decarboxylase and cyclooxygenase by UVB in human skin cells. Carcinogenesis. 1999;20:727–732. doi: 10.1093/carcin/20.4.727. [DOI] [PubMed] [Google Scholar]
- SUN J., FARIAS L.A., MARKOV A.K. Fructose 1,6 diphosphate prevents intestinal ischemic reperfusion injury and death in rats. Gastroenterology. 1990;98:117–126. doi: 10.1016/0016-5085(90)91299-l. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI K., CAO-DANH H., FRIEHS I., GLYNN P., D'AGOSTINO D., SIMPLACEANU E., MCGOWAN F.X., DEL NIDO P.J. Administration of fructose-1,-6-diphosphate during early reperfusion significantly improves recovery of contractile function in the postischemic heart. J. Thorac. Cardiovasc. Surc. 1998;116:335–343. doi: 10.1016/s0022-5223(98)70135-7. [DOI] [PubMed] [Google Scholar]
- TAVAZZI B., STARNES J.W., LAZZARINO G., DI PIERRO D., NUUTINEN E.M., GIARDINA B. Exogenous fructose-1,6-bisphosphate is a metabolizable substrate for the isolated normoxic rat heart. Basic Res. Cardiol. 1992;87:280–289. doi: 10.1007/BF00804337. [DOI] [PubMed] [Google Scholar]
- THUJII M., DUBOIS R.N. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- TYRRELL R.M. Activation of mammalian gene expression by the UV component of sunlight-from models to reality. Bioessays. 1996;18:139–148. doi: 10.1002/bies.950180210. [DOI] [PubMed] [Google Scholar]
- WILGUS T.A., ROSS M.S., PARRETT M.L., OBERYSZYN T.M. Topical application of a selective cyclooxygenase inhibitor suppresses UVB mediated cutaneous inflammation. Prostaglandins Other Lipid Mediat. 2000;62:367–384. doi: 10.1016/s0090-6980(00)00089-7. [DOI] [PubMed] [Google Scholar]


