Abstract
The pharmacological characteristics of muscarinic receptors in male and female mouse urinary bladder smooth muscle from different strains (C57Bl/6, 129/SvJ and hybrid backcross N1F2) were studied.
(+)-Cis-dioxolane, oxotremorine-M, acetylcholine, carbachol and pilocarpine induced concentration-dependent contractions of the urinary bladder smooth muscle (range for pEC50=6.4–6.6, 6.2–6.7, 6.2–6.4, 5.4–6.0 and 0.0–5.1, Tmax=1.9–4.7 g, 1.3–3.4 g, 1.0–3.0 g, 1.4–2.4 and 0.0–0.3 g, respectively, n=4–6 depending on the gender and the strain). In females, these contractions were competitively antagonized by a range of muscarinic receptor antagonists (pKB value range, depending on the strain): atropine (8.0–8.9), pirenzepine (6.1–6.4), 4-DAMP (7.6–8.4), methoctramine (5.6–6.1), p-F-HHSiD (7.5–7.7), zamifenacin (7.7–8.4) and darifenacin (8.2–8.7).
In recontraction studies, in which the muscarinic M3 receptor population was decreased, and conditions optimized to study M2 receptor activation, methoctramine exhibited an affinity estimate consistent with muscarinic M3 receptors (pKB=6.26±0.08, pA2=6.31±0.07; pKB=6.09±0.22, pA2=6.08±0.01 for female inbred strain 129/SvJ and hybrid backcross N1F2, respectively) or intermediate between the one expected for this compound at M2 and M3 receptors, (pKB=6.66±0.08, pA2=7.00±0.27 for female inbred strain C57BL/6).
These data study suggest that muscarinic M3 receptors are the predominant, if not the exclusive, subtype mediating contractile responses to muscarinic agonists in female mouse urinary bladder smooth muscle, with strain differences.
Keywords: Muscarinic receptors, M3-receptor, urinary bladder smooth muscle, gender, mouse strain
Introduction
Muscarinic receptors consist of five subtypes, M1, M2, M3, M4 and M5 defined pharmacologically and encoded by five different genes (Eglen et al., 1996; Caufield & Birdsall, 1998; see Eglen & Nahorski, 2000 for review). Muscarinic receptors are distributed widely and play a key physiological role in peripheral organs, including the urinary bladder. In most smooth muscle preparations (Eglen et al., 1996), the muscarinic M3 receptor subtype subtype, which forms only 20–30% of the receptor population in the bladder for example, has been demonstrated to play dominant roles in eliciting contraction in vitro (despite the fact that the M2 receptor subtype accounts for 70–80% of the receptor population). Therefore, it was proposed that muscarinic M3 receptor activation primarily causes direct contraction of the smooth muscle and the muscarinic M2 receptor contracts the tissue indirectly, by reversing sympathetically mediated relaxation (Thomas et al., 1993; Thomas & Ehlert, 1994; Hegde et al., 1997).
Muscarinic receptors mediating contraction of rat (Longhurst et al., 1995; Hegde et al., 1997), rabbit (Tobin & Sjögren, 1995; Choppin et al., 1998), guinea-pig (Noronha-Blob et al., 1989) and human (Newgreen & Naylor, 1996) detrusor muscle have been pharmacologically characterized. Recently, the availability of mutant mouse strains that lack functional receptors has provided the means to examine the physiological role of muscarinic receptors in native tissue. Nevertheless, only few investigations of the muscarinic receptors mediating contractions of wild-type mouse bladder have been undertaken (Durant et al., 1991; Paravicini et al., 2000; Welsh et al., 2000; Choppin & Eglen, 2001; Eglen & Choppin, 2001), but suggest a major role of the muscarinic M3 receptor in the contractile response, with the role of the M2 receptor, if any, being unresolved.
Since the gender and the genetic background of transgenic mice is rarely the same across studies, the objective of the present study was to examine, using a range of defining antagonists, the pharmacological characteristics of muscarinic receptors present in mouse urinary smooth muscle from both gender and from different strains, in isolated tissue studies.
Methods
In vitro contractile studies
Mice (both gender, inbred strains C57B1/6, 129/SvJ or hybrid N1F2; 25–30 g; 6–8-weeks-old; Jackson laboratories) were euthanized by CO2 asphyxiation. The urinary bladder was isolated, cleared of adhering adipose tissue and placed in oxygenated Krebs solution (composition in mM: NaCl 118.2, KCl 4.6, CaCl2 2.5, MgSO4. 7H2O 1.2, KH2PO4 1.2, NaHCO3 24.8 and dextrose 10.0). The physiological solution contained indomethacin (10 μM) in order to reduce prostaglandin-induced spontaneous activity of the tissues. Four strips of urinary bladder smooth muscle were cut from the supratrigonal portion of the bladder (longitudinal section). The tissues were mounted in 10 ml organ baths (Radnoti Glass Technology Inc, Monrovia, CA, U.S.A.) containing Krebs solution, maintained at 37°C and constantly aerated with 95% O2 /5% CO2 (pH=7.4). Grass FT03 transducers were used to measure changes in isometric tension of the tissues, which were displayed on a Grass 7E polygraph. The tissues were maintained at a resting tension of 1 g during an equilibration period of 60 min. Tension adjustments were made as necessary. The tissues were washed every 15 min.
The viability of each tissue was assessed by determining the contractile response to KCl (30 mM) at the start of the experimental protocol. After washing, tissues were re-equilibrated for 10 min and allowed to regain baseline tension. Cumulative concentration–effect curves to agonists ((+)-cis-dioxolane, oxotremorine-M, acetylcholine, carbachol and pilocarpine; 1 nM–0.1 mM) were then constructed in each tissue. Thereafter, tissues were equilibrated in either the absence (time control) or presence of antagonist for a 90 min period during which tissues were washed every 10 min. Subsequently, a second concentration–effect curve to the same agonist was constructed.
Recontraction experiments
After an initial concentration–response curve to (+)-cis-dioxolane was established, the tissues were washed and equilibrated with 4-DAMP mustard (40 nM) for 60 min in the presence of methoctramine (0.3 μM). This procedure enabled selective alkylation of M3 but not M2 receptors (Eglen et al., 1994; Ehlert, 1996; Hegde et al., 1997; Braverman & Ruggieri, 1999; Choppin & Eglen, 2001). 4-DAMP mustard was then removed from the tissues by overflow with Krebs solution containing methoctramine (0.3 μM) every 10 min for 60 min and subsequently with methoctramine-free Krebs solution every 10 min for 90 min. The tissues were then contracted with 90 mM of KCl and subsequently relaxed with isoproterenol (30 μM). Once the tissues had relaxed to baseline, a cumulative concentration–effect curve to (+)-cis-dioxolane (1 nM–0.1 mM) was constructed.
Effects of an M2 antagonist (methoctramine) on the recontractile responses to (+)-cis-dioxolane
After constructing two concentration–effect curves to (+)-cis-dioxolane under conditions described above, a third cumulative concentration effect curve to (+)-cis-dioxolane (1 nM–0.3 mM) was constructed after equilibration of tissue in absence (time control) or presence of methoctramine (0.1–1.0 μM) for 90 min.
Data analysis
Contractions were recorded as changes in tension from baseline and expressed as a percentage of the maximum response of the first agonist concentration–effect curve. Agonist concentration–response curves were fitted using a nonlinear iterative fitting program (Origin, Microcal Software, Inc., Northampton, MA, U.S.A.) using the relationship of Parker & Waud (1971). Agonist potencies and maximum response are expressed as pEC50 (− logarithm of the molar concentration of agonist producing 50% of the maximum response) and Tmax, respectively. Concentration ratios (CRs) were determined from EC50 values in the presence and absence of antagonist. Antagonist affinity estimates (pKB values) were determined with the equation described by Furchgott (1972); pKB=−log ([antagonist]/CR-1)) or using the method of Arunlakshana & Schild (1959) using at least three concentrations of the antagonist (pA2 values). In cases where the slope of the linear regression was not significantly different from unity, the slope was constrained to unity and the data expressed as the pKB value. All data are expressed as mean±s.e.mean. Pearson correlation coefficients (r) and associated P-values were calculated using the method described by Dixon & Massey (1983). The sum of squares of differences in affinity estimates for each plot (Σ (y−x)2, noted ssq) defines the proximity of the data points to the line of identity (y=x).
Compounds used
Atropine sulphate, indomethacin and isoproterenol were obtained from Sigma Chemical Co (MO, U.S.A.). (+)-Cis-dioxolane, acetylcholine, carbachol, oxotremorine-M, pilocarpine, pirenzepine dihydrochloride, methoctramine hydrochloride, 4-diphenylacetoxy-N-methylpiperidine (4-DAMP) methiodide, 4-DAMP mustard and para fluoro hexahydrosiladifenidol (p-F-HHSiD) hydrochloride were obtained from Research Biochemicals Inc. (MA, U.S.A.). Darifenacin hydrobromide and zamifenacin fumerate were generously provided by Pfizer Central Research (Sandwich, Kent, U.K.).
All compounds were diluted in distilled water except indomethacin, which was diluted in polyethylene glycol.
Results
Characterization of muscarinic receptors mediating contractions of the mouse isolated urinary bladder smooth muscle
(+)-Cis-dioxolane, oxotremorine-M, acetylcholine, carbachol and pilocarpine induced concentration-dependent contractions of the mice urinary bladder smooth muscle (pEC50 and Tmax are summarized in Table 1, n=4–6). Two consecutive concentration–effect curves to these agonists could be constructed in the same tissue with no significant temporal change in the agonist potency and maximum response (time–control; Figure 1).
Table 1.
Potency and efficacy estimates for muscarinic agonists in mouse urinary bladder smooth muscle

Figure 1.

Effects of muscarinic agonists on female C57B1/6 mouse urinary bladder smooth muscle. Contractile effects were expressed as percentages of the maximum response of the control curve. The values shown are mean±s.e.mean, n=4 animals.
The muscarinic receptor(s) mediating direct contractions was identified by pharmacological determination of antagonist affinities. Atropine, pirenzepine, 4-DAMP, methoctramine, p-F-HHSiD, zamifenacin and darifenacin were tested for their ability to inhibit (+)-cis-dioxolane-induced responses. All these compounds surmountably antagonized cumulative agonist concentration–response curves, in a concentration-dependent fashion, with parallel rightward displacements. Their functional affinity estimates (pKB) are summarized in Table 2.
Table 2.
Affinity estimates (pKB) for muscarinic antagonists in mouse urinary bladder smooth muscle

Comparison of functional data for female mouse urinary bladder smooth muscle with binding data at human recombinant muscarinic receptors
The analysis between the affinities of the antagonists at muscarinic receptors in the female mouse urinary bladder smooth muscle and the affinities at human recombinant muscarinic receptors showed a significant correlation at m3 (r=0.93, P=0.002, ssq=7.95; r=0.76, p=0.05, ssq=6.10; r=0.92, P=0.004, ssq=6.30; for female 129/SvJ, C57BL/6 and N1F2, respectively). In contrast, poor correlations were observed at m1, m2, m4 and m5 (Figure 2).
Figure 2.
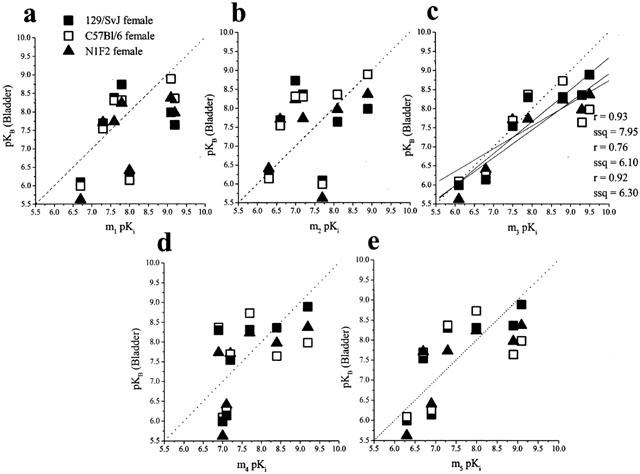
Correlation between the functional affinities (pKB values) of muscarinic antagonists at muscarinic receptor in female mouse isolated urinary bladder smooth muscle and binding affinities (pKi values) at human recombinant muscarinic receptors (m1–m5; a–e respectively). The binding data were taken from Dörje et al., 1991; Eglen et al., 1997; Hegde et al., 1997; Nilvebrant et al., 1996. The broken line is the line of identity (x=y) while the solid line is the correlation plot (the inserts give the correlation factors (r) and the sum of squares values (ssq)).
Characterization of muscarinic receptors mediating the recontractions in female mouse urinary bladder smooth muscle
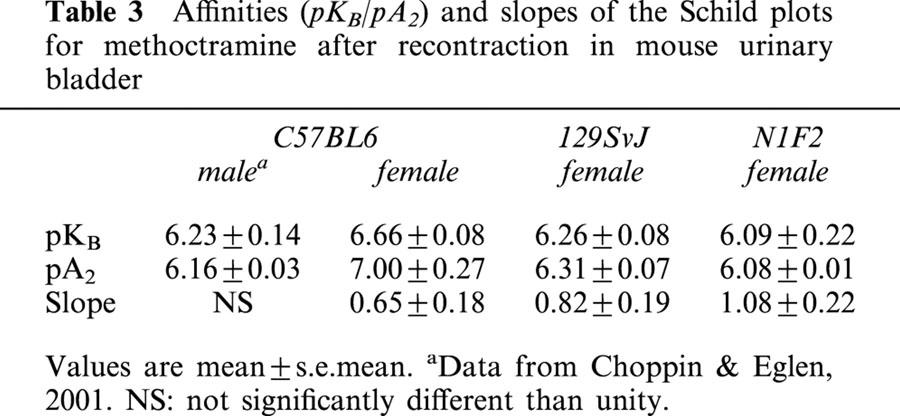
(+)-Cis-dioxolane produced concentration–dependent contractions of female mouse urinary bladder smooth muscle under control conditions. After preferential alkylation of M3 receptor (exposure to 4-DAMP mustard in presence of methoctramine), (+)-cis-dioxolane produced recontractile (reversal of contraction) responses of KCl-precontracted tissues, which were relaxed with isoproterenol. No time-dependent changes in agonist sensitivity were observed during the construction of two consecutive concentration-recontractile effect curves. As shown in Figure 3 for female N1F2 mice, methoctramine produced surmountable antagonism of the recontractile response to (+)-cis-dioxolane. The affinity estimates (pKB/pA2) for methoctramine and the slope of the Schild plots (not significantly different than unity) are summarized in Table 3.
Figure 3.

Recontraction experiments in female N1F2 mouse urinary bladder smooth muscle: effect of methoctramine on the recontractile concentration-effect to (+)-cis-dioxolane obtained after elevation of adenylyl cyclase activity following preferential alkylation of muscarinic M3 receptors (n=4). (a) Time control; (b) +0.3 μM methoctramine; (c) +1.0 μM methoctramine; (d) +3.0 μM methoctramine.
Table 3.
Affinities (pKB/pA2) and slopes of the Schild plots for methoctramine after recontraction in mouse urinary bladder
Discussion
Muscarinic antagonists are still the first line of treatment for overactive bladder, which occurs predominantly in females (Wein, 1995). Although gender differences in responsiveness of the bladder to muscarinic stimulation have been reported in rat (Chun et al., 1990; Longhurst et al., 1992; Longhurst & Levendusky, 2000), the receptor subtypes that are responsible have not been identified. Previous studies using mouse urinary bladder (Durant et al., 1991; Lundbeck & Sjögren, 1992) have also demonstrated a muscarinic-induced contractile response but did not characterize the receptor subtype(s) involved. Thus, the aim of this study was to examine the influence of gender as well as strains on muscarinic responsiveness of mouse bladder strips.
Using five different muscarinic agonists (including the partial agonist pilocarpine), the potencies and maximal responses obtained in mouse urinary bladder smooth muscle suggest gender and strain differences (range for pEC50=6.4–6.6, 6.2–6.9, 6.2–6.7, 5.4–6.0 and 0.0–5.8, Tmax=1.9–4.7 g, 1.3–3.4 g, 1.0–3.0 g, 1.4–2.7 and 0.0–0.9 g, for (+)-cis-dioxolane, oxotremorine-M, acetylcholine, carbachol and pilocarpine, respectively, n=4–6, depending on the gender and the strain; see Table 1). Amongst these agonists, (+)-cis-dioxolane was the only one producing bladder contractions of similar potency and maximal response in both gender and strains. Therefore, it was used to determine the affinity estimates of a range of muscarinic antagonists (see Table 2): the agonist-induced concentration-dependent contractions were inhibited in a competitive fashion. The pKB values of these antagonists correlated most strikingly with the binding affinities of the antagonists at m3 recombinant muscarinic receptors (pKi are: atropine 9.5; pirenzepine 6.8; 4-DAMP DAMP 9.3; methoctramine 6.1; p-F-HHSiD 7.5; zamifenacin 7.9 and darifenacin 8.8; Hegde et al., 1997; Loury et al., 1999; Since ionic strength influences antagonist binding affinity, it is important to mention that these values were measured in Tris–Krebs buffer; Pedder et al., 1991). This finding suggests the exclusive involvement of M3 muscarinic receptors in the direct contractile response to muscarinic agonists in female mouse bladder. This accords with preliminary results described by Paravicini et al. (2000) but also in male mice (Choppin & Eglen, 2001) and other species: rabbit (Tobin, 1995; Tobin & Sjögren, 1995), rat (Longhurst et al., 1995; Hegde et al., 1997) and human (Newgreen & Naylor, 1996) bladder.
If present, a functional role of M2 receptors could be unmasked in recontraction experiments (with preferential alkylation of M3 receptors and elevated adenylyl cyclase). Under these conditions, methoctramine exhibited a low affinity in the mouse strains tested (pA2=6.31 and 6.08 in female 129/SvJ and N1F2, respectively), which argues against the involvement of an M2 receptor. As previously described in male mouse bladder (Choppin & Eglen, 2001), no indirect contractile role of M2 receptors was revealed by functional studies (recontraction experiments) in female mouse bladder, and only M3 receptor activation induced bladder contraction. The remaining response observed with (+)-cis-dioxolane after recontraction is likely due to an incomplete alkylation of muscarinic M3 receptors. However, in female C57BL/6, methoctramine yielded a pA2 value (7.00) intermediate between the one expected for this compound at M2 and M3 receptors, suggesting the involvement of both of these subtypes. Similar observations have been seen in rat urinary bladder (Hegde et al., 1997) and several gastrointestinal smooth muscle tissues.
Recently, knockout male mice for M2 (Stengel et al., 2000; Zhou et al., 2002) and M3 (Matsui et al., 2000) receptors were reported. Data from these studies suggest, on one hand, that muscarinic M2 receptors play a minor role in carbachol-induced contraction of isolated bladder smooth muscle (the potency of muscarinic agonists was only modestly reduced, and the maximal response unaffected; Stengel et al., 2000). On the other hand, Matsui et al. (2000) demonstrated a predominant involvement of muscarinic M3 receptor in bladder contractions, as these contractions in vitro were virtually abolished and urinary retention was marked in male mice in vivo, in transgenic mice lacking the muscarinic M3 receptor. These data suggest that a dominant, if not exclusive, role of this subtype prevails and are consistent with the findings obtained in the present study. Similarly in rat bladder, it has been shown that gender influences the sensitivity to muscarinic stimulation (Longhurst & Levendusky, 2000).
Conclusions
The present data suggest that only M3 receptors play a role in mouse urinary bladder smooth muscle contraction, in accord with emerging data from knockout animals. The pharmacological antagonist profile of the muscarinic receptors present in the mouse bladder equates most closely with the M3 muscarinic receptor, with however gender and strain differences. It thus appears that one should be careful when comparing results obtained from different studies using various strains.
Abbreviations
- 4-DAMP
4-diphenylacetoxy-N-methylpiperidine
- p-F-HHSiD
para fluoro hexahydrosiladifenidol
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAVERMAN A.S., RUGGIERI M.R. Selective alkylation of rat urinary bladder muscarinic receptors with 4-DAMP mustard reveals a contractile function for the M2 muscarinic receptor. J. Recept. Signal Transduct. Res. 1999;19:819–833. doi: 10.3109/10799899909042875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUFIELD M.P., BIRDSALL N.J.M. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998;58:279–290. [PubMed] [Google Scholar]
- CHOPPIN A., EGLEN R.M. Pharmacological characterisation of muscarinic receptors in mouse isolated urinary bladder smooth muscle. Br. J. Pharmacol. 2001;133:1035–1040. doi: 10.1038/sj.bjp.0704165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN A., EGLEN R.M., HEGDE S.S. Pharmacological characterisation of muscarinic receptors in rabbit isolated iris sphincter muscle and urinary bladder smooth muscle. Br. J. Pharmacol. 1998;124:883–888. doi: 10.1038/sj.bjp.0701920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUN A.L., WEIN A.J., HARKAWAY R., LEVIN R.M. Comparison of urinary bladder function in sexually mature and immature male and female rats. J. Urol. 1990;143:1267–1271. doi: 10.1016/s0022-5347(17)40252-7. [DOI] [PubMed] [Google Scholar]
- DIXON W.J., MASSEY F.J. Introduction to statistical analysis 1983New York: McGraw-Hill Publishing Company; 4th edn [Google Scholar]
- DÖRJE F., WESS J., LAMBRECHT G., TACKE R., MUTSCHLER E., BRANN M.R. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J. Pharmacol. Exp. Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- DURANT P.A., SHANKLEY N.P., WELSH N.J., BLACK J.W. Pharmacological analysis of agonist-antagonist interactions at acetylcholine muscarinic receptors in a new urinary bladder assay (1991) Br. J. Pharmacol. 1991;104:145–150. doi: 10.1111/j.1476-5381.1991.tb12399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., BONHAUS D.W., CALIXTO J.J., CHOPPIN A., LEUNG E., LOEB M., LOURY D., MOY T., WILDA M., HEGDE S.S. Characterization of the interaction of tolterodine at muscarinic receptor subtypes in vitro and in vivo. Br. J. Pharmacol. 1997;120:63P. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., CHOPPIN A. Pharmacological characterisation of muscarinic receptors in mouse urinary bladder smooth muscle. Life Sci. 2001;68:39P. doi: 10.1038/sj.bjp.0704165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., HEGDE S.S., WATSON N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- EGLEN R.M., NAHORSKI S.R. The muscarinic M5 receptor: a silent or emerging subtype. Br. J. Pharmacol. 2000;130:13–21. doi: 10.1038/sj.bjp.0703276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., REDDY H., WATSON N. Selective inactivation of muscarinic receptor subtypes. Int. J. Biochem. 1994;26:1357–1368. doi: 10.1016/0020-711x(94)90178-3. [DOI] [PubMed] [Google Scholar]
- EHLERT F.J. The interaction of 4-DAMP mustard with subtypes of the muscarinic receptor. Life Sci. 1996;58:1971–1978. doi: 10.1016/0024-3205(96)00187-7. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Catecholamines, Handbook of Experimental Pharmacology 1972Vol. 33Berlin, Heidelberg, New York: Springer; 283–335.ed. Blaschko, H. & Muscholl, E [Google Scholar]
- HEGDE S.S., CHOPPIN A., BONHAUS D., BRIAUD S., LOEB M., MOY T.M., LOURY D., EGLEN R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGHURST P.A., EIKA B., LEGGETT R.E., LEVIN R.M. Comparison of urinary bladder function in 6 and 24 month male and female rats. J. Urol. 1992;148:1615–1620. doi: 10.1016/s0022-5347(17)36981-1. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., LEGGETT R.E., BRISCOE J.A.K. Characterization of functional muscarinic receptors in the rat urinary bladder. Br. J. Pharmacol. 1995;116:2279–2285. doi: 10.1111/j.1476-5381.1995.tb15065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGHURST P.A., LEVENDUSKY M. Influence of gender and the oestrous cycle on in vitro contractile responses of the rat urinary bladder to cholinergic stimulation. Br. J. Pharmacol. 2000;131:177–184. doi: 10.1038/sj.bjp.0703551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOURY D.N., HEGDE S.S., BONHAUS D.W., EGLEN R.M. Ionic strength of assay buffers influences antagonist binding affinity estimates at muscarinic M1–M5 cholinoceptors. Life Sci. 1999;64:6P. [Google Scholar]
- LUNDBECK F., SJÖGREN C. A pharmacological in vitro study of the mouse urinary bladder at the time of acute change in bladder reservoir function after irradiation. J. Urol. 1992;148:179–182. doi: 10.1016/s0022-5347(17)36548-5. [DOI] [PubMed] [Google Scholar]
- MATSUI M., MOTOMURA D., KARASAWA H., FUJIKAWA T., JIANG J., KOMIYA Y., TAKAHASHI S., TAKETO M.M. Multiple functional deficits in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWGREEN D.T., NAYLOR A.M. Characterization of functional muscarinic receptors in human bladder. Br. J. Pharmacol. 1996;119:45P. [Google Scholar]
- NILVEBRANT L., SUNDQUIST S., GILLBERG P.-G.Tolterodine is not subtype (m1–m5) selective but exhibits functional bladder selectivity in vivo Neurourol. Urodyn. 19961534(abstract) [Google Scholar]
- NORONHA-BLOB L., LOWE V.C., PATTON A., CANNING B., COSTELLO D., KINNIER W.J. Muscarinic receptors: relationships among phosphoinositide breakdown, adenylate cyclase inhibition, in vitro detrusor muscle contractions and in vivo cystometrogram studies in guinea-pig bladder. J. Pharmacol. Exp. Ther. 1989;249:843–851. [PubMed] [Google Scholar]
- PARAVICINI T., PENNEFATHER J.N., LAU W.A.K., MA S., PATAK E. Muscarinic receptors mediating contraction of the urinary bladder from the female mouse. Proc. Aust. Soc. Clin. Exp. Pharmacol. Toxicol. 2000;7:1–12P. [Google Scholar]
- PARKER R.B., WAUD D.R. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation. I. Agonists. J. Pharmacol. Exp. Ther. 1971;177:1–12. [PubMed] [Google Scholar]
- PEDDER E.K., EVELEIGH P., POYNER D., HULME E.C., BIRDSALL N.J. Modulation of the structure-binding relationships of antagonists for muscarinic acetylcholine receptor subtypes. Br. J. Pharmacol. 1991;103:1561–1567. doi: 10.1111/j.1476-5381.1991.tb09827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENGEL P.W., GOMEZA J., WESS J., COHEN M.L. M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J. Pharmacol. Exp. Ther. 2000;292:877–885. [PubMed] [Google Scholar]
- THOMAS E.A., BAKER S.A., EHLERT F.J. Functional role for the M2 muscarinic receptor in smooth muscle of guinea pig ileum. Mol. Pharmacol. 1993;44:102–110. [PubMed] [Google Scholar]
- THOMAS E.A., EHLERT F.J. Pertussis toxin blocks M2 muscarinic receptor-mediated effects on contraction and cyclic AMP in the guinea pig ileum, but not M3-mediated contractions and phosphoinositide hydrolysis. J. Pharmacol. Exp. Ther. 1994;271:1042–1050. [PubMed] [Google Scholar]
- TOBIN G. Muscarinic receptor subtypes in the submandibular gland and the urinary bladder of the rabbit: in vivo and in vitro functional comparisons of receptor antagonists. J. Auton. Pharmacol. 1995;15:451–463. doi: 10.1111/j.1474-8673.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]
- TOBIN G., SJÖGREN C. In vivo and in vitro effects of muscarinic receptor antagonists on contractions and release of [3H]-acetylcholine in the rabbit urinary bladder. Eur. J. Pharmacol. 1995;281:1–8. doi: 10.1016/0014-2999(95)00221-6. [DOI] [PubMed] [Google Scholar]
- WEIN A.J. Pharmacology of Incontinence. Urol. Clin. North Am. 1995;22:557–577. [PubMed] [Google Scholar]
- WELSH N.J., EGLEN R.M., SHANKLEY N.P. Pharmacological comparison of the muscarinic receptors mediating contraction of the guinea-pig left atrium, gastric smooth muscle and mouse urinary bladder. Br. J. Pharmacol. 2000;131:57P. [Google Scholar]
- ZHOU H., MEYER A., STARKE K., GOMEZA J., WESS J., TRENDELENBURG A.-U. Heterogeneity of release-inhibiting muscarinic autoreceptors in heart atria and urinary bladder: a study with M2- and M4-receptor-deficient mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;365:112–122. doi: 10.1007/s00210-001-0517-7. [DOI] [PubMed] [Google Scholar]


