Abstract
The anti-inflammatory activity of the endogenous fatty acid amide palmitoylethanolamide and its relationship to cyclo-oxygenase (COX) activity, nitric oxide (NO) and oxygen free radical production were investigated in the rat model of carrageenan-induced acute paw inflammation and compared with the nonsteroidal anti-inflammatory drug (NSAID) indomethacin.
Palmitoylethanolamide (1, 3, 5, 10 mg kg−1; p.o.) and indomethacin (5 mg kg−1; p.o.) were administered daily after the onset of inflammation for three days and the paw oedema was measured daily; 24 h after the last dose (fourth day) the rats were killed and the COX activity and the content of nitrite/nitrate (NO2−/NO3−), malondialdehyde (MDA), endothelial and inducible nitric oxide synthase (eNOS and iNOS) were evaluated in the paw tissues.
Palmitoylethanolamide had a curative effect on inflammation, inhibiting the carrageenan-induced oedema in a dose- and time-dependent manner. This effect was not reversed by the selective CB2 receptor antagonist (N-[(1S)-endo-1,3,3-trimethylbicyclo[2.2.1]heptan-2yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)pyrazole-3 carboxamide) (SR144528), 3 mg kg−1 p.o. On the fourth day after carrageenan injection, COX activity and the level of NO2−/NO3−, eNOS and MDA were increased in the inflamed paw, but iNOS was not present. Palmitoylethanolamide (10 mg kg−1) and indomethacin markedly reduced these increases.
Our findings show, for the first time, that palmitoylethanolamide has a curative effect in a model of acute inflammation. The inhibition of COX activity and of NO and free radical production at the site of inflammation might account for this activity.
Keywords: Cannabinoid, palmitoylethanolamide, inflammation, therapy, carrageenan, cyclo-oxygenase, nitric oxide, oxygen free radicals
Introduction
The endogenous fatty acid amide palmitoylethanolamide behaves like an autacoid, modulating the mast-cell response to inflammation (Aloe et al., 1993), so it may be a potential new anti-inflammatory agent. Since this original report, the use of this compound as an anti-inflammatory drug has been extensively investigated by Mazzari et al. (1996), who reported that palmitoylethanolamide given orally to rats reduced substance P-induced mast-cell degranulation and plasma extravasation, passive cutaneous anaphylaxis-induced extravasation, carrageenan-, formalin- and dextran-induced oedema and carrageenan-induced hyperalgesia. Jaggar et al. (1998a, b) showed the anti-hyperalgesic action of both palmitoylethanolamide and the endocannabinoid anandamide in visceral and somatic inflammatory pain. Unlike anandamide, however, palmitoylethanolamide did not prevent the viscero–visceral hyperreflexia associated with inflammation of the rat urinary bladder, suggesting that it becomes effective as an analgesic only when an inflammatory state is established (Jaggar et al., 1998b). Although there is no doubt about the anti-inflammatory effect of palmitoylethanolamide in rodents, the molecular mechanism underlying this action is a puzzle. The involvement of CB1 and CB2 cannabinoid receptors seems unlikely, since palmitoylethanolamide is unable to bind with high affinity to both cannabinoid receptor subtypes (Devane et al., 1992; Griffin et al., 2000), even though its pharmacological effects were reversed by the selective CB2 receptor antagonist (N-[(1S)-endo-1,3,3-trimethylbicyclo [2.2.1] heptan-2yl] -5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl) pyrazole-3 carboxamide) (SR144528) (Calignano et al., 1998; 2001; Conti et al., 2002). It also cannot be excluded that palmitoylethanolamide potentiates the action of endogenous anandamide by minimizing its degradation by fatty acid amide hydrolase (Lambert & Di Marzo, 1999).
To date, the anti-inflammatory efficacy of palmitoylethanolamide has only been assessed when the drug was administered before the inflammatory stimulus, and nothing is known about its curative effect once inflammation has set in. Thus, the present study was designed to investigate whether palmitoylethanolamide reduced oedema when therapeutically administered in a model of acute inflammation induced by carrageenan in the rat. This inflammation is characterized by vasodilatation, plasma extravasation and polymorphonuclear neutrophil (PMN) influx. Carrageenan-induced oedema in the rat hindpaw is due to various mediators such as histamine, 5-hydroxytryptamine (5-HT), bradykinin (di rosa et al., 1971), prostaglandins (PG) (di rosa & Willoughby, 1971; di rosa et al., 1971) which operate in parallel to produce this inflammatory response. It has also been attributed to the activation of inducible cyclo-oxygenase (COX-2) in the hindpaw (Siebert et al., 1994). Another important mediator in acute inflammation induced by carrageenan is nitric oxide (NO) which is produced in physiopathological conditions by three distinct isoforms of nitric oxide synthase (NOS): endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS) (Moncada et al., 1991). The first two are expressed constitutively, whereas iNOS is expressed in response to various inflammatory stimuli, such as cytokines and lipopolysaccharide. At the site of inflammation NO is formed by a number of different cells, including leukocytes, endothelial cells and sensory nerve cells (Handy & Moore, 1998). NO is a potent vasodilator and its involvement during an inflammatory response may be related to its ability to increase vascular permeability and oedema through changes in local blood flow (Moncada et al., 1991; Moncada & Higgs, 1993).
Local neutrophil infiltration and activation contribute to inflammatory responses by producing, among other mediators, oxygen-derived free radicals such as superoxide anion (O2−) and hydroxyl radicals (Fantone & Ward, 1982). NO can also interact with O2− to form peroxynitrite (ONOO−), a potent oxidizing molecule that can induce lipid peroxidation and cellular damage (Beckman et al., 1990; Radi et al., 1991; Rubbo et al., 1994). A further aim of the study was therefore to verify whether repeated doses of palmitoylethanolamide influence COX activity, NO and lipid peroxide production, measured ex vivo at the site of inflammation.
In all these studies indomethacin was used for comparison as a representative, well-known nonsteroidal anti-inflammatory drug (NSAID).
Methods
Animals
Male Wistar rats (100–120 g, Harlan, Italy) aged 30–35 days were used. They were housed in a room with controlled temperature (22±1°C), humidity (60±10%) and light (12 h per day) for at least a week before being used. Food and water were available ad libitum. Animal care was in accordance with Italian State regulations governing the care and treatment of laboratory animals (Permission n° 94/2000A).
Drugs
Drugs were obtained from the following companies: λ-carrageenan (Sigma-Aldrich, Milano, Italy), palmitoylethanolamide (Cayman Chemical, Ann Arbor, MI, U.S.A.), indomethacin (Sigma-Aldrich, Milano, Italy), SR144528 (kindly supplied by Sanofi-Synthelabo, Montpellier, France).
Experimental design
Acute inflammation was induced by intraplantar (i.pl.) injection of 0.1 ml carrageenan (1% w v−1 in saline) into the right paw. Palmitoylethanolamide (1, 3, 5, 10 mg kg−1) and indomethacin (5 mg kg−1), or an appropriate volume of vehicle (carboxymethylcellulose 1.5% w v−1 in saline) were administered orally (0.5 ml hg−1) 2 h after carrageenan on the first day and again on the second and third days at the same time as the first injection. The doses of palmitoylethanolamide and indomethacin were chosen since we reported (Conti et al., 2002) that they were effective in preventing the development of carrageenan oedema in the rat paw. Control animals received an i.pl. injection of saline (0.1 ml) and an oral dose of drug vehicle.
In the antagonism studies SR144528 3 mg kg−1 p.o. was given 1 h before palmitoylethanolamide (10 mg kg−1 p.o.) every day for three days. The SR144528 dose was chosen in view of our previous results indicating that this dose reversed the anti-inflammatory effect of palmitoylethanolamide in the same model (Conti et al., 2002).
Evaluation of oedema
The paw volume was measured with a plethysmometer (Ugo Basile, Varese, Italy). On the first day, the volume was measured immediately before the injection of carrageenan or saline and then 1, 3, 4, 5 h after drugs or vehicle. On the next three days, paw volumes were recorded just before drug or vehicle injection and on the last day (the fourth) just before euthanasia.
Biochemical studies
On the fourth day after the carrageenan injection, animals were killed by cervical dislocation, paws were cut at the level of the calcaneus bone, weighed, crushed at 0–4°C and used for determination of COX activity and the levels of nitrite/nitrate (NO2−/NO3−), iNOS and eNOS protein, and malondialdeyde (MDA).
COX activity
Tissue was homogenized with a T25, 18N Ultra-Turrax in 1 : 4 (w v−1) Tris HCl buffer (20 mM, pH 7.6). The homogenate was centrifuged (9000×g, 10 min, 4°C) and the supernatant was ultracentrifuged at 100,000×g for 1 h to obtain the microsomal fraction. Microsomes were resuspended in 500 μl Tris HCl (0.1 M, pH 7.4). The COX activity of this preparation was determined by measuring oxygen consumption with a Clark-type polarographic electrode in a 600 μl reaction vessel (Yellow Springs Instruments Co.). The assay mixture contained 100 mM Tris HCl pH 8.1, 1 μM haeme, 1 μM phenol. A sample of 200 μl microsomal suspension was incubated for 30 s to equilibrate with haeme and the reaction was started by adding arachidonic acid (10 mM). The reaction was essentially complete after 1 min. The specific activity was expressed as natom O min−1 (g wet weight tissue)−1 (Gierse et al., 1999).
Nitrite/nitrate assay
NO production was assessed on the basis of NO2−/NO3− which are the oxidation end-products of NO. NO2−/NO3− concentrations were measured using the method of Misko et al. (1993) based on the acid catalysed ring closure of 2,3-diaminonaphthalene (DAN) with nitrite to form the highly fluorescent product 1-(H)-naphthotriazole. Tissue was homogenized with a T25, 18N Ultra-Turrax in 1 : 4 (w v−1) Tris HCl buffer (20 mM, pH 7.6). The homogenate was centrifuged (9000×g, 10 min, 4°C) and the supernatant was filtered through Microcon YM-10 (Millipore) (10,000×g, 30 min, 4°C) to remove the proteins. Nitrate in the samples (20 μl) was converted to nitrite by incubation with 9 μl nitrate reductase of Aspergillus niger (14 mU) and with 30 μl of the reduced form of β-nicotinamide adenine dinucleotide phosphate (1 mM), in a final volume of 150 μl of 20 mM Tris-HCl pH 7.6, for 5 min at 21°C. The reaction was terminated by dilution (1 : 2) with distilled water and all the samples were incubated at 21°C for 10 min with 150 μl EDTA (0.01 M) and 30 μl DAN (0.05 mg ml−1) in HCl (0.62 M). The reaction was terminated by adding 15 μl NaOH (2.8 N). Formation of 1-(H)-naphthotriazole was measured using a FP-777 spectrofluorimeter (Jasco, Lecco, Italy) with excitation at 365 nm and emission read at 450 nm. NO2−/NO3− content in the paw was calculated using a standard curve, and expressed as nmol (g wet weight tissue)−1.
Determination of eNOS and iNOS by Western blot analysis
Tissue was homogenized in 1 : 4 (w v−1) Tris-HCl (50 mM)-EDTA (0.1 mM) buffer, pH 7.4, containing a protease inhibitor cocktail (1 tablet for 10 ml) (Roche Diagnostics, Milano, Italy). The homogenate was centrifuged at 9000×g for 10 min at 4°C and the supernatant was ultracentrifuged at 100,000×g for 1 h at 4°C. The microsomal and cytosolic fractions were stored at −80°C until eNOS and iNOS assay. Microsomes and cytosol were diluted in Laemli buffer (0.3 M Tris-HCl, pH 6.8, containing 10% sodium dodecylsulphate (SDS), 50% glycerol, 5% dithiothreitol and 0.05% bromophenol blue) to obtain 20 μg and 100 μg protein, respectively. The proteins were fractionated on 10/20 cm separating SDS–PAGE gel containing 7.5% acrylamide. Proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, BAS 85) with the semidry method for 45 min and 90 min respectively for mini-gels or standard gels at 15 V at room temperature. The membranes were incubated overnight at room temperature with 5% non-fat dry milk in phosphate buffer saline (PBST : Na2HPO4 16 mM, NaH2PO4 1.9 mM, NaCl 6.7 M pH 7.5, 0.1% Tween 20) (blocking solution). Nitrocellulose membranes were then washed five times (5, 5, 15, 5 and 5 min) with PBST and incubated with primary antibodies (rabbit anti-iNOS polyclonal antibody from Chemicon Int., Temecula, CA, U.S.A. and rabbit anti-eNOS polyclonal antibody from Transduction Laboratories, Lexington, KY, U.S.A., 1 : 1000 in blocking solution) for 2 h at room temperature, with shaking. The primary antibodies were then removed and membranes were washed as described previously. Secondary antibody (anti-rabbit IgG, peroxidase linked F(ab′)2 fragment from Amersham Pharmacia Biotech, Milano, Italy, 1 : 1500 in 3% blocking solution) was then added and incubated for 1 h, with shaking; the secondary antibody was removed and membranes were washed with PBST, as above.
The immunoreactive proteins were detected by enhanced chemioluminescence (ECL) using Hyperfilm (Amersham Pharmacia Biotech, Milano, Italy) and ECL reagent (Roche Diagnostics, Monza, Italy). The grey level of the bands was quantified by an image analysis system, consisting of a videocamera (Hamamazsu) connected to an Apple Macintosh personal computer. The public domain image 1.47 software was used (National Institutes of Health, U.S.A.). Each band was traced with the mouse cursor control and the light transmittance was determined as the grey level. The grey level for densitometric measurements was calculated after subtraction of the film background density. Grey levels obtained with densitometric analysis were expressed as a percentage of the respective control (non inflamed).
Lipid peroxide assay
Lipid peroxide was determined by measuring malondialdehyde (MDA) in hind paw homogenate prepared with a T25, 18N Ultra-Turrax in a ratio of 1 g wet tissue to 9 ml potassium phosphate (50 mM)-EDTA (0.1 mM) buffer, pH 7.4. The lipid peroxide level was established spectrophotometrically at 532 nm by the thiobarbituric acid test of Ohkawa et al. (1979) employing 0.156 μM−1 cm−1 as the extinction coefficient, and was expressed as nmol malondialdehyde (g wet weight tissue)−1.
Statistical analysis
The results were expressed as the means±s.e.mean. The data were analysed using one-way repeated measures analysis of variance (ANOVA) followed by Tukey's test for parametric values and Kruskal-Wallis ANOVA followed by Rayan's test for non-parametric values (percentages). Differences were considered significant at P<0.05. Linear regression analysis was done using GraphPAD Software (San Diego, CA, U.S.A.).
Results
Effect of palmitoylethanolamide on paw volume
The i. pl. injection of carrageenan led to an increase in paw volume that remained high throughout the four days (Figure 1a,b). On the first day, palmitoylethanolamide (10 mg kg−1) and indomethacin (5 mg kg−1) both reduced oedema by about 50% (Figure 1a).
Figure 1.
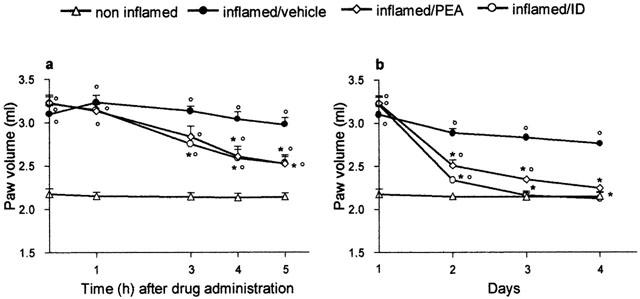
Effect on oedema of palmitoylethanolamide (PEA) 10 mg kg−1 and indomethacin (ID) 5 mg kg−1, p.o. in the rat after the onset of inflammation. (a) Time-course of the inhibition of oedema by PEA and ID on the first day of treatment (time zero represents paw volume evaluation 2 h after carrageenan or saline). (b) Inhibitory effect of PEA and ID during treatment (day 1 represents paw volume evaluation 2 h after carrageenan or saline). Each point represents the mean±s.e.mean of 8–12 animals. °P<0.05 vs non-inflamed; *P<0.05 vs inflamed/vehicle.
Subsequent daily doses of palmitoylethanolamide resulted in a time-dependent decrease of oedema (Figure 1b); on the second and third days the paw volume of animals treated with palmitoylethanolamide was significantly different from that of inflamed and non-inflamed animals; on the fourth day palmitoylethanolamide abolished the inflammation, bringing the paw volume down to that of non-inflamed animals. Indomethacin also had a time-related anti-oedema effect, completely inhibiting the oedema from the third day (Figure 1b). Repeated treatment with palmitoylethanolamide (1, 3, 5, 10 mg kg−1) reduced the oedema, evaluated on the fourth day, in a dose-related fashion with r2=0.909, P<0.0001, y=1.363–0.3672x (data not shown).
Effect of SR144528 on palmitoylethanolamide'santi-oedema effect
SR144528 3 mg kg−1 p.o. had no effect on the inflamed paw volume and did not reverse the anti-oedema effect of palmitoylethanolamide 10 mg kg−1 p.o. (Figure 2).
Figure 2.
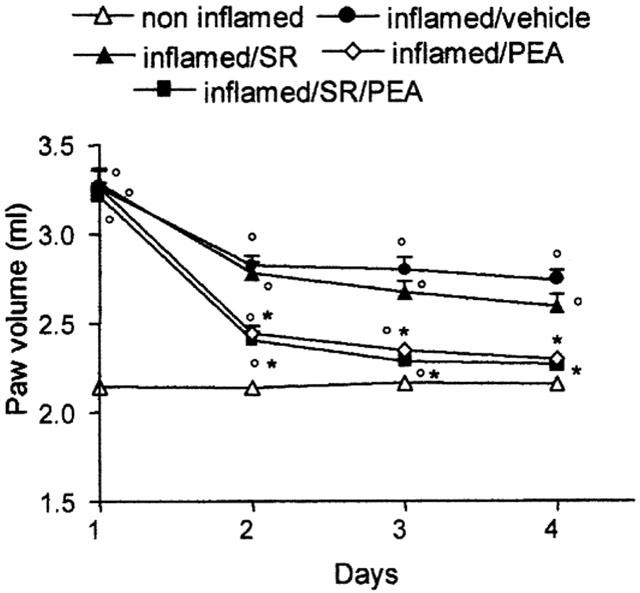
Effect of SR144528 (SR) 3 mg kg−1 p.o. repeated pretreatment on the anti-oedema action of palmitoylethanolamide (PEA) 10 mg kg−1 during treatment (day 1 represents paw volume evaluation 2 h after carrageenan or saline). Each point represents the mean±s.e.mean of 4–5 animals. °P<0.05 vs non-inflamed; *P<0.05 vs inflamed/vehicle.
Effect of palmitoylethanolamide on COX activity
Four days after carrageenan, COX activity, measured ex vivo in the paw tissues injected with carrageenan, was significantly increased (by 46%) compared with the paw injected with saline; 10 mg kg−1 palmitoylethanolamide and indomethacin brought COX activity down to the level of non-inflamed tissues (Figure 3).
Figure 3.
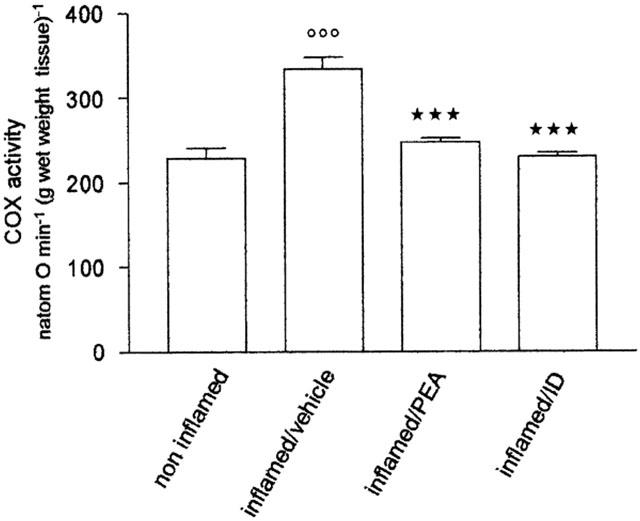
Effect of repeated treatment with palmitoylethanolamide (PEA) 10 mg kg−1 and indomethacin (ID) 5 mg kg−1 on cyclo-oxygenase activity in microsomes from paw tissue four days after carrageenan injection. Each bar represents the mean±s.e.mean of 8–12 animals. °°°P<0.001 vs non-inflamed; ***P<0.001 vs inflamed/vehicle.
Effect of palmitoylethanolamide on NO2−/NO3− and MDA
Four days after carrageenan, inflammation was associated with an elevated production of NO2−/NO3− and MDA in the paw tissues. NO2−/NO3− and MDA were respectively eight times and twice the levels in non-inflamed tissue. Palmitoylethanolamide (10 mg kg−1) and indomethacin brought NO2−/NO3− and MDA down to physiological levels (Figure 4a,b).
Figure 4.
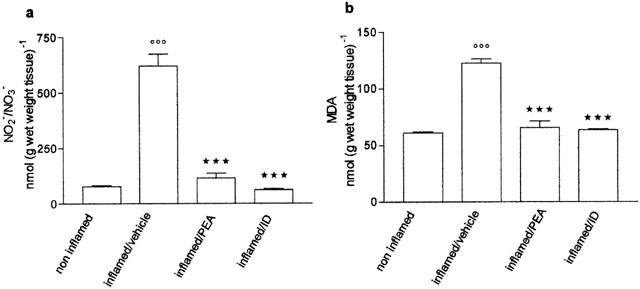
Effect of repeated treatment with palmitoylethanolamide (PEA) 10 mg kg−1 and indomethacin (ID) 5 mg kg−1 on (a) nitrite/nitrate (NO2−/NO3−) and (b) malondialdehyde (MDA) formation, measured in paw homogenate four days after carrageenan injection. Each bar represents the mean±s.e.mean of 8–12 animals. °°°P<0.001 vs non-inflamed; ***P<0.001 vs inflamed/vehicle.
Effect of palmitoylethanolamide on eNOS and iNOS
Four days after carrageenan, the production of NO2−/NO3− in inflamed paw tissues was associated with an increase in the level of protein corresponding immunologically to the 140 kDalton endothelial isoform of NOS (Figure 5a). Densitometric analysis of blots indicated that the amount of eNOS doubled compared to the content of non-inflamed tissues (Figure 5b). iNOS did not contribute to the production of NO2−/NO3−, since none was detectable. Repeated treatment with palmitoylethanolamide (10 mg kg−1) and indomethacin blocked the enhancement of eNOS and the levels did not differ from those of non-inflamed tissues (Figure 5a,b).
Figure 5.
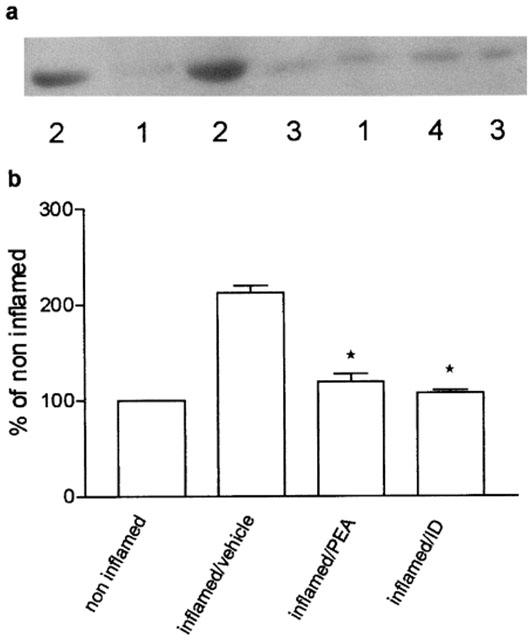
Effect of repeated treatment with palmitoylethanolamide (PEA) 10 mg kg−1 and indomethacin (ID) 5 mg kg−1 on eNOS protein levels in the microsomal fraction of inflamed paw tissues four days after carrageenan injection. (a) Representative immunoreactive bands: (1) non-inflamed; (2) inflamed/vehicle; (3) inflamed/PEA; (4) inflamed/ID. (b) Relative levels of expression with densitometric analysis of the gray levels expressed as a percentage of non-inflamed. Each bar represents the mean±s.e.mean of 8–12 determinations. *P<0.05 vs inflamed/vehicle.
Previous work showed that iNOS protein was detectable earlier in inflammation induced by carrageenan (Salvemini et al., 1996; Osborne & Coderre, 1999; Omote et al., 2001). To verify whether palmitoylethanolamide affected the levels of this isoform we performed a time-course study, evaluating the level of iNOS at different times after carrageenan. iNOS was already detected at 3 h, was higher at 6 h and at 10 h, but at 24 h it was considerably reduced (data not shown). Since at 6 h the protein levels of iNOS were very high, we chose this time to study the influence of palmitoylethanolamide (10 mg kg−1) and indomethacin. At the same time we also measured eNOS and the oxidation end-products of NO.
In inflamed paw tissues the levels of iNOS and eNOS were higher than in non-inflamed tissues (Figure 6a,b): the densitometric readings of blots showed a 6 fold increase in both isoforms (Figure 6c,d). A single dose of palmitoylethanolamide or indomethacin, 2 h after carrageenan, reduced the level of iNOS by 48% and by 76% (Figure 6c). The levels of eNOS were also reduced by palmitoylethanolamide and indomethacin (Figure 6d). At this interval, the increase in iNOS and eNOS in inflamed paw tissues was associated with an elevated production of NO2−/NO3−, whose content appeared higher (894±139 nmol (g wet weight tissue)−1) than in inflamed tissues four days after carrageenan (620±54 nmol (g wet weight tissue)−1); palmitoylethanolamide and indomethacin significantly inhibited this enhancement (Figure 7).
Figure 6.
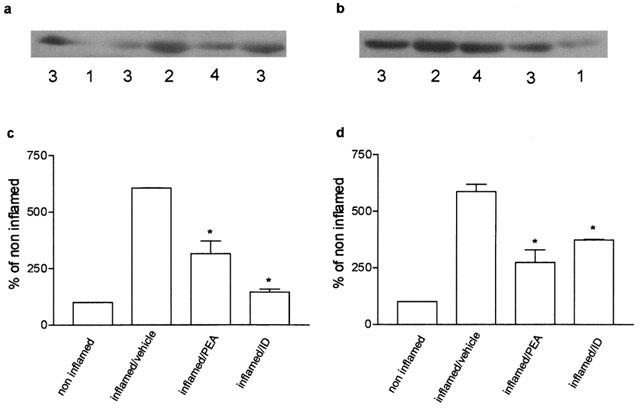
Effect of treatment with palmitoylethanolamide (PEA) 10 mg kg−1 and indomethacin (ID) 5 mg kg−1 on iNOS and eNOS protein levels in the microsomal fraction of inflamed paw tissues 6 h after carrageenan injection. (a) Representative immunoreactive bands of iNOS protein: (1) non-inflamed; (2) inflamed/vehicle; (3) inflamed/PEA; (4) inflamed/ID. (b) Representative immunoreactive bands of eNOS protein: (1) non-inflamed; (2) inflamed/vehicle; (3) inflamed/PEA; (4) inflamed/ID. (c) and (d) Relative levels of expression with densitometric analysis of the gray levels expressed as a percentage of non-inflamed. Each bar represents the mean±s.e.mean of 8–12 determinations. *P<0.05 vs inflamed/vehicle.
Figure 7.
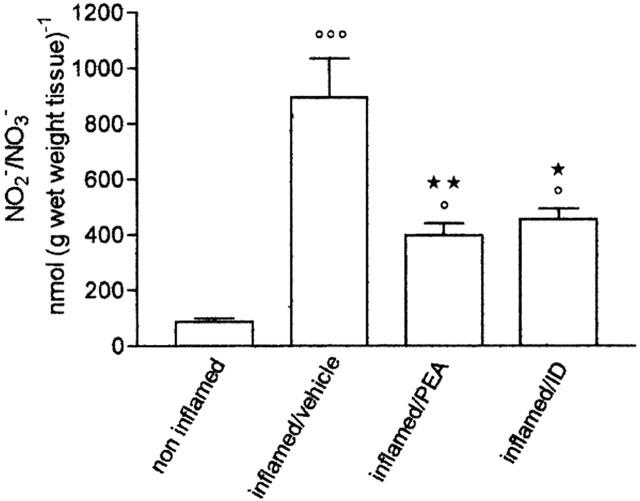
Effect of treatment with palmitoylethanolamide (PEA) 10 mg kg−1 and indomethacin (ID) 5 mg kg−1 on nitrite/nitrate (NO2−/NO3−) formation in paw homogenate 6 h after carrageenan injection. Each bar represents the mean±s.e.mean of 8–12 animals. °P<0.05, °°°°°°P<0.001 vs non-inflamed; *P<0.05, **P<0.01 vs inflamed/vehicle.
Discussion
The fatty acid amide palmitoylethanolamide was previously found to act as an anti-inflammatory agent in various rodent models of inflammation in a preventive protocol (Mazzari et al., 1996; Conti et al., 2002). The present study shows, for the first time, that palmitoylethanolamide reduces the oedema induced by carrageenan, when the compound was given after inflammation was established. Palmitoylethanolamide had dose- and time-dependent action on the oedema, leading to earlier recovery of the inflamed paw, and the highest dose, 10 mg kg−1, caused disappearance of the oedema within four days. The anti-oedema effect was not associated with any changes in overt behaviour, indicating that all doses were well tolerated; the body weight gain of palmitoylethanolamide-treated rats was similar to that of control animals (data not shown).
These findings extend our knowledge of the therapeutic anti-inflammatory efficacy of cannabinoids and related compounds. To date, this information is restricted to a synthetic cannabinoid acid, 11-11-dimethylheptyl-Δ8-tetrahydrocannabinol-11 oic acid (CT3), a derivative of Δ9-tetrahydrocannabinol, and to cannabidiol, one of the major non-psychoactive components of Cannabis sativa. Zurier et al. (1998) reported that CT3 significantly reduced the accumulation of leukocytes in the pouch fluid induced by IL-1β and TNF-α and reduced the severity of adjuvant-induced polyarthritis. More recently, Malfait et al. (2000) showed that, in murine collagen-induced arthritis, cannabidiol administered after the onset of clinical symptoms, blocked the progression of arthritis, protected the joints against severe damage, reduced type II collagen-specific proliferation and interferon-γ (INF-γ) production from lymph node cells, and reduced the release of TNF-α by knee synovial cells. Palmitoylethanolamide and anandamide both attenuated the viscero–visceral hyperreflexia induced by inflammation of the rat urinary bladder when administered after the inflammatory response in the bladder wall (Jaggar et al., 1998a).
So far we have demonstrated the curative efficacy of palmitoylethanolamide only in a model of acute inflammation; further studies will verify whether palmitoylethanolamide, as like CT3 and cannabidiol, is also active in chronic inflammation. This effect was not reversed by the specific CB2 antagonist SR144528, so the curative effect of palmitoylethanolamide seems not to be mediated by a CB2-sensitive mechanism, even if its preventive effects appear to involve the CB2 receptor (Conti et al., 2002; Calignano et al., 2001).
NSAIDs act inhibiting COX activity. Two isoforms of COX are known: the constitutive enzyme COX-1 is responsible for the physiological production of eicosanoids that help maintain normal renal function, gastric mucosa integrity and hemostasis (Griswold & Adams, 1996), and the inducible isoform COX-2 is upregulated in inflamed tissue and is therefore thought to be responsible for the enhanced production of eicosanoids.
We found that four days after induction of inflammation the residual paw oedema was still associated with an increase in tissue COX activity. Palmitoylethanolamide (10 mg kg−1), like indomethacin, brought COX activity down to the same value as in non-inflamed tissues, indicating that the anti-inflammatory activity of palmitoylethanolamide might depend, at least in part, on suppression of eicosanoid synthesis in the tissue (Pairet & Engelhardt, 1996). It is surprising that in the inflamed paw indomethacin, a non-selective COX inhibitor, failed to lower COX activity to below that found in non-inflamed tissues. However, when the same dose of indomethacin was given orally to control rats it inhibited the basal paw COX activity by 40% (data not shown); thus we suggest that the indomethacin concentrations reached in inflamed paw tissue were too low to reduce the COX activity below the basal value. Future studies are now in progress to better define the effect of palmitoylethanolamide on the COX system.
Other mediators of inflammation include NO and activated oxygen species which per se or together with NO are involved in membrane lipid peroxidation and in the consequent tissue damage in inflammatory conditions. NO in particular appears to be involved in the acute inflammatory response to injection of carrageenan into the rat hindpaw: NOS inhibitors attenuated the oedema (Ialenti et al., 1992), the oedema was associated with an increase in NO2−/NO3− in the paw exudate, and iNOS mRNA and iNOS protein were enhanced respectively between 3 and 10 h and 6 and 10 h after carrageenan in inflamed tissue (Salvemini et al., 1996). We found that four days after carrageenan injection the levels of NO end-products (NO2−/NO3−) were still high. This was associated with an increase of eNOS content; at this time no iNOS was expressed, so we suggest that NO2−/NO3− derive solely from the eNOS isoform. The iNOS isoform was upregulated in earlier phases of inflammation: Western blot analysis 3, 6 and 10 h after carrageenan showed that iNOS was detectable at 3 h, increased at 6 and 10 h and had a very low level at 24 h. Comparing the levels of NO end-products on the fourth day and 6 h after carrageenan, we found higher production of NO at 6 h. Since at this time eNOS too was higher in inflamed paw tissues than in controls, we assume that at 6 h the NO products derive from both isoforms. The involvement of the iNOS isoform 6–8 h after carrageenan was shown by other authors (Osborne & Coderre, 1999; Omote et al., 2001), employing selective iNOS inhibitors. Palmitoylethanolamide (10 mg kg−1), like indomethacin, reduced the increase of eNOS and iNOS protein and NO2−/NO3− levels both at 6 h and on the fourth day, indicating that the decrease in NO products could be due to a reduction of NOS protein content.
For the first time we have shown that palmitoylethanolamide inhibits NO production in vivo; this finding agrees with Ross et al. (2000) who demonstrated in vitro the inhibitory effect of palmitoylethanolamide on lipopolysaccharide mediated NO release in the murine macrophage cell line RAW264.7.
Salvemini et al. (1996) reported that neutrophil infiltration in response to i.pl. carrageenan injection, and following activation of NADPH oxidase, generates an oxygen respiratory burst giving rise to oxygen-derived free radicals which promote lipid peroxidation.
In this study, 4 days after carrageenan injection the levels of MDA, a final product of lipid peroxidation, were still high. Palmitoylethanolamide, like indomethacin, abolished this enhancement, suggesting that the anti-inflammatory effect of palmitoylethanolamide may partially depend on its ability to lower the content of oxygen free radicals in inflamed tissues. It remains to be ascertained whether this effect can be ascribed to an antioxidant effect of palmitoylethanolamide, as shown for other cannabinoids (Hampson et al., 1998), and/or to its ability to block NO production.
In summary, our findings extend previous observations of palmitoylethanolamide's anti-inflammatory activity, showing its curative efficacy in a rat model of acute inflammation induced by carrageenan, and indicate that this anti-inflammatory action may be linked to its effects on various mediators involved in inflammation. Moreover, since palmitoylethanolamide may possibly behave as an autacoid, locally modulating mast-cell activation in response to inflammatory stimuli, we intend to study whether modulators of endogenous palmitoylethanolamide might serve as anti-inflammatory agents.
Acknowledgments
This work was supported by grants from the Italian Ministry for Education, University and Research (M.I.U.R.). The authors thank Dr Francis Barth (Sanofi-Synthelabo, Montpellier, France) for the gift of SR144528 and J.D. Baggott for editing.
Abbreviations
- COX
cyclo-oxygenase
- 5-HT
5-hydroxytryptamine
- INF
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- NO
nitric oxide
- NOS
nitric oxide synthase
- NSAID
nonsteroidal anti-inflammatory drug
- PG
prostaglandin
- PMN
polymorphonuclear neutrophil
- SDS
sodium dodecylsulphate
- TNF
tumor necrosis factor
References
- ALOE L., LEON A., LEVI-MONTALCINI R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Action. 1993;39:C145–C147. doi: 10.1007/BF01972748. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN T.W., MARSHALL P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implication of endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., GIUFFRIDA A., PIOMELLI D. Control of pain initiation by endogenous cannabinoid. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., PIOMELLI D. Antinociceptive activity of the endogenous fatty acid amide, palmitoylethanolamide. Eur. J. Pharmacol. 2001;419:191–198. doi: 10.1016/s0014-2999(01)00988-8. [DOI] [PubMed] [Google Scholar]
- CONTI S., COSTA B., COLLEONI M., PAROLARO D., GIAGNONI G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br. J. Pharmacol. 2002;135:181–187. doi: 10.1038/sj.bjp.0704466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI ROSA M., GIROUD J.P., WILLOUGHBY D.A. Studies of mediators of acute inflammatory response induced in rats in different sites by carrageenin and turpentine. J. Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- DI ROSA M., WILLOUGHBY D.A. Screens for anti-inflammatory drugs. J. Pharm. Pharmacol. 1971;23:297–300. doi: 10.1111/j.2042-7158.1971.tb08661.x. [DOI] [PubMed] [Google Scholar]
- FANTONE J.C., WARD P.A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am. J. Pathol. 1982;110:963–968. [PMC free article] [PubMed] [Google Scholar]
- GIERSE J.K., KOBOLDT C.M., WALKER M.C., SIEBERT K., ISAKSON P.C. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem. J. 1999;339:607–614. [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN G., TAO Q., ABOOD M.E. Cloning and pharmacological characterization of the rat CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- GRISWOLD D.E., ADAMS J.L. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): rationale for selective inhibition and progress to date. Med. Res. Rev. 1996;16:181–186. doi: 10.1002/(SICI)1098-1128(199603)16:2<181::AID-MED3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- HANDY R.L.C., MOORE P.K. A comparison of the effect of L-NAME, 7-NI and L-NIL on carrageenan-induced hindpaw oedema and NOS activity. Br. J. Pharmacol. 1998;123:1119–1126. doi: 10.1038/sj.bjp.0701735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPSON A.J., GRIMALDI M., AXELROD J., WINK D. Cannabidiol and Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGGAR S.I., HASNIE F.S., SELLATURAY S., RICE A.S.C. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998a;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- JAGGAR S.I., SELLATURAY S., RICE A.S.C. The endogenous cannabinoid anandamide, but not the CB2 ligand palmitoylethanolamide, prevents the viscero-visceral hyperreflexia associated with inflammation of the rat urinary bladder. Neurosci. Lett. 1998b;253:123–126. doi: 10.1016/s0304-3940(98)00621-1. [DOI] [PubMed] [Google Scholar]
- IALENTI A., IANARO A., MONCADA S., DI ROSA M. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharmacol. 1992;211:177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- LAMBERT D.M., DI MARZO V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic. Curr. Med. Chem. 1999;6:757–773. [PubMed] [Google Scholar]
- MALFAIT A.M., GALLILY R., SUMARIWALLA P.F., MALIK A.S., ANDREAKOS E., MECHOULAM R., FELDMAN M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZARI S., CANNELLA R., PETRELLI L., MARCOLONGO G., LEON A. N-(2-hydroxyethyl) hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996;300:227–236. doi: 10.1016/0014-2999(96)00015-5. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., SCHILLING R.J., SALVEMINI D., MOORE W.M., CURRIE M.G. A fluorimetric assay for the measurement of nitrite in biological samples. Anal. Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- MONCADA S., HIGGS A. The L-arginine-nitric oxide pathway. New Engl. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–141. [PubMed] [Google Scholar]
- OHKAWA H., OHISHI N., YAGI K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- OMOTE K., HAZAMA K., KAWAMATA T., KAWAMATA M., NAKAYAKA Y., TORIYABE M., NAMIKI A. Peripheral nitric oxide in carrageenan-induced inflammation. Brain Res. 2001;912:171–175. doi: 10.1016/s0006-8993(01)02733-0. [DOI] [PubMed] [Google Scholar]
- OSBORNE M.G., CODERRE T.J. Effects of intrathecal administration of nitric oxide synthase inhibitors on carrageenan-induced thermal hyperalgesia. Br. J. Pharmacol. 1999;126:1840–1846. doi: 10.1038/sj.bjp.0702508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAIRET M., ENGELHARDT G. Distinct isoforms (COX-1 and COX-2) of cyclooxygenase: possible physiological and therapeutic implications. Fundam. Clin. Pharmacol. 1996;10:1–15. doi: 10.1111/j.1472-8206.1996.tb00144.x. [DOI] [PubMed] [Google Scholar]
- RADI R., BECKMAN J.S., BUSH K.M., FREEMAN B.A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., BROCHIE H.C., PERTWEE R.G. Inhibition of nitric oxide production in RAW 264.7 macrophages by cannabinoids and palmitoylethanolamide. Eur. J. Pharmacol. 2000;401:121–130. doi: 10.1016/s0014-2999(00)00437-4. [DOI] [PubMed] [Google Scholar]
- RUBBO H., RADI R., TRUJILLO M., TELLERI R., KALYANARAMAN B., BARNES S., KIRK M., FREEMAN B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P.S., BOURDON D.M., MARINO M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEBERT K., ZHANG Y., LEAHY K., HAUSER S., MASFERRER J., PERKINS W., LEE L., ISAKSON P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZURIER R.B., ROSSETTI R.G., LANE J.H., GOLDBERG J.M., HUNTER S.A.H., BURSTEIN S.H. Dimethylheptyl-THC-11 oic acid a nonpsychoactive anti-inflammatory agent with a cannabinoid template structure. Arthritis Rheum. 1998;41:163–170. doi: 10.1002/1529-0131(199801)41:1<163::AID-ART20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]


