Abstract
Gingerols, the pungent constituents of ginger, were synthesized and assessed as agonists of the capsaicin-activated VR1 (vanilloid) receptor.
[6]-Gingerol and [8]-gingerol evoked capsaicin-like intracellular Ca2+ transients and ion currents in cultured DRG neurones. These effects of gingerols were blocked by capsazepine, the VR1 receptor antagonist.
The potency of gingerols increased with increasing size of the side chain and with the overall hydrophobicity in the series.
We conclude that gingerols represent a novel class of naturally occurring VR1 receptor agonists that may contribute to the medicinal properties of ginger, which have been known for centuries. The gingerol structure may be used as a template for the development of drugs acting as moderately potent activators of the VR1 receptor.
Keywords: Ginger, gingerols, DRG neurones, VR1 receptor, intracellular calcium, plasma membrane currents
Introduction
Ginger (Zingiber officinale) has been used extensively for more than 2500 years in China for conditions including headaches, nausea and colds (Grant & Lutz, 2000) and in Ayurvedic (Sharma & Clark, 1998) and Western herbal medicine practice for the treatment of arthritis, rheumatological conditions and muscular discomfort (Blumenthal & Werner, 1998). Its use in inflammatory conditions is consistent with anti-inflammatory activities of its components in vitro (Kiuchi et al., 1982; Mascolo et al., 1989). The moderate pungency of ginger has been attributed to the mixture of gingerol derivatives in the oleoresin fraction of processed ginger (Mustafa et al., 1993). Gingerols possess the vanillyl moiety (Figure 1, region A), which is considered important for activation of the VR1 receptor expressed in nociceptive sensory neurones (Walpole et al., 1993a). Recently it was found that molecules lacking the vanillyl structure also activate the VR1 receptor in DRG neurones. These molecules include N-arachidonoyl-dopamine and its 3-O-methyl analogue (Huang et al., 2002), anandamide, an endogenous ligand of the neuronal cannabinoid receptor (CB1) (Smart & Jerman, 2000; Smart et al., 2000; Zygmunt et al., 1999), lipoxygenase metabolites (Hwang et al., 2000; Craib et al., 2001; Piomelli, 2001), and the naturally occurring sesquiterpene dialdehydes (Szallasi et al., 1998). The VR1 receptor has recently been cloned and suggested to integrate chemical and thermal nociceptive stimuli (Tominaga et al., 1998; Caterina et al., 1997; 2000). Therefore, direct activation/deactivation of the VR1 receptor at the site where pain is generated during inflammation and other painful conditions provides a new strategy for the development of a new class of peripheral analgesics devoid of the well characterized side effects of currently available analgesics (Kress & Zeilhofer, 1999; Roufogalis & Dedov, 1999). Furthermore, VR1-expressing neurones have recently been found throughout the whole neuroaxis (Mezey et al., 2000), opening up a new and so far unexplored area of VR1-related drug development. We report here for the first time to our knowledge that the pungent principle of ginger, [6]-gingerol and [8]-gingerol, activate the VR1 receptor in capsaicin-sensitive neurones and that activation is blocked by the VR1 antagonist, capsazepine.
Figure 1.
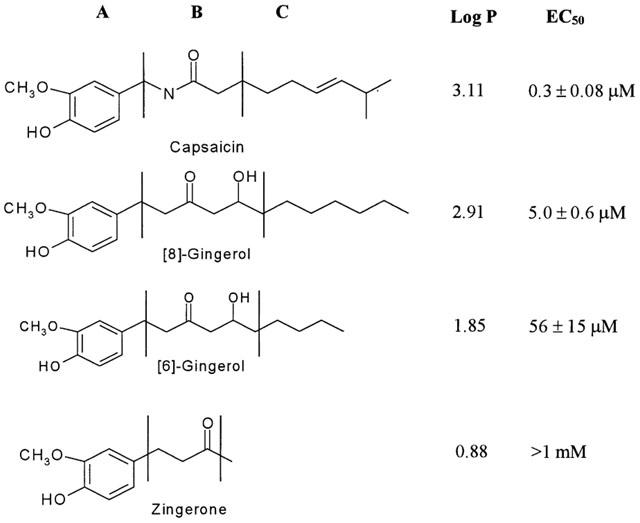
Comparison of capsaicin, [6]-gingerol, [8]-gingerol and zingerone. The molecules are divided into ‘A', ‘B' and ‘C' regions, as described for capsaicin by Walpole et al. (1993a, 1993b, 1993c). Log P values were calculated using Molecular Modeling Pro software (ChemSW, version 3.23). EC50 values for plasma membrane current flow were determined as described in the Results.
Methods
Materials
Fura-2 and Fluo-4 were obtained from Molecular Probes Inc. (Eugene, U.S.A.), DMEM and Neurobasal medium were from GIBCO (Gaithersburg, U.S.A.), NGF from ICN Biochemicals (Costa Mesa, U.S.A.). Other agents were obtained from Sigma (St. Louis, U.S.A.). All reagents were of analytical grade. Racemic [6]-gingerol (5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)decan-3-one) and [8]-gingerol (5-hydroxy-1-(4-hydroxy-3-methoxyphenyl) dodecan-3-one) were prepared as described in the literature (Denniff et al., 1981). Log P values of the compounds were calculated using Molecular Modeling Pro software (ChemSW, version 3.23).
Preparation of rat DRG neurones
This study was approved and carried out in accordance with the guidelines of the Animal Ethics Committee of The University of Sydney (AEC approval No: L24/11-99/2/3047). Isolated DRG from neonatal (3–5-day-old) rats were incubated in Hanks CMF saline with 0.05% collagenase and 0.25% trypsin for 25 min at 37°C. Neurones were obtained by trituration of ganglia with fire polished Pasteur pipettes of decreasing diameters and afterwards the cellular suspension was washed twice in DMEM medium supplemented with 10% foetal calf serum (FCS) and 2 mM glutamine. Freshly isolated neurones were plated onto collagen-coated coverslips (for intracellular Ca2+ assay) or on plastic petri dishes (for electrophysiological studies). Neurones were cultured in: (i) Neurobasal medium with B27 supplement, 50 ng ml−1 2.5 S nerve growth factor (NGF) for intracellular Ca2+ assay; (ii) DMEM medium supplied with 10% foetal calf serum for electrophysiological studies. All media contained 2 mM glutamine, 100 u ml−1 penicillin and 100 μg ml−1 streptomycin. All cultures of neurones were kept at 37°C with 5% CO2 overnight.
Measurement of intracellular Ca2+ transients in rat DRG neurones using Fura-2 AM
Intracellular Ca2+ ([Ca2+]i) was measured as described previously (Dedov & Roufogalis, 1998; 2000). Briefly, DRG neurones on coverslips were incubated with 5 μM Fura-2 AM for 30 min at 37°C. The coverslips were then mounted on a chamber attached to a rapid sample perfusion system. Single cell recordings were made on the stage of a Nikon Diaphot inverted microscope fitted with a Nikon 40×Fluo (NA 0.85) DL Ph3 or 40×Fluo (NA 1.3) oil objective. [Ca2+]i was calculated from dual excitation wavelength (340/380 nm) fluorescence measurements following an intracellular calibration procedure by the Grynkiewicz equation (Grynkiewicz et al., 1985; Kao, 1994) using MCID M2/M4 v.3.0 software (Imaging Res. Inc. St. Catharines, Ont., Canada). Cells were continuously perfused with a solution consisting of (in mM) NaCl 140, CaCl2 2, KCl 5, HEPES 20, glucose 10, pH 7.4. The cytoplasmic localization of Fura-2 was confirmed with the Mn2+ quenching technique (Dedov & Roufogalis, 1998). All experiments were performed at room temperature (20–22°C). A minimum of three separate cultures of 10–20 neurones was used for each experiment.
Measurement of intracellular Ca2+ transients in rat DRG neurones using Fluo-4 probe
Fluo-4 was used in a TCS SP2 Leica imaging system to measure intracellular Ca2+ transients in DRG neurones in capsazepine antagonism experiments with lower concentrations of capsaicin (100 nM). DRG cultures on coverslips were incubated with 10 μM Fluo-4 for 15 min at 37°C and mounted onto the Leica microscope using a laminar flow chamber, as described in the Fura-2 measurements above. Fluorescence changes of Fluo-4 were recorded every 2 s on the Leica system fitted with HC×PL APO 63x/1.20 W CORR water-objective. The perfusion system was similar to that described for Fura-2 measurements. In each experiment neurones were first exposed to a low dose of capsaicin (100 nM) for 5–10 s, followed by addition of [6]-gingerol (10 μM) or [8]-gingerol (10 μM), then KCl (50 mM) was added. A 3-min wash was employed between each addition of the drugs. This low concentration and short duration capsaicin exposure regime was designed to identify capsaicin-sensitive neurones and to minimize desensitization of neurones upon repeated applications of the drugs. In the capsazepine antagonism experiment neurones were first exposed to capsaicin (100 nM) followed by a 3-min wash, then capsazepine, gingerol and capsaicin were added in sequence. Neurones were finally washed with incubation buffer then KCl (50 mM) was added. The Ca2+-dependent fluorescence changes were calibrated at the end of experiments by adding ionomycin (10 μM) in the presence of 20 mM extracellular Ca2+ to obtain Fmax (saturated levels of fluorescence), followed by cell lysis in the presence of 50 μM digitonin to obtain Fmin (background fluorescence). The Ca2+ transients were represented as a ratio F/Fmax versus time. All experiments were performed at 20–22°C.
Electrophysiological studies
DRG neurones were prepared as described in Fura-2 AM experiments. Voltage-clamp recordings were made with whole-cell patch-clamp techniques using an AxoPatch 1D patch-clamp amplifier (Axon Instruments, Foster City, CA, U.S.A.). Micropipettes were pulled from borosilicate glass capillary tubing (7052 Glass, A-M Systems, Everett, WA) and had d.c. resistance of 0.8–2.0 MΩ. To record macroscopic currents, micropipettes were filled with a solution of the following composition (in mM): CsCl 130, NaCl 5, N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid (HEPES) 10, EGTA 10, CaCl2 2, MgATP 5 and NaGTP 0.3, with the pH adjusted to 7.3 with CsOH, osmolarity approximately 285 mosml. The external bathing solution contained (in mM): NaCl 140, MgCl2 1.5, CaCl2 2.5, KCl 2.5, glucose 10, HEPES 10, with the pH adjusted to 7.3 with NaOH and osmolarity approximately 330 mosml. Data were recorded at room temperature (20–22°C). Concentration response data was collected at a holding current of +40 mV, reversal potentials were determined by means of a ramp I/V, from +40 mV to −60 mV, as shown in Figure 4A. The liquid junction potential between internal and external solutions was approximately 4.5 mV, and all data were compensated for this value. Stimulation and recording were both controlled by a pClamp 5.5 data acquisition system (Axon Instruments). Data were filtered at 200 Hz (4 pole low-pass Bessel filter) and digital sampling rates were between 1 and 5 kHz, depending on the voltage protocol length. Capacitive currents were manually nulled and series resistance compensation of at least 80% was used for all cells. Concentration-response relationships were obtained by exposing cells to increasing concentrations of test compound at approximately 2-min intervals. Compounds were perfused until the current reached equilibrium, and the currents were compared to those caused by application of a maximally effective concentration of capsaicin (10 μM) applied at the end of the experiment. Application of drugs to the cells was carried out via a series of flow pipes in a similar procedure as described by Piper et al. (1999). Cells were voltage clamped at +40 mV to reduce calcium entry through VR1. Desensitization at +40 mV was negligible even after 2-min applications of capsaicin and this was much less than at −60 mV.
Figure 4.
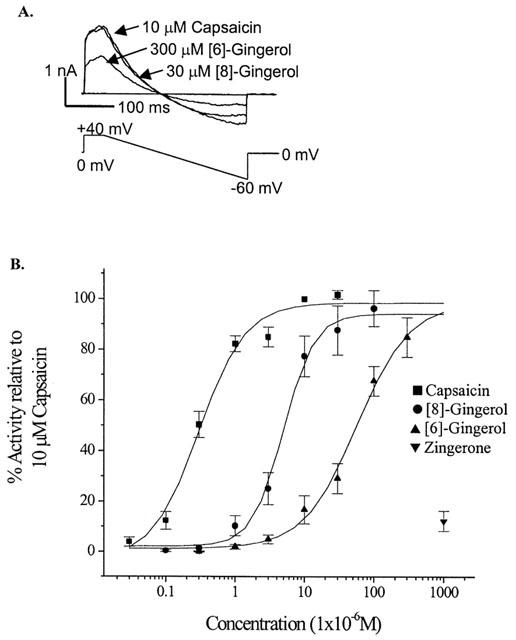
Ion currents evoked by capsaicin and gingerols in whole-cell patch clamped DRG neurones. (A) Voltage ramp was applied to patch-clamped DRG neurons in the presence of 10 μM capsaicin, 30 μM [8]-gingerol and 300 μM [6]-gingerol. Note the same I/V characteristics for all three drugs. (B) Ion current dose response to capsaicin, [8]-gingerol, [6]-gingerol and zingerone calculated as percentage to the amplitude of current evoked by 10 μM capsaicin. Data are presented as mean±s.d of at least six neurones per data point.
Results
Measurement of Ca2+ transients in Fura-2 loaded dorsal root ganglion cells
To determine whether gingerols activate the VR1 receptor, we have synthesized two gingerols representative of those present in natural ginger ([6]-gingerol and [8]-gingerol) and a gingerol degradation product (zingerone) devoid of the hydrophobic side chain (Figure 1, region C). Despite the obvious similarity of gingerol and capsaicin structures, the activity of gingerols on the VR1 receptor has yet to be reported to our knowledge.
Application of capsaicin to cultured DRG neurones loaded with Fura-2 evoked [Ca2+]i transients in the capsaicin-sensitive population of DRG neurones (Figure 2A, trace 1). These [Ca2+]i transients are due to influx of extracellular Ca2+ upon activation of the VR1 receptor and serve as characteristic markers of capsaicin-sensitive DRG neurones (Caterina et al., 1997; Cholewinski et al., 1993; Dedov & Roufogalis, 1998; 2000). A capsaicin-insensitive population of DRG neurones did not respond to capsaicin, but responded to the application of 50 mM KCl (Figure 2A, trace 2), which evoked extracellular Ca2+ influx via voltage-operated Ca2+ channels (VOCC) in all DRG neurones (Kostyuk & Verkhratsky, 1995). Application of capsaicin inhibited responses to the subsequent application of KCl in capsaicin-sensitive DRG neurones (Figure 2A–C), likely due to inhibition of VOCCs by capsaicin (Kopanitsa et al., 1995). KCl-insensitive cells were considered to be non-neuronal cells. Neither capsaicin nor gingerols evoke [Ca2+]i elevation in these cells (results not shown). A further increase in [Ca2+]i upon application of 2 μM ionomycin following the agonist application indicated that these responses were not limited by saturation of the Fura-2 signal (Figure 2A).
Figure 2.
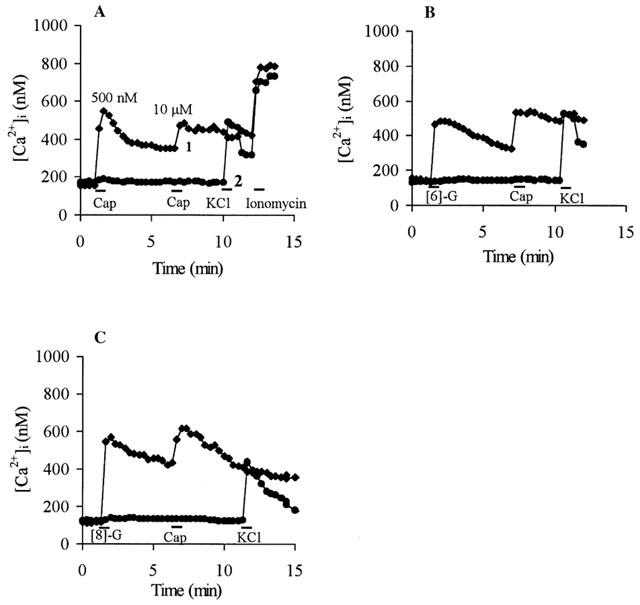
[Ca2+]i transients in Fura-2 loaded DRG neurones evoked by capsaicin and constituents of ginger. Drugs were applied for 1 min where indicated by bars, following extensive wash out with physiological solutions. Successive drug applications were: (A) 500 nM capsaicin (Cap), 10 μM capsaicin, 50 mM KCl, 2 μM ionomycin; (B) 10 μM [6]-gingerol ([6]-G), 10 μM capsaicin, 50 mM KCl; (C) 10 μM [8]-gingerol ([8]-G), 10 μM capsaicin, 50 mM KCl. Data from Fura-2 loaded DRG neurones typical of 10–20 cells from at least three separate cultures represent capsaicin and gingerol evoked [Ca2+]i transients (trace 1) and capsaicin and gingerol-insensitive [Ca2+]i transients (trace 2).
Typical traces showing that [6]-gingerol (10 μM) and [8]-gingerol (10 μM) evoked [Ca2+]i transients exclusively in the capsaicin-sensitive DRG neurones are shown in Figure 2B,C. In all cases (over 180 neurones analysed in 12 separate experiments) cells that responded to gingerols also responded to subsequent application of 10 μM capsaicin and vice versa, whereas capsaicin-insensitive cells responded neither to gingerol nor to capsaicin (Figure 2B,C).
Gingerol evoked [Ca2+]i transients in capsaicin-sensitive neurones was also demonstrated in rat DRG neurones loaded with Fluo-4, as shown in Figure 3A,C. Under the experimental conditions described, gingerols induced a rapid rise in intracellular calcium, similar to that produced by capsaicin. The continued rise in [Ca2+]i transients (F/Fmax ratios) after cessation of application of drug has been observed in previous studies (Cholewinski et al., 1993) where there is a lag phase between activation and inactivation of the VR-1 receptors in response to capsaicin. The continued rise in [Ca2+]i after removal of drug may result from the difficulty of washing out drugs from the membrane localization, Ca2+-dependent signalling processes or Ca2+ entry via VOCC's (Kostyuk & Verkhratsky, 1995). It is worth noting that repeated applications of capsaicin at 100 nM showed little or no desensitization of neurones (over 80 neurons in 24 separate experiments) under conditions described in these experiments (results not shown). The activation of DRG neurones by gingerols was again only shown to occur in neurones that are sensitive to capsaicin. This agonistic activity was blocked by capsazepine, a capsaicin antagonist, as shown in Figure 3B,D. Addition of capsaicin (100 nM) to neurones previously treated with capsazepine showed no [Ca2+]i response.
Figure 3.
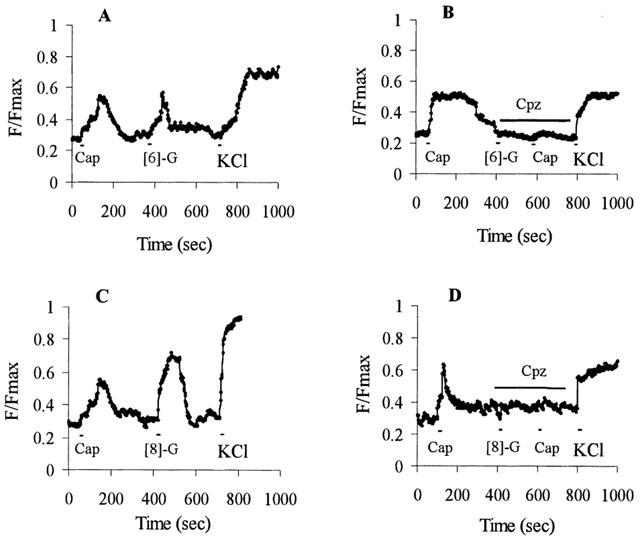
Effect of pre-treatment of DRG neurones with capsaicin and capsazepine on gingerol [Ca2+]i transient responses. [Ca2+]i transients are represented as an increase in fluorescence ratio (F/Fmax; basal amplitude=0.3±0.09) of Fluo-4 loaded DRG neurones (typical of 20–30 cells from at least three separate cultures) evoked by capsaicin, [6]-gingerol and [8]-gingerol. Drugs were applied for 5–10 s, as shown by bars in the Figure, following a 3-min wash out with physiological solutions between additions. Capsazepine applications are shown as indicated by bars. Successive drug applications were: (A) 100 nM capsaicin (Cap), 10 μM [6]-gingerol ([6]-G) and 50 mM KCl; (B) 100 nM capsaicin, 10 μM capsazepine (Cpz), 10 μM [6]-gingerol, 100 nM capsaicin and 50 mM KCl; (C) 100 nM capsaicin, 10 μM [8]-gingerol ([8]-G) and 50 mM KCl; (D) 100 nM capsaicin, 10 μM capsazepine, 10 μM [8]-gingerol, 100 nM capsaicin and 50 mM KCl.
These data suggest that gingerols exhibit agonist activity towards the VR1 receptor in rat DRG neurones similar to that of capsaicin. However, the absolute efficacy of gingerols at the VR1 receptor could not be determined from this study as gingerol-induced elevations of [Ca2+]i may represent both calcium entry through VR1 receptors and calcium entry resulting from membrane depolarization and opening of VOCCs. Moreover, primary DRG neurons in culture differ in holding potential and [Ca2+]i handling capacities (Kostyuk & Verkhratsky, 1995). This makes it impossible to study relative efficacy of gingerols by this technique and Figures 2 and 3 represent gingerol effects only qualitatively.
The patch-clamp technique, however, allows normalization of plasma membrane holding potentials and, therefore, comparative study of drug efficiencies. Hence the plasma membrane currents induced by capsaicin and gingerols in the presence and absence of capsazepine were also examined, as follows.
Measurement of plasma membrane currents
Plasma membrane currents induced by capsaicin in DRG neurones were outward at positive membrane potentials and inward at negative potentials, and reversed polarity at −6±1 mV (n=9), with an EC50 of 0.3±0.08 μM (Figure 4). Both [6]-gingerol and [8]-gingerol induced increases in the plasma membrane conductance exclusively in capsaicin-sensitive DRG neurones, as in all cells examined neurones that did not respond to capsaicin did not respond to the application of gingerols (results not shown). Currents evoked by gingerols mimicked those evoked by capsaicin, with reversal potential of −6±2 mV (n=6) for [8]-gingerol and −5±1 mV (n=7) for [6]-gingerol (Figure 4A). Concentration-response curves were determined by application of agonist to neurones at increasing concentrations at a voltage clamped at +40 mV (to minimize Ca2+ entry and desensitisation as explained in experimental section), and the currents were normalized to those produced by 10 μM capsaicin in each cell. [8]-gingerol (EC50 5.0±0.6 μM) was more potent than [6]-gingerol (EC50 56±15 μM) in inducing plasma membrane conductance. In contrast zingerone, even at high concentration (1 mM), produced a current only approximately 10% of the maximum current induced by capsaicin. [8]-Gingerol and [6]-gingerol produced maximum currents similar in magnitude to that of capsaicin (10 μM) (Figure 4B). Currents evoked by 1 μM [8]-gingerol, 10 μM [6]-gingerol and 1 μM capsaicin were almost completely blocked by pre-application of 10 μM capsazepine (Table 1), which corresponds to a >99% reduction in the current, if considered on currents normalized in each cell. All the neurones where the capsazepine block of current was tested had been previously shown to be sensitive to capsaicin or one of the gingerols. It should be noted that under the experimental conditions chosen desensitisation of capsaicin response was minimal. Capsaicin (10 μM) was applied three times, each separated by 3 min, to DRG neurones which were clamped at voltage of +40 mV to minimize calcium entry. The second application produced a current 103±3% of the first, the third application produced a current 106±6% of the first (n=6).
Table 1.
The effect of capsaicin and gingerols on plasma membrane current of capsaicin sensitive DRG neurones in the presence and absence of capsazepine
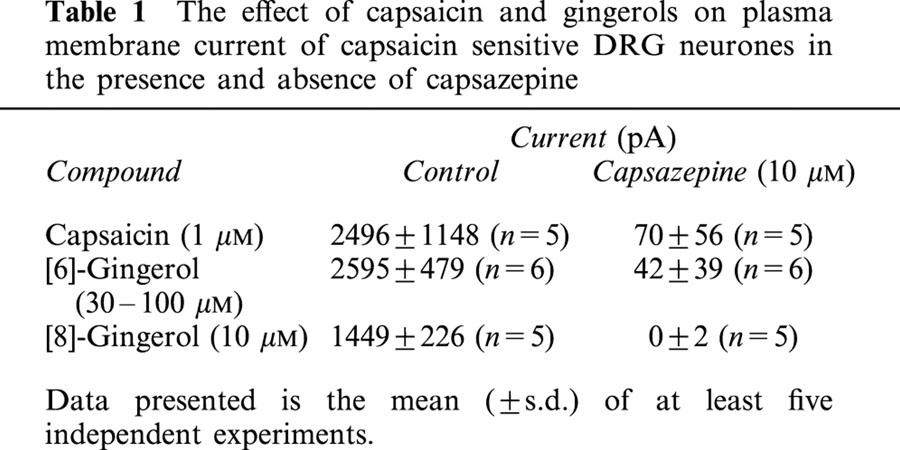
Direct measurement of channel currents of the agonists at a ligand-gated ion channel reflects the efficacy of the agonists. [8]-Gingerol gave a current at the highest concentration tested of 96±7 % the current caused by application of a maximal concentration of capsaicin. This indicates that the efficacy of the two compounds at VR1 is very similar and that [8]-gingerol is probably a full agonist. These data indicate that the gingerols activate capsaicin-like membrane conductance and may be considered as full agonists of the VR1 receptor.
Discussion
In this study we have shown for the first time that gingerol constituents of ginger are relatively potent and efficacious agonists of the VR1 receptor. The activity of gingerols depends on the size of the side chain, which also determines their hydrophobicity. Increasing the number of carbons from 6 to 8 and the hydrophobicity index from 1.90 to 2.88 in the transition from [6]-gingerol to [8]-gingerol coincided with about a 10 fold increase in potency in inducing plasma membrane currents (Figure 4B). Absence of the side chain in zingerone, a ketone product of gingerol degradation, dramatically reduced its activity towards the VR1 receptor by a magnitude of at least 100 fold compared to that of [6]-gingerol (Figure 4B). This finding was further supported by a study by Liu & Simon (1996) which showed that zingerone weakly activated inward current with a threshold concentration of 1 mM, which was approximately 10,000 fold less potent than capsaicin (threshold concentration of 0.1 μM) in inducing inward current in rat trigeminal ganglion cells (Liu & Simon, 1996).
Structure activity studies of capsaicin-like compounds have previously divided the capsaicin molecule into three regions, as shown in Figure 1; the aromatic ‘A' region (Walpole et al., 1993a), the amide bond ‘B' region (Walpole et al., 1993b), and the hydrophobic side-chain ‘C' region (Walpole et al., 1993c). In this study [8]-gingerol exhibited lower potency than capsaicin in activation of VR1 receptor, despite both having approximately the same number of carbons in the side chain. The presence of the hydroxyl moiety at C5 in the ‘B' region of [8]-gingerol could be significant in altering the potency of activation of the VR1 receptor. Substitution of an amide function in capsaicin appears to increase its hydrophobicity (Log P=3.11), and this coincides with a 20 fold higher potency of capsaicin in comparison to [8]-gingerol.
Discovery of gingerols as potent VR1 receptor agonists serves to explain the traditional and recent use of ginger for pain relief in rheumatic and inflammatory conditions (Blumenthal & Werner, 1998; Srivastava & Mustafa, 1992). Moreover, the presence of VR1 receptors throughout the brainstem (Mezey et al., 2000), where the nausea centre is located, may conceivably be associated in part with the common use of ginger as antiemetic medicine (Ernst & Pittler, 2000; Mustafa et al., 1993). The therapeutic value of capsaicin as a medicinal agent is limited by its high pungency and neurotoxicity (Wood, 1993). Topical application of a capsaicin-containing lotion has resulted in the loss of epidermal nerve fibres (Nolano et al., 1999). It appears that the toxic effects of capsaicin are associated with VR1 receptor mediated extracellular Ca2+ influx (Chard et al., 1995) and release of intracellular Ca2+ from internal stores in the endoplasmic reticulum (Olah et al., 2001), as well as the effect of capsaicin on mitochondria in DRG neurones (Dedov & Roufogalis, 2000). Therefore, further exploring the functional groups on the side chain of gingerol structures would allow for development of more desirable pharmacological compounds acting via the VR1 receptor for controlling pain.
Acknowledgments
Financial support was provided by Australian Research Council and Thursday Plantation P/L in a Strategic Partnership with Industry Research and Training project.
Abbreviations
- CAP
capsaicin
- CBI
cannabinoid receptor
- CPZ
capsazepine
- DRG
dorsal root ganglion neurones
- [6]-G
[6]-gingerol
- [8]-G
[8]-gingerol
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- [Ca2+]i
intracellular calcium
- NGF
nerve growth factor
- VR1
vanilloid receptor subtype-1
- VOCC
voltage-operated Ca2+ channels
References
- BLUMENTHAL M., WERNER B.R. The complete German Commission E monographs: therapeutic guide to herbal medicines. Austin, Tex.: American Botanical Council; Boston, MA.: Integrative Medicine Communications; 1998. [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- CHARD P.S., BLEAKMAN D., SAVIDGE J.R., MILLER R.J. Capsaicin-induced neurotoxicity in cultured dorsal root ganglion neurons: involvement of calcium-activated proteases. Neuroscience. 1995;65:1099–1108. doi: 10.1016/0306-4522(94)00548-j. [DOI] [PubMed] [Google Scholar]
- CHOLEWINSKI A., BURGESS G.M., BEVAN S. The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neuroscience. 1993;55:1015–1023. doi: 10.1016/0306-4522(93)90315-7. [DOI] [PubMed] [Google Scholar]
- CRAIB S.J., ELLINGTON H.C., PERTWEE R.G., ROSS R.A. A possible role of lipoxygenase in the activation of vanilloid receptors by anandamide in the guinea-pig bronchus. Br. J. Pharmacol. 2001;134:30–37. doi: 10.1038/sj.bjp.0704223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEDOV V.N., ROUFOGALIS B.D. Rat dorsal root ganglion neurones express different capsaicin-evoked Ca2+ transients and permeabilities to Mn2+ Neurosci. Lett. 1998;248:151–154. doi: 10.1016/s0304-3940(98)00351-6. [DOI] [PubMed] [Google Scholar]
- DEDOV V.N., ROUFOGALIS B.D. Mitochondrial calcium accumulation following activation of vanilloid (VR1) receptors by capsaicin in dorsal root ganglion neurons. Neuroscience. 2000;95:183–188. doi: 10.1016/s0306-4522(99)00423-6. [DOI] [PubMed] [Google Scholar]
- DENNIFF P., MACLEOD I., WHITING D.A. Syntheses of the (±)-[n]-gingerols (pungent principles of ginger) and related compounds through regioselective aldol condensations: relative pungency assays. J. Chem. Soc. Perkin Trans. 1981;1:82–87. [Google Scholar]
- ERNST E., PITTLER M.H. Efficacy of ginger for nausea and vomiting: a systematic review of randomised clinical trials. Br. J. Anaesth. 2000;84:367–371. doi: 10.1093/oxfordjournals.bja.a013442. [DOI] [PubMed] [Google Scholar]
- GRANT K.L., LUTZ R.B. Ginger. Am. J. Health Syst. Pharm. 2000;57:945–947. doi: 10.1093/ajhp/57.10.945. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., TREVISANI M., AL-HAYANI A., DE PETROCELLIS L., FEZZA F., TOGNETTO M., PETROS T.J., KREY J.F., CHU C.J., MILLER J.D., DAVIES S.N., GEPPETTI P., WALKER J.M., DI MARZO V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG S.W., CHO H., KWAK J., LEE S-Y., KANG C-J., JUNG J., CHO S., MIN K.H., SUH Y-G., & OH U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAO J.P. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- KIUCHI F., IWAKAMI S., SHIBUYA M., HANAOKA F., SANKAWA U. Inhibitors of prostaglandin biosynthesis from ginger. Chem. Pharm. Bull. 1982;30:754–757. doi: 10.1248/cpb.30.754. [DOI] [PubMed] [Google Scholar]
- KOPANITSA M.V., PANCHENKO V.A., MAGURA E.I., LISHKO P.V., KRISHTAL O.A. Capsaicin blocks Ca2+ channels in isolated rat trigeminal and hippocampal neurons. NeuroReport. 1995;6:2338–2340. doi: 10.1097/00001756-199511270-00016. [DOI] [PubMed] [Google Scholar]
- KOSTYUK P.G., VERKHRATSKY A.N. Calcium signaling in the nervous system. Chichester; New York: J. Wiley and Sons; 1995. Ca2+ influx through the plasmalemma; pp. 1–38. [Google Scholar]
- KRESS M., ZEILHOFER H.U. Capsaicin, protons and heat: new excitement about nociceptors. Trends Pharmacol. Sci. 1999;20:112–118. doi: 10.1016/s0165-6147(99)01294-8. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Similarities and differences in the currents activated by capsaicin, piperine, and zingerone in rat trigeminal ganglion cells. J. Neurophysiol. 1996;76:1858–1869. doi: 10.1152/jn.1996.76.3.1858. [DOI] [PubMed] [Google Scholar]
- MASCOLO N., JAIN R., JAIN S.C., CAPASSO F. Ethnopharmacologic investigation of ginger (Zingiber officinale) J. Ethnopharmacol. 1989;17:129–140. doi: 10.1016/0378-8741(89)90085-8. [DOI] [PubMed] [Google Scholar]
- MEZEY E., TOTH Z.E., CORTRIGHT D.N., ARZUBI M.K., KRAUSE J.E., ELDE R., GUO A., BLUMBERG P.M., SZALLASI A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSTAFA T., SRIVASTAVA K.C., JENSEN K.B. Drug development report: 9. Pharmacology of ginger, Zingiber officinale. J. Drug Dev. 1993;6:25–39. [Google Scholar]
- NOLANO M., SIMONE D.A., WENDELSCHAFER-CRABB G., JOHNSON T., HAZEN E., KENNEDY W.R. Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–145. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- OLAH Z., KARAI L., IADAROLA M.J. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J. Biol. Chem. 2001;276:31163–31170. doi: 10.1074/jbc.M101607200. [DOI] [PubMed] [Google Scholar]
- PIOMELLI D. The ligand that came from within. Trends Pharmacol. Sci. 2001;22:17–19. doi: 10.1016/s0165-6147(00)01602-3. [DOI] [PubMed] [Google Scholar]
- PIPER A.S., YEATS J.C., BEVAN S., DOCHERTY R.J. A study of the voltage dependence of capsaicin-activated membrane currents in rat sensory neurones before and after acute desensitization. J. Physiol. 1999;518:721–733. doi: 10.1111/j.1469-7793.1999.0721p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUFOGALIS B.D., DEDOV V.N.Capsaicin receptor mechanism and future trends for analgesics Herbal medicinal products for the treatment of pain 1999Lismore: Southern Cross University Press; 94–98.ed. Chrubasik, S. & Roufogalis, B.D. pp [Google Scholar]
- SZALLASI A., BIRO T., MODARRES S., GARLASCHELLI L., PETERSEN M., KLUSCH A., VIDARI G., JONASSOHN M., DE ROSA S., STERNER O., BLUMBERG P.M., KRAUSE J.E. Dialdehyde sesquiterpenes and other terpenoids as vanilloids. Eur. J. Pharmacol. 1998;356:81–89. doi: 10.1016/s0014-2999(98)00514-7. [DOI] [PubMed] [Google Scholar]
- SHARMA H., CLARK C. Contemporary Ayurveda: medicine and research in Maharishi Ayur-Veda. New York: Churchill Livingstone; 1998. Active ingredients, free radicals and the herbal pharmacopeia; pp. 75–95. [Google Scholar]
- SMART D., JERMAN J.C. Anandamide: an endogenous activator of the vanilloid receptor. Trends Pharmacol. Sci. 2000;21:134. doi: 10.1016/s0165-6147(00)01459-0. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRIVASTAVA K.C., MUSTAFA T. Ginger (Zingiber officinale) in rheumatism and musculoskeletal disorders. Med. Hypotheses. 1992;39:342–348. doi: 10.1016/0306-9877(92)90059-l. [DOI] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- WALPOLE C.S.J., WRIGGLESWORTH R., BEVAN S., CAMPBELL E.A., DRAY A., JAMES I.F., PERKINS M.N., REID D.J., WINTER J. Analogues of capsaicin with agonist activity as novel analgesic agents; Structure-activity studies: 1. The Aromatic ‘A-region'. J. Med. Chem. 1993a;36:2362–2372. doi: 10.1021/jm00068a014. [DOI] [PubMed] [Google Scholar]
- WALPOLE C.S.J., WRIGGLESWORTH R., BEVAN S., CAMPBELL E.A., DRAY A., JAMES I.F., MASDIN K.J., PERKINS M.N., WINTER J. Analogues of capsaicin with agonist activity as novel analgesic agents; Structure-activity studies: 2. The amide bond ‘B-region'. J. Med. Chem. 1993b;36:2373–2380. doi: 10.1021/jm00068a015. [DOI] [PubMed] [Google Scholar]
- WALPOLE C.S.J., WRIGGLESWORTH R., BEVAN S., CAMPBELL E.A., DRAY A., JAMES I.F., MASDIN K.J., PERKINS M.N., WINTER J. Analogues of capsaicin with agonist activity as novel analgesic agents; Structure-activity studies: 3. The hydrophobic side-chain ‘C-region'. J. Med. Chem. 1993c;36:2381–2389. doi: 10.1021/jm00068a016. [DOI] [PubMed] [Google Scholar]
- WOOD J.N. Capsaicin in the study of pain 1993New York: Academic press; ed. [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H.H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


