Abstract
The P2 receptors that mediate contraction of the rat isolated small (SPA, 200–500 μm i.d.) and large (LPA, 1–1.5 mM i.d.) intrapulmonary arteries were characterized.
In endothelium-denuded vessels the contractile order of potency was α,β-methyleneATP (α,β-meATP)>>UDP=UTP=ATP=2-methylthioATP>ADP in the SPA and α,β-meATP=UTP⩾UDP>2-methylthioATP, ATP>>ADP in the LPA. α,β-meATP, 2-methylthioATP and ATP had significantly greater effects in the SPA than the LPA (P<0.001), but there was no difference in the potency of UTP or UDP between the vessels.
In the SPA, P2X1 receptor desensitisation by α,β-meATP (100 μM) inhibited contractions to α,β-meATP (10 nM–300 μM), but not those to UTP or UDP (100 nM–300 μM). In the LPA, prolonged exposure to α,β-meATP (100 μM) did not desensitize P2X receptors.
Pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), suramin and reactive blue 2 (RB2) (30–300 μM) inhibited contractions evoked by α,β-meATP. UTP and UDP were potentiated by PPADS, unaffected by RB2 and inhibited, but not abolished by suramin. 1 and 3 mM suramin produced no further inhibition, indicating suramin-resistant components in the responses to UTP and UDP.
Thus, both P2X and P2Y receptors mediate contraction of rat large and small intrapulmonary arteries. P2Y agonist potency and sensitivity to antagonists were similar in small and large vessels, but P2X agonists were more potent in small arteries. This indicates differential expression of P2X, but not P2Y receptors along the pulmonary arterial tree.
Keywords: Pulmonary artery, P2X receptors, P2Y receptors, ATP, UTP, UDP
Introduction
P2X and P2Y receptors mediate the extracellular actions of nucleotides such as adenosine 5′-triphosphate (ATP) and uridine 5′-triphosphate (UTP) and can play an important role in the control of vascular tone (Boarder & Hourani, 1998). P2X receptors are ligand-gated cation channels, seven mammalian subtypes (P2X1–7) of which have been cloned (Khakh et al., 2001). The P2X1 appears to be the predominant P2X subunit expressed in vascular smooth muscle, although mRNA and/or antibody staining for other P2X subunits has also been detected, in a vessel-specific manner (Collo et al., 1996; Vulchanova et al., 1996; Bo et al., 1998a, b; Nori et al., 1998; Phillips et al., 1998; Phillips & Hill, 1999; Hansen et al., 1999; Gitterman & Evans, 2000; Lewis et al., 2000). Smooth muscle P2X1 receptors mediate a vasoconstriction that depends upon the influx of extracellular calcium ions through the P2X1 receptor ion channel and via L-type calcium channels opened by cell depolarization (Kennedy et al., 1997). P2Y receptors are heptahelical, G protein-coupled receptors and seven subtypes (P2X1,2,4,6,11,12,13) have been cloned in mammals (Boarder & Webb, 2001; Communi et al., 2001). Of these, mRNA for the P2Y1,2,4,6 subtypes has been detected in vascular smooth muscle (Erlinge et al., 1998; Harper et al., 1998; Hartley et al., 1998; Lewis et al., 2000). The most common responses to vascular P2Y receptor activation are contraction via smooth muscle receptors and vasodilation via endothelial receptors (Burnstock & Kennedy, 1986; McLaren et al., 1998a; Ralevic & Burnstock, 1998).
The pulmonary circulation is a low-pressure, low-resistance circuit that regulates blood flow to the gas exchanging surfaces in the lung. P2 receptor agonists are vasoactive in the pulmonary vascular bed of a variety of species, including humans (Barnes & Liu, 1995). In rat lung, P2 receptor agonists evoke vasoconstriction at resting tone and vasodilation if muscle tone is first raised (McCormack et al., 1989; Hasséssian & Burnstock, 1995; Rubino & Burnstock, 1996). Similarly, in large diameter branches of rat isolated intrapulmonary artery, P2 receptor agonists are contractile at resting tone and relaxant at raised tone (Liu et al., 1989; Rubino et al., 1999). However, which P2 receptor subtypes mediate these effects is unclear. Furthermore, there are inconsistencies in the published data. For example, ATP was reported to be equipotent with UTP as a vasoconstrictor in the perfused rat lung (Rubino & Burnstock, 1996), but a weaker agonist in rat large intrapulmonary artery in vitro (Rubino et al., 1999). Also, the P2 receptor antagonist suramin had no effect on the responses to UTP in the perfused lung and isolated large intrapulmonary artery, whereas it inhibited contractions evoked in isolated small intrapulmonary artery (Hartley et al., 1998). These differences are likely to reflect the differential expression of multiple P2 receptor subtypes in smooth muscle and endothelial cells along the pulmonary arterial tree.
In order to understand fully the vasoactive effects of P2 receptors in the intact pulmonary vascular bed, it is clearly essential to know which P2 subtypes are expressed in different regions of the vascular tree and what type of response they mediate. Therefore, the aim of this study was to characterize and compare under identical conditions the properties of the P2 receptors present in isolated vessels from different regions of the rat pulmonary vascular tree. To do so, we isolated segments of small (SPA, 200–550 μm i.d.) and large (LPA, 1.0–1.5 mm i.d.) intrapulmonary arteries and determined the effects of the agonists ATP, UTP, uridine 5′-diphosphate (UDP), adenosine 5′-diphosphate (ADP), α,β-methyleneATP (α,β-meATP) and 2-methylthioATP (2-meSATP), which between them act at all known P2 receptor subtypes. Also, we determined the effects of the P2 receptor antagonists, pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), suramin and reactive blue 2 (RB2) against each agonist. A preliminary account of these results has been published (Chootip et al., 2000).
Methods
Male Sprague-Dawley rats (150–250 g) were sacrificed by cervical dislocation and exsanguination. Following thoracotomy, the heart and lungs were removed en bloc, the lungs separated and intrapulmonary arteries of internal diameter 200–500 μm (SPA) and 1.0–1.5 mm (LPA) dissected out. The arteries were cleaned of connective tissue, cut into rings 5 mm long and mounted horizontally in 1 ml baths on a pair of intraluminal wires. Tissues were allowed to equilibrate under a resting tension of 0.5 g (SPA) and 1.0 g (LPA) for 60 min at 37°C in a salt solution composed of (mM): NaCl 122; KCl 5; N-[2-hydroxyethyl]piperazine-N′-[2-ethane-sulphonic acid] (HEPES) 10; KH2PO4 0.5; NaH2PO4 0.5; MgCl2 1; glucose 11; CaCl2 1.8, titrated to pH 7.3 with NaOH and bubbled with air (21% O2, 5% CO2, 74% N2). Tension was recorded with Grass FT03 isometric force transducers connected to a MacLab/4e system, using Chart 3.3 software (AD Instruments).
After equilibration, the integrity of the smooth muscle was determined by applying 200 nM phenylephrine twice. The presence of a functional endothelium was assessed by determining the degree of relaxation to acetylcholine (10 μM) in vessels precontracted with phenylephrine. When appropriate the endothelium was removed by gently rubbing the vessel lumen with a stainless steel wire. Removal of the endothelium was confirmed by loss of the relaxant response to acetylcholine.
Experimental protocols
Cumulative concentration–response curves to α,β-meATP, 2-meSATP, ATP, UTP, UDP and ADP (O'connor et al., 1990) and to uridine and adenosine (10 nM–300 μM) were obtained in endothelium-intact and -denuded rings at resting tone. Contractions generally took 1–4 min to reach a plateau and were expressed as a percentage of the contraction induced by 40 mM K+, which was applied by replacement of HEPES–buffer solution in the same preparation.
To induce desensitization of P2X receptors, 100 μM α,β-meATP was applied, evoking a large contraction. Once tension had returned to baseline, a further 100 μM α,β-meATP was added. α,β-meATP was then washed out, and when re-applied 2 min later, no contraction or contraction less than 10% of that to 40 mM K+ was seen, confirming that the P2X receptors were desensitized. α,β-meATP was again washed out and 2 min later the test agonist administered. The control responses to a test agonist were obtained at the same time in separate pulmonary artery rings from the same animal, but without pre-exposure to α,β-meATP. The effects of P2 receptor antagonists were investigated by incubating tissues for 30 min with PPADS, suramin or RB2 (30–300 μM) and P2 receptor agonists were then applied. Control responses to the agonists were obtained in separate tissues from the same animal. In a series of experiments designed to determine the effects of high concentrations of suramin, three consecutive contractions were obtained to 300 μM UTP or UDP. Suramin (1 or 3 mM) was then applied to the tissue for 30 min and the agonist then reapplied.
Drugs and solutions
Phenylephrine hydrochloride, acetylcholine chloride, ATP (magnesium salt), ADP (potassium salt), UDP (sodium salt), UTP (sodium salt), adenosine hemisulfate and uridine (Sigma, U.K.), α,β-meATP (lithium salt), suramin hexasodium, 2-meSATP tetrasodium, PPADS tetrasodium (RBI, U.S.A.) and RB2, also known as Basilen Blue E-3G (Tocris, U.K.) were dissolved in distilled water as 10 or 100 mM stock solutions. Isotonic 40 mM K+ solution was prepared by replacing NaCl with an equimolar amount of KCl in HEPES-buffered solution to maintain osmolarity of the solution.
Data analysis
Where possible, log concentration–response plots were fitted to the data by logistic (Hill equation), non-linear regression analysis (Graphpad Prism, San Diego, U.S.A.). Except for α,β-meATP, the agonist concentration–response curves did not reach a maximum and so EC50 values could not be calculated. Therefore, the potency of these agonists was compared at the level of contraction equivalent to 40% of the response to 40 mM K+ (EC40K). Values in the text and figures refer to mean±s.e.mean or geometric mean with 95% confidence limits for EC50 and EC40K values. Data were compared by paired or unpaired t-tests or one-way analysis of variance and Tukey's comparison as appropriate. Differences were considered significant when P<0.05.
Results
Contractions to P2 receptor agonists
In endothelium-intact SPA at resting tone, all P2 agonists tested evoked concentration–dependent contractions, with α,β-meATP being the most potent (EC50=0.93 μM, 95% confidence limits=0.48–1.80 μM). UTP, ATP and 2-meSATP were equipotent at the EC40K level, but about 50 times less potent than α,β-meATP (Table 1). UDP was 227 times less potent than α,β-meATP, whilst ADP was even less potent, and at the highest concentration used (300 μM) evoked a contraction that was only 34±7% (n=4) of the response to 40 mM K+. Removal of the endothelium had no significant effect on contractions evoked by any P2 receptor agonist except UDP, which was potentiated by about 5 fold (P<0.01). Thus, in the absence of the endothelium the rank order of potencywas α,β-meATP>>UDP=UTP=ATP=2-meSATP>ADP (Figure 1a, Table 1).
Table 1.
The potency of P2 receptor agonists in evoking contractions of SPA and LPA
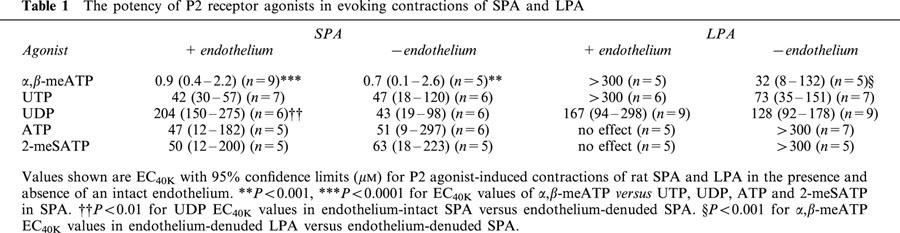
Figure 1.
Contractions of rat isolated SPA and LPA to P2 receptor agonists. Cumulative concentration-response curves are shown for contractions to α,β-meATP (10 nM–300 μM), UTP (0.1–300 μM), UDP (0.1–300 μM), ATP (0.1–300 μM) and 2-meSATP (0.1–300 μM) at resting tone in endothelium-denuded rat (a) SPA and (b) LPA. Each point is the mean of 5–8 experiments. Vertical bars indicate s.e.mean and are smaller than the symbol in some cases.
In the endothelium-intact LPA at resting tone, α,β-meATP, UTP and UDP evoked relatively small contractions and only those to UDP consistently reached the EC40K level. 2-meSATP, ATP and ADP were essentially inactive (Table 1). Removal of the endothelium substantially and significantly (P<0.05) increased the amplitude of contractions evoked by α,β-meATP (100–300 μM), UTP (300 μM) and UDP (300 μM) such that the EC40K level was now reached by all three agonists (Figure 1b, Table 1). Contractions to 2-meSATP (100–300 μM), ATP (300 μM) (Figure 1b) and phenylephrine (200 nM, n=5, not shown) were also significantly (P<0.05) increased, but the nucleotides' concentration–response curves did not reach the EC40K level. ADP remained inactive (n=4). Thus, the rank order of agonist potency in the LPA in the absence of endothelium was α,β-meATP=UTP⩾UDP>2-meSATP, ATP>>ADP.
In endothelium-denuded vessels α,β-meATP, 2-meSATP and ATP had significantly greater effects in the SPA than the LPA (P<0.001), but there was no significant difference in the potency of UTP or UDP between the vessels (Table 1). Neither adenosine nor uridine (0.01–300 μM), breakdown products of ATP and UTP/UDP, evoked contractions in endothelium-intact or -denuded SPA and LPA (n=4 each). These data indicate that both P2X and P2Y receptors are expressed in the smooth muscle of rat SPA and LPA and that the potency of P2X agonists is greater in the SPA.
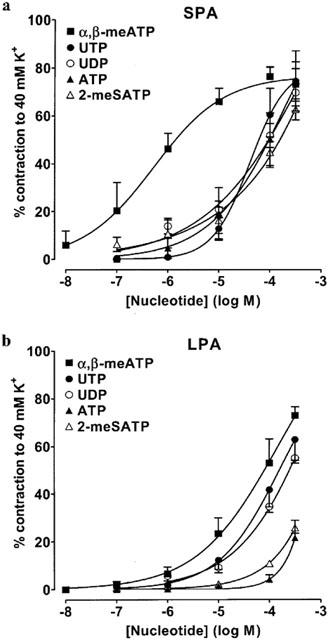
Effects of prolonged administration of α,β-meATP
α,β-meATP induces desensitization of the P2X1 receptor, the predominant P2X receptor subtype present in smooth muscle (see Kennedy, 2001). In the SPA, the contraction evoked by prolonged exposure to α,β-meATP (100 μM) decayed to basal tension over 10–15 min (Figure 2a). Further application of α,β-meATP (100 μM) had no effect. When α,β-meATP was washed out and reapplied 2 min later, there was still no response, indicating that it had desensitized the P2X receptors in the SPA. Contractions to subsequent application of 10 nM–10 μM α,β-meATP were abolished and those to 100–300 μM virtually abolished. Following the same protocol, responses to ATP (Figure 2c), 2-meSATP (0.1–300 μM, not shown), and ADP (1–300 μM, not shown) were inhibited in a similar manner. However, contractions evoked by UTP (Figure 2c) and UDP (1–300 μM, not shown) were unaffected by P2X receptor desensitization. This is consistent with the expression of both P2X and P2Y receptors in rat SPA.
Figure 2.
Effects of prolonged exposure to α,β-meATP in rat SPA and LPA. (a) SPA: α,β-meATP (100 μM), added as indicated by the horizontal bars, evoked a contraction that was not maintained. Once tension had returned to baseline, further addition of α,β-meATP (100 μM) had no effect. The drug was washed out and when α,β-meATP (100 μM) was added 2 min later no contraction was seen, confirming that the P2X1 receptors had been desensitized. (b) LPA: α,β-meATP (100 μM) evoked a contraction that was sustained for at least 30 min. The drug was washed out and when α,β-meATP (100 μM) was re-added, a maintained contraction of similar amplitude was seen. (c) Cumulative concentration-response curves are shown for contractions to UTP and ATP (0.1–300 μM) at resting tone in SPA before (closed symbols) and after (open symbols) prolonged exposure to α,β-meATP. Each point is the mean of 5–6 experiments. Vertical bars indicate s.e.mean and are smaller than the symbol in some cases.
In contrast to the SPA, α,β-meATP (100 μM) evoked a contraction in the LPA, which reached a peak within 2–3 min and was reasonably well maintained for at least 30 min (Figure 2b). Further application of α,β-meATP (100 μM) induced further contraction. After the agonist was washed out, re-application of α,β-meATP still induced a sustained contraction. Similar responses were seen in endothelium-intact (n=5) and -denuded (n=6) LPA. Thus, in this tissue, α,β-meATP acts mainly at P2X receptors that do not desensitize and so the effects of P2X receptor desensitization on the responses to the other agonists was not studied.
Effects of P2 receptor antagonists in SPA
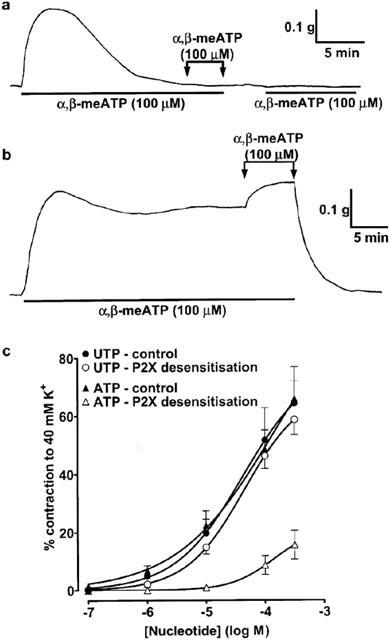
To further characterize the P2 receptors expressed in rat SPA and LPA, we next determined the ability of several P2 receptor antagonists to inhibit contractions evoked by α,β-meATP, which only acts at P2X receptors, and UTP and UDP, which only act at P2Y receptors. Thirty and 100 μM PPADS (Figure 3a), suramin (Figure 4a) and RB2 (Figure 5a) caused a progressive rightward shift of the concentration–response curve to α,β-meATP and significantly increased its EC40K value (Table 2). The maximum response to α,β-meATP also appeared to decrease. Three hundred μM PPADS and suramin evoked a further rightward shift and depression of the α,β-meATP curve, such that the EC40K level was not reached. Higher concentrations of RB2 were not used as these also depressed contractions evoked by phenylephrine (not shown). Thus, α,β-meATP evokes contraction of the SPA via a receptor that is sensitive to PPADS, suramin and RB2.
Figure 3.
The effect of PPADS on contractions evoked by α,β-meATP, UTP and UDP in rat SPA. Cumulative concentration-response curves are shown for contractions of rat SPA evoked by (a) α,β-meATP (10 nM–300 μM), (b) UTP (0.1–300 μM) and (c) UDP (1–300 μM) in the absence and presence of PPADS (30–300 μM). Each point is the mean of 4–6 experiments. Vertical bars indicate s.e.mean. The effects of α,β-meATP and UTP were studied in endothelium-intact tissues, whereas endothelium-denuded tissues were used when studying UDP.
Figure 4.
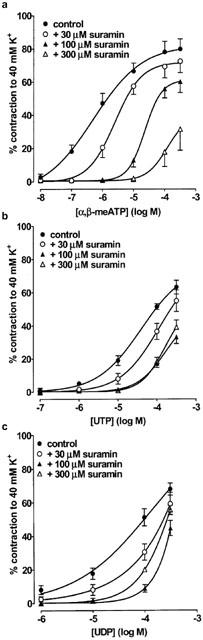
The effect of suramin on contractions evoked by α,β-meATP, UTP and UDP in rat SPA. Cumulative concentration-response curves are shown for contractions of rat SPA evoked by (a) α,β-meATP (10 nM–300 μM), (b) UTP (0.1–300 μM) and (c) UDP (1–300 μM) in the absence and presence of suramin (30–300 μM). Each point is the mean of 4–10 experiments. Vertical bars indicate s.e.mean. The effects of α,β-meATP and UTP were studied in endothelium-intact tissues, whereas endothelium-denuded tissues were used when studying UDP.
Figure 5.
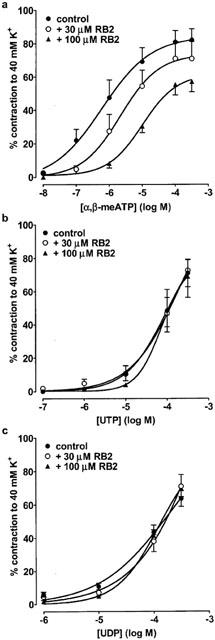
The effect of RB2 on contractions evoked by α,β-meATP, UTP and UDP in rat SPA. Cumulative concentration-response curves are shown for contractions of rat SPA evoked by (a) α,β-meATP (10 nM–300 μM), (b) UTP (0.1–300 μM) and (c) UDP (1–300 μM) in the absence and presence of RB2 (30–100 μM). Each point is the mean of 4–6 experiments. Vertical bars indicate s.e.mean. The effects of α,β-meATP and UTP were studied in endothelium-intact tissues, whereas endothelium-denuded tissues were used when studying UDP.
Table 2.
Effect of P2 receptor antagonists on responses to α,β-meATP, UTP and UDP in rat SPA
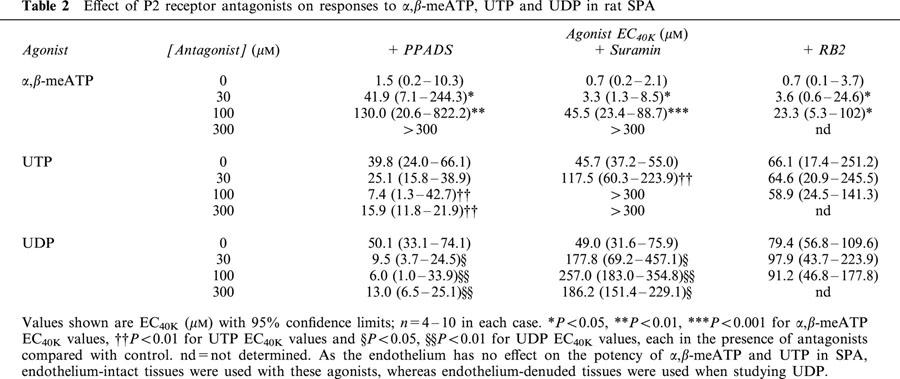
In contrast to α,β-meATP, the vasoconstrictor responses to UTP and UDP (Figure 3b,c) were potentiated by PPADS (30–300 μM) and their EC40K values were significantly reduced (Table 2). Suramin (30–100 μM) caused a progressive rightward shift of their concentration–contraction curves (Figure 4b,c) and significantly increased their EC40K values (Table 2), although increasing the concentration of suramin to 300 μM (Figure 4b,c) and to 1 or 3 mM produced no further inhibition (n=5–7, not shown). Finally, RB2 (30–100 μM) had no effect on contractions evoked by UTP and UDP (Figure 5b,c, Table 2). Thus, both UTP and UDP evoke contraction of the SPA via two receptors, one suramin-sensitive and the other suramin-insensitive, neither of which are antagonised by PPADS or RB2.
Effects of P2 receptor antagonists in LPA
The effects of PPADS, suramin and RB2 against each agonist in the LPA were qualitatively the same as those in the SPA, although there were some quantitative differences with α,β-meATP (Table 3). Thus, PPADS again caused a progressive rightward shift of the concentration–response curve to α,β-meATP, but produced a greater depression of the curve such that in the presence of 100 and 300 μM PPADS the EC40K level was not reached. However, PPADS potentiated the contractions to UTP and UDP to a similar extent in the LPA as in the SPA. Suramin also caused a greater depression of the concentration–response curve to α,β-meATP in the LPA and 300 μM suramin abolished the contractions. As in the SPA, suramin (100 and 300 μM) inhibited contractions to UTP and UDP to a similar extent, and increasing the concentration of suramin to 1 and 3 mM produced no further inhibition. Finally, similar to PPADS and suramin, RB2 produced a greater depression of the concentration–response curve to α,β-meATP in the LPA compared with the SPA. However, RB2 was ineffective against UTP and UDP.
Table 3.
Effect of P2 receptor antagonists on responses to α,β-meATP, UTP and UDP in rat LPA
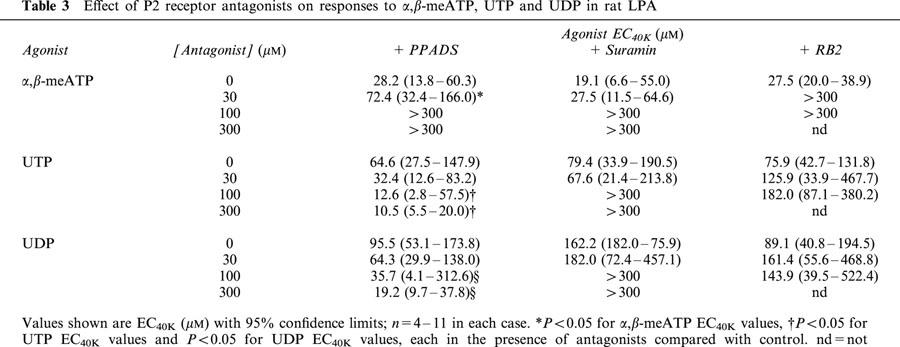
Thus, α,β-meATP evokes contraction of the LPA via a receptor that is sensitive to PPADS, suramin and RB2, but the antagonists depress the agonist concentration–response curve to a greater extent than in the SPA. Similar to the SPA, UTP and UDP evoke contraction of the LPA via two receptors, one suramin-sensitive and the other suramin-insensitive, neither of which is antagonized by PPADS or RB2.
Discussion
This study shows that purine and pyrimidine nucleotides evoke contraction of the rat SPA and LPA via multiple P2X and P2Y receptor subtypes. Contractions evoked by the P2X receptor agonist α,β-meATP were transient in the SPA, but tonic in the LPA. The P2Y receptor agonists UTP and UDP both appeared to act via two receptors in both vessels, although it is not clear if they were acting at the same receptors. Also, α,β-meATP was about 30 times more potent in the SPA than in the LPA, whereas UTP and UDP were equipotent in the SPA and LPA. Thus, the relative functional expression of P2X and P2Y receptors varies along the pulmonary arterial tree.
P2X receptors in rat pulmonary arteries
In this study, P2X receptor agonists appeared to act at the P2X1 receptor to evoke vasoconstriction of the SPA. The rank order of potency was α,β-meATP>>2-meSATP=ATP>ADP, and responses to each agonist were abolished or virtually abolished by prolonged exposure to α,β-meATP. This is essentially the pharmacological profile of the P2X receptor in whole tissues, as originally described by Burnstock & Kennedy (1985). Also, PPADS, suramin and RB2 all caused substantial antagonism. Similar responses have been reported in the rat perfused pulmonary vascular bed (McCormack et al., 1989; Hasséssian & Burnstock, 1995; Rubino & Burnstock, 1996).
Of the known recombinant P2X receptors, α,β-meATP is an agonist at the P2X1 and P2X3 subunits only, and PPADS and suramin are antagonists at both of these (Ralevic & Burnstock, 1998; Khakh et al., 2001). However, the P2X3 subunit has a very restricted distribution and is absent from smooth muscle cells (Collo et al., 1996). In contrast, the P2X1 subunit is expressed highly in arterial smooth muscle (Collo et al., 1996; Vulchanova et al., 1996; Bo et al., 1998a, b; Nori et al., 1998; Phillips et al., 1998; Phillips & Hill, 1999; Hansen et al., 1999; Gitterman & Evans, 2000; Lewis et al., 2000). Thus, the P2X1 receptor is most likely to mediate the vasoconstriction evoked by P2X receptor agonists in SPA.
In the LPA the same rank order of agonist potency was seen, and PPADS, suramin and RB2 all antagonized contractions to α,β-meATP. However, the agonists were substantially less potent in the LPA than in the SPA. Furthermore, α,β-meATP induced a maintained contraction of the LPA. This was an unexpected finding, as in most arteries prolonged exposure to α,β-meATP induces P2X receptor desensitization (Kennedy et al., 1997). Non-desensitizing responses to α,β-meATP have been reported in only a few cases; in the human placenta arterial bed (Dobronyi et al., 1997; Ralevic et al., 1997) and rat renal vessels (Inscho et al., 1998; van der giet et al., 1999).
Which P2X receptor subunit(s) mediates these maintained responses is unclear as α,β-meATP is not an agonist at the non-desensitizing recombinant P2X receptors (P2X2,4,5,6,7) (Khakh et al., 2001). It may be that the P2X1 subunit is non-desensitizing in the LPA due to a post-translational modification or expression of a splice variant. That this could occur is implied by the finding that inhibiting phosphorylation of the P2X2 subunit converts it from a rapidly- to a slowly-desensitizing form (Boué-grabot et al., 2000). Alternatively, the P2X1 subunit may form a non-desensitizing heteropolymer with another subunit. A precedent for this mechanism is the report that when co-expressed, the P2X1 and P2X5 subunits form a channel that is activated by α,β-meATP in a slowly desensitizing manner and antagonized by PPADS and suramin (Torres et al., 1998; Haines et al., 1999). However, to date, the presence of only P2X1, P2X2, and P2X4 subunits has been established in rat pulmonary artery smooth muscle (Nori et al., 1998; Hansen et al., 1999). The presence of P2X5 receptors has not been investigated, although low levels of P2X5 mRNA and protein have been reported in other arteries (Bo et al., 1998b; Phillips et al., 1998; Phillips & Hill, 1999; Gitterman & Evans, 2000; Lewis et al., 2000). Finally, an alternative possibility is that α,β-meATP may act in the LPA at a P2X receptor subunit that has yet to be cloned.
P2Y receptors in rat pulmonary arteries
The current study showed that UTP and UDP both act via P2Y receptors, but not P2X receptors, to elicit contraction of rat SPA and LPA. We have reported previously that high concentrations of UTP activates P2X1 receptors in rat tail artery (McLaren et al., 1998a, b), but here the UTP- and UDP-evoked contractions of rat SPA were unaffected by P2X1 receptor desensitization. Also, PPADS and RB2 inhibited responses to α,β-meATP, but not UTP or UDP in both SPA and LPA. This is consistent with previous reports of PPADS-, RB2- and α,β-meATP-resistant contractions to UTP and UDP in the rat perfused pulmonary bed (Rubino & Burnstock, 1996) and rat isolated pulmonary artery branches (Hartley et al., 1998; Rubino et al., 1999). Thus, UTP and UDP only act via P2Y receptors in rat SPA and LPA.
In this study UTP and UDP both acted via two P2Y receptors, although it is not clear if they acted at the same two P2Y receptors. In either case, suramin is an antagonist at one site, but not the other, and neither is antagonized by PPADS or RB2. Of the recombinant rat P2Y receptors UTP and UDP are agonists at the P2Y2, P2Y4 and P2Y6 subtypes only (Boarder & Webb, 2001). UTP is a potent agonist and UDP is inactive at the P2Y2 and P2Y4 receptors, while UDP is a potent agonist at the P2Y6 subtype. UTP has been reported to be an agonist at the P2Y6 receptor, although it is substantially less potent than UDP (Nicholas et al., 1996; Filippov et al., 1999) and can also act indirectly at the P2Y6 subtype after dephosphorylation to UDP (Nicholas et al., 1996).
Our data are consistent with the P2Y2 receptor as one of the sites of action of UTP, as it is suramin-sensitive and PPADS-insensitive (Charlton et al., 1996a; Chen et al., 1996a), as was seen here. The identity of the suramin-resistant UTP receptor is less clear. Both the P2Y4 (Charlton et al., 1996b; Bogdanov et al., 1998) and P2Y6 receptors (Chang et al., 1995; Robaye et al., 1997) are largely insensitive to suramin and so are possible sites of action. On the other hand, PPADS and RB2 are reported to antagonize both of these (Chang et al., 1995; Robaye et al., 1997; Bogdanov et al., 1998), but did not inhibit UTP-evoked contractions in rat SPA and LPA. Indeed, in our study PPADS potentiated the responses to UTP (and UDP). This was probably due to PPADS inhibiting their breakdown, as it is known to inhibit ecto-nucleotidases present in smooth muscle (Khakh et al., 1995) and other tissues (Chen et al., 1996b; Grobben et al., 2000). Consistent with this idea, PPADS and the ecto-nucleotidase inhibitor ARL 67156 potentiated contractions to UTP in the rat isolated tail artery in a similar manner (McLaren et al., 1998a).
A similar lack of inhibition of UTP by PPADS and suramin was seen in the rat perfused pulmonary bed (Rubino & Burnstock, 1996) and PPADS, suramin and RB2 were ineffective in rat isolated pulmonary artery branches (Rubino et al., 1999). This is consistent with our conclusion that none of the cloned P2Y receptors appears to have the same antagonist sensitivity as the second site of action of UTP, which we report in rat SPA and LPA. Thus, UTP may act at a novel, uncloned receptor in the rat SPA and LPA. However, it should be noted that in most cases the interaction of putative antagonists with cloned P2Y receptors has not been extensively characterized and there are numerous contradictions in the published literature (see Suarez-Huerta et al., 2001 for examples). Further studies are required and the P2Y4 and P2Y6 receptors are still potential candidates for the second site of action of UTP in rat SPA and LPA.
The present data indicate that the P2Y6 receptor is probably the suramin-insensitive site of action of UDP in rat SPA and LPA, as UDP is an agonist at the cloned, suramin-insensitive P2Y6 subtype (Border & Webb, 2001). Consistent with this, Hartley et al. (1998) identified mRNA for the P2Y6 receptor in rat SPA. However, the same reservations regarding the lack of antagonism by PPADS and RB2 discussed above have to be noted. The identity of the suramin-sensitive site of action of UDP is less clear, since as noted above, of the recombinant P2Y receptors, UDP only acts at the P2Y6 subtype. Thus, UDP may act at a novel, uncloned receptor in the rat SPA and LPA. Alternatively, the suramin-insensitive effects of UDP may be mediated by the P2Y2 receptor after extracellular conversion to UTP by ecto-nucleoside diphosphokinase (eNDPK) in vascular smooth muscle. At present, selective eNDPK inhibitors are not available and so this possibility cannot be tested. However, such a conversion of UDP to UTP has been proposed to explain, in part, apparent agonist actions of UDP at P2Y2 receptors expressed in a cell line (Nicholas et al., 1996).
Ideally, native receptors should be characterized on the basis of the actions of stable and selective agonists and antagonists, but at present such compounds are not available for P2Y2,4,6 receptors. Nonetheless, these studies indicate that UTP most likely acts via suramin-sensitive P2Y2 and suramin-insensitive P2Y4 or P2Y6 receptors to elicit contraction of rat SPA and LPA. Likewise, UDP probably acts via P2Y6 receptors and P2Y2 receptors. However, for both agonists an action at a novel, uncloned P2Y receptor cannot be ruled out.
In summary, the results of the present study indicate the presence of multiple subtypes of contractile P2X and P2Y receptors in rat SPA and LPA smooth muscle cells and suggests that the functional expression of these receptors varies along the pulmonary arterial tree. A similar conclusion was reached for the rat mesenteric arterial bed, although in that case the potency of both P2X and P2Y receptor agonists varied (Gitterman & Evans, 2000). The responses of the rat perfused lung to P2X receptor agonists, particularly the desensitization evoked by α,β-meATP (McCormack et al., 1989; Rubino & Burnstock, 1996), more closely resembles those seen in the isolated SPA than in the LPA in the present study. This is consistent with small vessels exerting a greater effect than large vessels on pulmonary arterial pressure (Barnes & Liu, 1995). Thus, although studies on isolated arteries, particularly large arteries, have provided a substantial amount of information about receptor expression and mechanisms of action in general, care must be taken in extrapolating the data to the intact vascular bed.
Acknowledgments
This work was supported by funding from the Thailand Government and Naresuan University, Thailand (K. Chootip), the Wellcome Trust (C. Kennedy) and British Heart Foundation (A.M. Gurney).
Abbreviations
- α,β-meATP
α,β-methyleneATP
- 2-meSATP
2-methylthioATP
- ADP
adenosine 5′-diphosphate
- ATP
adenosine 5′-triphosphate
- EC40K
40% of the contraction to 40 mM K+
- LPA
large intrapulmonary artery
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
- RB2
reactive blue 2
- SPA
small intrapulmonary artery
- UDP
uridine 5′-diphosphate
- UTP
uridine 5′-triphosphate
References
- BARNES P.J., LIU S.F. Regulation of pulmonary vascular tone. Pharmacol. Rev. 1995;47:87–131. [PubMed] [Google Scholar]
- BO X., KAROON P., NORI S.L., BARDINI M., BURNSTOCK G. P2X purinoceptors in postmortem human cerebral arteries. J. Card. Pharmacol. 1998a;31:794–799. doi: 10.1097/00005344-199805000-00020. [DOI] [PubMed] [Google Scholar]
- BO X., SEXTON A., XIANG Z., NORI S.L., BURNSTOCK G. Pharmacological and histochemical evidence for P2X receptors in human umbilical vessels. Eur. J. Pharmacol. 1998b;353:59–65. doi: 10.1016/s0014-2999(98)00383-5. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI M.O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends in Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., WEBB T.E.P2Y receptors: structure and function Handbook of Experimental Pharmacology 151/1; Purinergic and Pyrimidinergic Signalling I: Molecular, Nervous and Urogenitary System Function 2001Berlin: Springer; 65–88.ed. Abbracchio, M.P. & Williams, M., pp [Google Scholar]
- BOGDANOV Y.D., WILDMAN S.S., CLEMENTS M.P., KING B.F., BURNSTOCK G. Molecular cloning and characterisation of rat P2Y4 nucleotide receptor. Br. J. Pharmacol. 1998;124:428–439. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUÉ-GRABOT E., ARCHAMBAULT V., SÉGUÉLA P. A protein kinase C site highly conserved in P2X subunits controls the desensitization kinetics of P2X2 ATP-gated channels. J. Biol. Chem. 2000;275:10190–10195. doi: 10.1074/jbc.275.14.10190. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., KENNEDY C. Is there a basis for distinguishing two types of P2-purinoceptor. Gen. Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., KENNEDY C. A dual function for adenosine triphosphate in the regulation of vascular tone: excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ. Res. 1986;58:319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- CHANG K., HANAOKA K., KUMADA M., TAKUWA Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J. Biol. Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- CHARLTON S.J., BROWN C.A., WEISMAN G.A., TURNER J.T., ERB L., BOARDER M.R. PPADS and suramin as antagonists at cloned P2Y- and P2U-purinoceptors. Br. J. Pharmacol. 1996a;118:704–710. doi: 10.1111/j.1476-5381.1996.tb15457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARLTON S.J., BROWN C.A., WEISMAN G.A., TURNER J.T., ERB L., BOARDER M.R. Cloned and transfected P2Y4 receptors: characterization of a suramin and PPADS-insensitive response to UTP. Br. J. Pharmacol. 1996b;119:1301–1303. doi: 10.1111/j.1476-5381.1996.tb16038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Z.P., KRULL N., XU S., LEVY A., LIGHTMAN S.L. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinol. 1996a;137:1833–1840. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- CHEN B.C., LEE C.M., LIN W.W. Inhibition of ecto-ATPase by PPADS, suramin and reactive blue in endothelial cells, C6 glioma cells and RAW 264.7 macrophages. Br. J. Pharmacol. 1996b;119:1628–1634. doi: 10.1111/j.1476-5381.1996.tb16082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOOTIP K., KENNEDY C., GURNEY A.M. Characterisation of P2 receptors mediating contraction of the rat isolated pulmonary vasculature. Br. J. Pharmacol. 2000;131:167P. [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMUNI D., SUAREZ GONZALEZ N., DETHEUX M., BREZILLON S., LANNOY V., PARMENTIER M., BOEYNAEMS J.M. Identification of a novel human ADP receptor coupled to Gi. J. Biol. Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- DOBRONYI I., HUNG K.S., SATCHELL D.G., MAGUIRE M.H. Evidence for a novel P2X purinoceptor in human placental chorionic surface arteries. Eur. J. Pharmacol. 1997;320:61–64. doi: 10.1016/s0014-2999(96)00967-3. [DOI] [PubMed] [Google Scholar]
- ERLINGE D., HOU M., WEBB T.E., BARNARD E.A., MÖLLER S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT–PCR. Biochem. Biophys. Res. Comm. 1998;248:864–870. doi: 10.1006/bbrc.1998.9083. [DOI] [PubMed] [Google Scholar]
- FILIPPOV A.K., WEBB T.E., BARNARD E.A., BROWN D.A. Dual coupling of heterologously-expressed rat P2Y6 nucleotide receptors to N-type Ca2+ and M-type K+ currents in rat sympathetic neurones. Br. J. Pharmacol. 1999;126:1009–1017. doi: 10.1038/sj.bjp.0702356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GITTERMAN D.P., EVANS R.J. Properties of P2X and P2Y receptors are dependent on artery diameter in the rat mesenteric bed. Br. J. Pharmacol. 2000;131:1561–1568. doi: 10.1038/sj.bjp.0703760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROBBEN B., CLAES P., ROYMANS D., ESMANS E.L., VAN ONCKELEN H., SLEGERS H. Ecto-nucleotide pyrophosphatase modulates the purinoceptor-mediated signal transduction and is inhibited by purinoceptor antagonists. Br. J. Pharmacol. 2000;130:139–145. doi: 10.1038/sj.bjp.0703289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAINES W.R., TORRES G.E., VOIGT M.M., EGAN T.M. Properties of the novel ATP-gated ionotropic receptor composed of the P2X1 and P2X5 isoforms. Mol. Pharmacol. 1999;56:720–727. [PubMed] [Google Scholar]
- HANSEN M.A., DUTTON J.L., BALCAR V.J., BARDEN J.A., BENNETT M.R. P2X (purinergic) receptor distributions in rat blood vessels. J. Auton. Nerv. Sys. 1999;75:147–155. doi: 10.1016/s0165-1838(98)00189-1. [DOI] [PubMed] [Google Scholar]
- HARPER S., WEBB T.E., CHARLTON S.J., NG L.L., BOARDER M. Evidence that P2Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br. J. Pharmacol. 1998;124:703–710. doi: 10.1038/sj.bjp.0701895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY S.A., KATO K., SALTER K.J., KOZLOWSKI R.Z. Functional evidence for a novel suramin-insensitive pyrimidine receptor in rat small pulmonary arteries. Circ. Res. 1998;83:940–946. doi: 10.1161/01.res.83.9.940. [DOI] [PubMed] [Google Scholar]
- HASSÉSSIAN H., BURNSTOCK G. Interacting roles of nitric oxide and ATP in the pulmonary circulation of the rat. Br. J. Pharmacol. 1995;114:846–850. doi: 10.1111/j.1476-5381.1995.tb13281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INSCHO E.W., COOK A.K., NAVAR L.G. Direct assessment of renal microvascular responses to P2-purinoceptor agonists. Am. J. Physiol. 1998;274:F718–F727. doi: 10.1152/ajprenal.1998.274.4.F718. [DOI] [PubMed] [Google Scholar]
- KENNEDY C.The role of purines in the peripheral nervous system Handbook of Experimental Pharmacology 151/1; Purinergic and Pyrimidinergic Signalling I: Molecular, Nervous and Urogenitary System Function 2001Berlin: Springer; 289–304.ed. Abbracchio, M.P. & Williams, M. pp [Google Scholar]
- KENNEDY C., MCCLAREN G.J., SNEDDON P.ATP as a cotransmitter with noradrenaline in sympathetic nerves – a target for hypertensive therapy Purinergic Approaches in Experimental Therapeutics 1997New York: John Wiley; 173–184.ed. Jacobson, K. & Jarvis, M. pp [Google Scholar]
- KHAKH B.S., BURNSTOCK G., KENNEDY C., KING B.F., NORTH R.A., SEGUELA P., VOIGT M., HUMPHREY P.P.A. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- KHAKH B.S., MICHEL A.D., HUMPHREY P.P.A. Inhibition of ectoATPase and Ca-ATPase in rat vas deferens by P2-purinoceptor antagonists. Br. J. Pharmacol. 1995;115:2P. doi: 10.1111/j.1476-5381.1995.tb16336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS C.J., ENNION S.J., EVANS R.J. P2 purinoceptor-mediated control of rat cerebral (pial) microvasculature: contribution of P2X and P2Y receptors. J. Physiol. 2000;527:315–324. doi: 10.1111/j.1469-7793.2000.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU S.F., MCCORMACK D.G., EVANS T.W., BARNES P.J. Characterization and distribution of P2-purinoceptor subtypes in rat pulmonary vessels. J. Pharmacol. Exp. Ther. 1989;251:1204–1210. [PubMed] [Google Scholar]
- MCCORMACK D.G., BARNES P.J., EVANS T.W. Purinoceptors in the pulmonary circulation of the rat and their role in hypoxic vasoconstriction. J. Pharmacol. Exp. Ther. 1989;251:1204–1210. doi: 10.1111/j.1476-5381.1989.tb12606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAREN G.J., BURKE K.S., BUCHANAN K.J., SNEDDON P., KENNEDY C. Evidence that ATP acts at two sites to evoke contraction in the rat isolated tail artery. Br. J. Pharmacol. 1998a;124:5–12. doi: 10.1038/sj.bjp.0701772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAREN G.J., SNEDDON P., KENNEDY C. Comparison of the actions of ATP and UTP at the P2X1-receptor in smooth muscle cells of the rat tail artery. Eur. J. Pharmacol. 1998b;351:139–144. doi: 10.1016/s0014-2999(98)00294-5. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A., WATT W.C., LAZAROWSKI E.R., LI Q., HARDEN T.K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: Identification of UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- NORI S., FUMAGALLI L., BO X., BOGDANOV Y., BURNSTOCK G. Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: An in situ hybridization and RT-PCR study. J. Vasc. Res. 1998;35:179–185. doi: 10.1159/000025582. [DOI] [PubMed] [Google Scholar]
- O'CONNOR S.E., WOOD B.E., LEFF P. Characterization of P2X-receptors in rabbit isolated ear artery. Br. J. Pharmacol. 1990;101:640–644. doi: 10.1111/j.1476-5381.1990.tb14133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS J.K., HILL C.E. Neuroreceptor mRNA expression in the rat mesenteric artery develops independently of innervation. Int. J. Devl. Neurosci. 1999;17:377–386. doi: 10.1016/s0736-5748(99)00032-5. [DOI] [PubMed] [Google Scholar]
- PHILLIPS J.K., MCLEAN A.J., HILL C.E. Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentry. Br. J. Pharmacol. 1998;124:1403–1412. doi: 10.1038/sj.bjp.0701976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–491. [PubMed] [Google Scholar]
- RALEVIC V., BURRELL S., KINGDOM J., BURNSTOCK G. Effects of purine and pyrimidine nucleotides on vascular tone of human placental cotyledons. Br. J. Pharmacol. 1997;121:1121–1126. doi: 10.1038/sj.bjp.0701262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBAYE B., BOEYNAEMS J.M., COMMUNI D. Slow desensitization of the human P2Y6 receptor. Eur. J. Pharmacol. 1997;329:231–236. [PubMed] [Google Scholar]
- RUBINO A., BURNSTOCK G. Evidence for a P2 purinoceptor mediator mediation vasoconstriction by UTP, ATP and related nucleotides in the isolated pulmonary vascular bed of the rat. Br. J. Pharmacol. 1996;118:1415–1420. doi: 10.1111/j.1476-5381.1996.tb15554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINO A., ZIABARY L., BURNSTOCK G. Regulation of vascular tone by UTP and UDP in isolated rat intrapulmonary arteries. Eur. J. Pharmacol. 1999;370:139–143. doi: 10.1016/s0014-2999(99)00150-8. [DOI] [PubMed] [Google Scholar]
- SUAREZ-HUERTA N., POUILLON V., BOEYNAEMS J.M., ROBAYE B. Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur. J. Pharmacol. 2001;416:197–202. doi: 10.1016/s0014-2999(01)00875-5. [DOI] [PubMed] [Google Scholar]
- TORRES G.E., HAINES W.R., EGAN T.M., VOIGT M.M. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol. Pharmacol. 1998;54:989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- VAN DER GIET M., CINKILIC O., JANKOWSKI J., TEPEL M., ZIDEK W., SCHLÜTER H. Evidence for two different P2X-receptors mediating vasoconstriction of Ap5A and Ap6A in the isolated perfused rat kidney. Br. J. Pharmacol. 1999;127:1463–1469. doi: 10.1038/sj.bjp.0702667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VULCHANOVA L., ARVIDSSON U., RIEDL M., WANG J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]


