Abstract
Among components of oxidized low density lipoproteins, cholesterol derivatives oxidized in position 7 inhibit endothelium-dependent arterial relaxation by decreasing the release of the main endothelium-derived relaxing factor, nitric oxide (NO). The aim of the present study was to bring new insights into the molecular mechanism by which 7-ketocholesterol can block the endothelium-dependent arterial relaxation.
Superoxide dismutase did not prevent the inhibitory effect of 7-ketocholesterol on endothelium-dependent relaxation, and consistent observations were made whether superoxide dismutase was conjugated or not to polyethylene glycol. In addition, neither glutathione supplementation, nor oxypurinol, i.e. a xanthine oxidase inhibitor could reverse the effect of 7-ketocholesterol, indicating that NO was not inactivated by superoxide anion.
A direct alteration of the activity of the calcium-dependent NO synthase could also be ruled out, since identical relaxing effects of the calcium ionophore A23187 were observed whether arterial rings were treated or not with 7-ketocholesterol.
Whereas the above observations come in support of an early, inhibitory action of 7-ketocholesterol, the specific blockade of one given subtype of membrane receptors could be discarded, and similar inhibitions were observed when either muscarinic or purinergic receptors were stimulated.
Finally, the blockade of protein kinase C activity by chelerythrine arose as the sole relevant tool in preventing the effect of 7-ketocholesterol on the endothelium-dependent relaxation of rabbit aortic rings. In addition, complementary studies on cultured bovine aortic endothelial cells came in direct support of the ability of 7-ketocholesterol to activate PKC.
In conclusion, 7-ketocholesterol that is present in human hypercholesterolaemic plasma, in atherosclerotic arteries, and in many processed foods can block the release of NO by vascular endothelial cells through its ability to activate PKC.
Keywords: Cholesterol oxides, endothelium-dependent relaxation, protein kinase C
Introduction
Recent studies demonstrated that alterations in vascular reactivity are associated with cardiovascular disorders. In particular, arteries from hypercholesterolaemic and atherosclerotic patients exhibit marked attenuation of the endothelium-dependent arterial relaxation (Zeiher et al., 1991; Reddy et al., 1994). Although the impairment of arterial relaxation at an early stage of the atherosclerotic process may constitute a crucial step in the disease progression, the related molecular mechanisms remain unclear. Oxidized low density lipoproteins have been implicated in the occurrence of endothelial dysfunction (Simon et al., 1990; Plane et al., 1992; Cox & Cohen, 1996) and their deleterious effect was shown to result mainly from the accumulation of two lipid derivatives, i.e. lysophosphatidylcholine and cholesterol oxides (Kugiyama et al., 1990; Deckert et al., 1997; Mougenot et al., 1997). Cholesterol oxides are stable end-products of the lipid oxidation cascade, and detectable amounts were reported in plasma, arterial wall and food products (for review Brown & Jessup, 1999). Interestingly, they were found to be abnormally elevated in hypercholesterolaemic plasma (Hodis et al., 1991) and atherosclerotic aortas (Carpenter et al., 1993; Breuer et al., 1996; Maor et al., 2000; Yasunobu et al., 2001; Garcia-Cruset et al., 2001), and increased plasma levels of 7β-hydroxycholesterol were recently associated with an increased risk of atherosclerosis in humans (Salonen et al., 1997; Zieden et al., 1999; Yasunobu et al., 2001). In previous studies, we have shown that cholesterol derivatives oxidized in position 7 (7β-hydroxycholesterol and 7-ketocholesterol) can mimic the ability of oxidized LDL to inhibit endothelium-dependent arterial relaxation. Their inhibitory effect on arterial relaxation was found to be independent of their known cytotoxic effect (Lizard et al., 1996), and studies in human umbilical vein endothelial cells (HUVEC) revealed that 7β-hydroxycholesterol and 7-ketocholesterol can reduce the histamine-activated release of nitric oxide (Deckert et al., 1998), the main endothelium-derived relaxing factor (Palmer et al., 1987; Ignarro et al., 1987).
The aim of the present study was to understand further the molecular mechanism by which cholesterol oxides alter the endothelium-dependent relaxation. For that purpose, we focused our attention on the effect of 7-ketocholesterol that has been shown to constitute the main cholesterol derivative formed after LDL oxidation, and the major oxysterol present in arterial macrophages (Maor et al., 2000), in hypercholesterolaemic plasma and in advanced atherosclerotic lesions (for review see Brown & Jessup, 1999). In order to determine the precise implication of 7-ketocholesterol in the cascade that leads to NO synthesis and release, several hypotheses have been tested: abnormal receptor function, NO synthase substrate deficiency, decreased NO bioavailability due to increased breakdown by oxygen-containing free radicals, and activation of protein kinase C.
Methods
Determination of vascular reactivity
Preparation of blood vessels
Animal experiments were performed under the framework of the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Blood vessels were prepared according to the method previously described (Deckert et al., 1997). Briefly, the abdominal aorta from 3–3.5 kg New Zealand White rabbits was removed and transferred into a Krebs solution bubbled with 95% O2 and 5% CO2. Aortic rings were suspended horizontally between two wire hooks in organ baths containing oxygenated Krebs' solution maintained at 37°C (Deckert et al., 1997). Changes in isometric tension were monitored continuously on a Mac Lab 8 system (AD Instruments Ltd). The resting tension of the rings was set to 2 g. After a 30-min equilibration period, the contractile response to KCl was first obtained to check the contractile response of the vascular smooth muscle.
Experimental protocols
The effect of cumulative concentrations of various relaxing agents, i.e. acetylcholine (ACh), adenosine diphosphate (ADP), or calcium ionophore (A23187) was determined on distinct aortic rings which were precontracted by 0.3 μM norepinephrine (NE). ACh, ADP and A23187 were used in the 1 nM to 0.03 mM, 10 nM to 0.1 mM and 1 nM to 0.1 mM concentration ranges, respectively. Since ADP is known to exert a relaxing effect in rabbit aorta through both a direct action on smooth muscle cell and an indirect action on endothelial cells (Furchgott, 1983), paired rings from each rabbit aorta were treated in parallel as follows: one arterial ring was relaxed by ADP in the absence of Nω-nitro-L-arginine (0.01 mM) in order to measure the total relaxing response to ADP; the other one was relaxed by ADP in the presence of Nω-nitro-L-arginine (0.01 mM) in order to measure the endothelium-independent relaxation. The endothelium-dependent relaxation was calculated as the difference between the relaxation obtained under the two distinct conditions.
Following each initial contraction/relaxation cycle, aortas were repeatedly washed out and incubated for 2 h in the absence or in the presence of 7-ketocholesterol (90 μg ml−1). At the end of the incubation period, aortic segments were precontracted with NE (0.3 μM) and subsequently relaxed by either ACh or ADP. Since in the case of calcium ionophore A23187 tachyphylaxis does not allow to perform two consecutive response curves (Furchgott, 1983), the effect of 7-ketocholesterol on A23187-induced relaxation was evaluated by parallel comparison of rings that were treated or not.
Preparation of 7-ketocholesterol
As indicated by the supplier, 7-ketocholesterol was ⩾98% pure, and the high degree of purity was confirmed in our laboratory by capillary gas chromatography analysis (Deckert et al., 1997). Prior to be added into the organ bath, 7-ketocholesterol (500 μg) was dissolved in ethanol (30 μl) and then mixed at a 1 : 1 molar ratio with a solution of fatty acid-poor BSA dissolved in Krebs' buffer. Control segments with no 7-ketocholesterol added were incubated in Krebs' buffer containing ethanol and BSA only.
Specific pretreatments of arterial segments
In experiments with oxypurinol (final concentration, 1 mM) or superoxide dismutase conjugated to polyethylene glycol (PEG-SOD, 150 U ml−1), pretreatments were applied for 45 and 15 min, respectively, and pretreated rings were subsequently incubated with 7-ketocholesterol prior to the second contraction/relaxation cycle. In experiments with chelerythrine (10 nM), glutathione (5 mM) or unconjugated SOD (150 U ml−1), compounds were added 15 min (unconjugated SOD) or 30 min (other compounds) before the second contraction/relaxation cycle.
Determination of protein kinase C activity in cultured endothelial cells
Cell culture
Bovine aortic endothelial cells (BAEC) were obtained from the European Collection of Cell Cultures (ECACC, Salisbury, U.K.). The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 2 mM glutamine, and supplemented with 10% foetal bovine serum and antibiotics (100 U ml−1 penicillin, 100 μg ml−1 streptomycin). The cells were plated into 75-cm2 culture flasks (Falcon) and incubated at 37°C in a humidified 5%–95% CO2/air atmosphere. Confluent monolayers of cells at 4 days of culture (107 cells) were used in subsequent experiments.
Cell treatments and protein kinase C assay
7-ketocholesterol was first dissolved in ethanol, and then mixed with 30 ml of culture medium, yielding final concentrations ranging from 30 to 90 μg ml−1. BAEC were incubated from 1 min to 2 h at 37°C with culture medium in the presence or absence of 7-ketocholesterol. At the end of the incubation period, BAEC were washed in ice-cold phosphate buffer saline (PBS) and scraped into 1-ml cold buffer containing (in mM) Tris-HCl 50, benzamidine 10, EDTA 5, EGTA 10, β-mercaptoethanol 50, phenylmethylsulphonyl fluoride (PMSF) 1, pH 7.5. BAEC were sonicated on ice at intermediate settings (five times for 10 s each) and centrifugated for 1 h at 100,000×g. Protein kinase C activity was determined in the supernatant by using a non-radioactive Protein Kinase Assay Kit (Calbiochem-Novabiochem Corporation, La Jolla, CA, U.S.A.) according to the manufacturer's recommendations. Briefly, in a first step, a synthetic pseudosubstrate peptide that is covalently bound to the microwells of the titration plate is phosphorylated by PKC. In a second step, the phosphorylated form of the pseudosubstrate is specifically recognized by a biotinylated monoclonal antibody. In a last step, peroxidase-conjugated streptavidin is added, and the colour development is made with o-phenylenediamine. Protein kinase C activity is proportional to the absorbence units as measured at 490 nm in a multiwell spectrophotometer (Victor II, Wallac).
Data analysis
The maximal relaxations (Emax) induced by either ACh, ADP or A23187, and expressed as the percentage of the contraction to NE (0.3 μM) were determined from experimental data. pD2 values corresponding to the negative logarithm of the agonist concentration required to produce a half maximal relaxing effect (EC50), were calculated after fitting each curve according to a sigmoid equation of the form:
in which Y is the relaxing effect, expressed as a percentage of the contraction to NE (0.3 μM); X is the corresponding agonist concentration; P1, the lower plateau response; P2, the range between the lower and the maximal plateaus of the concentration-effect curve; P3, a negative curvature index indicating the slope independently of the range; and P4, log EC50 (Deckert et al., 1994).
Data are expressed as mean±s.e.mean. Statistical comparison of data means was performed by use of either ANOVA or student's t-test.
Drugs and solutions
ACh, 7-ketocholesterol, NE, calcium ionophore A23187, ADP, chelerythrine, oxypurinol, SOD, PEG-SOD, Nω-nitro-L-arginine, fatty acid-poor BSA, glutathione, DMEM containing 2 mM glutamine, PBS, PMSF, and β-mercaptoethanol were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Foetal bovine serum, penicillin-streptomycin were purchased from GIBCO-BRL.
All drugs were dissolved in distilled water except A23187, oxypurinol, and chelerythrine which were dissolved in dimethylsulphoxide. Nω-nitro-L-arginine and glutathione were added directly to Krebs solution.
Results
Effect of 7-ketocholesterol on the endothelium-dependent relaxation of rabbit aorta induced by ACh, ADP or A23187
ACh which activates endothelial NO synthase through a receptor-dependent mechanism relaxed NE-precontracted rabbit aorta in a concentration-dependent manner (Figure 1A). The subthreshold concentration required to produce the relaxation of untreated aortic rings was 0.01 μM, and the maximal relaxation was obtained with 3 μM ACh. The pretreatment of the arteries with 7-ketocholesterol (90 μg ml−1; 2 h) modified significantly endothelium-dependent relaxation induced by ACh (Figure 1A). The maximal arterial relaxation (Emax) was significantly reduced by the pretreatment with 7-ketocholesterol, whereas no significant alteration in the sensitivity of rabbit aorta to ACh (EC50 values) was observed (Table 1).
Figure 1.
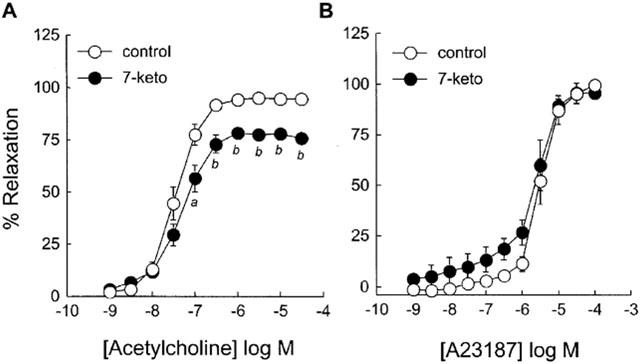
Effect of 7-ketocholesterol on ACh- and A23187-mediated endothelium-dependent relaxation of rabbit aorta. Graphs show concentration-response curves to ACh (A) and A23187 (B) of arteries precontracted with 0.3 μM NE and preincubated for 2 h without (control) or with (7-keto) 90 μg ml−1 of 7-ketocholesterol. Each point represents the mean±s.e.mean of 5–8 distinct experiments. Significantly different from control: aP<0.05, bP<0.001.
Table 1.
Effects of 7-ketocholesterol on maximal relaxation (Emax) and sensitivity (pD2) of rabbit aortic rings to ACh, ADP and A23187
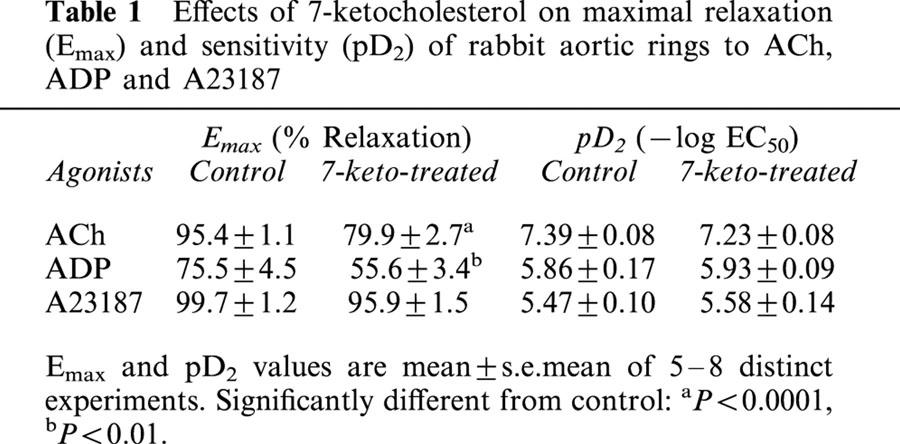
In contrast to ACh, the calcium ionophore A23187 stimulated endothelial NO synthase through a receptor-independent pathway. In the present studies, A23187 relaxed NE-precontracted rabbit aorta in a concentration-dependent manner (Figure 1B); the subthreshold concentration was 0.3 μM, and the maximal relaxation of rabbit aorta was obtained with the 0.1 mM dose. Unlike ACh-mediated relaxation, the receptor-independent relaxation induced by A23187 was not significantly modified by 7-ketocholesterol, and both Emax and EC50 values remained unchanged whether arterial rings were pretreated or not with 7-ketocholesterol (Table 1).
ADP relaxes NE-precontracted rabbit aorta through its dual action on endothelial cells and smooth muscle cells. The subthreshold concentration required to produce the relaxation of untreated aortic rings was 0.3 μM, and the maximal relaxation was obtained with 30 μM ADP (Figure 2A). The pretreatment of the arteries with 7-ketocholesterol (90 μg ml−1; 2 h) significantly inhibited the relaxation induced by 30 μM of ADP (% relaxation: 84.4±2.8% in 7-ketocholesterol-treated arteries vs 100.4±3.0% in control arteries, (P<0.01); Figure 2A). When the endothelial effect of ADP was blocked with the addition of LNA (NO synthase inhibitor), a direct relaxing effect of ADP on precontracted smooth muscle cells was observed, however with no significant effect of 7-ketocholesterol in this case (Figure 2A). As calculated from the concentration-response curves obtained with or without NO synthase inhibition (i.e. with or without LNA, respectively), the endothelium-dependent relaxation to ADP of arteries was significantly reduced by 7-ketocholesterol (90 μg ml−1; 2 h) (Figure 2B, Table 1).
Figure 2.
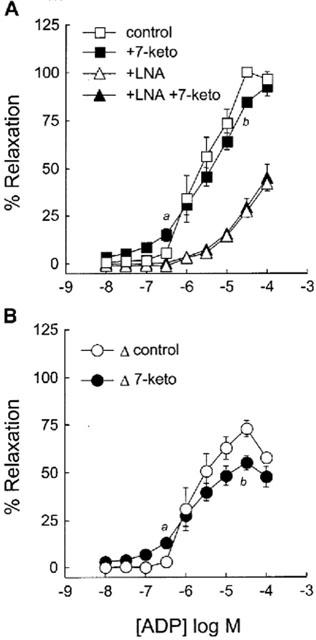
Effect of 7-ketocholesterol on ADP-mediated relaxation of rabbit aorta. Graph A shows concentration-response curves to ADP of arteries precontracted with 0.3 μM NE in the presence or in the absence of LNA (0.01 mM) and preincubated for 2 h without (control) or with (7-keto) 90 μg ml−1 of 7-ketocholesterol. Concentration-response curves obtained in the absence of LNA represent the total relaxing response (endothelium-independent plus endothelium-dependent relaxation) of arteries to ADP whereas those obtained in the presence of LNA represent exclusively the endothelium-independent relaxation induced by ADP. Graph B shows calculated endothelium-dependent relaxing response to ADP resulting from the difference between the total relaxation (A-squares) and the endothelium-independent relaxation (A-triangles). Data in Graph B are expressed as delta of % Relaxation. Each point represents the mean±s.e.mean of six distinct experiments. Significantly different from control: aP<0.05, bP<0.01.
Effect of superoxide dismutase, oxypurinol and glutathione on 7-ketocholesterol-induced inhibition of endothelium-dependent arterial relaxation
To investigate whether the 7-ketocholesterol-mediated impairment of endothelium-dependent relaxation of aortic rings could relate to an increased breakdown of NO by O2−, the effect of superoxide dismutase (SOD) was investigated. As shown in Figure 3, SOD (150 U ml−1) did not improve the relaxing response to ACh of 7-ketocholesterol-treated arteries, again with a significant decrease in the maximal relaxation as compared to untreated control rings. Similar observations were made when SOD was conjugated to polyethylene glycol (PEG-SOD), that allows to assess the effect of 7-ketocholesterol on the O2− production at the intracellular level (Beckman et al., 1988; Figure 3 and Table 2). These results indicate that the effect of 7-ketocholesterol does not relate to alterations in the level of O2−, neither in the intra-, nor in the extracellular compartment.
Figure 3.
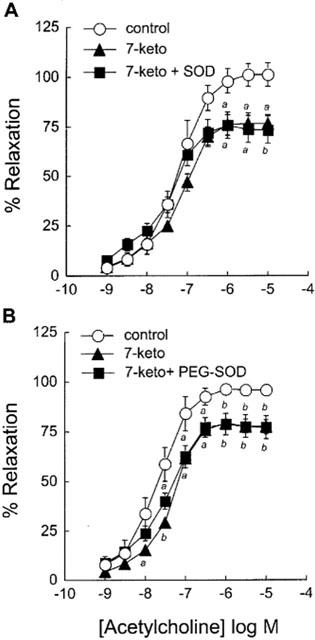
Effect of SOD and PEG-SOD on 7-ketocholesterol-induced inhibition of endothelium-dependent relaxation to ACh. Graphs show concentration-response curves to ACh of arteries precontracted with 0.3 μM NE. Arteries were preincubated for 2 h without (control) or with (7-keto) 90 μg ml−1 of 7-ketocholesterol. PEG-SOD (150 U ml−1) was added 15 min before preincubation with 7-ketocholesterol; SOD (150 U ml−1) was added 15 min before the contraction/relaxation cycle was performed. Each point represents the mean±s.e.mean of 4–8 distinct experiments. Significantly different from control: aP<0.05, bP<0.01.
Table 2.
Effect of various antagonists on 7-ketocholesterol-induced inhibition of ACh-mediated relaxation of rabbit aorta
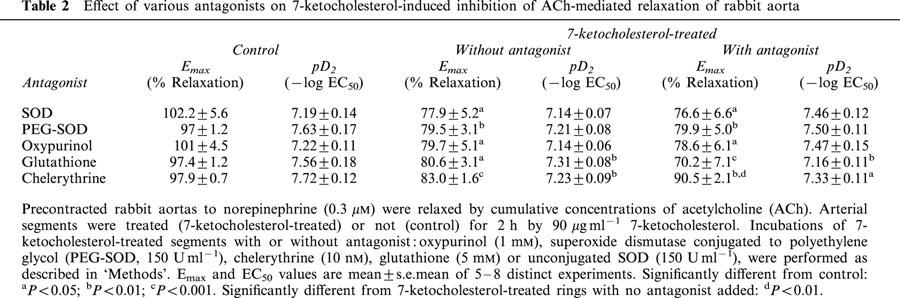
Since the activation of endothelial xanthine oxidase has been reported to contribute to the generation of O2− in endothelial cells (Tan et al., 1993), the effect of oxypurinol, i.e. a specific xanthine oxidase inhibitor was investigated. As shown in Figure 4, oxypurinol (1 mM) was not able to restore a normal endothelium-dependent relaxation of rabbit aortic rings that were incubated with 7-ketocholesterol (Figure 4, Table 2).
Figure 4.
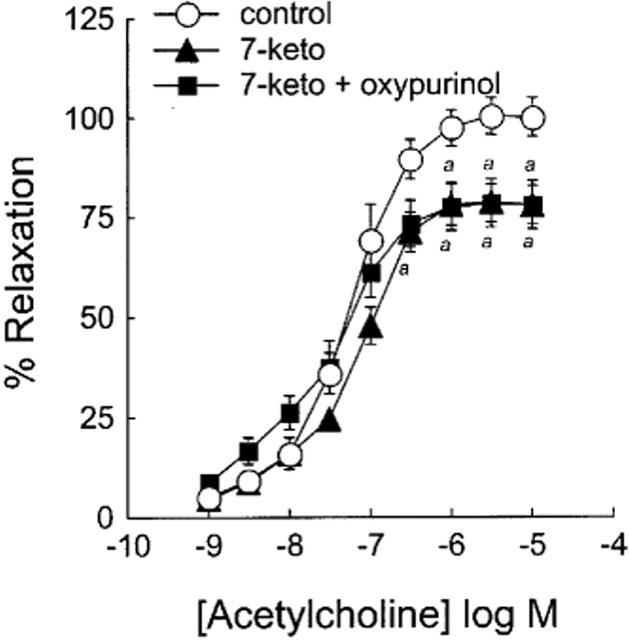
Effect of oxypurinol on 7-ketocholesterol-induced inhibition of endothelium-dependent relaxation to ACh. Graph shows concentration-response curves to ACh of arteries precontracted with 0.3 μM NE. Arteries were pretreated or not with oxypurinol (1 mM) for 45 min before addition of 7-ketocholesterol (90 μg ml−1) for 2 h. Each point represents the mean±s.e.mean of seven distinct experiments. Significantly different from control: aP<0.05.
Since glutathione constitutes a major cellular antioxidant system in addition to SOD, it can protect endothelial cell from oxygen free radicals, and its effect on 7-ketocholesterol-pretreated rings was investigated. As shown in Figure 5 and Table 2, and in agreement with observations made above with SOD and oxypurinol, the pretreatment of rabbit aorta with glutathione (5 mM) did not prevent the inhibitory effect of 7-ketocholesterol on arterial endothelium-dependent relaxation.
Figure 5.
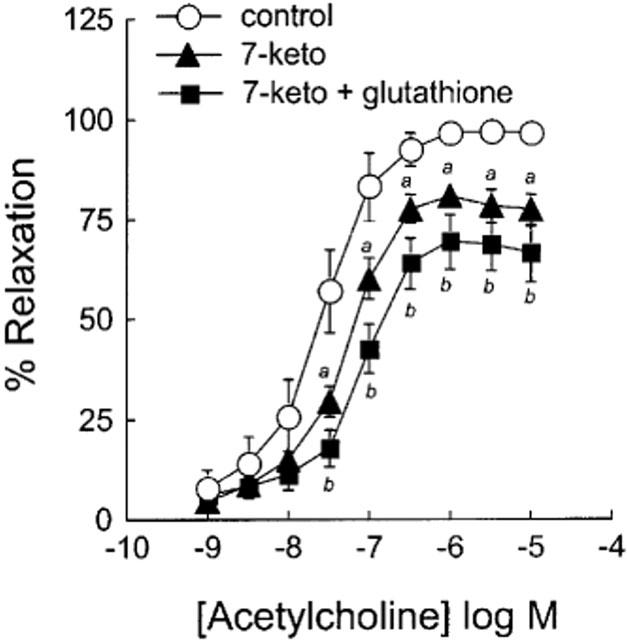
Effect of glutathione on 7-ketocholesterol-induced inhibition of endothelium-dependent relaxation to ACh. Graph shows concentration-response curves to ACh of arteries precontracted with 0.3 μM NE and preincubated for 2 h without (control) or with (7-keto) 90 μg ml−1 of 7-ketocholesterol. Glutathione (5 mM) was subsequently added 30 min before the contraction/relaxation cycle was performed. Each point represents the mean±s.e.mean of six distinct experiments. Significantly different from control: aP<0.05, bP<0.01.
Effect of chelerythrine on 7-ketocholesterol-induced inhibition of endothelium-dependent relaxation
To determine the possible involvement of protein kinase C (PKC) in mediating the inhibitory effect of 7-ketocholesterol on vasorelaxation, we examined the effect of the treatment of aortic rings with chelerythrine (10 nM), i.e. a potent PKC inhibitor. As shown in Figure 6, the deleterious effect of 7-ketocholesterol on endothelium-dependent relaxation was significantly counteracted by chelerythrine, with Emax values that became intermediate between control and 7-ketocholesterol-treated arteries. In the absence of 7-ketocholesterol, chelerythrine did not modify ACh-mediated relaxation (Figure 6).
Figure 6.
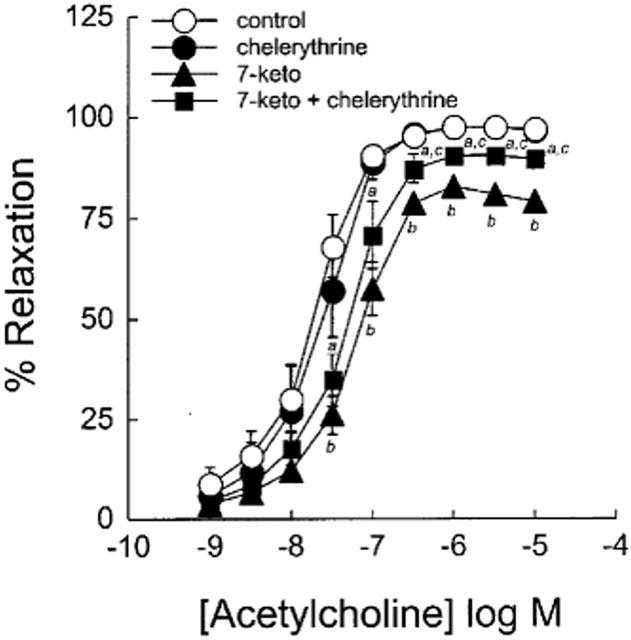
Effect of chelerythrine on ACh-mediated endothelium-dependent relaxation in the presence and in the absence of 7-ketocholesterol. Graph shows concentration-response curves to ACh of arteries precontracted with 0.3 μM NE and preincubated for 2 h without or with 90 μg ml−1 of 7-ketocholesterol. Chelerythrine (10 nM) was added 30 min before the contraction/relaxation cycle was performed. Each point represents the mean±s.e.mean of six distinct experiments. Significantly different from control: aP<0.05, bP<0.01. Significantly different from 7-ketocholesterol-treated rings. cP<0.05.
Effect of 7-ketocholesterol on PKC activity in cultured bovine aortic endothelial cells (BAEC)
On the basis of positive results that were obtained with the chelerythrine treatment (see above), we sought further for a direct evidence in favour of the effect of 7-ketocholesterol on PKC activity. To this end, cultured endothelial cells were treated with 7-ketocholesterol, and the effect of the treatment on PKC activity was directly assessed by using a specific immunoassay (see Methods). As compared to basal PKC activity measured in control mixture with no cholesterol oxide added (Absorbence at 490 nm: 0.746±0.043 (n=9)), 2-h treatments of BAEC with 7-ketocholesterol (concentration: 30, 60 and 90 μg ml−1) increased PKC activity in a concentration-dependent manner (Absorbence at 490 nm: 0.967±0.092 (n=6, ns vs control); 0.992±0.113 (n=6, P<0.05 vs control); and 1.033±0.063 (n=6, P<0.01 vs control), respectively). 7-ketocholesterol could exert its effect in a time-dependent manner, and a tendency towards increase in PKC activity was observed only 5 min after starting the incubation (P=0.0639 vs control) (Figure 7).
Figure 7.
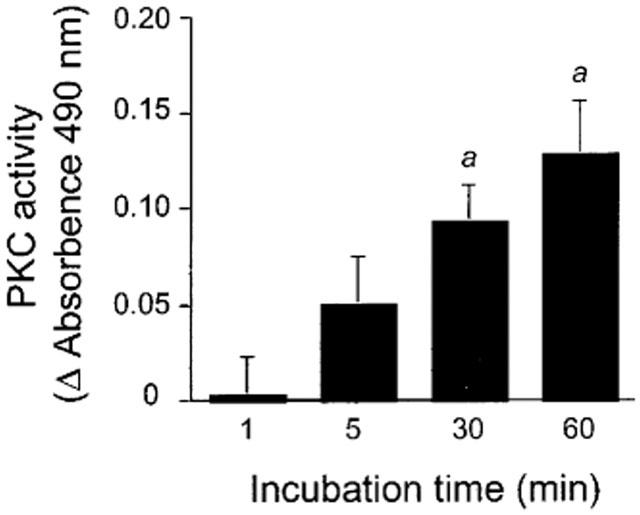
Effect of 7-ketocholesterol on PKC activity in cultured bovine endothelial aortic cells (BAEC). BAEC were incubated for 1, 5, 30 and 60 min at 37°C in culture medium containing or not 7-ketocholesterol. At the end of the incubation period, BAEC were washed in ice-cold PBS and cells were scraped for PKC assay (see Methods). Variations in PKC activity (Y axis) are expressed as compared to control homologous incubations with no 7-ketocholesterol added. Bars are means±s.e.mean of 6–7 determinations. Significantly different from homologous incubation with 7-ketocholesterol added. aP<0.02.
Discussion
Vascular tone homeostasis is impaired in hypercholesterolaemia and in the early stage of the atherogenic process, and the generation of cholesterol oxides was shown to account at least in part for this defect (Deckert et al., 1997; Mougenot et al., 1997). A direct effect of cholesterol oxides on the response of smooth muscle cells to NO donors was excluded (Deckert et al., 1997), and observations rather came in support of the ability of cholesterol oxides to promote endothelial dysfunction. However, the precise molecular mechanisms by which lipid derivatives impair endothelium-dependent relaxation remains incompletely understood. Data of the present study come in support of an early action of 7-ketocholesterol in increasing protein kinase C activity in vascular endothelial cells.
Evidence from both animal and human studies indicate that inactivation of NO by oxygen free radicals constitutes an important step in the pathogenesis of endothelial dysfunction, and an elevated generation of superoxide anion (O2−) was proposed to account for the impaired NO-dependent vasodilation in hypercholesterolaemic vessels (Minor et al., 1990; Ohara et al., 1993; White et al., 1994; Mügge et al., 1994; White et al., 1996). Interestingly, specific enzymatic systems can prevent NO inactivation, and for instance the activity of vascular extracellular superoxide dismutase (SOD), i.e. the major superoxide anion scavenger system in the arterial wall (Stralin et al., 1995) was recently shown to be reduced in patients with coronary artery disease (Landmesser et al., 2000). However, the latter mechanism is unlikely to account for the deleterious effect of 7-ketocholesterol on the endothelium function since the addition of extracellular SOD was not able to restore a normal endothelium-mediated relaxation in the present study. Besides its putative inactivation shortly after its release from the endothelium, NO is also susceptible to be inactivated within the endothelial cells, through intracellular O2− action. In order to take into account the latter point, SOD was also used in the present studies as a PEG-SOD conjugate that was reported to enhance the uptake of the enzyme by endothelial cells (Beckman et al., 1988). Again, and as observed with extracellular SOD supplementation, the PEG-SOD treatment did not improve the endothelial relaxation of 7-ketocholesterol-treated rabbit aorta. These results are supported by earlier studies which reported the lack of a significant effect of SOD supplementation on either the inhibition by oxidized LDL of the release of NO by endothelial cells (Chin et al., 1992) or the impairment of endothelium-dependent relaxation in patients with hypercholesterolaemia (Garcia et al., 1995). In further support against a direct implication of superoxide anion in mediating the deleterious effect of 7-ketocholesterol, the pretreatment of arterial rings with oxypurinol (i.e. a compound that inhibits the formation of superoxide anions through the blockade of xanthine oxidase (Ohara et al., 1993; Mügge et al., 1994)) could not prevent endothelial dysfunction. Finally, glutathione supplementation was not able to modify the inhibitory effect of 7-ketocholesterol on the ACh-mediated, endothelium-dependent relaxation. Since glutathione, the major antioxidant in the cell, has been shown to normalize the intracellular redox balance, to prevent the inactivation of NO in endothelium and to restore coronary vasodilation (Kugiyama et al., 1998), the ability of 7-ketocholesterol to inactivate NO in rabbit aorta could be ruled out.
In contrast to the receptor-dependent relaxation induced by ACh, the relaxation to calcium ionophore A23187, which is receptor-independent was not inhibited by 7-ketocholesterol. The normal response to calcium ionophore suggested therefore that in rabbit aorta treated with 7-ketocholesterol, the ability of NO synthase to generate NO from L-arginine was not significantly modified. This conclusion was in good agreement with our previous observations which demonstrated that 7-ketocholesterol does not inhibit the production of NO by cultured human endothelial cells that were stimulated with ionomycin, i.e. another calcium ionophore (Deckert et al., 1998). Moreover, L-arginine supplementation did not modify the 7-ketocholesterol-induced reduction of NO release (Deckert et al., 1998). Together, these observations came in support of an early action of 7-ketocholesterol, upstream of the intracellular calcium rise, a hypothesis that was consistent with the rapid effect of 7-ketocholesterol that became apparent in the present study after short incubation periods.
A selective blockade of muscarinic receptors by 7-ketocholesterol, similar to this one initially proposed by Bossaler et al. (1987) to explain the impairment of endothelium-dependent relaxation in atherosclerotic arteries, could be ruled out in the present study. Indeed, when we turned to ADP, a specific agonist of purinergic receptors, the 7-ketocholesterol-mediated impairment of arterial relaxation could still be observed, and the inhibition potency of 7-ketocholesterol was of the same magnitude as that observed through the ACh-mediated stimulation of muscarinic receptors. These observations led us to consider that 7-ketocholesterol exerts its action at an intermediate step that occurs after the stimulation of membrane muscarinic or purinergic receptors, but before the mobilization of intracellular calcium and the activation of NO synthase. Finally, our quest led us to consider the putative ability of 7-ketocholesterol to modulate the protein kinase C activity. Indeed, PKC plays an important role in the intracellular signalling pathway (Newton, 1995) and its activation is known to inhibit cell surface receptor-coupled signal transduction in many cell types, including endothelial cells (Lewis & Henderson, 1987; Cowan & Steffen, 1995). Interestingly, Cowan & Steffen reported that activation of protein kinase C by phorbol esters, such as phorbol 12-myristate 13-acetate, inhibits endothelium-dependent relaxation of rabbit abdominal aorta induced by ACh but not by A23187 (Cowan & Steffen, 1995). In the present study, we observed that chelerythrine, a potent inhibitor of protein kinase C (Herbert et al., 1990; Gopalakrishna & Jaken, 2000) could significantly prevent the inhibitory effect of 7-ketocholesterol, suggesting therefore that the mechanism by which 7-ketocholesterol modifies acetylcholine-mediated endothelium-dependent relaxation involves, at least in part protein kinase C activation. Confirmation of this hypothesis came from the measurement of PKC activity in cultured endothelial cells. Indeed, we observed that 7-ketocholesterol was able to increase significantly the PKC activity 30 min after starting the incubation, with a tendency that was visible after only a few minutes of incubation. Although the molecular mechanism of PKC activation, involving the translocation of the enzyme from the cytosol to the membrane and its subsequent activation is a complex, incompletely understood process, it has been shown to be strongly influenced by the physiochemical properties of the lipid membrane (Slater et al., 1994; Newton, 1995; Stubbs & Slater, 1996). Since oxysterols are known to promote drastic changes in the physicochemical properties of the plasma membrane (Morel & Lin, 1996; Verhagen et al., 1996), 7-ketocholesterol might facilitate PKC activation through the structural changes it induces in the plasma membrane.
In conclusion, the inhibitory effect of 7-ketocholesterol on endothelium-dependent relaxation of isolated rabbit aorta did not relate to an increased breakdown of NO by oxygen containing free radicals, to alteration in calcium fluxes, to the inhibition of NO synthase, or to the specific antagonism of one given membrane receptor subtype. The present data rather suggest that 7-ketocholesterol inhibits the early transmembrane signalling pathway in endothelial cells through protein kinase C activation. These observations bring new insights into the molecular mechanism by which cholesterol oxides, and among them 7-ketocholesterol are susceptible to promote endothelial dysfunction and atherosclerotic lesions in animals and humans.
Acknowledgments
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), the Fondation de France, the Université de Bourgogne and the Conseil Régional de Bourgogne.
Abbreviations
- ACh
acetylcholine
- ADP
adenosine diphosphate
- BSA
bovine serum albumine
- 7-keto
7-ketocholesterol
- LDL
low density lipoprotein
- NE
norepinephrine
- NO
nitric oxide
- PEG
polyethylene glycol
- PKC
protein kinase C
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
References
- BECKMAN J.S., MINOR R.L., WHITE C.W., REPINE J.E., ROSEN M.G., FREEMAN B.A. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J. Biol. Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
- BOSSALER C., HABIB G.B., YAMAMOTO H., WILLIAMS C., WELLS S., HENRY P.D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5′-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J. Clin. Invest. 1987;79:170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREUER O., DZELETOVIC S., LUND E., DICZFALUSY U. The oxysterols cholest-5-ene-3β,4α-diol, cholest-5-ene-3β,4β-diol and cholestane-3-β,5α,6α-triol are formed during in vitro oxidation of low density lipoprotein, and are present in human atherosclerotic plaques. Biochim. Biophys. Acta. 1996;1302:145–152. doi: 10.1016/0005-2760(96)00052-5. [DOI] [PubMed] [Google Scholar]
- BROWN A.J., JESSUP W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- CARPENTER K.L.H., TAYLOR S.E., BALLANTINE J.A., FUSSEL B., HALLIWELL B., MITCHINSON M.J. Lipids and oxidised lipids in human atheroma and normal aorta. Biochim. Biophys. Acta. 1993;1167:121–130. doi: 10.1016/0005-2760(93)90151-x. [DOI] [PubMed] [Google Scholar]
- CHIN J.H., AZHAR S., HOFMANN B.B. Inactivation of endothelium-derived relaxing factor by oxidized lipoproteins. J. Clin. Invest. 1992;89:10–18. doi: 10.1172/JCI115549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWAN C.L., STEFFEN R.P. Lysophosphatidylcholine inhibits relaxation of rabbit abdominal aorta mediated by endothelium-derived nitric oxide and endothelium-derived hyperpolarizing factor independent of protein kinase C activation. Arterioscler. Thromb. Vasc. Biol. 1995;15:2290–2297. doi: 10.1161/01.atv.15.12.2290. [DOI] [PubMed] [Google Scholar]
- COX D.A., COHEN M.L. Effects of oxidized low-density lipoprotein on vascular contraction and relaxation: clinical and pharmacological implications in atherosclerosis. Pharmacol. Rev. 1996;48:3–19. [PubMed] [Google Scholar]
- DECKERT V., BRUNET A., LANTOINE F., LIZARD G., MILLANVOYE-VAN BRUSSEL E., MONIER S., LAGROST L., DAVID-DUFILHO M., GAMBERT P., DEVYNCK M.-A. Inhibition by cholesterol oxides of NO release from human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1998;18:1054–1060. doi: 10.1161/01.atv.18.7.1054. [DOI] [PubMed] [Google Scholar]
- DECKERT V., PERSEGOL L., VIENS L., LIZARD G., ATHIAS A., LALLEMANT C., GAMBERT P., LAGROST L. Inhibitors of arterial relaxation among components of human oxidized low-density lipoproteins. Cholesterol derivatives oxidized in position 7 are potent inhibitors of endothelium-dependent relaxation. Circulation. 1997;95:723–731. doi: 10.1161/01.cir.95.3.723. [DOI] [PubMed] [Google Scholar]
- DECKERT V., PRUNEAU D., ELGHOZI J.-L. Mediation by 5-HT1D receptors of 5-hydroxytryptamine-induced contractions of rabbit middle and posterior cerebral arteries. Br. J. Pharmacol. 1994;112:939–945. doi: 10.1111/j.1476-5381.1994.tb13171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F. Role of endothelium in responses of vascular smooth muscle. Circ. Res. 1983;53:557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- GARCIA-CRUSET S., CARPENTER K.L., GUARDIOLA F., STEIN B.K., MITCHINSON M.J. Oxysterol profiles of normal human arteries, fatty streaks and advanced lesions. Free Radic. Res. 2001;35:31–41. doi: 10.1080/10715760100300571. [DOI] [PubMed] [Google Scholar]
- GARCIA C.E., CRESCENCE M., KILCOYNE R.N., CARDILLO C., CANNON R.O., QUYYUMI A.A., PANZA J.A. Evidence that endothelial dysfunction in patients with hypercholesterolemia is not due to increased extracellular nitric oxide breakdown by superoxide anions. Am. J. Cardiol. 1995;76:1157–1161. doi: 10.1016/s0002-9149(99)80327-0. [DOI] [PubMed] [Google Scholar]
- GOPALAKRISHNA R., JAKEN S. Protein kinase C signaling and oxidative stress. Free Rad. Biol. Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- HERBERT J.M., AUGEREAU J.M., GLEYE J., MAFFRAND J.P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- HODIS H.N., CRAWFORD D.W., SEVANIAN A. Cholesterol feeding increases plasma and aortic tissue cholesterol oxide levels in parallel: further evidence for the role of cholesterol oxidation in atherosclerosis. Atherosclerosis. 1991;89:117–126. doi: 10.1016/0021-9150(91)90051-4. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUGA G.M., WOOD K.S., BYRNS R.E., CHAUDHURI G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUGIYAMA K., KERNS S.A., MORRISETT J.D., ROBERTS R., HENRY P.D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990;344:160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- KUGIYAMA K., OHGUSHI M., MOTOYAMA T., HIRASHIMA O., SOEJIMA S., MISUMI K., YOSHIMURA M., OGAWA H., SUGIYAMA S., YASUE H. Intracoronary infusion of reduced glutathione improves endothelial vasomotor response to acetylcholine in human coronary circulation. Circulation. 1998;97:2299–2301. doi: 10.1161/01.cir.97.23.2299. [DOI] [PubMed] [Google Scholar]
- LANDMESSER U., MERTEN R., SPIEKERMANN S., BÜTTNER K., DREXLER H., HORNIG B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease. Circulation. 2000;101:2264–2270. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- LEWIS M.J., HENDERSON A.H. A phorbol ester inhibits the release of endothelium-derived relaxing factor. Eur. J. Pharmacol. 1987;137:167–171. doi: 10.1016/0014-2999(87)90218-4. [DOI] [PubMed] [Google Scholar]
- LIZARD G., DECKERT V., DUBREZ L., MOISANT M., GAMBERT P., LAGROST L. Induction of apoptosis in endothelial cells treated with cholesterol oxides. Am. J. Pathol. 1996;148:1625–1638. [PMC free article] [PubMed] [Google Scholar]
- MAOR I., KAPLAN M., HAYEK T., VAYA J., HOFFMAN A., AVIRAM A. Oxidized monocyte-derived macrophages in aortic atherosclerosis lesion from apolipoprotein E-deficient mice and from human carotid artery contain lipid peroxides and oxysterols. Biochem. Biophys. Res. Commun. 2000;269:775–780. doi: 10.1006/bbrc.2000.2359. [DOI] [PubMed] [Google Scholar]
- MINOR R.L., MYERS P.R., GUERRA R., BATES J.N., HARRISON D.G. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J. Clin. Invest. 1990;86:2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREL D.W., LIN C.Y. Cellular biochemistry of oxysterols derived from the diet or oxidation in vivo. J. Nutr. Biochem. 1996;7:495–506. [Google Scholar]
- MOUGENOT N., LESNIK P., RAMIREZ-GIL J.F., NATAF P., DICZFALUSY U., CHAPMAN J.M., LECHAT P. Effect of the oxidation state of LDL on the modulation of arterial vasomotor response in vitro. Atherosclerosis. 1997;133:183–192. doi: 10.1016/s0021-9150(97)00124-x. [DOI] [PubMed] [Google Scholar]
- MÜGGE A., BRANDES R.P., BÖGER R.H., DWENGER A., BODE-BÖGER S., KIENKE S., FRÖLICH J.C., LICHTEN P.R. Vascular release of superoxide radicals is enhanced in hypercholesterolemic rabbits. J. Cardiovasc. Pharmacol. 1994;24:994–998. doi: 10.1097/00005344-199424060-00019. [DOI] [PubMed] [Google Scholar]
- NEWTON A.C. Protein kinase C: structure, function, and regulation. J. Biol. Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- OHARA Y., PETERSON T.E., HARRISON D.G. Hypercholesterolemia increases endothelial superoxide anion production. J. Clin. Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.M.J., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PLANE F., BRUCKDORFER K.R., KERR P., STEUER A., JACOBS M. Oxidative modification of low-density lipoproteins and the inhibition of relaxations mediated by endothelium-derived nitric oxide in rabbit aorta. Br. J. Pharmacol. 1992;105:216–222. doi: 10.1111/j.1476-5381.1992.tb14237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY K.G., NAIR R.N., SHEEHAN H.M., HODGSON J.M. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J. Am. Coll. Cardiol. 1994;123:833–843. doi: 10.1016/0735-1097(94)90627-0. [DOI] [PubMed] [Google Scholar]
- SALONEN J.T., NYYSSÖNEN K., SALONEN R., PORKKALA-SARATAHO E., TUOMAINEN T.-P., DICZFALUSY U., BJÖRKHEM I. Lipoprotein oxidation and progression of carotid atherosclerosis. Circulation. 1997;95:840–845. doi: 10.1161/01.cir.95.4.840. [DOI] [PubMed] [Google Scholar]
- SIMON B.C., CUNNINGHAM L.D., COHEN R.A. Oxidized low density lipoproteins cause contraction and inhibit endothelium-dependent relaxation in the pig coronary artery. J. Clin. Invest. 1990;86:75–79. doi: 10.1172/JCI114718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLATER S.J., KELLY M.B., TADDEO F.J., HO C., RUBIN E., STUBBS C.D. The modulation of protein kinase C activity by membrane lipid bilayer structure. J. Biol. Chem. 1994;269:4866–4871. [PubMed] [Google Scholar]
- STRALIN P., KARLSSON K., JOHANSSON B.O., MARKLUND S.L. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 1995;15:2032–2036. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- STUBBS C.D., SLATER S.J. The effects of non-lamellar forming lipids on membrane protein-lipid interactions. Chem. Phys. Lipids. 1996;81:185–195. doi: 10.1016/0009-3084(96)02581-9. [DOI] [PubMed] [Google Scholar]
- TAN S., YOKOYAMA Y., DICKENS E., CASH T.G., FEEMAN B.A., PARKS D.A. Xanthine oxidase activity in the circulation of rats following hemorrhagic shock. Free Rad. Biol. Med. 1993;15:407–414. doi: 10.1016/0891-5849(93)90040-2. [DOI] [PubMed] [Google Scholar]
- VERHAGEN J.C., TER BRAAKE P., TEUNISSEN J., VAN GINKEL G., SEVANIAN A. Physical effects of biologically formed cholesterol oxidation products on lipid membranes investigated with fluorescence depolarization spectroscopy and electron spin resonance. J. Lipid. Res. 1996;37:1488–1502. [PubMed] [Google Scholar]
- WHITE R.C., BROCK T.A., CHANG L.Y., CRAPO J., BRISCOE P., KU D., BRADLEY W.A., GIANTURCO S.H., GORE J., FREEMAN B.A., TARPEY M.M. Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE C.R., DARLEY-USMAR V., BERRINGTON W.R., MCADAMS M., GORE J.Z., THOMPSON J.A., PARKS D.A., TARPEY M.T., FREEMAN B.A. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YASUNOBU Y., HAYASHI K., SHINGU T., YAMAGATA T., KAJIYAMA G., KAMBE M. Coronary atherosclerosis and oxidative stress as reflected by autoantibodies against oxidized low-density lipoprotein and oxysterols. Atherosclerosis. 2001;155:445–453. doi: 10.1016/s0021-9150(00)00581-5. [DOI] [PubMed] [Google Scholar]
- ZEIHER A.M., DREXLER H., WOLLSCHLÄGER H., JUST H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- ZIEDEN B., KAMINSKAS A., KRISTENSON M., KUCINSKIENE Z., VESSBY B., OLSSON A.G., DICZFALUSY U. Increased plasma 7β-hydroxycholesterol concentrations in a population with a high risk for cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 1999;19:967–971. doi: 10.1161/01.atv.19.4.967. [DOI] [PubMed] [Google Scholar]


