Abstract
The lipid mediator PAF plays an important role in the phagocytosis of particles, including bacteria, and consequent production of pro-inflammatory cytokines, such as TNF-α and IL-8.
Using a PAF receptor antagonist (UK-74,505) and PAF receptor knock-out mice, we have investigated the relevance of PAF for the inflammatory changes and lethality after pulmonary infection with the gram-negative bacteria Klebsiella pneumoniae in mice.
At an inoculum of 3×106 bacteria, there was marked pulmonary (bronchoalveolar lavage and lung) neutrophilia that started early (2.5 h after infection) and peaked at 48 h. All animals were dead by day 4 of infection. The chemokine KC and the pro-inflammatory cytokine TNF-α increased rapidly and persisted for 48 h in the lungs.
Pretreatment with UK-74,505 (30 mg kg−1 per day, p.o.) had no significant effects on the number of infiltrating neutrophils in BAL fluid or lung tissue, as assessed by histology and measuring myeloperoxidase, or on the concentrations of KC. In contrast, concentrations of TNF-α and the number of bacteria inside neutrophils were significantly diminished.
In order to support a role for the PAF during K. pneumoniae infection, experiments were also carried out in PAFR-deficient mice. In the latter animals, lethality occurred earlier than in wild-type controls. This was associated with greater number of bacteria in lung tissue and diminished percentage of neutrophils containing bacteria in their cytoplasm.
Our results suggest that PAF, acting on its receptor, plays a protective role during infection with K. pneumoniae in mice.
Keywords: Chemokines, lung inflammation, neutrophils, TNF-α, recruitment
Introduction
Platelet-activating factor (PAF, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a potent autacoid lipid mediator with various biological activities, including platelet and leukocyte activation. PAF acts by binding to a G protein-coupled seven transmembrane receptor, the PAF receptor (PAFR), and appears to regulate constitutively various physiological processes (Ishii & Shimizu, 2000). In addition to its role as a physiological mediator, PAF has been shown to play an important role in the pathophysiology of various inflammatory conditions (Ishii & Shimizu, 2000). Studies with PAFR antagonists or PAFR-deficient animals have shown an essential role of PAFR during systemic allergic anaphylaxis-associated shock in mice (Ishii & Shimizu, 2000; Montrucchio et al., 2000). Studies with PAFR antagonists or strategies that decrease PAF activity have also demonstrated an important role of PAF for lipopolysaccharide (LPS)-induced shock and lethality (e.g. Fukuda et al., 2000) and for the lung injury which follows a range of inflammatory stimuli (Miotla et al., 1998; Tavares-De-lima et al., 1998; de matos et al., 1999).
More recently, we have suggested an important role of PAFR for the protective immune response of the murine host against infection with an intracellular protozoan parasite, T. cruzi (Aliberti et al., 1999). In the latter system, PAF induced NO release by T. cruzi-infected macrophages in vitro and pretreatment of mice with PAFR antagonists increased blood parasitaemia and enhanced infection-associated lethality (Aliberti et al., 1999). These results are in line with the ability of leukocytes to produce PAF upon encounter with microorganisms or soluble particles and to engulf them in a PAF-dependent manner (Makristathis et al., 1993; Au et al., 2001). Moreover, exposure of leukocytes to endotoxin or bacteria may trigger PAF release (reviewed by Montrucchio et al., 2000; Makristathis et al., 1993). Thus, it is clear that PAF may have a dual role during bacterial infections. On one hand, PAF appears to play an important role in the ability of a host to deal with infections by facilitating phagocytosis and killing of engulfed microorganisms. On the other hand, PAFR activation may underlie the tissue injury and shock associated with the infection and endotoxin released.
In this study, we have investigated the relevance of PAF receptors in a model of pulmonary infection in mice caused by gram-negative bacteria. Thus, we have assessed the effects of the treatment with a PAFR antagonist, UK-74,505, on the lethality bacterial counts and inflammatory indices following pulmonary infection of mice with Klebsiella pneumoniae. UK-74,505 is a potent, specific, orally available and long-acting PAFR antagonist (Alabaster et al., 1991; Parry et al., 1994; Jezequel et al., 1996). For comparison, we also assessed the lethality and infection indices of PAFR−/− mice after infection with K. pneumoniae.
Methods
Animals
Balb/C (8 to 12 week-old) female mice obtained from the Bioscience unit of our Institution were housed in standard conditions and had free access to commercial chow and water. PAF receptor-deficient (PAFR−/−) mice were generated as previously described and backcrossed or at least 10 generations into a Balb/C background (Ishii et al., 1998). All procedures described here had prior approval from the animal ethics committee of Instituto de Ciências Biológicas (Belo Horizonte, Brazil).
Bacteria
The bacterium used was Klebsiella pneumoniae – ATCC 27 736 that has been kept in the Department of Microbiology, Universidade Federal de Minas Gerais. Before the experiments described herein, bacteria were made pathogenic by 10 passages in Balb/C mice (i.p. injection and collection in the spleen 24 h later) and kept frozen in a −70°C freezer at a concentration of 1×109 CFU ml−1 in tryptic soy broth containing 10% glycerol (v v−1) until use. Bacteria were frozen when in the log phase of growth.
Treatment with UK-74,505
The PAF receptor antagonist UK-74,505 (modipafant, a gift of Dr J. Parry, Pfizer, Sandwich, U.K.) was dissolved initially in 0.1 M HCl and further diluted 10 fold in saline. Control animals received an oral administration of vehicle (0.01 M HCl), whereas the test group received an oral administration of UK-74,505 at dose of 30 mg kg−1. The oral dose chosen was recommended by the supplier and has been previously shown to give good bioavailability for 24 h (Alabaster et al., 1991; Parry et al., 1994; Jezequel et al., 1996). For lethality experiments, the drug was administered 24 and 2 h prior to inoculation of bacteria and daily thereafter. For the experiments measuring infection and inflammatory indices, the drug was administered 24 and 2 h prior to inoculation of bacteria and animals sacrificed 24 h after inoculation.
K. pneumoniae inoculation
K. pneumoniae was grown in tryptic soy broth (Difco, Detroit, MI, U.S.A.) for 18 h at 37°C prior to inoculation. The concentration of bacteria in broth was routinely determined by serial 1 : 10 dilutions. One hundred microlitres of each dilution were plated on McConkey agar plates and incubated for 24 h at 37°C and then colonies were counted. Each animal was anaesthetized i.p. with 0.2 ml of a solution containing xylazin (0.002 mg ml−1), ketamin (50 mg ml−1) and saline in a proportion of 1 : 0.5 : 3, respectively. The trachea was exposed and 30 μl of a suspension containing 3×106 K. pneumoniae or saline was administered with a sterile 26-gauge needle. The skin incision was closed with surgical staples.
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) was performed to obtain leukocytes in the alveolar spaces. The trachea was exposed and a 1.7-mm-outside-diameter polyethylene catheter inserted. BAL was performed by instilling three 1-ml aliquots of PBS and approximately 2 ml of fluid was retrieved per mouse. The number of total leukocytes was determined by counting leukocytes in a modified Neubauer chamber after staining with Turk's solution. Differential counts were obtained from cytospin preparations by evaluating the percentage of each leukocyte on a slide stained with May-Grunwald-Giemsa. In some experiments, the percentage of BAL neutrophils that had phagocytosed at least one bacterium was evaluated in at least 200 cells.
Determination of myeloperoxidase activity
The extent of neutrophil accumulation in the lung tissue was measured by assaying myeloperoxidase activity as previously described (de matos et al., 1999). Using the conditions described below, this methodology is very selective for the determination of neutrophils over macrophages (data not shown). Briefly, a portion of left lungs of animals was removed and snap frozen in liquid nitrogen. Upon thawing, the tissue (0.1 g of tissue per 1.9 ml of buffer) was homogenized in pH 4.7 buffer (0.1 M NaCl, 0.02 M NaPO4, 0.015 M NaEDTA), centrifuged at 3000×g for 10 min and the pellet subjected to hypotonic lyses (1.5 ml of 0.2% NaCl solution followed 30 s later by addition of an equal volume of a solution containing NaCl 1.6% and glucose 5%). After a further centrifugation, the pellet was resuspended in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide (HTAB) and re-homogenized. One millilitre aliquots of the suspension were transferred into 1.5 ml-Eppendorf tubes followed by three freeze-thaw cycles using liquid nitrogen. The aliquots were then centrifuged for 15 min at 3000×g, the pellet was resuspended to 1 ml and samples of lung were diluted (1 : 20) prior to assay. Myeloperoxidase (MPO) activity in the resuspended pellet was assayed by measuring the change in optical density (O.D.) at 450 nm using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM). Results were expressed as ‘myeloperoxidase index' and were calculated by comparing the O.D. of tissue supernatant with the O.D. of mouse peritoneal neutrophils processed in the same way. To this end, neutrophils were induced in the peritoneum of mouse by injecting 3 ml of casein 5%. A standard curve of neutrophil (>95% purity) numbers versus O.D. was obtained by processing purified neutrophils as above and assaying for MPO activity.
Determination of plasma and lung K. pneumoniae colony forming units
At time of sacrifice, plasma was collected from the branchial plexus, the right ventricle was perfused with 3 ml of sterile saline and lungs were harvested. Tissues were then homogenized with a homogenizer in a vented hood. The homogenates and plasma were placed on ice, and serial 1 : 10 dilutions were made. One hundred microlitres of each dilution were plated on McConkey agar plates (Difco) and incubated for 24 h at 37°C and then the number of colony forming units (CFU) was counted. The detection limit of the assay was 100 bacteria ml−1 or 100 bacteria per 100 mg of tissue.
Harvesting of lungs and blood for cytokine analysis
At the designated time point, mice were anaesthetized with xylazin/ketamin/saline as above, blood collected from the brachial plexus and the animals sacrificed. Prior to lung removal, the pulmonary vasculature was perfused with 3 ml of PBS via the right ventricle. The right lung was then harvested for assessment of the various cytokine protein levels.
Measurement of cytokine concentrations in serum, BAL and lungs
The cytokine concentrations (TNF-α, KC, MCP-1/JE) were measured in serum, BAL and lung of animals using ELISA techniques with commercially available antibodies and according to the instructions supplied by the manufacturer (R&D Systems). Serum was obtained from coagulated blood (15 min at 37°C, then 30 min at 4°C) and stored at −20°C until further analysis. Serum and BAL samples were analysed at a 1 : 3 and 1 : 5 dilution in assay dilution buffer, respectively. One hundred milligrams of lung of controls and treated animals were homogenized in 1 ml of PBS (0.4 m NaCl and 10 mM NaPO4) containing anti-proteases (0.1 mM PMSF, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 10 min at 3000×g and the supernatant immediately used for ELISA assays at a 1 : 5 dilution in assay dilution buffer. The detection limit of the ELISA assays was 16 pg ml−1.
Determination of the levels of circulating leukocytes
The total number of circulating leukocytes and neutrophils were evaluated in blood samples obtained at the end of the experiments described in Figure 1. The number of total circulating leukocytes was determined by counting leukocytes in a modified Neubauer chamber after staining with Turk's solution and differential counts by evaluating the percentage of each leukocyte on blood films stained with May-Grunwald-Giemsa.
Figure 1.

Kinetics of the influx of neutrophils in the lungs of mice infected with K. pneumoniae. Animals were inoculated with 3×106 bacteria or vehicle (30 μl) and neutrophil influx in (A) in the bronchoalveolar lavage (BAL) fluid or (B) lungs assessed after 2.5, 24 and 48 h. Myeloperoxidase (MPO) activity in lungs was used as an index of neutrophil influx in that tissue. Results are shown as the number of neutrophils or leukocyte index and represent the mean±s.e.mean of six animals in each group. *P<0.01 when compared with uninfected animals.
Histology
Lungs were inflated with 2 ml phosphate-buffered 10% formalin, embedded in paraffin and 4 μm-thick sections obtained. The sections were then stained with haematoxylin and eosin and examined under a light microscope.
Statistical analysis
Results are shown as means±s.e.mean. Data sets were compared by using analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc analysis. Results were considered significant when P<0.05.
Results
Kinetics of the pulmonary inflammation and infection after intratracheal (i.t.) inoculation of K. pneumoniae
Initial experiments were carried out to characterize the kinetics of the pulmonary response after instillation of K. pneumoniae. Mice were injected with 30 μl of a saline suspension containing 3×106 bacteria, an inoculum shown to be optimal for inducing lung inflammation and survival of animals for at least 48 h (data not shown). Twenty-four hours after inoculation of the bacteria, animals developed signs of infection – lethargy, decreased food intake, piloerection and ruffled fur. Histological analysis showed a marked diffuse infiltration of neutrophils in the lungs of infected animals. Many neutrophils were found in the alveolar spaces and occasional neutrophil aggregates were observed. In addition, there was diffuse hyperaemia and scattered areas of haemorrhage (data not shown). Within the time frame of the experiment (48 h) and when compared to uninfected mice, there did not seem to be an increase in the number of mononuclear cells in lung tissues (data not shown). In order to quantify the influx of neutrophils into the lungs of infected animals, we assessed the number of neutrophils (PMN) in BAL fluid and tissue MPO activity. In BAL fluid, there was an accumulation of PMN that was substantial at 24 h and was sustained until 48 h after infection (Figure 1A). Similarly, there was a marked infiltration of neutrophils in the lung parenchyma, as assessed by tissue MPO activity (Figure 1B). Neutrophils were first observed at 24 h after infection and greater numbers were observed after 48 h (Figure 1B). With an exception of a neutrophilia observed at 2.5 h, there were few modifications in total or differential leukocyte counts in blood (data not shown).
The concentration of bacteria in the lungs of infected mice rapidly increased after the inoculation of K. pneumoniae (Figure 2). The total number of bacteria was already greater than 1×108 CFU per lung 2.5 h after infection. These concentrations increased rapidly and were not countable at 48 h at the dilutions used (Figure 2). In contrast, we failed to observe any dissemination of the infection, as no K. pneumoniae colony could be determined in plasma in any of the time points until 48 h after infection (data not shown).
Figure 2.
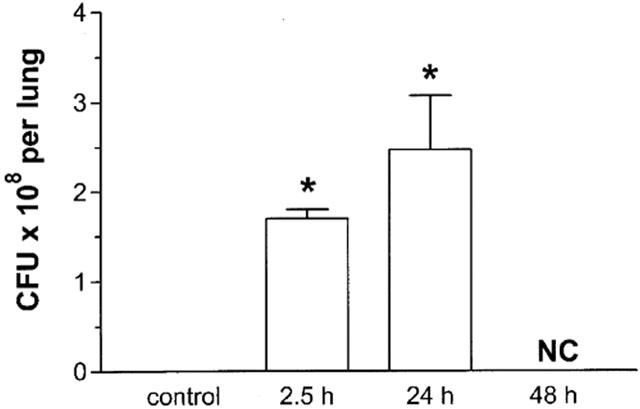
Kinetics of the number of colony-forming units (CFU) in the lungs of mice infected with K. pneumoniae. Animals were inoculated with 3×106 bacteria or vehicle (30 μl and the number of CFU in lung tissue evaluated after 2.5, 24 and 48 h. At 48 h, the number of CFU was not countable (NC). Results are shown as the mean±s.e.mean of five animals in each group. *P<0.01 when compared with uninfected animals.
KC and TNF-α are known to play a role during gram negative bacterial infection in mice (Tsai et al., 1998; Greenberger et al., 1995; Laichalk et al., 1996). Experiments evaluating the kinetics of expression of KC and TNF-α in lung homogenates after K. pneumoniae are shown in Figure 3. Markedly elevated concentrations of both cytokines in lungs were already detectable at 2.5 h after infection. The concentrations were still elevated at 24 h and levels started to fall at 48 h (Figure 3). No TNF-α could be detected in plasma samples in any of the time points measured and KC was detectable in plasma at 2.5 h and 24 h after infection but not at 48 h (control, below detection limit; 2.5 h, 759.35±209.95 pg ml−1; 24 h, 121.4±92.0 pg ml−1; 48 h, below detection limit; n=6). In all further experiments, samples were harvested at 24 h after infection, as tissue inflammation was marked at this time point, bacteria levels in tissues were elevated and all animals were alive but with signs of infection.
Figure 3.
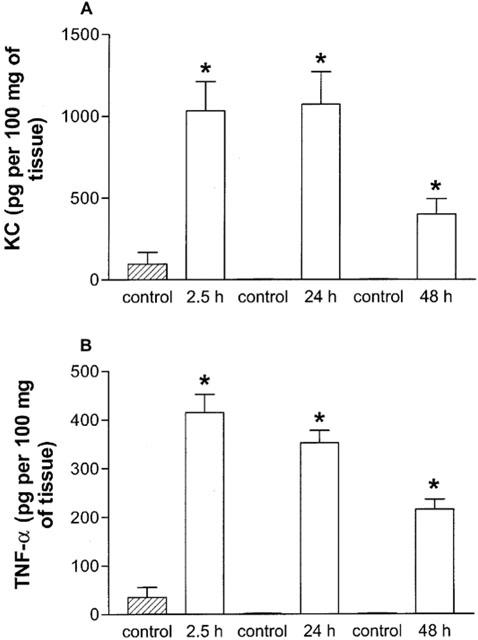
Kinetics of TNF-α and KC protein production in lung homogenates after infection with K. pneumoniae. Animals were inoculated with 3×106 bacteria or vehicle (30 μl) and the concentration of KC (A) and TNF-α (B) in lung tissue evaluated by ELISA after 2.5, 24 and 48 h. Results are shown as the mean±s.e.mean of six animals in each group. *P<0.01 when compared with uninfected animals.
Effects of the treatment with the PAF receptor antagonist UK-74,505 on the course of K. pneumoniae infection
Treatment with UK-74,505 had no significant effect on the number of neutrophils which were recruited into the airspaces of infected animals (infected animals, 34.0±17.7×105 neutrophils; infected and UK-74,505-treated, 42.8±13.7×105, n=6). Similarly, the recruitment of neutrophils in the lung of UK-74,505-treated mice, as assessed by MPO assay (Infected, 1.2±0.2; Infected+UK-74,505, 0.9±0.2×106 neutrophils 100 mg−1 of tissue, n=6), was not significantly different from vehicle-treated K. pneumoniae-infected animals. Histological analysis of lungs of infected animals treated with vehicle or UK-74,505 showed a marked diffuse infiltration of neutrophils, hyperaemia and areas of haemorrhage. There did not appear to be any qualitative differences between the two groups (data not shown).
In agreement with the lack of effect of the PAFR antagonist on neutrophil recruitment into the lung tissue, the tissue and BAL fluid concentrations of the neutrophil-active chemokine KC was not different in UK-74,505-treated and untreated mice (Figure 4A,B). Similarly, pulmonary tissue concentrations of MCP-1 were not significantly different in both groups of animals (Infected, 1516.0±194.0 pg per 100 mg of tissue; infected and UK-74,505-treated, 1129.4±103.4 pg per 100 mg of tissue; n=6). In contrast, treatment with UK-74,505 significantly decreased the concentrations of TNF-α detected in the lung and BAL fluid of K. pneumoniae-infected mice (Figure 4C,D).
Figure 4.
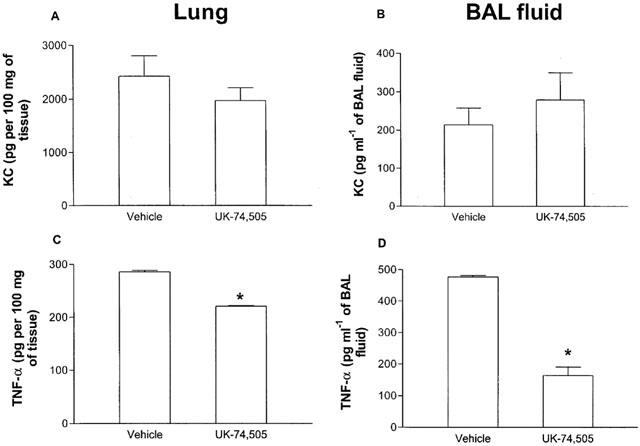
Effect of pre-treatment with the PAF receptor antagonist UK 74,505 on the production of KC and TNF-α after infection with K. pneumoniae. Animals were inoculated with 3×106 bacteria or vehicle (30 μl) and the concentration of KC (A,B) and TNF-α (C,D) in lung tissue (A,C) or BAL fluid (B,D) evaluated by ELISA after 24 h. Results are shown as the mean±s.e.mean of six animals in each group. *P<0.01 when compared with animals treated with vehicle.
The effects of daily treatment of K. pneumoniae-infected mice with UK-74,505 on bacterial counts is shown in Figure 5. Treatment with UK-74,505 resulted in a significant increase in the number of CFU in the lungs of K. pneumoniae-infected mice (Figure 5A). Similarly, infection of PAFR−/− mice with K. pneumoniae resulted in a larger number of CFU in the lungs when compared to wild-type controls (Figure 5B).
Figure 5.
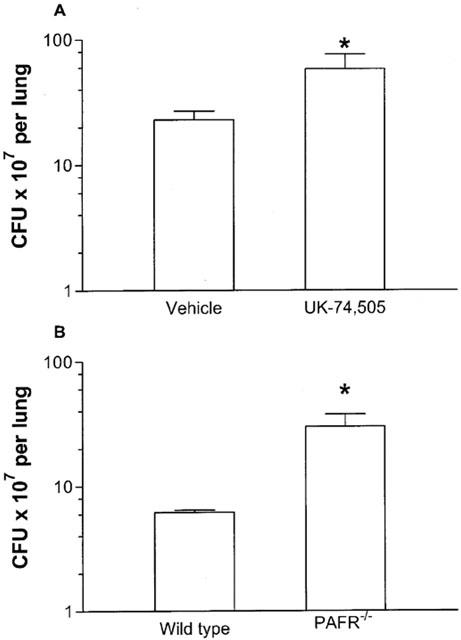
Number of colony-forming units (CFU) after infection with K. pneumoniae (A) after the pre-treatment with the PAF receptor antagonist UK 74,505 or in (B) PAFR−/− mice. Animals were inoculated with 3×106 bacteria or vehicle (30 μl) and the number of CFU in lung homogenates evaluated after 24 h. PAFR−/− mice were compared to their respective wild-type controls. Results are shown as the mean±s.e.mean of five animals in each group. *P<0.01 when compared with animals treated with vehicle-treated wild-type controls.
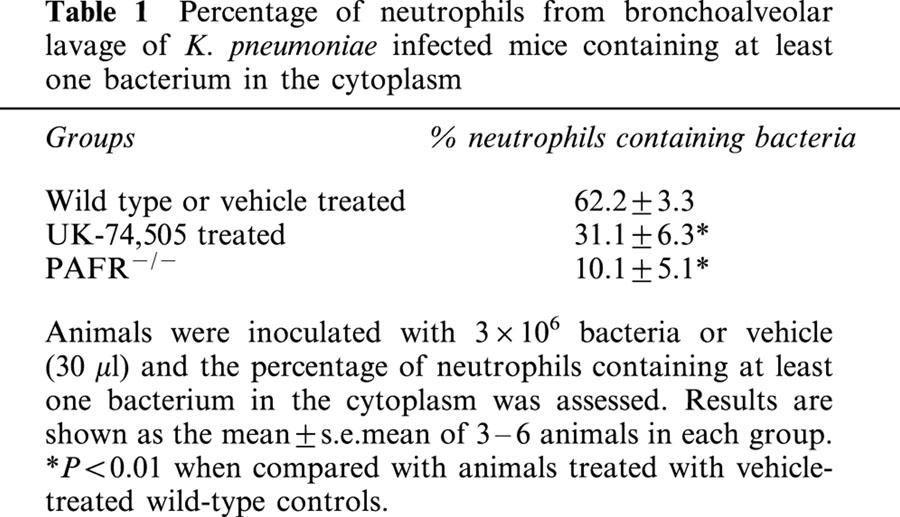
The percentage of BAL neutrophils that had ingested at least one bacterium was evaluated 24 h after infection. As seen in Table 1, pre-treatment with UK-74,505 was accompanied by a 50% inhibition of the ability of BAL neutrophils to phagocytose K. pneumoniae. Similarly, there was a marked suppression of K. pneumoniae uptake by BAL neutrophils from PAFR−/− mice when compared to their wild type controls (Table 1).
Table 1.
Percentage of neutrophils from bronchoalveolar lavage of K. pneumoniae infected mice containing at least one bacterium in the cytoplasm
Survival of mice infected with K. pneumoniae
Subsequent experiments were performed to examine the contribution of PAF to the survival of mice infected with K. pneumoniae. As shown in Figure 6, all untreated K. pneumoniae-infected animals were alive by 72 h of infection after which time mortality increased substantially, with 100% lethality noted by day 5 after inoculation. The treatment with UK-74,505 resulted in earlier lethality with 30 and 85% of animals dead at 72 and 96 h, respectively (Figure 6). Similarly, infection of PAFR−/− mice with K. pneumoniae resulted in significantly earlier lethality when compared to wild-type animals (Figure 6). Of note, 40% of animals were dead at 48 h after infection.
Figure 6.

Role of the PAF receptor for the survival of animals after infection with K. pneumoniae. Four groups of animals were evaluated (eight animals in each group): infected animals (inoculated with 3×106 bacteria) which were PAF receptor-deficient (PAFR−/−), wild-type (Infected WT) or wild-type treated with the PAF receptor antagonist UK 74,505 (WT+UK 74,505) and animals which received only vehicle (30 μl, not infected). The number of dead animals was evaluated every 12 h and results are shown as per cent survival. Survival of PAFR−/− and UK 74,505-treated animals was significantly different from that of infected WT animals (P<0.05).
Discussion
The host defence against acute bacterial infection requires the generation of a vigorous inflammatory response that predominantly involves recruitment and activation of neutrophils. Although in the majority of cases this response is sufficient to control infection, an overt inflammatory response may cause marked tissue injury, haemodynamic shock and death (Teixeira et al., 2001). PAF is a biologically active phospholipid mediator known to be important for the ability of leucocytes to phagocytose foreign particles and kill microorganisms (Ishii & Shimizu, 2000; Au et al., 2001). On the other hand, several studies have demonstrated the role of PAF and its receptor in mediating tissue injury, shock and lethality following endotoxin challenge and other acute inflammatory stimuli (Miotla et al., 1998; Fukuda et al., 2000; Ishii and Shimizu, 2000; Montrucchio et al., 2000; Souza et al., 2000). Thus, during acute bacterial infection, activation of PAFR may be important for the ability of the host to deal with infection but could underlie or contribute to the systemic inflammatory response observed in the most severe cases. These possible dual effects of PAFR activation led us to investigate the functional role of the receptor in a model of lung infection with K. pneumoniae.
In our model of lung infection, we chose to use K. pneumoniae for several reasons. This gram-negative aerobic organism is an important cause of community-acquired pneumonia in individuals with impaired pulmonary defences and is a major pathogen for nosocomial pneumonia (Granton & Grossman, 1993; Maloney & Jarvi, 1995). Importantly, preliminary studies showed that after intratracheal (i.t.) inoculation with K. pneumoniae mice developed pneumonia with features resembling human disease. Moreover, there is a reproducible relationship between the size of the inoculum and lethality of infection (Toews et al., 1979). A dose of 3×106 bacteria was chosen for all experiments as this dose allowed for the development of substantial inflammation within 24 to 48 h without excessive mortality. Untreated animals receiving this dose of K. pneumoniae were unable to clear the bacteria and all animals ultimately died. Therefore, this dose allowed for assessment of the inflammatory mediators and bacterial growth, as well as the effects on survival.
In our experiments, the inoculation of K. pneumoniae induced a time-dependent increase of infiltration of neutrophils and pro-inflammatory cytokines in the lungs of infected mice. The number of neutrophils increased rapidly and was markedly elevated 24 h after the infection. The increase in neutrophil numbers in BAL fluid and lungs was preceded by an increase in the concentrations of TNF-α and KC. Of note, these pro-inflammatory mediators have been shown to play important roles in the control of bacterial infection and lung inflammation following pulmonary infection with K. pneumoniae (Laichalk et al., 1996; Tsai et al., 1998; 2000). Interestingly, at the inoculum used and at time points observed, the inflammatory response seemed to be mostly compartmentalized in the lungs, as the number of leukocytes, and the concentration of cytokines (with the exception of KC) and bacteria were not significantly elevated in the plasma of infected animals. Only later was the control of the infection lost and lethality occurred. Overall, our experiments are in good agreement with observations of other studies examining pulmonary inflammation after K. pneumoniae infection (e.g.: Laichalk et al., 1996; Tsai et al., 1998; 2000).
Daily treatment with the PAFR antagonist UK-74,505 had no relevant effect on the influx of neutrophils, as assessed by the number of these cells in BAL fluid, MPO activity and histological analysis of lung tissue. Previous studies have suggested a role for neutrophil-active (CXC) chemokines and chemokine receptors for the migration of neutrophils into the lungs of mice infected with bacteria (Greenberger et al., 1996; Moore et al., 2000; Tsai et al., 2000; Fillion et al., 2001). In addition to the lack of effect of PAFR blockade on neutrophil influx, UK-74,505 had little effect on the tissue concentrations of the CXC chemokine KC. These results suggest that the early generation of PAF does not play a role in the generation of the neutrophil-active chemokine KC and subsequent sequestration of neutrophils into the lungs of infected mice. Similarly, PAF failed to affect the recruitment of neutrophils to the lungs after i.v. challenge with endotoxin (Miotla et al., 1998) or after acid-induced lung injury (Nagase et al., 1999) in mice. Thus, whereas PAFR activation may play a role in phagocytosis-dependent production of the CXC chemokine IL-8 after stimulation with a particulate stimulus (zymosan) (Au et al., 2001), production of KC after bacterial infection in vivo seemed to be independent of PAFR activation.
Previous studies (Laichalk et al., 1996) have shown a critical role of TNF-α as part of the pulmonary host defense in a murine model of infection with K. pneumoniae. In our model, there was a marked pulmonary production of TNF-α that peaked early after infection and was maximal around 24 h. Pretreatment with UK-74,505 significantly reduced the concentrations of TNF-α both in BAL fluid and pulmonary tissue extracts after K. pneumoniae infection. These effects of PAF on TNF-α production seemed to be of physiopathological relevance, as demonstrated by the increase in the number of CFU in the lungs of K. pneumoniae-infected mice. The increase in bacterial counts in the lungs were reflected in the severity of the disease, as UK-74,505-treated animals died significantly faster than vehicle-treated controls. Our lethality results are in contrast with a previously published study evaluating the effect of a distinct, shorter-acting PAFR antagonist (WEB2170) in a model of K. pneumoniae infection in NMRI mice (Makristathis et al., 1993). In this latter study, WEB2170 treatment was accompanied by a small, dose-independent increase in survival and a marginal, dose-independent decrease in bacterial counts. Moreover, statistical analysis was not provided in that study (Makristathis et al., 1993).
The role of PAFR for the ability of the host to mount an effective immune response was even more markedly appreciated when PAFR-deficient animals were used. In these animals, significant lethality was already noticeable 48 h after infection. Thus, our results clearly suggest that the ability of PAFR to modulate TNF-α production in the lungs of mice may be relevant for an effective innate immune response against K. pneumoniae pulmonary infection.
An important role for PAFR in the phagocytosis of particulate stimuli and ensuing pro-inflammatory cytokine production has been demonstrated in several studies (Au et al., 1994, 2001; Aliberti et al., 1999; Owaki et al., 2000). Interestingly, the phagocytosis of K. pneumoniae by neutrophils is accompanied by the release of PAF from neutrophils (Makristathis et al., 1993). One possibility that stems from the latter observations is that, in our model, the blockade of PAFR may have prevented the ability of phagocytes to engulf bacteria and produce pro-inflammatory cytokines, such as TNF-α, in response to the phagocytic stimulus. In this regard, results of the pre-treatment with UK-74,505 or experiments with PAFR−/− mice showed that fewer neutrophils that migrated to the lungs after K. pneumoniae infection ingested bacteria. Moreover, a role of intracellular PAF for the production of TNF-α by macrophages has been previously demonstrated in vitro (Yamada et al., 1999; Tsuyuki et al., 2002). Overall, the results above argue for an important role of PAFR activation in mediating the phagocytosis of bacteria. The latter possibility is being actively investigated in our laboratory.
The CC chemokine MCP-1 appears to play an important role in the pulmonary anti-fungal response after Aspergillus fumigatus and Cryptococcus neoformans infection in mice (Huffnagel et al., 1995; Blease et al., 2001). Similarly, administration of MCP-1 prior to a systemic infection with Pseudomonas aeruginosa enhanced survival, an effect associated with enhanced bacterial phagocytosis and killing in vitro (Nakano et al., 1994). Nevertheless, several studies have now shown that MCP-1 may shift the balance in favour of anti-inflammatory cytokine production after endotoxin challenge or during septic peritonitis (Matsukawa et al., 2000; Zisman et al., 1997; Hogaboam et al., 1998). There was a marked increase in pulmonary MCP-1 concentrations 24 h after infection with K. pneumoniae. Pretreatment with UK-74,505 failed to affect MCP-1 concentrations significantly in our system, excluding a role for PAFR in modulating MCP-1 production.
In conclusion, our results suggest that PAF, by acting on its receptor, plays little role in the local production of chemokines and recruitment of leukocytes during K. pneumoniae infection in mice. However, the PAFR appears to contribute to the local production of TNF-α and to the ability of leukocytes to deal with the infecting bacteria. The latter roles of the PAFR may underlie the increased lethality observed in animals treated with a PAFR antagonist or in PAFR−/− mice. Thus, whereas PAFR antagonism appears to be an effective strategy to control the lung injury associated with a range of acute inflammatory stimuli, this receptor is also part of an effective innate immune response against bacterial infection in the lungs. The latter effects of PAFR antagonists may be relevant in humans and could limit any beneficial effects of the drugs in acute inflammatory conditions, such as sepsis.
Abbreviations
- BAL
bronchoalveolar lavage
- CFU
colony forming units
- KC
keratinocyte-derived chemokine
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- MPO
myeloperoxidase
- PAF
platelet activating factor
- PAFR
PAF receptor
- PAFR−/−
PAF receptor-deficient mice
- PBS
phosphate buffered saline
- PMN
polymorphonuclear leukocyte
- TNF-α
tumour necrosis factor-α
References
- ALABASTER V.A., KEIR R.F., PARRY M.J., DE SOUZA R.N. UK-74,505, a novel and selective PAF antagonist, exhibits potent and long lasting activity in vivo. Agents Actions Suppl. 1991;34:221–227. [PubMed] [Google Scholar]
- ALIBERTI J.C.S., MACHADO F.S., GAZZINELLI R.T., TEIXEIRA M.M., SILVA J.S. Platelet-activating factor induces nitric oxide synthesis in Trypanosoma cruzy-infected macrophages and mediates resistance to parasite infection in mice. Infect. Immun. 1999;67:2810–2814. doi: 10.1128/iai.67.6.2810-2814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AU B.T., TEIXEIRA M.M., COLLINS P.D., WILLIAMS T.J. Blockade of PAF receptors controls interleukin-8 production by regulating the activation of neutrophil CD11/CD18. Eur. J. Pharmacol. 2001;425:65–71. doi: 10.1016/s0014-2999(01)01141-4. [DOI] [PubMed] [Google Scholar]
- AU B.T., WILLIAMS T.J., COLLINS P.D. Zymosan-induced IL-8 release from human neutrophils involves activation via the CD11b/CD18 receptor and endogenous platelet-activating factor as an autocrine modulator. J. Immunol. 1994;152:5411–5419. [PubMed] [Google Scholar]
- BLEASE K., MEHRAD B., LUKACS N.W., KUNKEL S.L., STANDIFORD T.J., HOGABOAM C.M. Antifungical and airway remodeling roles for murine monocyte chemoattractant protein-1/CCL2 during pulmonary exposure to Aspergillus fumigatus conidia. J. Immunol. 2001;166:1832–1842. doi: 10.4049/jimmunol.166.3.1832. [DOI] [PubMed] [Google Scholar]
- DE MATOS I.M., SOUZA D.G., SEABRA D.G., FREIRE-MAIA L., TEIXEIRA M.M. Effects of tachykinin NK1- or PAF-receptor blockade on the lung injury induced by scorpion venom. Eur. J. Pharmacol. 1999;376:293–300. doi: 10.1016/s0014-2999(99)00382-9. [DOI] [PubMed] [Google Scholar]
- FILLION I., OUELLET N., SIMARD M., BERGERON Y., SATO S., BERGERON M.G. Role of chemokine and formyl peptides in pneumococcal pneumonia-induced monocyte/macrophage reruitment. Am. Association of Immun. 2001;166:7353–7361. doi: 10.4049/jimmunol.166.12.7353. [DOI] [PubMed] [Google Scholar]
- FUKUDA Y., KAWASHIMA H., SAITO K., INOMATA N., MATSUI M., NAKANISHI T. Effect of human plasma-type platelet-activating factor acethylhydrolase in two anaphilactic shock models. Eur. J. Pharmacol. 2000;390:203–207. doi: 10.1016/s0014-2999(99)00920-6. [DOI] [PubMed] [Google Scholar]
- GRANTON J.T., GROSSMAN R.F. Community-acquired pneumonia in the elderly patient. Clinical features, epidemiology, and treatment. Clin. Chest. Med. 1993;14:537–553. [PubMed] [Google Scholar]
- GREENBERGER M.J., STIETER R.M., KUNKEL S.L., DANFORTH J.M., GOODMAN R.E., STANDIFORD T.J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J. Infect. Dis. 1995;155:722–729. [PubMed] [Google Scholar]
- GREENBERGER M.J., STRIETER R.M., KUNKEL S.L., DANFORTH J.M., LAICHALK L.L., MCGILLICUDDY D.C., STANDIFORD T.J. Neutralization of Macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella plenumonia. J. Infect. Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- HOGABOAM C.Y., STEINHAUSER M.L., SCHOCK H., LUKACS N., STRIETER R.M., STANDIFORD T., KUNDEL S.L. Therapeutic effects of nitric oxide inhibition during experimental fecal peritonitis: Role of interleukin-10 and monocyte chemoattractant protein 1. Effect. Immun. 1998;66:650–655. doi: 10.1128/iai.66.2.650-655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUFFNAGEL G.B., STRIETER R.M., STANDIFORD T.J., MCDONALD R.A., BURDICK M.D., KUNKEL S.L., TOEWS G.B. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- ISHII S., KUWAKI T., NAGOSE T., MAKI K., TASHIRO F., SUNAGA S., CAO W., KUME K., FUKUCHI Y., IKUTA K., MIYAZAKI J., KUMADA M., SHIMIZU T. Impaired anaphylactic response with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J. Exp. Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII S., SHIMIZU T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- JEZEQUEL S.G., UDEN S., WASTALL P. Modipafant, a new PAF antagonist: pharmacokinetics and disposition in rat, dog and man. Xenobiotica. 1996;26:963–975. doi: 10.3109/00498259609052498. [DOI] [PubMed] [Google Scholar]
- LAICHALK L.L., KUNKEL S.L., STRIETER R.M., DANFORTH J.M., BAILE M.B., STANDIFORD T.J. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect. Immun. 1996;64:5211–5218. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKRISTATHIS A., STAUFFER F., FEISTAUER S.M., GEORGOPOULOS A. Bacteria induce release of platelet-activating factor (PAF) from polymorphonuclear neutrophil granulocytes: possible role for PAF in pathogenesis of experimentally induced bacterial pneumonia. Infect. Immun. 1993;61:1996–2002. doi: 10.1128/iai.61.5.1996-2002.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALONEY S.A., JARVI W.R. Epidemic nosocomial pneumonia in the intensive care unit. Clin. Chest Med. 1995;16:209–223. [PubMed] [Google Scholar]
- MATSUKAWA A., HOGABOAM C.M., LUKACS N.W., LINCOLN P.M., STRIETER R.M., KUNKEL S.L. Endogenous MCP-1 influences systemic cytokine balance in a murine model of acute septic peritonitis. Exp. Mol. Pathol. 2000;68:77–84. doi: 10.1006/exmp.1999.2296. [DOI] [PubMed] [Google Scholar]
- MIOTLA J.M., JEFFERY P.K., HELLEWELL P.G. Platelet-activating factor plays a pivotal role in the induction of experimentally lung injury. Am. J. Cell Mol. Biol. 1998;18:197–204. doi: 10.1165/ajrcmb.18.2.2846. [DOI] [PubMed] [Google Scholar]
- MONTRUCCHIO G., ALLOATTI G., CAMUSSI G. Role of platelet-activating factor in vascular cardiovascular pathophysiology. Physiol. Rev. 2000;80:1669–1699. [Google Scholar]
- MOORE T.A., NEWSTEAD M.W., STRIETER R.M., MEHRAD B., BEAMAN B.L., STANDIFORD T.J. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonare Nocardia asteroides infection. J. Immunol. 2000;164:908–915. doi: 10.4049/jimmunol.164.2.908. [DOI] [PubMed] [Google Scholar]
- NAGASE T., ISHII S., KUME K., UOZUMI N., IZUMI Y., OUCHI Y., SHIMIZU T. Platelet-activating factor mediates acid-induced lung injury in genetically engineered mice. J. Clin. Invest. 1999;104:1071–1076. doi: 10.1172/JCI7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKANO Y., KASAHARA T., MUKAIDA N., KO Y.C., NAKANO M., MATSUSHIMA K. Protection against lethal bacterial infection in mice by monocyte-chemotactic and -activating factor. Infect. Immunol. 1994;62:377–383. doi: 10.1128/iai.62.2.377-383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWAKI T., MENESHIAN A., MAEMURA K., TAKAO S., WANG D., FUH K.C., BULKLEY G.B., KLEIN A.S. Endothelial cells potentiate phagocytic killing by macrophages via platelet-activating factor release. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H269–H276. doi: 10.1152/ajpheart.2000.278.1.H269. [DOI] [PubMed] [Google Scholar]
- PARRY M.J., ALABASTER V.A., CHEESEMAN H.E., COOPER K., DE SOUZA R.N., KEIR R.F. Pharmacological profile of UK-74,505, a novel and selective PAF antagonist with potent and prolonged oral activity. J. Lipid. Mediat. Cell Signal. 1994;10:251–268. [PubMed] [Google Scholar]
- SOUZA D.G., CARA D.C., CASSALI G.D., COUTINHO S.F., SILVEIRA M.R., ANDRADE S.P., POOLE S., TEIXEIRA M.M. Effects of the PAF receptor antagonist UK 74,505 on local and remote reperfusion injuries following ischemia of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2000;131:1800–1808. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAVARES-DE-LIMA W., STEIL A.A., RUSSO M., STAROBINAS N., TEIXEIRA C.F.P., JANCAR S. Lipid mediators, tumor necrosis factor and nitric oxide and their interactions in immune-complex-induced lung injury. Eur. J. Pharmacol. 1998;358:69–75. doi: 10.1016/s0014-2999(98)00594-9. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., CUNHA F.Q., FERREIRA S.H.Editorial. Response to an infectious insult Braz. J. Med. Biol. Res. 200134:1ppreceding 555 [PubMed] [Google Scholar]
- TOEWS G.B., GROSS G.N., PIERCE A.K. The relationship of inoculum size to lung bacterial clearance and phagocytic cell response in mice. Am. Rev. Resp. Dis. 1979;120:559–566. doi: 10.1164/arrd.1979.120.3.559. [DOI] [PubMed] [Google Scholar]
- TSAI W.C., STRIETER R.M., MEHRAD B., NEWSTEAD M.W., ZENG X., STANDIFORD T.J. CXC chemokine receptor CXCR@ is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 2000;68:4289–4296. doi: 10.1128/iai.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAI W.C., STRIETER R.M., WILKOWSKI J.M., BUCKNELL K.A., BURDICK M.D., LIRA S.A., STANDIFORD T.J. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J. Immunol. 1998;161:2435–2440. [PubMed] [Google Scholar]
- TSUYUKI K., ICHINOWATARI G., TANIMOTO A., YAMADA M., YAGINUMA H., OHUCHI K. Possible participation of intracellular platelet-activating factor in NF-kappaB activation in rat peritoneal macrophages. Biochim. Biophys. Acta. 2002;1583:26–34. doi: 10.1016/s1388-1981(02)00161-0. [DOI] [PubMed] [Google Scholar]
- YAMADA M., TANIMOTO A., ICHINOWATARI G., YAGINUMA H., OHUCHI K. Possible participation of intracellular platelet-activating factor in tumor necrosis factor-α production by rat peritoneal macrophages. Eur. J. Pharmacol. 1999;374:341–350. doi: 10.1016/s0014-2999(99)00337-4. [DOI] [PubMed] [Google Scholar]
- ZISMAN D.A., KUNKEL S.L., STRIETER R.M., TSAI W.C., BUCKNELL K., WILKOWSKI J. MCP-1 protects mice in lethal endotoxemia. J. Clin. Invest. 1997;99:2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]


