Abstract
The endogenous fatty acid anandamide (AEA) is a partial agonist at cannabinoid CB1 receptors and has been reported to be a full agonist at the recombinant vanilloid receptor, VR1.
Whole cell voltage clamp techniques were used to examine the efficacy of AEA and related analogues methanandamide and N-(4-hydroxyphenyl)-arachidonylamide (AM404) at native VR1 receptors in acutely isolated mouse trigeminal neurons.
Superfusion of the VR1 agonist capsaicin onto small trigeminal neurons voltage clamped at +40 mV produced outward currents in most cells, with a pEC50 of 6.3±0.1 (maximum currents at 10–30 μM).
AEA produced outward currents with a pEC50 of 5.6±0.1. Maximal AEA currents (30–100 μM) were 38±2% of the capsaicin maximum. AEA currents were blocked by the VR1 antagonist capsazepine (30 μM), but unaffected by the CB1 antagonist SR141716A (1 μM).
Methanandamide and AM404 were less potent than AEA at activating VR1. Methanandamide (100 μM) produced currents 37±6% of the capsaicin maximum, the highest concentration of AM404 tested (100 μM) produced currents that were 55±9% of the capsaicin maximum.
Capsazepine abolished the currents produced by AM404 (100 μM) and strongly attenuated (>70%) those produced by methanandamide (100 μM).
Co-superfusion of AEA (30 μM, methanandamide (100 μM) or AM404 (100 μM) with capsaicin (3 μM) resulted in a significant reduction of the capsaicin current.
These data indicate that AEA, methanandamide and AM404 activate native VR1 receptors, but that all three compounds are partial agonists when compared with capsaicin.
Keywords: VRI, capsaicin, efficacy, methanandamide, AM404, nociception
Introduction
The endogenous fatty acid anandamide (AEA, Devane et al., 1992), activates cannabinoid CB1 receptors in sensory neurons, resulting in inhibition of nociception. (Calignano et al., 1998; Richardson et al., 1998). AEA also activates the vanilloid receptor VR1 (Caterina et al., 1997; Zygmunt et al., 1999), a protein known to be primarily activated by noxious stimuli, including heat, hydrogen ions and capsaicin, the pungent ingredient found in chili peppers. The possible concurrent activation of these two receptors, which may be expressed on the same primary afferent fibers (Ahluwalia et al., 2000; Tognetto et al., 2001), raises interesting questions regarding the physiological role of AEA in nociceptive function and its effect on sensory neuronal activity. In order to address these questions, it is important that the interactions of AEA with its cellular effectors such as CB1 receptors and VR1 be unambiguously characterized.
AEA is considered to be a partial agonist at CB1 receptors (Mackie et al., 1993; Burkey et al., 1997; Glass & Northup, 1999), although it can exhibit similar maximal effectiveness to efficacious synthetic cannabinoids at native CB1 receptors (Shen et al., 1996; Twitchell et al., 1997; Morisset & Urban, 2001). AEA and capsaicin appear to share a similar binding site on VR1 (Jordt & Julius, 2002) but the relative efficacy of AEA at VR1 has been the subject of some controversy. Initial reports suggested that AEA was a partial agonist at recombinant VR1 (Zygmunt et al., 1999; Hwang et al., 2000) while a number of subsequent studies have demonstrated that AEA is apparently a full agonist at recombinant rat and human VR1 (Smart et al., 2000; de petrocellis et al., 2000; Ralevic et al., 2001; Ross et al., 2001). The latter studies have used measurements of elevations of intracellular calcium ([Ca2+]i) or 45Ca2+ uptake as an indirect method of determining VR1 receptor activation. Elevations of [Ca2+]i resulting from VR1 activation are likely to be significantly amplified in any cell, including heterologous expression systems (see Discussion), so it seemed possible that the high potency and efficacy of AEA reported in these studies may not be a true reflection of the efficacy and potency of AEA at VR1. In order to better define the potency and efficacy of AEA at VR1, we have used whole cell patch clamp techniques to examine the effectiveness of AEA in activating native VR1 ion channels in isolated mouse trigeminal sensory neurons. We found that AEA, and its analogues methanandamide and AM404, all act as partial agonists when compared with the prototypical agonist of VR1, capsaicin.
Methods
All experiments in this study were conducted in accordance with protocols approved by the University of Sydney Animal Ethics Committee. C57B16/J male mice were anaesthetized with halothane (4%), and killed by decapitation. The trigeminal ganglia were removed and placed in ice-cold physiological saline containing (mM) NaCl 126; KCl 2.5; CaCl2 2.5; MgCl2 10; NaH2PO4 1.2; NaHCO3 24; and glucose 10, gassed with 95% O2-5% CO2. Cells were prepared as previously described (Borgland et al., 2002). Briefly, ganglia were cut up with iridectomy scissors and incubated at 32–34°C for 20 min in physiological saline. The ganglia were then transferred to physiological saline containing collagenase for 10 min. The ganglia were then transferred to oxygenated modified HEPES buffered saline (HBS) containing 20 units ml−1 papain and incubated at 32–34°C for 20 min. The modified HBS contained (mM): NaCl 140; KCl 2.5; CaCl2 2.5; MgCl2 10; HEPES 10; glucose 10; pH 7.3 (NaOH); 330±5 mosmol. The digestion was terminated with addition of HBS containing 1 mg ml−1 bovine serum albumin (BSA) and 1 mg ml−1 trypsin inhibitor. Minced ganglia were washed free of enzyme and enzyme inhibitors with room temperature modified HBS. Cells were released by gentle trituration through decreasing bore, silanized Pasteur pipettes with fire-polished tips. The cells were plated onto plastic culture dishes and kept at room temperature in modified HBS. Cells remained viable for up to 10 h after dissociation.
Electrophysiological recording
Ionic currents from mouse trigeminal neurons were recorded in the whole-cell configuration of the patch-clamp method (Hamill et al., 1981) at room temperature (22–24°C). Dishes were continually perfused with HBS containing (mM) NaCl 140; KCl 2.5; CaCl2 2.5; MgCl21; HEPES 10; glucose 10; pH 7.3 (NaOH), 330±5 mosmol l−1. Except where noted, extracellular solutions contained BSA (0.05%) to facilitate reversal of AEA effects. BSA was not included in solutions containing AEA unless noted. For recording of currents through Ca2+ channels (ICa), the extracellular solution contained (mM) tetraethylammonium chloride (TEACI) 140; CsCl 2.5; HEPES 10; MgCl2 1, BaCl22; glucose 10, pH 7.2 (CsOH) 330±5 mosmol l−1. Recordings were made with fire polished borosilicate pipettes of resistance approximately 2 MOhm when filled with intracellular pipette solution which contained (in mM) CsCl 40; EGTA 10; HEPES 10; Csmethanesulfonate 90; MgATP 5; pH 7.3 (with CsOH), 290±5 mosmol l−1.
Recordings were made using an Axopatch 1D or Axopatch 200B amplifier using pCLAMP, and Axotape acquisition software (Axon Instruments, Union City, CA, U.S.A.). For ICa recordings currents were sampled at 20–50 KHz, recordings of currents through VR1 were sampled at 500 Hz. Currents were recorded on hard disk for later analysis. Series resistance ranged from 2–7 MOhm and was compensated by 80% in all experiments. Cell capacitance was compensated manually by nulling the capacitive transient evoked by a 20 mV pulse from −90 mV and ranged from 6–20 pF.
We have previously characterized several populations of trigeminal neurons in mice that are defined by the presence or absence of a prominent low voltage activated ‘T-type' ICa (LVA ICa, Borgland et al., 2001; 2002). Type 1 cells do not express a prominent LVA ICa and are usually capsaicin sensitive whereas Type 2 cells have a prominent LVA ICa and are never sensitive to capsaicin. Therefore, the types of ICa in each cell was determined by stepping the membrane potential from a holding potential of −90 mV to test potentials between −60 mV and +60 mV in 10 mV increments. Type 2 cells were defined as having an ICa evoked by step from −90 to −40 mV greater than 15% of the maximal inward current and were discarded. The remaining cells were then voltage clamped at +40 mV to measure currents through VR1. A holding potential of +40 mV was chosen to minimize calcium entry, as previous studies have demonstrated that desensitization of capsaicin evoked currents in dorsal root ganglion neurons was significantly less at holding potentials of +40 mV versus a more physiological −60 mV (Piper et al., 1999). Under our recording conditions VR1 receptor desensitization in response to brief capsaicin exposures was minimal. Three consecutive applications of 10 μM capsaicin applied 3 min apart produced currents 102±10 and 106±20% of the first application (n=6). Cells were exposed to drugs via a series of flow pipes positioned about 200 μM from the cells. Drug solutions were switched using a Warner SF 77B rapid solution exchanger, which produced solution exchange times of less than 100 ms.
Data analysis
Currents produced by AEA and other test drugs were normalized to the currents produced by maximally effective concentrations of capsaicin (10–30 μM) in the same cells. Concentration response relationships were constructed using either consecutive or cumulative addition of 2–3 concentrations of a single agonist to each cell. Normalized concentration-response data was pooled and fitted to a logistic equation using the software package GraphPad Prism v.3. Significant differences between means were tested, using a paired or unpaired, two tailed Student's t-test as noted. All data are expressed as mean±s.e.mean unless otherwise indicated.
Drugs and chemicals
Capsaicin, capsazepine and anandamide (in water soluble emulsion) were from Tocris Cookson (Bristol, U.K.). R-1 Methanandamide and AM404 were from Cayman Chemical Company (Ann Arbor MI, U.S.A.). BSA and trypsin inhibitor (chicken egg white ovomucoid, type II-O) were from Sigma Australia. Papain and Collagenase were from Worthington Biochemical Corporation (Freehold, NJ, U.S.A.). Buffer salts were from either BDH or Sigma. SR141716A (N-piperidino-5-(4-chlorophenyl)-I-(2,4-dichlorophenyl)-4-methyl-3-pyrazole carboxamide) was kindly donated by Sanofi Recherche. OL-53 (1-(oxazolo[4,5-b]pyridin-2-yl)-1-oxo-6-phenylhexane (Boger et al., 2000) was kindly donated by Dr Dale L. Boger (The Scripps Research Institute, La Jolla, CA, U.S.A.).
Results
Anandamide activates VR1 receptors in sensory neurons
Application of AEA (30 nM–100 μM) to Type 1 trigeminal neurons voltage clamped at +40 mV resulted in outward currents in 56 of 61 cells (see Methods). All of these cells subsequently responded to the VR1 agonist capsaicin. The activation of outward currents by AEA was relatively slow, and could take up to 2 min to reach a steady state (Figure 1a). Wash of AEA from cells was also very slow (taking up to 15 min) and could only be achieved by washing the cells with buffer containing BSA (0.05%, data not shown). Inclusion of BSA (0.05% in the perfusion buffer containing AEA resulted in a dramatic reduction of the effectiveness of AEA (1–10 μM, P<0.005), but not capsaicin (100 nM–10 μM), to activate outward currents in sensory neurons (Figure 2, n=6 for each). The currents induced by capsaicin (10 μM) in the presence of BSA were 3300±500 pA (n=8) in the absence of BSA they were 2800±450 (n=9).
Figure 1.
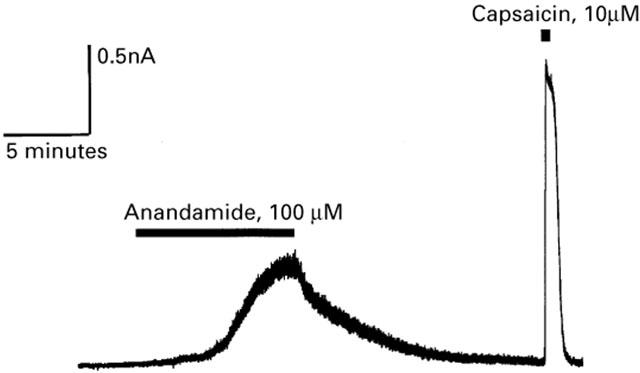
Anandamide activates outward currents in capsaicin-sensitive mouse trigeminal sensory neurons. Selected trigeminal neurons were voltage clamped at +40 mV, as described in Methods. Superfusion of AEA (100 μM) produced a slowly developing outward current that reversed slowly on wash. Subsequent superfusion of capsaicin produced a large, rapidly developing outward current in the same neuron. This experiment is typical of 56 small trigeminal neurons challenged with both AEA and capsaicin, five additional cells did not respond to either agonist.
Figure 2.
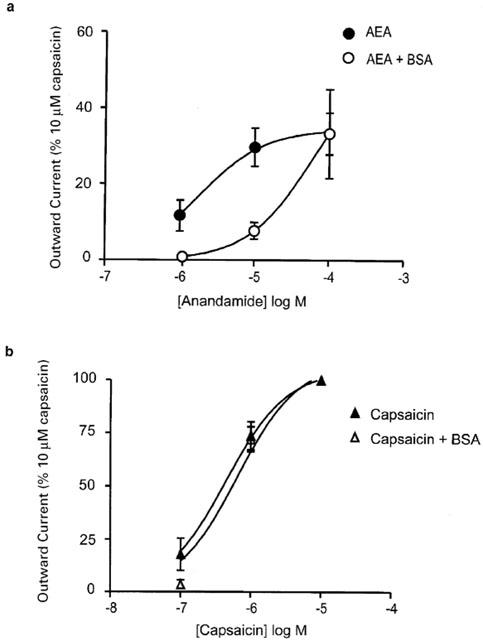
The activity of anandamide, but not capsaicin, is antagonized by bovine serum albumin. Trigeminal sensory neurons were voltage clamped at +40 mV and superfused with anandamide and capsaicin in the presence or absence of 0.05% BSA. (a) Concentration-response relationships for AEA in the absence and presence of BSA (0.5 mg ml−1). BSA strongly inhibited the outward currents produced by submaximally effective concentrations of AEA. Each point represents the mean±s.e.mean of six cells, the currents produced by AEA were normalized to that produced by 10 μM capsaicin. (b) Concentration-response relationships for capsaicin in the absence and presence of BSA (0.5 mg ml−1). Capsaicin currents were not affected by the inclusion of BSA. Each point represents the mean±s.e.mean of six cells.
Outward currents produced by AEA (30 μM) were completely blocked following a 2 min pre-application of the vanilloid receptor antagonist capsazepine (30 μM Figure 3a, n=6). AEA is also an agonist at the cannabinoid CB1 receptor and we examined whether the CB1 receptor activity of AEA played any role in the AEA-induced activation of VR1. The CB1 antagonist SR141716 (1 μM) was applied 2 min before the co-application of SR141716 and AEA (10 μM). The addition of the CB1 antagonist did not alter outward currents produced by AEA (Figure 3b, n=6). Superfusion of SR141716 (1 μM) and capsaicin (300 nM) after a 2-min pre-incubation with SR141716 (1 μM) produced an outward current that was similar to that produced by superfusion of 300 nM capsaicin alone in the same cells (Figure 3c). Superfusion of SR141716 alone did not produce any currents.
Figure 3.
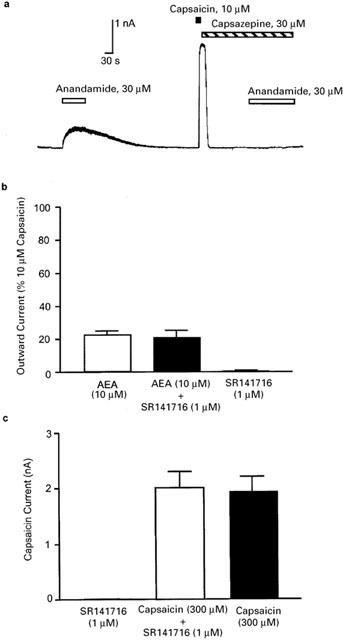
Anandamide acts via vanilloid and not cannabinoid receptors to produce outward currents in mouse trigeminal neurons. Trigeminal sensory neurons were voltage clamped at +40 mV and superfused with AEA and capsaicin alone or in the presence of the VR1 antagonist capsazepine or the CB1 receptor antagonist SR141716. (a) Representative trace showing that capsazepine (30 μM) blocks AEA-induced outward currents in a AEA and capsaicin-sensitive neuron. Typical of six similar experiments. (b) Summary data of the effect of a 2-min preincubation of SR141716 on currents induced by AEA (10 μM). The currents were normalized to those produced by 10 μM capsaicin, each bar represents the mean±s.e.mean of six cells. (c) Summary data of the effect of a 2-min preincubation of SR141716 on currents induced by capsaicin. The same cells were challenged with capsaicin, then superfused with SR141716 and rechallenged with SR141716+capsaicin. The bars represent the mean±s.e.mean of six cells.
Anandamide has a lower maximum effect than capsaicin at VR1 receptors in trigeminal neurons
The relative efficacy and potency of AEA and capsaicin at VR1 in mouse trigeminal neurons was investigated by constructing concentration-response curves, as outlined in the Methods. Capsaicin activated VR1 with an EC50 of 300 nM (pEC50 6.3±0.1) with maximal capsaicin currents observed at 10–30 μM (Figure 4). AEA activated VR1 currents were normalized to maximum currents induced by capsaicin (10–30 μM) in the same cell. AEA activated VR1 currents with an EC50 of 3 μM (pEC50 5.3±0.1, Figure 4). The maximum outward current produced by AEA (30–100 μM) was 38±2% of the maximal capsaicin response.
Figure 4.
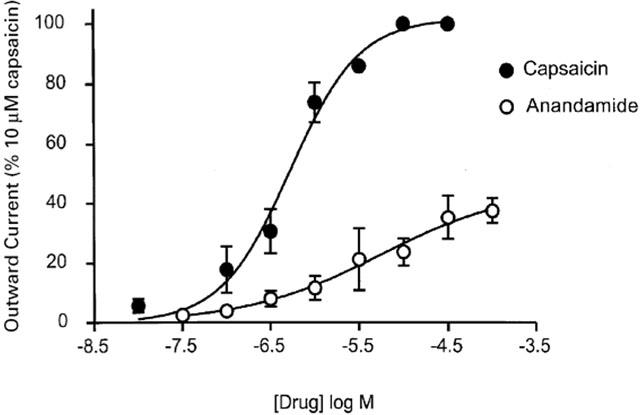
Anandamide activation of VR1 is concentration-dependent, but less efficacious than capsaicin. Selected trigeminal neurons were voltage clamped at +40 mV, as described in Methods. Concentration-response curves for capsaicin and AEA to activate VR1 in trigeminal neurons. In each cell, capsaicin or AEA responses were normalized to that produced by 10–30 μM capsaicin in that cell. The EC50 for capsaicin was 300 nM, for AEA 3 μM. AEA produced maximal currents 38±2% of maximal capsaicin currents. Each point represents the mean±s.e.mean of at least six cells, error bars are contained within the symbol for several points.
We also examined the effects of the cannabinoid analogues methanandamide and AM404 on VR1 receptors in trigeminal neurons. Superfusion of methanandamide or AM404 produced concentration-dependent outward currents at +40 mV in trigeminal neurons, although both compounds were less potent than AEA or capsaicin (Figure 5a). The maximal current produced by methanandamide was similar to that of AEA (37±6% of the capsaicin maximum). The outward currents produced by highest concentration of AM404 tested was 55±9% of that produced by high concentrations of capsaicin, and was significantly greater than the maximum currents produced by AEA or methanandamide (P<0.002, Figure 5a). A 2-min preapplication of capsazepine (30 μM, Figure 5b) significantly attenuated the currents produced by methanandamide (100 μM, currents were 27% of control methanandamide currents, n=5, P<0.01, unpaired t-test, Figure 5c) and AM404 (100 μM, currents were 0.1% of control AM404 currents, n=5, P<0.01, unpaired t-test, Figure 5c).
Figure 5.
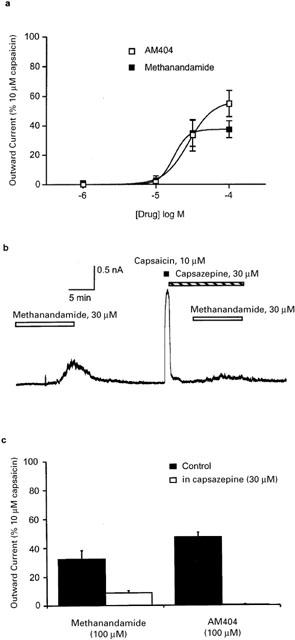
Anandamide analogs methanandamide and AM404 activates VR1 in sensory neurons. Trigeminal sensory neurons were voltage clamped at +40 mV and superfused with methanandamide or AM404 and the currents produced compared with capsaicin (10 μM). (a) Concentration-response curves for methanandamide and AM404 to activate outward currents in trigeminal neurons, each response was normalized to that produced by 10–30 μM capsaicin in the same cell. Methanandamide produced maximum currents of 37±6% of maximal capsaicin currents, at the highest concentration of AM404 tested the currents were 55±9% of the capsaicin maximum. Each point represents the mean±s.e.mean of at least six cells. (b) Representative trace showing that capsazepine (30 μM) blocks most of the methanandamide-induced outward currents in a capsaicin-sensitive neuron. (c) Summary data of the effect of a 2-min preincubation of capsazepine (30 μM) on currents produced by methanandamide (100 μM) and AM404 (100 μM), determined as illustrated in (b). Capsazepine significantly (P<0.01) blocked the currents produced by both agonists. Outward currents were normalized to those produced by 10 μM capsaicin, each bar represents the mean±s.e.mean of six cells.
Anandamide is a partial agonist at VR1 receptors in trigeminal neurons
Preceding experiments suggest that anandamide acts as a partial agonist at VR1 receptors in sensory neurons. To explicitly test this, cells were exposed to 3 μM capsaicin, followed by co-application of 30 μM AEA+capsaicin (3 μM) (Figure 6a). Co-application of AEA with capsaicin significantly reduced the currents produced by capsaicin (n=6, P<0.01, paired t-test, Figure 6b). Subsequent application of 3 μM capsaicin following wash of AEA produced similar currents to the first application, indicating that decrease in current produced by AEA was not simply due to VR1 desensitization (Figure 6a). Co-application of methanandamide (100 μM) or AM404 (100 μM) with capsaicin (3 μM) produced similar results (n=5–6 for each, Figure 6b). These results confirm that AEA, and related analogues methanandamide and AM404 act as partial agonists at native mouse VR1 receptors.
Figure 6.
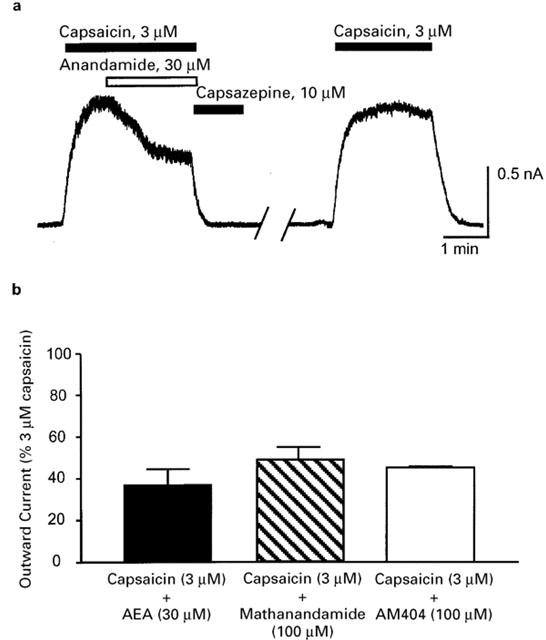
Anandamide, methanandamide and AM404 inhibit capsaicin-stimulated VR1 currents. Trigeminal sensory neurons were voltage clamped at +40 mV and superfused with capsaicin (3 μM) alone until the outward current reached a steady state and then AEA (30 μM), methanandamide (100 μM) or AM404 (100 μM) were co-superfused with capsaicin. (a) An example trace illustrating the effects of co-superfusion of AEA (30 μM) with capsaicin (3 μM). Approximately 4 min of wash have been removed for clarity. This is typical of six cells. (b) Bar chart summarizing the effect of co-superfusion AEA, methanandamide and AM404 on the currents induced by capsaicin. Each drug significantly (P<0.01) reduced the capsaicin (3 μM) current. Each bar represents the mean±s.e.mean of 5–6 cells.
Discussion
This study demonstrates that the endogenous cannabinoid anandamide acts as a partial agonist at native VR1 receptors in mouse trigeminal ganglion neurons. This conclusion is based on two main findings. Firstly, the currents induced by maximally effective concentrations of AEA were only about 40% of those induced by high concentrations of capsaicin. Secondly, co-superfusion of high concentrations of AEA significantly reduced the currents produced by a high concentration of capsaicin. Two AEA analogues, methanandamide and AM404, also appear to be partial agonists at VR1 receptors in trigeminal ganglion neurons. A previous study demonstrated that AEA apparently had a substantially lower efficacy than capsaicin to activate single VR1 channels, but the effectiveness of the two substances were not directly compared (Hwang et al., 2000). This study provides the first direct comparison of the efficacy of AEA and capsaicin at native VR1 receptors and the results suggest that previous uncertainty about its efficacy may have resulted, at least in part, from the indirect methods used to measure VR1 activation.
The effects of capsaicin, AEA and AM404 were completely inhibited by capsazepine. Although capsazepine has been reported to inhibit ICa and calcium release activated currents (ICRAC) with an EC50 of less than 10 μM (Docherty et al., 1997; Fischer et al., 2001), these currents are not directly activated by vanilloid agonists, nor is their activity likely to contribute significantly to background currents recorded at a holding potential of +40 mV. The effects of capsaicin, AEA and AM404 were thus likely mediated via VR1 receptors. The effects of methanandamide were largely (>70%) but not completely inhibited by capsazepine. It is possible that capsazepine does not fully compete with the methanandamide binding site on VR1, although there is no other evidence that methanandamide activates VR1 at a site distinct from AEA or capsaicin. We have preliminary data from mice in which the VR1 receptor has been deleted which indicate that high concentrations of methanandamide produce a small, capsazepine-insensitive outward current in the same population of cells as examined in this study (Roberts, Christie & Connor, unpublished observations). The nature of this additional current is under investigation.
Although VR1 can be activated by a number of very different stimuli such as heat, low pH and vanilloids (Tominaga et al., 1998), AEA and capsaicin appear to share a binding site on VR1 that is at least partially distinct from regions of the receptor that mediate activation by heat or pH (Jordt & Julius, 2002). Thus the partial agonist properties of AEA with respect to capsaicin are not likely to arise from a distinct mechanism of VR1 activation by the two agonists, but reduced capacity of AEA to promote the conformational change in VR1 necessary for activation of the channel.
Anandamide was identified as a VR1 agonist through its ability to cause a capsazepine-sensitive relaxation of visceral blood vessels, a process mediated by VR1-dependent release of the neuropeptide CRGP from sensory neuron nerve terminals (Zygmunt et al., 1999). Capsaicin, AEA and methanandamide each produced complete relaxation of the preconstricted blood vessels, but electrophysiological experiments with the cloned VR1 receptors expressed in HEK293 cells and Xenopus oocytes led the authors to estimate the efficacy of AEA at about 30–50% of that of capsaicin (Zygmunt et al., 1999), and methanandamide as an even lower efficacy agonist. As discussed by Zygmunt et al. (1999), the simplest explanations for the differences between the agonist efficacies in native tissues and at the cloned receptors was that in the native tissues there could be a significant receptor reserve for VR1-dependent relaxation, a significant cellular amplification of the VR1 receptor activation and/or proteins associated with native VR1 receptors that changed their responses to AEA and analogues. Later studies have shown that AEA can be as efficacious as capsaicin in activating VR1-dependent processes in some (Ralevic et al., 2001), but not all sensory nerves (Tucker et al., 2001). We have used a direct measurement of VR1 activation to conclude that AEA is a partial agonist; determining the current passed by the AEA-activated VR1 channel.
The studies reporting high efficacy for AEA at recombinant human (Smart et al., 1999; de petrocellis et al., 2000) or rat (Ralevic et al., 2001) VR1 have used indirect measures of channel activity, usually elevation of total [Ca2+]i following stimulation of VR1 in HEK293 or CHO cells. There are several possible explanations for the apparently higher efficacy of AEA at VR1 expressed in heterologous expression systems, and these are essentially the same as those advanced by Zygmunt et al. (1999), and outlined above. Firstly, VR1 pharmacology may differ between HEK293 or CHO cells and native sensory neurons, and AEA may be a full agonist at VR1 in these cells. Although expression of VR1 by itself is sufficient to form a heat and capsaicin activated channel (Caterina et al., 1997), native sensory neurons likely contain other transient receptor potential-superfamily channels that interact with VR1 and may give rise to a native pharmacology different to that of VR1 expressed in heterologous systems (Smith et al., 2002). However, as the binding site for AEA and capsaicin on VR1 appears to be the same (Jordt & Julius, 2002), it is likely that AEA and capsaicin potency and efficacy would change in parallel across different systems. Differences in post-translational modification such as glycosylation could also lead to differences in channel properties in sensory neurons vs cell lines or oocytes (Kedei et al., 2001).
A second difference between the present study and those that utilize indirect measurements of VR1 activity is that there is likely to be significant signal amplification in these studies. Activation of VR1 will produce an elevation of [Ca2+]i that is an integrated signal from a number of processes, only some of which are directly proportional to initial agonist activation of VR1. VR1 activation will lead to Ca2+ entry via plasma membrane VR1 channels, and also Ca2+ release from intracellular stores in the endoplasmic reticulum, where functional VR1 is located in both sensory neurons and cells heterologously expressing VR1 (Jahnel et al., 2001; Olah et al., 2001b). The release of Ca2+ from the intracellular stores will likely stimulate Ca2+-permeable plasma membrane channels that are expressed in HEK-293 cells (Wu et al., 2000; 2002), and perhaps further release of Ca2+ by Ca2+-mediated stimulation of phospholipase C. These processes will amplify the initial Ca2+ signal through VR1. Considering this, it is notable that the EC50 for capsaicin in the indirect assays of VR1 activation is generally of the order of 10–80 nM (Smart et al., 2000; Szallasi et al., 1999; de petrocellis et al., 2000; Jerman et al., 2000; Ralevic et al., 2001), which is up to 50 times less than the potency for capsaicin to maximally activate VR1 currents at the recombinant receptor expressed in the same cells (100–600 nM, Tominaga et al., 1998; Gunthorpe et al., 2000; Shin et al., 2001). Interestingly, when the capsaicin-mediated elevation of [Ca2+]i was determined during the first 10–20 s of the drug exposure, a time when signal amplification and receptor desensitization is likely to be minimized, capsaicin's potency for elevating [Ca2+]i was essentially identical to that for activating VR1 currents (350 v 200 nM) in the same cells (McIntyre et al., 2001). Unless there is considerable signal amplification or saturation of the Ca2+ reporter system, it is difficult to understand how maximum elevations of [Ca2+]i can be achieved at concentrations of agonist significantly lower than those which result in maximum ligand-dependent activation of VR1 currents.
Another study compared radioligand binding and 45Ca2+-uptake in CHO cells transfected with rat VR1 and concluded that AEA and AM404 were low potency, low efficacy VR1 agonists, despite AEA and AM404 producing a similar maximal 45Ca2+-uptake to capsaicin and resiniferatoxin (Ross et al., 2001). Methanandamide was virtually inactive in stimulating 45Ca2+-uptake. Similar results for AEA were obtained in a study of 45Ca2+-uptake in dorsal root ganglion cells and various heterologous systems (Olah et al., 2001a), but in these experiments AEA was only substantially active at low pH (less than 6.5), which is in contrast to the present findings.
The results of this study have also reinforced the role of another possible confounding factor in determining AEA potency and efficacy, namely the influence of serum albumin on AEA responses. BSA is routinely used in many laboratories, including ours, to inhibit the non-specific absorption of drugs to glass and plastic. However, BSA has long been known to be a carrier protein for fatty acids, particularly the AEA precursor arachidonic acid (Spector, 1975), and it seems likely that BSA also sequesters AEA (di marzo et al., 1994). Consistent with this, addition of BSA to assay buffer dramatically inhibits AEA stimulated increases in [Ca2+]i in VR1-expressing HEK-293 cells (de petrocellis et al., 2001). BSA at concentrations from 0.1% to 0.5% has been included in the assay buffers of virtually all studies that have examined the effects of AEA on VR1 or CB1 receptors, and this is likely to have resulted in a significant underestimation of both the potency and efficacy of AEA at its effectors (e.g. Burkey et al., 1997; Twitchell et al., 1997; Glass & Northup, 1999; Smart et al., 2000; Olah et al., 2001a; Tognetto et al., 2001). The routine inclusion of a functional antagonist of AEA in indirect assays of AEA activity further underscores the likelihood of significant signal amplification in the assays of [Ca2+]i which utilized 0.2% BSA. We were only able to obtain a significant reversal of AEA responses in trigeminal neurons if BSA was included in the wash buffer (data not shown), similar to a recent study examining the effect of AEA on TASK-1 K channels (Maingret et al., 2001). The extremely slow reversal of AEA currents in HBS without BSA suggested that under our recording conditions AEA metabolism in sensory neurons was minimal. This could be due to low levels of the principal enzyme responsible for AEA degradation, fatty acid amide hydrolase (FAAH, Cravatt et al., 1996; PNAS), in sensory neurons or low FAAH activity at room temperature. The potent inhibitor of FAAH, OL-53 (Boger et al., 2000) did not potentiate the currents produced by low concentrations of AEA under our recording conditions (Roberts, Christie and Connor, unpublished observations).
AEA activation of VR1 in sensory neuron nerve endings in vitro results in a variety of effects (e.g. Zygmunt et al., 1999; Tognetto et al., 2001). Clearly, even though AEA is a partial agonist at VR1, it has sufficient efficacy to produce significant depolarization of nerve terminals and increases in [Ca2+]i that lead to release of neuropeptides and glutamate (Tognetto et al., 2001). AEA interaction with other ion channels may also contribute to these processes, as recent studies have demonstrated that AEA inhibits several types of K channel (Van den Bosche & Vanheel, 2000; Maingret et al., 2001) and each of the cloned T-type calcium channels (Chemin et al., 2001). The EC50s for AEA to inhibit these channels are between 300 nM and 4 μM, so it is likely that if in vivo concentrations of AEA were sufficient to activate VR1, AEA would be simultaneously affecting cellular excitability via these other proteins, as well as through actions at CB1 receptors.
Acknowledgments
This work was supported by NH&MRC of Australia Grant #153911 to M. Connor. M.J. Christie was supported by the University of Sydney Medical Foundation. The donation of SR141716 by Sanofi Recherche is gratefully acknowledged.
Abbreviations
- AEA
anandamide
- AM404
N-(4-hydroxyphenyl)-arachidonylamide
- BSA
bovine serum albumin
- [Ca2+]i
intracellular calcium concentration
- FAAH
fatty acid amide hydrolase
- HBS
Hepes buffered saline
- ICa
voltage-dependent calcium channel current
- ICRAC
calcium release activated current
- LVA ICa
low voltage activated calcium channel current
- OL-53
1-(oxazolo[4,5-b]pyridin-2-yl)-1-oxo-6-phenylhexane
- SR141716
N-piperidino-5-(4-chlorophenyl)-I-(2,4-dicholorphenyl)-4-methyl-3-pyrazole carboxamide
- VR1
vanilloid receptor 1
References
- AHLUWALIA J., URBAN L., CAPOGNA M., BEVAN S., NAGY I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- BOGER D.L., SATO H., LERNER A.E., HEDRICK M.P., FECIK R.A., MIYAUCHI H., WILKIE G.D., AUSTIN B.J., PATRICELLI M.P., CRAVATT B.F. Exceptionally potent inhibitors of fatty acid amide hydrolase: the enzyme responsible for degradation of endogenous oleamide and anandamide. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5044–5049. doi: 10.1073/pnas.97.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORGLAND S.L., CONNOR M., CHRISTIE M.J. Nociceptin inhibits calcium channel currents in a subpopulation of small nociceptive trigeminal ganglion neurons in mouse. J. Physiol. (Lond) 2001;536:35–47. doi: 10.1111/j.1469-7793.2001.t01-1-00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORGLAND S.L., CONNOR M., RYAN R.M., BALL H., CHRISTIE M.J. Prostaglandin E2 inhibits calcium current in two subpopulations of acutely isolated mouse trigeminal sensory neurons. J. Physiol. (Lond) 2002;539:433–444. doi: 10.1113/jphysiol.2001.013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKEY T.H., QUOCK R.M., CONSROE P., EHLERT F.J., HOSOHATA Y., ROESKE W.R., YAMAMURA H.I. Relative efficacies of cannabinoid CB1 receptor agonists in the mouse brain. Eur. J. Pharmacol. 1997;336:295–298. doi: 10.1016/s0014-2999(97)01255-7. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., LARANA G., GIUFFRIDA A., PIOMELLI D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHEMIN J., MONTEIL A., PEREZ-REYES E., NARGEOT J., LORY P. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 2001;20:7033–7040. doi: 10.1093/emboj/20.24.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAVATT B.F., GIANG D.K., MAYFIELD S.P., BOGER D.L., LERNER R.A., GILULA N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., DAVIS J.B., PERTWEE R.G., DI MARZO V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., DAVIS J.B., DI MARZO V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001;506:253–256. doi: 10.1016/s0014-5793(01)02934-9. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DOCHERTY R.J., YEATS J.C., PIPER A.S. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurons in culture. Br. J. Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI MARZO V., FONTANA A., CADAS H., SCHINELLI S., CIMINO G., SCHWARTZ J.-C., PIOMELLI D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- FISCHER B.S., QUIN D., KIM K., MCDONALD T.V. Capsaicin inhibits Jurkat T-cell activation by blocking calcium entry current ICRAC. J. Pharmacol. Exp. Ther. 2001;299:238–246. [PubMed] [Google Scholar]
- GLASS M., NORTHUP J.K. Agonist selective regulation of G proteins by cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- GUNTHORPE M.J., HARRIES M.H., PRINJHA R.K., DAVIS J.B., RANDALL A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1) J. Physiol. (Lond.) 2000;525:747–759. doi: 10.1111/j.1469-7793.2000.t01-1-00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HWANG S.W., CHO H., KWAK J., LEE S.-Y., KANG C.-J., JUNG J., CHO S., MIN K.H., SUH Y.-G., KIM D., OH U. Direct activation of capsaicin receptors by products of lipoxygenases; endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAHNEL R., DREGER M., GILLEN C., BENDER O., KURRECK J., HUCHO F. Biochemical characterization of the vanilloid receptor 1 expressed in a dorsal root ganglia derived cell line. Eur. J. Biochem. 2001;268:5489–5496. doi: 10.1046/j.1432-1033.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- JERMAN J.C., BROUGH S.J., HARRIES M.H., DAVIS J.B., SMART D. Characterization using FLIPR of rat vanilloid receptor (rVR1) pharmacology. Br. J. Pharmacol. 2000;130:916–922. doi: 10.1038/sj.bjp.0703390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDT S.-E., JULIUS D. Molecular basis for species-specific sensitivity to ‘hot' chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- KEDEI N., SZABO T., LILE J.D., TREANOR J.J., OLAH Z., IADAROLA M.J., BLUMBERG P.M. Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- MACKIE K., DEVANE W.A., HILLE B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol. Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- MAINGRET F., PATEL A.J., LAZDUNSKI M., HONORE E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCINTYRE P., MCLATCHIE L.M., CHAMBERS A., PHILLIPS E., CLARKE M., SAVIDGE J., TOMS C., PEACOCK M., SHAH K., WINTER J., WEERASAKERA N., WEBB M., RANG H.P., BEVAN S., JAMES I.F. Pharmacological differences between the human and rat vanilloid receptor (VR1) Br. J. Pharmacol. 2001;132:1084–1094. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORISSET V., URBAN L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J. Neurophysiol. 2001;86:40–48. doi: 10.1152/jn.2001.86.1.40. [DOI] [PubMed] [Google Scholar]
- OLAH Z., KARAI L., IADAROLA M.J. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J. Biol. Chem. 2001a;276:31163–31170. doi: 10.1074/jbc.M101607200. [DOI] [PubMed] [Google Scholar]
- OLAH Z., SZABO T., KARAI L., HOUGH C., FIELDS R.D., CAUDLE R.M., BLUMBERG P.M., IADAROLA M.J. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J. Biol. Chem. 2001b;276:11021–11030. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]
- PIPER A.S., YEATS J.C., BEVAN S., DOCHERTY R.J. A study of the voltage dependence of capsaicin-activated membrane currents in rat sensory neurones before and after acute desensitization. J. Physiol. (Lond) 1999;528:721–733. doi: 10.1111/j.1469-7793.1999.0721p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., JERMAN J.C., MIDDLEMISS D.N., SMART D. Cannabinoid activation of recombinant and endogenous vanilloid receptors. Eur. J. Pharmacol. 2001;424:211–219. doi: 10.1016/s0014-2999(01)01153-0. [DOI] [PubMed] [Google Scholar]
- RICHARDSON J.D., KILO S., HARGREAVES K.M. Cannabinoids reduce peripheral hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., BROCKIE H.C., LESLIE M., PASHMI G., CRAIB S.J., DI MARZO V., PERTWEE R.G. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN M., PISER T.M., SEYBOLD V.S., THAYER S.A. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J. Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIN J.S., WANG M.-H., HWANG S.W., CHO H., CHO S.Y., KWON M.J., LEE S.-Y., OH U. Differences in sensitivity of vanilloid receptor 1 transfected to human embryonic kidney cells and capsaicin-activated channels in cultured rat dorsal root ganglion neurons to capsaicin receptor agonists. Neurosci. Lett. 2001;299:135–139. doi: 10.1016/s0304-3940(00)01777-8. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.A., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH G.D., GUNTHORPE M.J., KELSELL K.E., HAYES P.D., REILLY P., FACER P., WRIGHT J.E., JERMAN J.C., WALHIN J.-P., OOI L., EGRTON J., CHARLES K.J., SMART D., RANDALL A.D., ANAND P., DAVIS J.B. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- SPECTOR A.A. Fatty acid binding to plasma albumin. J. Lipid Res. 1975;16:165–179. [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M., ANNICELLI L.L., KRAUSE J.E., CORTRIGHT D.N. The cloned rat vanilloid receptor VR1 mediates both R-type binding and C-type calcium response in dorsal root ganglion neurons. Mol. Pharmacol. 1999;56:581–587. doi: 10.1124/mol.56.3.581. [DOI] [PubMed] [Google Scholar]
- TOGNETTO M., AMADESI S., HARRISON S., CREMINON C., TREVISANI M., CARRERAS M., MATERA M., GEPPETTI P., BIANCHI A. Anandamide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. J. Neurosci. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- TUCKER R.C., KAGAYA M., PAGE C.P., SPINA D. The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. Br. J. Pharmacol. 2001;132:1127–1135. doi: 10.1038/sj.bjp.0703906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TWITCHELL W., BROWN S., MACKIE K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- VAN DEN BOSCHE I., VANHEEL B. Influence of cannabinoids on the delayed rectifier in freshly dissociated smooth muscle cells of the rat aorta. Br. J. Pharmacol. 2000;131:85–93. doi: 10.1038/sj.bjp.0703521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU X., BABNIGG G., VILLEREAL M.L. Functional significance of human trp1 and trp3 in store-operated Ca2+ entry in HEK-293 cells. Am. J. Physiol. 2000;278:C526–C536. doi: 10.1152/ajpcell.2000.278.3.C526. [DOI] [PubMed] [Google Scholar]
- WU X., BABNIGG G., ZAGRANICHNAYA T., VILLEREAL M.L. The role of endogenous human Trp4 in regulating carbachol-induced calcium oscillations in HEK-293 cells. J. Biol. Chem. 2002;277:13597–13608. doi: 10.1074/jbc.M110881200. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H.H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


