The principal biologically active constituent of cannabis, Δ9-tetrahydrocannabinol (THC) and its synthetic analogues decrease neuronal signalling in the central, peripheral and enteric nervous systems (Martin, 2002). Some neural effects produced by cannabinoids have potential clinical benefits, such as their analgesic and antiemetic actions. In the past decade, several arachidonic acid derivatives such as anandamide and 2-arachidonoylglycerol have been identified as endogenous activators of two G protein-coupled cannabinoid receptors. Postsynaptic activity stimulates endocannabinoid synthesis, resulting in a retrograde action of endocannabinoids on cannabinoid receptors located on presynaptic terminals. Through the use of cannabinoid receptor knockout animals and the development of selective receptor antagonists, it has been possible to link many of the behavioural and peripheral effects of cannabinoids to actions on the endocannabinoid system.
Although cannabinoid receptors have been cloned and are well-characterized, pharmacological studies with cannabinoid ligands have continued to present conundrums. Some of the neuronal, cardiovascular and gastrointestinal actions of cannabinoids have been ascribed to mechanisms independent of cannabinoid CB1 and CB2 receptors (Kunos et al., 2000; Zygmunt et al., 2002). Moreover, anandamide appears to act as an agonist at vanilloid VR1 receptors (Smart et al., 2000). Finally, the cannabimimetic eicosanoids, THC derivatives (such as CP-55,940) and aminoalkylindole-based cannabinoids (WIN-55,212-2 is an example) inhibit 5-HT evoked depolarization of rat nodose ganglion neurones. This effect could not be attributed to cannabinoid receptors or indeed to a G protein-coupled receptor, but rather to the ionotropic 5-HT3 receptor (Fan, 1995).
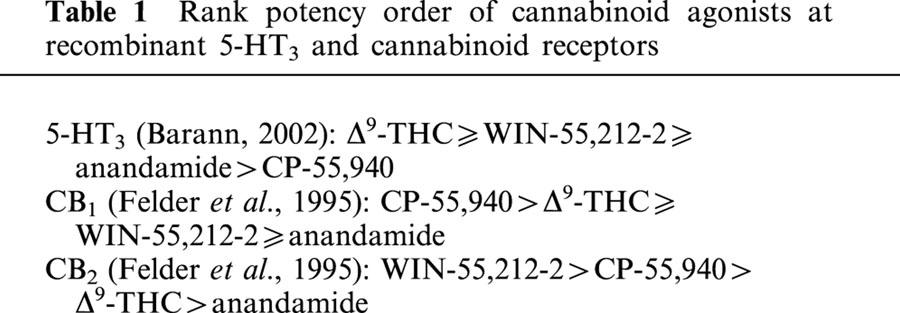
Using 5-HT3A receptor subunits expressed in HEK 293 cells, Barann et al. (this issue, 2002) report compelling evidence that cannabinoids allosterically modulate this ligand-gated ion channel. Chemically dissimilar cannabinoid agonists inhibited 5-HT-induced cation conductance with a rank order of potency that differed from their potencies in stimulating cannabinoid receptors (Table 1). Moreover, the inhibitory actions of WIN-55,212 were resistant to the cannabinoid CB1 receptor antagonist, SR-141716A and the presence of specific cannabinoid binding sites was not detected in HEK 293 cells. These results suggest that the inhibitory actions of cannabinoids are not mediated by cannabinoid receptors, yet their actions were potent and stereoselective. Thus, these lipophilic drugs appear to exert their actions directly on the 5-HT3A receptor through a novel modulatory site.
Table 1.
Rank potency order of cannabinoid agonists at recombinant 5-HT3 and cannabinoid receptors
This modulatory action is non-competitive in nature, as pre-treatment of the recombinant 5-HT3A receptor with the cannabinoid agonist WIN55,212-2 results in a suppression of the maximal current evoked by 5-HT. The mechanism of this antagonism is still not clear, although the required pre-incubation and the lack of use dependence would seem to rule out an open channel block. The allosteric site is distinct from the 5-HT binding domain because the cannabinoids did not alter the binding properties of the selective 5-HT3 receptor ligand [3H]-GR-65630. Given the high lipid solubility of cannabinoid agonists, it seems reasonable to assume that these compounds partition into the lipid bilayer and interact with the transmembrane domains of this ionotropic receptor (Figure 1). The 5-HT3 receptor is structurally related to the nicotinic acetylcholine, type A γ-aminobutyric acid and glycine receptors. Each of these ligand-gated ion channels have allosteric modulatory sites (Belelli et al., 1999; Bullock et al., 1997) and it would not be surprising if the 5-HT3 receptor possesses a similar site for non-competitive receptor modulation. It is also possible that cannabinoids may act through analogous allosteric sites in some of these other ligand-gated channels to influence their ionic conductances.
Figure 1.
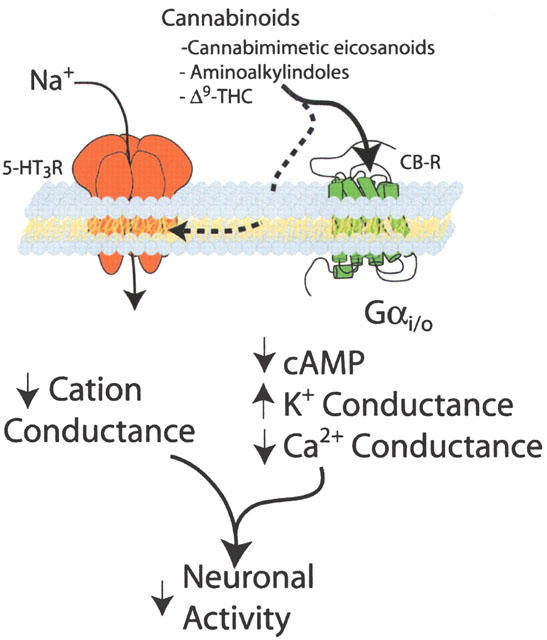
Proposed model of the actions of cannabinoids on neuronal excitability mediated by G protein-coupled cannabinoid receptors (CB-R) and ionotropic 5-HT3 receptors (5-HT3-R). cAMP, cyclic AMP; Gαi/o, alpha subunit of inhibitory heterotrimeric G protein; Δ9-THC, Δ9-tetrahydrocannabinol.
The obvious question arises as to whether these cannabinoid receptor-independent effects are relevant in a physiological setting. Both cannabinoid and 5-HT3 receptors are present in neuronal systems mediating similar functions. For example, both synthetic Δ9-THC and 5-HT3 receptor antagonists such as ondansetron decrease nausea and vomiting (Hornby, 2001). Indeed, the modulatory interaction observed here may represent a component of cannabinoid activity in vivo. Cannabinoid actions mediated by 5-HT3 receptors might be particularly pronounced in cannabinoid receptor knock-out animals. Ultimately, the physiological importance of 5-HT3 receptor modulation by cannabinoids will be buttressed by additional lines of evidence, such as the demonstration that biochemical mechanisms for endocannabinoid synthesis and degradation are located in close proximity to the 5-HT3 receptor. Nevertheless, exogenous cannabinoids are capable of modulating 5-HT-evoked currents at concentrations within the range of doses producing psychoactive and therapeutic effects and it will be interesting to ascertain whether 5-HT3 receptors participate in mediating their actions.
Although it remains to be determined whether the interaction of cannabinoids with 5-HT3 receptors is physiologically significant, the pharmacological specificity of this interaction raises the exciting possibility that a novel modulatory site on the 5-HT3 receptor exists. This site may serve as a target for drugs having unique antiemetic, analgesic or other activities. It is clear that the complicated pharmacological characteristics of cannabinoids continue to puzzle and fascinate.
Abbreviations
- 5-HT
5-hydroxytryptamine
- THC
Δ9-tetrahydrocannabinol
References
- BARANN M., MOLDERINGS G., BRÜSS M., BÖNISCH H., URBAN B.W., GÖTHERT M.Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site Brit. J. Pharmacol. 2002. in press [DOI] [PMC free article] [PubMed]
- BELELLI D., PISTIS M., PETERS J.A., LAMBERT J.J. The interaction of general anaesthetics and neurosteroids with GABA(A)and glycine receptors. Neurochem. Int. 1999;34:447–452. doi: 10.1016/s0197-0186(99)00037-6. [DOI] [PubMed] [Google Scholar]
- BULLOCK A.E., CLARK A.L., GRADY S.R., ROBINSON S.F., SLOBE B.S., MARKS M.J., COLLINS A.C. Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes. J. Neurochem. 1997;68:2412–2423. doi: 10.1046/j.1471-4159.1997.68062412.x. [DOI] [PubMed] [Google Scholar]
- FAN P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J. Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., MANSOURI J., MACKIE K., BLOND O., LAI Y., MA A.L., MITCHELL R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- HORNBY P.J. Central neurocircuitry associated with emesis. Am. J. Med. 2001;111 Suppl 8A:106S–112S. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- KUNOS G., JARAI Z., BATKAI S., GOPARAJU S.K., ISHAC E.J., LIU J., WANG L., WAGNER J.A. Endocannabinoids as cardiovascular modulators. Chem. Phys. Lipids. 2000;108:159–168. doi: 10.1016/s0009-3084(00)00194-8. [DOI] [PubMed] [Google Scholar]
- MARTIN B.R. Identification of the endogenous cannabinoid system through integrative pharmacological approaches. J. Pharmacol. Exp. Ther. 2002;301:790–796. doi: 10.1124/jpet.301.3.790. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Brit. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., ANDERSSON D.A., HOGESTATT E.D. Delta 9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J. Neurosci. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


