Abstract
Leptin, a pleiotropic hormone believed to regulate body weight, has recently been associated with inflammatory states and immune activity. Here we have studied the effect of leptin on expression of IFN-γ-induced nitric oxide synthase (iNOS) and cyclo-oxygenase-2 (COX-2), both prominent markers of macrophage activation, using the murine macrophage J774A.1 cell line.
After 24 h of incubation, leptin (1–10 μg ml−1) potently synergized with IFN-γ (100 U ml−1) in nitric oxide (NO) release, evaluated as nitrite and nitrate (NOx), and prostaglandin E2 (PGE2) production in culture medium.
The observed increase of NO and PGE2 was related to enhanced expression of the respective inducible enzyme isoforms, measured in mRNA and protein by RT–PCR and Western blot analysis, respectively.
When cells were stimulated only with leptin, a weak induction of NO and PGE2 release and of the expression of related inducible enzymes was observed.
Moreover IFN-γ increased the expression of the functional form of leptin receptor (Ob-Rb) and this effect was potentiated by leptin in a concentration-dependent manner.
These data suggest that macrophages, among the peripheral immune cells, represent a target for leptin and confirm the relevance of this hormone in the pathophysiology of inflammation.
Keywords: Leptin, macrophage, interferon-γ, inducible nitric oxide synthase, cyclo-oxygenase-2, leptin receptor
Introduction
Recent studies clearly indicate that leptin, originally related only to the regulation of body weight and energy expenditure, plays a role as an immunoregulatory hormone (Fantuzzi & Faggioni, 2000). Leptin stimulates the inflammatory response, T-lymphocyte proliferation, and Th1 cytokine production during fasting in normal and in fed ob/ob mice, indicating that leptin is an important link between nutrition and the immune system (Lord et al., 1998). Studies of rodents with genetic abnormalities in leptin or leptin receptors revealed obesity-related deficits in macrophage phagocytosis and expression of pro-inflammatory cytokines both in vivo and in vitro, which were reversed by exogenous leptin treatment (Loffreda et al., 1998). On the other hand, a variety of cytokines, such as tumour necrosis factor (TNF), leukaemia inhibitory factor (LIF) and interleukin-1 (IL-1) induced leptin synthesis (Grunfeld et al., 1996; Sarraf et al., 1997; Janik et al., 1997), contributing to anorexia and weight loss in several inflammatory diseases (Sarraf et al., 1997; Janik et al., 1997; Schwartz & Seeley, 1997).
Moreover, immunocompetent cells, such as lymphocytes and macrophages (Bennet et al., 1996; Bouloumie et al., 1998; Gainsford et al., 1996; O'rourke et al., 2001), express the transmembrane form of leptin receptor (Ob-R) and the primary structure of this receptor shows homologies to the class I cytokine receptor family (Tartaglia et al., 1995; Tartaglia, 1997).
Macrophages modulate inflammatory and immune responses by synthesizing several cytokines and mediators, which alone or in combination with LPS have been found to induce the co-expression of pro-inflammatory enzymes such as nitric oxide synthase (iNOS) and cyclo-oxygenase-2 (COX-2) (di rosa et al., 1996). These inducible isoform enzymes are responsible for the production of large amounts of nitric oxide (NO) and prostaglandins (PGs) at the inflammatory site (Nussler & Billiar, 1993; Lee et al., 1992).
In light of the involvement of leptin in immune and inflammatory response, we have investigated the pro-inflammatory effect of this hormone in in vitro studies.
In particular, the aim was to study the effect of leptin alone or in combination with IFN-γ on the induction of the expression of iNOS and COX-2 and the synthesis of their metabolites, NO and PGE2, using the macrophage cell line J774A.1, where functional leptin receptor (Ob-Rb) is expressed (O'rourke et al., 2001). It is well known that IFN-γ, whose production is induced by leptin in lymphocytes (Lord et al., 1998), stimulates innate cell-mediated immunity and, above all, the activation of macrophages.
Methods
Materials
The murine macrophage cell line J774A.1 was obtained from the European Collection of Animal Cell Cultures (Salisbury, Wiltshire, U.K.). Recombinant mouse leptin was from NIDDK's National Hormone & Peptide Program. Foetal bovine serum (FBS), tissue culture media, and supplements were purchased from Hy-Clone (Road Logan, UT, U.S.A.). Bovine serum albumin, Bio-Rad protein assay, 3-(4,5dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and anti-β- actin antibody (clone AC-15) were from Sigma (St. Louis, MO, U.S.A.). Recombinant mouse IFN-γ was obtained from Calbiochem (La Jolla, CA, U.S.A.). PCR primers were synthesized by ‘Servizio di Biologia Molecolare, Stazione Zoologica A. Dohrn' (Naples, Italy). iNOS and COX-2 were detected on Western blot with monoclonal antibodies (N39120, clone 54, and C22420, clone 33, respectively) from Transduction Laboratories (Lexington, KY, U.S.A.); the polyclonal antibody against the extracellular membrane portion of leptin receptor (Ob-R) raised in rabbit was obtained from Affinity Bioreagents (Golden, CO, U.S.A.); the peroxidase conjugated secondary antibodies were purchased from Jackson (West Grove, PA, U.S.A.). Agarose conjugate (Protein A/G Plus-agarose) was obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, U.S.A.). EIA kit for prostaglandin E2 was provided by Cayman (Ann Arbor, MI, U.S.A.); Detoxi-gelTM endotoxin removing gel columns were obtained from Pierce (Rockford, IL, U.S.A.).
Cell culture
The murine macrophage J774A.1 cell line was cultured in 75-cm2 flasks in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin, 130 μg ml−1 pyruvate, 25 mM HEPES at 37°C under 5% CO2 humidified air.
Cell treatments
J7774A.1 cells were plated in complete medium (10% FBS) in P-60 well plates (1.5×106 dish). After 2 h to allow adhesion, cells were treated with leptin (1, 3 and 10 μg ml−1) alone or in combination with IFN-γ (100 U ml−1) in medium at 5% FBS. Before use, leptin solutions were filtered with Detoxi-gelTM columns in order to remove pyrogens. After 24 h of incubation, thereafter the supernatants were separated and kept frozen at −80°C until assayed for NO and PGE2 measurement, while cells were lysed. In another set of experiments cells were stimulated as before and mRNAs were extracted after 16 h of incubation.
NOx and PGE2 assay
NO3− and NO2− (NOx), stable metabolites of NO, present in the supernatant of cells, were measured according to Thomsen et al. (1991). After reducing nitrate (NO3−) to nitrite (NO2−) with the use of acid-washed cadmium powder, NO2− amounts were measured by Griess reaction. In brief, 100 μl of cell culture medium were mixed with 100 μl of Griess reagent (equal volumes of 1% (w v−1) sulphanilamide in 5% (v v−1) phosphoric acid and 0.1% (w v−1) naphtylethylenediamine-HCl) and incubated at room temperature for 10 min, and then the absorbance at 550 nm was measured in a microplate reader Titertek (Dasit, Cornaredo, Milan, Italy). The amount of NO2− in the samples (in micromolar units) was calculated from a sodium nitrite standard curve.
PGE2 levels in macrophage supernatants were quantified by EIA kit according to manufacturer's instructions.
MTT assay for cell viability
Proliferation studies were performed in a 96-well plate. J774A.1 cells were plated 60,000 well−1 and, after 120 min, treated with leptin at increasing concentrations (0.03, 0.1, 0.3, 1, 3 and 10 μg ml−1) in DMEM 5% FBS. Before use, leptin solutions were filtered with Detoxi-gelTM columns in order to remove pyrogens. After 24 h of incubation at 37°C, 3-(4,5dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, 5 mg ml−1) was added to each well; 3 h later the cells were lysed with lysis buffer 20% SDS and 50% DMF, pH 4.7). After an incubation of 18 h at 37°C, the optical densities (OD620) for the serial dilutions of leptin were compared to the OD of the control wells to assess cell viability (Mosmann, 1983).
RT–PCR analysis
Total RNA was extracted by a modified method of Chomczynski & Sacchi (1987), using TRIzol Reagent (Life Technologies, Milan, Italy) according to the manufacturer's instructions. Reverse transcription was performed by a standard procedure (Brenner et al., 1989) using 2 μg of total RNA. After reverse transcription, 2 μl of RT products were diluted in 48 μl of PCR mix, to give a final concentration of 50 U ml−1 of Taq DNA polymerase (Life Technologies, Milan, Italy), 4 μM of 5′ and 3′ primers, 50 μM of each dNTP, 1.5 mM MgCl2, and 1×PCR buffer (20 mM Tris-HCl, pH 8.4, 50 mM KCl). cDNAs underwent the number of cycles as indicated: 35 cycles for COX-2, 28 cycles for iNOS and β-actin, and 34 cycles for Ob-Rb transcript, each one performed at 94°C for 1 min, Tm°C for 1 min, and 72°C for 1 min. After this treatment 10 μl of RT–PCR products were separated by 1.5% agarose gel electrophoresis in TBE 1×(Tris-base 0.089 M, boric acid 0.089 M) containing 0.2 μg ml−1 of ethidium bromide. Fragments of DNA were seen under UV light. β-actin was used as an internal reference.
Specific primer sequences were as follows:
 |
Western blot analysis
After 24 h of incubation, cells were washed twice with ice cold phosphate-buffered saline (PBS), harvested, and resuspended in Tris-HCl (20 mM pH 7.5), 10 mM NaF, 150 mM NaCl, 1% Nonidet P-40, 1 mM phenylmethylsulphonyl fluoride, 1 mM Na3VO4, leupeptin and trypsin inhibitor (10 μg ml−1). After 1 h, cell lysates were obtained by centrifugation at 100,000 g for 15 min at 4°C. Protein concentrations were estimated by the Bio-Rad protein assay using bovine serum albumin as standard.
Fifty micrograms of protein (cell lysates) were subjected to 8% SDS–PAGE and transferred to polyvinylidene difluoride membrane. The filter was then blocked with 1×PBS, 5% non fat dried milk and incubated with specific antibodies in 1×PBS, 5% non fat dried milk, 0.1% Tween 20 overnight at 4°C. We used the specific mAbs against iNOS (1 : 10,000) or COX-2 (1 : 500). Leptin receptor (Ob-R) was demonstrated by a polyclonal antibody (1 : 2000. Thereafter, filters were incubated with the secondary antibody (anti-mouse IgG or anti-rabbit IgG peroxidase conjugate 1 : 10,000 dilution) for 1 h at room temperature. Subsequently, blots were developed using enhanced chemiluminescence detection reagents (Amersham), and exposed to Kodak X-Omat film. To ascertain that blots were loaded with equal amounts of protein lysates, they were also incubated in the presence of the antibody against the β-actin protein. The protein bands of iNOS (∼130 kDa), and COX-2 (∼70 kDa) on X-ray film were scanned and densitometrically analysed with a model GS-700 imaging densitometer (Bio-Rad Laboratories, Segrate, Milan, Italy).
Immunoprecipitation
To evaluate leptin-induced iNOS expression, immunoprecipitation of iNOS was performed on lysates (0.5 mg 0.3 ml−1) from leptin-stimulated cells (1, 3, 10 μg ml−1). Each lysate was precleared with normal mouse serum (1 μg) and 20 μl of agarose conjugate (Protein A/G Plus-agarose, Santa Cruz Biotechnology, Inc.) for 2 h at 4°C. Precleared lysates were then incubated overnight with 1 μg of anti-iNOS at 4°C. Subsequently 50 μl of agarose conjugate were added and incubated 2 h at 4°C. The immunoprecipitates were washed and resuspended in Laemmli's sample buffer, subjected to 6% SDS-polyacrylamide gel and immunoblotted as described above.
Data analysis
Data are reported as mean±s.e.mean values of independent experiments, which were done at least three times, each time with three or more independent observations. Statistical analysis was performed by ANOVA test, and multiple comparisons were made by Bonferroni's test.
Results
As shown in Figure 1A, leptin (1–10 μg ml−1) induced a weak concentration-dependent increase in NOx accumulation in J774A.1 cell supernatant, which became significant at 10 μg ml−1 (P<0.05). Incubation of macrophages with increasing concentrations of leptin potentiated significantly IFN-γ (100 U ml−1)-induced NO release at 3 and 10 μg ml−1 (P<0.05 and P<0.001, respectively; Figure 1B). Moreover, leptin induced an increase of PGE2 production (P<0.05 at 10 μg ml−1; Figure 2A) and potentiated IFN-γ-induced PGE2 release (Figure 2B). This effect was significant at 3 and 10 μg ml−1 leptin (P<0.05, and P<0.001, respectively vs IFN-γ).
Figure 1.
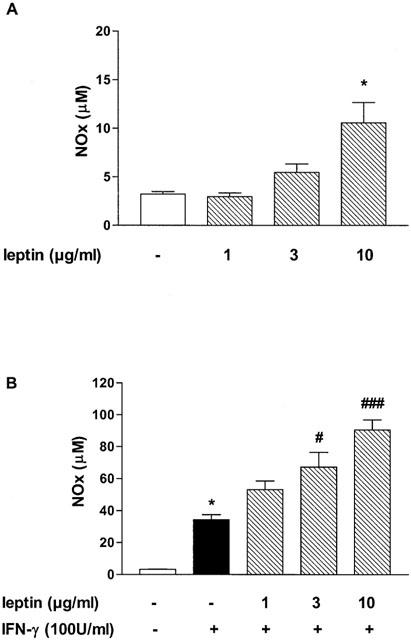
Effect of leptin on NO release. Leptin significantly increases NOx production (A) and enhances NOx induced by IFN-γ in J774A.1 macrophage supernatant (B). Data are expressed as mean±s.e.mean of at least three experiments; *P<0.05 vs control; #P<0.05, and ###P<0.001 vs IFN-γ.
Figure 2.
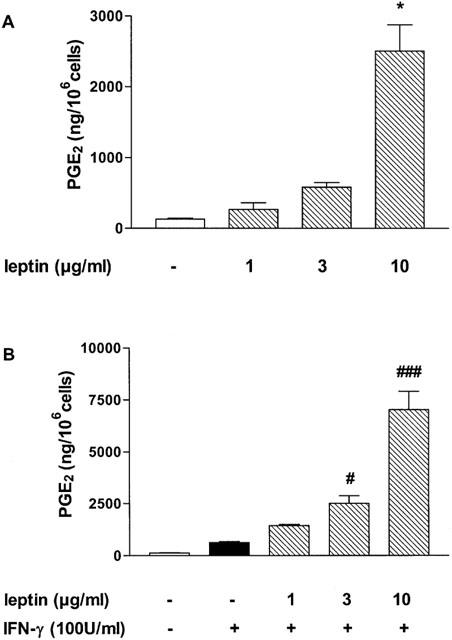
Effect of leptin on PGE2 release. Leptin alone (A) or in combination with IFN-γ (B) increases PGE2 production in J774A.1 macrophage supernatant. Data are expressed as mean±s.e.mean of at least three experiments; *P<0.05 vs conrol; #P<0.05, and ###P<0.001 vs IFN-γ.
Since NO and PGE2 production is proportional to cell number, to exclude a possible interference of leptin proliferative effect on mediator release, leptin activity on cell viability was seen. Following leptin incubation (0.03–10 μg ml−1), no change in cell proliferation was seen. An inhibitory effect on cell proliferation was seen only at the highest concentration used (22.85±2.64% of inhibition, P<0.01 vs controls).
Since elevated production of NOx and PGE2 in activated macrophages requires induction of iNOS and COX-2 enzymes, we have evaluated their expression on proteins and mRNA. Lysates, prepared from the same cells used to generate the data presented in Figures 1 and 2, were subjected to SDS–PAGE and analysed for iNOS and COX-2 expression by Western blot analysis (Figure 3A). For iNOS, no bands were observed in lysates from unstimulated or leptin-stimulated cells. Predominant expression of iNOS (130 kDa) was observed in cell lysates prepared from cells stimulated with IFN-γ that was upregulated when cells were treated with increasing concentrations of leptin. The densitometric analysis was performed on three different experiments. IFN-γ-induced iNOS expression was increased by 95.8±33.8%, 338.0±73.4%, and 546.7±48.2% when cells were incubated with leptin 1, 3 or 10 μg ml−1, respectively (P<0.001 at 10 μg ml−1). A similar pattern was observed for COX-2 expression (70 kDa), even if the hormone alone already induced the enzyme expression. Densitometric analysis, performed on three different experiments, showed an increase of COX-2 expression by leptin (1, 3 and 10 μg ml−1) of 42.0±11.0%, 56.3%±6.2%, and 149.0±22.8% vs IFN-γ (P<0.01 at 10 μg ml−1). Using semi-quantitative RT–PCR analysis, we showed a weak concentration-dependent increase in iNOS (383 bp) and COX-2 (371 bp) mRNA expression by leptin stimulation. Moreover, leptin potentiated IFN-γ-induced expression of both enzyme mRNA (Figure 3B).
Figure 3.
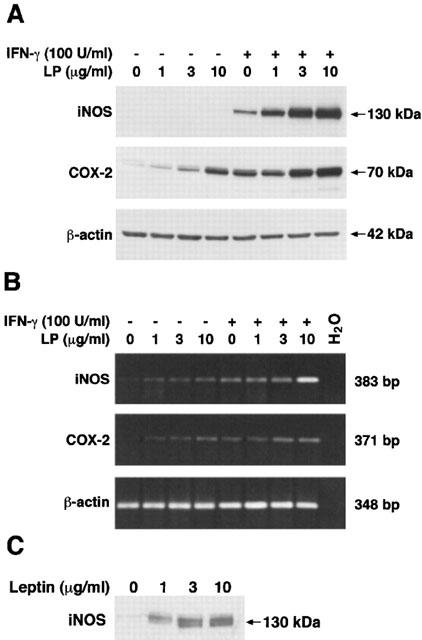
Leptin induction of iNOS and COX-2 expression in macrophage J774A.1. Western blot analysis (A): leptin modulates IFN-γ-induced iNOS in a concentration-dependent manner. The hormone induces COX-2 and increases the enzyme expression induced by IFN-γ. Representative blots show untreated and leptin-stimulated cells in presence or not of IFN-γ. Similar results were obtained in three additional independent experiments. RT–PCR analysis (B): Leptin, alone and in combination with IFN-γ, modulates both iNOS and COX-2 mRNA expression in a concentration-dependent manner. β-actin was used as internal control. The cells were extracted and reverse-transcribed as described in the Methods section. Similar results were obtained in four additional separate experiments. No bands were observed in absence of cDNA. Immunoprecipitation of leptin-induced iNOS (C). A representative blot of three independent experiments is shown. After incubation with leptin, cells were lysated and 0.5 mg of each lysate was immunoprecipitated and blotted with anti-iNOS antibody.
Since iNOS bands were not detected by Western blot on crude lysates prepared from leptin-stimulated cells, we performed an immunoprecipitation using an antibody against iNOS; the immune-complexes were then subjected to SDS–PAGE and blotted with the anti-iNOS. iNOS expression was now well evident at all concentrations used (Figure 3C).
To verify whether the mechanism behind the synergistic effect of leptin with IFN-γ might be related to an increase of Ob-R expression, we evaluated its modulation by the different cell treatments. With Western blot analysis no bands of the long form of Ob-R (∼125 kDa) were revealed in unstimulated or leptin-treated cells, while, when the cells were stimulated with IFN-γ, Ob-Rb was expressed and upregulated by leptin. No modulation was seen in short form expression of Ob-R (∼100 kDa) (Figure 4A). RT–PCR with a specific primer for Ob-Rb (Caprio et al., 1999), showed the leptin receptor in untreated and leptin (10 μg ml−1)-treated cells and confirmed the upregulation of Ob-Rb by IFN-γ stimulation and its potentiation by leptin.
Figure 4.
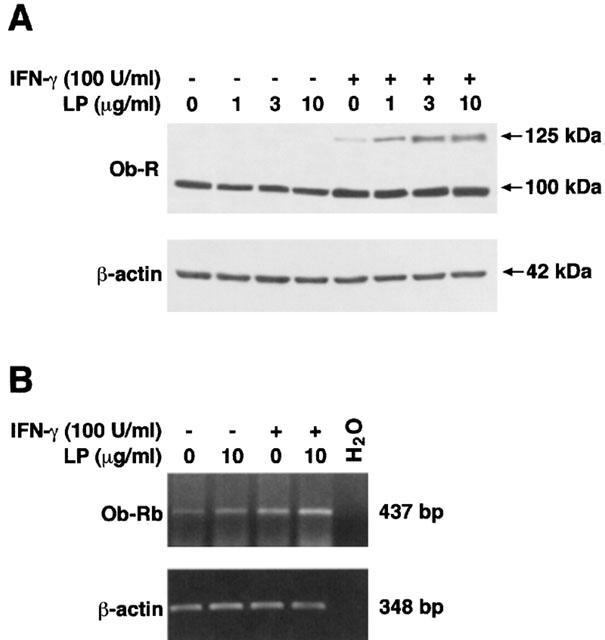
Western blot analysis of Ob-R (A). Long form of Ob-R (∼125 kDa) is induced by IFN-γ and upregulated by leptin in a concentration-dependent manner. No modulation of short form expression of Ob-R (∼100 kDa) was evident. Lysates were obtained from untreated or 24-h-stimulated cells with IFN-γ and/or leptin at the indicated concentrations. A representative immunoblot from three separate experiments is shown. RT–PCR analysis of Ob-Rb (B). Leptin and IFN-γ induced Ob-Rb mRNA expression in J774A.1 macrophages. A synergistic effect is well evident when cells were stimulated with their combination. β-actin was used as internal control and no bands were observed in absence of cDNA. RT–PCR analysis is referred to a single experiment representative of four separate experiments.
Discussion
During inflammation, such as in the process of host defence, endotoxins and cytokines induce rapid alterations in cellular immediate-early gene expression leading to the de novo synthesis of COX-2 (Maier et al., 1990; Mitchell et al., 1995) and iNOS (Moncada et al., 1991). Co-induction of iNOS and COX-2 has been shown in several cell types, including murine macrophages (Salvemini et al., 1993; Akarasereenont et al., 1994; Swierkosz et al., 1995) with similarities in the signal transduction pathways. It is now evident that NOS and COX pathways are interrelated and the cross-talk between the two pathways is important in the regulation of the inflammatory process.
Several in vivo studies show that different inflammatory stimuli, endotoxin, and cytokines induce the expression of leptin or an increase of circulating levels of this hormone (Sarraf et al., 1997; Gualillo et al., 2000; Grunfeld et al., 1996). Although originally believed to be expressed exclusively by adipocytes, leptin mRNA and protein have recently been shown to be expressed in other areas (Masuzaki et al., 1997; Hoggard et al., 1997; Bado et al., 1998) and several studies have recently shown that this hormone plays a regulatory role in immunity, inflammation and hematopoiesis (Fantuzzi & Faggioni, 2000; Lord et al., 1998; Loffreda et al., 1998). Leptin up-regulates murine macrophagic phagocytic activity and enhances the secretion of inflammatory cytokines such as TNF-α, IL-6 and IL-12 from rat isolated macrophages and human monocytes in response to lipopolysaccharide (Loffreda et al., 1998; Santos-Alvarez et al., 1999). In addition, leptin promotes CD4+ helper T-cell (Th) activity in vitro, which orchestrates most immune responses. In particular leptin increases the proliferation of naive T cells, increases Th1 (IFN-γ and IL-2) and suppresses Th2 (IL-4) cytokine production (Lord et al., 1998).
Here we have demonstrated that in vitro leptin induces selected inflammatory mediators. Moreover, leptin markedly increases IFN-γ-induced NO and PGE2 production in a concentration-dependent manner by increasing iNOS and COX-2 expression in J774 macrophages. During inflammation, activated T-lymphocytes could be the main source of different inflammatory cytokines such as IFN-γ, TNF-α, and IL-6. Thus, leptin, released following the inflammatory state, could induce in co-operation with IFN-γ, NO and PGE2 release, contributing to sustaining the ongoing inflammatory response. Actually leptin exhibits a more potent activity on COX-2 than on iNOS expression. Anyway the capability to induce both enzymes could also involve other features besides those common to the two pathways implied to their expression. This major effect on COX-2 pathway activation is more evident when leptin is combined with IFN-γ where the synergic effect on PGE2 production appears stronger than that on NOx release. This preferential activity of leptin on PGE2 release confers to leptin a clear pro-inflammatory role.
Moreover, we showed that IFN-γ induces Ob-Rb expression in J774A.1 cells, which posses the molecular machinery required to respond to leptin stimulation. Evidence indicates that leptin signals inside the cell when the long form (Ob-Rb) of this receptor is expressed (Bauman et al., 1996; Banks et al., 2000; Kim et al., 2000). A previous report clearly established that Ob-Rb is the major form of leptin receptor present in J774 macrophages and that signalling pathways downstream of leptin receptor are activated in these cells (O'rourke et al., 2001). Also the short form Ob-Ra, which mediates transport of leptin, is capable of leptin-mediated signalling but much less effective than the full length receptor (Murakami et al., 1997).
The mechanism underlying the observed synergic effect could be due to the increased expression of Ob-Rb: IFN-γ by itself was able to induce an upregulation of Ob-Rb expression and this effect was potentiated by leptin in a concentration-dependent manner.
Our observation that leptin synergized with IFN-γ in inflammatory mediators release and Ob-Rb induction suggests that leptin is an important inflammatory signal between endocrine and immune response, conferring a novel role for this hormone in the pathophysiology of wasting and in the proper functioning of the immune system in infectious and inflammatory diseases.
Acknowledgments
We thank NIDDK's National Hormone & Peptide Program and the Scientific Director A.F. Parlow for providing recombinant mouse leptin. This study was supported in part by a grant from the Ministero dell'Istruzione, dell'Università e della Ricerca, Italy.
Abbreviations
- COX-2
cyclo-oxygenase-2
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- IFN-γ
interferon-γ
- iNOS
inducible nitric oxide synthase
- MTT
3-(4,5dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NO
nitric oxide
- Ob-R
leptin receptor
- PBS
phosphate-buffered saline
- PGE2
prostaglandin E2
- TNF-α
tumour necrosis factor-α
References
- AKARASEREENONT P., MITCHELL J.A., THIEMERMANN C., VANE J.R. Involvement of tyrosine kinase in the induction of cyclooxygenase and nitric oxide synthase by endotoxin in cultured cells. Br. J. Pharmacol. 1994;113:1522–1528. doi: 10.1111/j.1476-5381.1994.tb17169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADO A., LEVASSEUR S., ATTOUB S., KERMORGANT S., LAIGNEAU J.P., BORTOLUZZI M.N., MOIZO L., LEHY T., GUERRE-MILLO M., LEMARCHAND-BRUSTEL Y., LEWIN M.J. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- BANKS A.S., DAVIS S.M., BATES S.H., MYERS M.G. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- BAUMAN H., MORELLA K.K., WHITE D.W., DEMBSKI M., BAILON P.S., KIM H., LAI C., TARTAGLIA L.A. The full-length leptin receptor has signalling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNET B., SOLAR G., YUAN J., MATHIAS J., THOMAS G., MATTHEWS W. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 1996;6:1170–1180. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- BOULOUMIE A., DREXLER H.C.A., LAFONTAN M., BUSSE R. Leptin, the product of Ob gene, promotes angiogenesis. Circ. Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- BRENNER C.A., TAM A.W., NELSON P.A., ENGELMAN E.G., SUSUKI N., FRY K.E., LARRICK J.W. Message amplification phenotyping: a technique to simultaneously measure multiple mRNAs from small numbers of cells. Biotechniques. 1989;7:1096–1103. [PubMed] [Google Scholar]
- CAPRIO M., ISIDORI A.M., CARTA A.R., MORETTI C., DUFAU M.L., FABBRI A. Expression of functional leptin receptor in rodent Leydig cells. Endocrinology. 1999;140:4939–4947. doi: 10.1210/endo.140.11.7088. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DI ROSA M., IALENTI A., IANARO A., SAUTEBIN L. Interaction between nitric oxide and cyclooxygenase pathways. Prostaglandins Leukot. Essent. Fatty Acids. 1996;54:229–238. doi: 10.1016/s0952-3278(96)90053-8. [DOI] [PubMed] [Google Scholar]
- FANTUZZI G., FAGGIONI R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- GAINSFORD T., WILLSON T.A., METCALF D., HANDMAN E., MCFARLANE C., NG A., NICOLA N.A., ALEXANDER W.S., HILTON D.J. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. USA. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNFELD C., ZHAO C., FULLER J., POLLACK A., MOSER A., FRIEDMAN J., FEINGOLD K.R. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J. Clin. Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUALILLO O., EIRAS S., LAGO F., DIEGUEZ C., CASANEUVA F.F. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 2000;67:2433–2441. doi: 10.1016/s0024-3205(00)00827-4. [DOI] [PubMed] [Google Scholar]
- HOGGARD N., HUNTER L., DUNKAN J.S., WILLIAM L.M., TRAYHURN P., MERCER J.G. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc. Natl. Acad. Sci. USA. 1997;94:11073–11078. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANIK J.E., CURTIS B.D., CONSIDINE R.V., RAGER H.C., POWERS G.C., ALVORD W.G., SMITH J.W., GAUSE B.L., KOPP W.C. Interleukin 1 alpha increases serum leptin concentrations in humans. J. Clin. Endocrinol. Metab. 1997;82:3084–3086. doi: 10.1210/jcem.82.9.4214. [DOI] [PubMed] [Google Scholar]
- KIM Y., UOTANI S., PIERROZ D.D., FLIER J.S., KAHN B.B. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues overlapping but distinct pathways from insulin. Endocrinology. 2000;141:2328–2339. doi: 10.1210/endo.141.7.7536. [DOI] [PubMed] [Google Scholar]
- LEE S.H., SOYOOLA E., CHANMUGAM P., HART S., SUN W., ZHONG H., LIOU S., SIMMONS D., HWANG D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- LOFFREDA S., YANG S.Q., LIN H.Z., KARP C.L., BRENGMAN M.L., WANG D.J., KLEIN A.S., BULKLEY G.B., BAO C., NOBLE P.W., LANE M.D., DIEHL A.M. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- LORD G.M., MATARESE G., HOWARD J.K., BAKER R.J., BLOOM S.R., LECHLER R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;294:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- MAIER J.A., HLA T., MACIAG T. Cyclooxygenase in an immediate-early gene induced by interleukin-1 in human endothelial cells. J. Biol. Chem. 1990;265:12231–12234. [PubMed] [Google Scholar]
- MASUZAKI H., OGAWA Y., SAGAWA N., HOSODA K., MATSUMOTI T., MISE H., NISHIMURA H., YOSHIMASA Y., TANAKA I., MORI T., NAKAO K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nature Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.A., LARKIN S., WILLIAMS T.J. Cyclooxygenase-2: regulation and relevance in inflammation. Biochem. Pharmacol. 1995;50:1535–1542. doi: 10.1016/0006-2952(95)00212-x. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–141. [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- MURAKAMI T., YAMASHITA T., IIDA M., KUWAJIMA M., SHIMA K. A short form of leptin receptor performs signal transduction. Biochem. Biophys. Res. Commun. 1997;231:26–29. doi: 10.1006/bbrc.1996.6030. [DOI] [PubMed] [Google Scholar]
- NUSSLER A.K., BILLIAR T.R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J. Leuk. Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- O'ROURKE L., YEAMAN S.J., SHEPHERD P.R. Insulin and leptin acutely regulate cholesterol ester metabolism in macrophages by novel signaling pathways. Diabetes. 2001;50:955–961. doi: 10.2337/diabetes.50.5.955. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J.L., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenasse enzymes. Proc. Natl. Acad. Sci. USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTOS-ALVAREZ J., GOBERNA R., SANCHEZ-MARGALET B. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- SARRAF P., FREDERICH R.C., TURNER E.M., MA G., JASKOWIAK N.T., RIVET D.J., III, FILIER J.S., LOWELL B.B., FRAKER D.L., ALEXANDE H.R. Multiple cytokines and acute inflammation raise mouse leptin levels. Potential role in inflammatory anorexia. J. Exp. Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ M.W., SEELEY R. Neuroendocrine responses to starvation and weight loss. N. Engl. J. Med. 1997;336:1802–1811. doi: 10.1056/NEJM199706193362507. [DOI] [PubMed] [Google Scholar]
- SWIERKOSZ T.A., MITCHELL J.A., WARNER T.D., BOTTING R.M., VANE J.R. Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostaglandins. Br. J. Pharmacol. 1995;114:1335–1342. doi: 10.1111/j.1476-5381.1995.tb13353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARTAGLIA L.A. The leptin receptor. J. Biol. Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- TARTAGLIA L.A., DEMBSKI M., WENG X., DENG N., CULPEPPER J., DEVOS R., RICHARDS G.J., CAMPFIELD L.A., CLARK F.T., DEEDS J. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- THOMSEN L.L., CHING L.M., ZUANG L., GAVIN J.B., BAGULEY B.C. Tumor-dependent increased plasma nitrate concentrations as an indication of the antitumor effect of flavone-8-acetic acid and analogues in mice. Cancer Res. 1991;51:71–81. [PubMed] [Google Scholar]


