Abstract
Activation of vanilloid receptors on sensory nerve terminals in the commissural nucleus of the solitary tract (cNTS) of rats with capsaicin, produces respiratory slowing. In this study, we used microinjection techniques employing pungent and non-pungent vanilloids to further characterize vanilloid receptors in the cNTS.
Microinjection of the pungent vanilloid, resiniferatoxin (RTX), into the cNTS of urethane-anaesthetized rats, dose-dependently reduced respiratory rate without affecting tidal volume. RTX was 20 fold more potent at slowing respiration (∼ED50, 100 pmol) than capsaicin (∼ED50, 2 nmol). Doses of RTX greater than 100 pmol caused either irregular (dyspnoeic) breathing or terminal apnoea (>250 pmol). The respiratory slowing response to RTX (75 pmol), was dose-dependently attenuated by injecting RTX (but not vehicle) into the same site 60 min earlier.
The non-pungent phorbol derivative of RTX, phorbol 12-phenylacetete 13-acetate 20-homovanillate (PPAHV, 0.1–1 nmol), also slowed respiration (ED50, ∼1 nmol) and almost abolished response to RTX (75 pmol) injected into the same site 60 min later.
In contrast to RTX, PPAHV and capsaicin, the putative endogenous vanilloid receptor agonist, arachidonyl ethanolamide (AEA), and non-pungent capsaicin derivative, olvanil, had no direct effect on respiration. However, both AEA and olvanil dose-dependently reduced the respiratory response to injection of RTX (75 pmol) 60 min later into the same site (EC50s, for AEA and olvanil, ∼2 and 0.2 nmol, respectively).
These studies suggest that both pungent and non-pungent vanilloids interact with vanilloid receptors in the cNTS. However, whereas RTX and PPAHV activate and subsequently desensitize vanilloid receptors on sensory nerve terminals in the cNTS, olvanil and AEA fail to activate despite readily desensitizing responses to RTX in this region.
Keywords: Nucleus of the solitary tract, tachykinins, vanilloid, desensitization, C-fibre
Introduction
Vanilloids, including capsaicin and resiniferatoxin (RTX), are thought to act via specific vanilloid receptors located on the central and peripheral terminals of unmyelinated C-fibres and a subset of thinly myelinated Aδ-fibres. These agents have been proposed as non-steroidal analgesic and anti-inflammatory agents since they desensitize C- and Aδ-fibres with prolonged or repeated administration (for review, see Szolcsányi, 1993; Dray & Urban, 1996; Szallasi & Blumberg, 1999). Vanilloid receptors are widely distributed throughout the central nervous system (Mezey et al., 2000). However, autoradiographic, immunocytochemical and in-situ hybridization studies suggest that the highest vanilloid receptor densities are in the dorsal horn of the spinal cord and discrete regions of the brain stem, including the nucleus of the solitary tract (NTS; Szallasi et al., 1995; Guo et al., 1999).
Vanilloid receptors are activated by a variety of agents. RTX is a highly potent vanilloid receptor agonist, which displays greater efficacy for inducing desensitization than for causing pain, relative to capsaicin (Blumberg et al., 1993; Szallasi & Blumberg, 1996; Appendino & Szallasi, 1997). However, RTX may still initially cause pain and a number of other undesirable side-effects, including respiratory depression. Thus, several RTX and capsaicin analogues have been synthesized which display a reduced ability to evoke pain (i.e., they are less pungent). For example, the RTX analogue, phorbol 12-phenylacetete 13-acetate 20-homovanillate (PPAHV), is only mildly pungent and is devoid of side effects at doses that protect against neurogenic inflammation (Appendino et al., 1996). Olvanil, a capsaicin derivative, similarly lacks pungency and is an effective analgesic in rodent models of pain (Brand et al., 1987). In addition, recent studies suggest that the cannabinoid receptor agonist, arachydonyl ethanolamide (AEA, anandamide), may also be an endogenous vanilloid receptor agonist (Zygmunt et al., 1999).
The NTS receives input from many sources, including peripheral chemo- and baroreceptor, and vagal pulmonary afferents, i.e., slowly adapting stretch receptors, rapidly adapting (irritant) receptors and bronchopulmonary C-fibres (Kalia & Mesulam, 1980; Jordan & Spyer, 1986), many of which are capsaicin-sensitive (Lukovic et al., 1987; Matsumoto et al., 1997; Ho et al., 2001). Since activation of these capsaicin-sensitive visceral afferent nerves evokes profound respiratory effects (for example, see Lee & Lundberg, 1994), we reasoned (in a previous study) that vanilloid receptors in the NTS could be characterized using an in vivo model which enabled measurements of respiratory responses following delivery of agents to the NTS of anaesthetized rats. In that study, we demonstrated that capsaicin produces a dose-dependent bradypnoea (and apnoea at higher doses) when microinjected into the commissural subnucleus of the NTS (cNTS) of the rat (Mazzone & Geraghty, 1999). Moreover, the capsaicin-induced bradypnoea was attenuated by the competitive vanilloid receptor antagonist, capsazepine, and by tachykinin NK2 and NK3 receptor antagonists (SR 48968 and SR 142801, respectively) supporting the hypothesis that capsaicin evokes neuropeptide release from the central terminals of capsaicin-sensitive visceral afferents which terminate at this site.
The aim of the present study was to further investigate the characteristics of vanilloid receptors in the rat cNTS. Using our previously described bioassay (Mazzone & Geraghty, 1999), we monitored respiratory responses evoked following microinjection of capsaicin, RTX, olvanil, PPAHV, and the putative endogenous vanilloid receptor agonist, AEA, into the cNTS of anaesthetized, spontaneously-breathing rats. Furthermore, we compared the potency of each agonist at evoking respiratory depression and desensitization of vanilloid receptor-dependent responses in the cNTS. The results of the study suggest that vanilloid agonists display differential potencies for activating versus desensitizing vanilloid receptors in the rat brain stem.
Methods
Surgery
All experimental procedures were approved by the University of Tasmania Ethics Committee (Animal Experimentation; project A5710). Male Hooded Wistar rats (240–310 g; n=97) were anaesthetized using urethane (1–1.5 g kg−1 i.p.) and allowed to breathe room air throughout all procedures. This dose of urethane provides a deep and stable level of anaesthesia for up to 9 h, although experiments rarely lasted longer than 4 h. The level of anaesthesia was regularly assessed by monitoring limb withdrawal and head-shake reactions. There were no instances where supplementary injections of urethane were required during an experiment. Body core temperature was kept constant at 37°C by placing the rat in the prone position on a thermostatically controlled waterbed. The animal's head was stabilized in a Kopf stereotaxic apparatus and the dorsal aspect of the brain stem and cerebellum was exposed by a midline incision and partial occipital craniotomy. The dura mater was temporarily left intact. Animals were allowed to stabilize for 20 min before continuing.
Respiration, which was spontaneous and rhythmic, was recorded using a modification of the method previously described by our laboratory (Mazzone et al., 1998; Mazzone & Geraghty, 1999; 2000a, 2000b). Briefly, respiratory movements were recorded using subcutaneous electrodes (inserted along the sixth intercostal space) and an impedance converter (UFI, Morro Bay, California, U.S.A.). Output from the impedance converter was collected using an 8SP PowerLab data acquisition system and displayed on a Pentium III computer operating Chart4 software (ADInstruments, Sydney, Australia). At the end of each experiment, animals were killed by an intracardiac injection of sodium pentobarbitone. Injections sites were confirmed in histological brain stem sections (stained with neutral red) selected at random from each data set (refer to Figure 1). There were no instances where animals needed to be excluded from a data set due to an incorrect placement of the micropipette.
Figure 1.
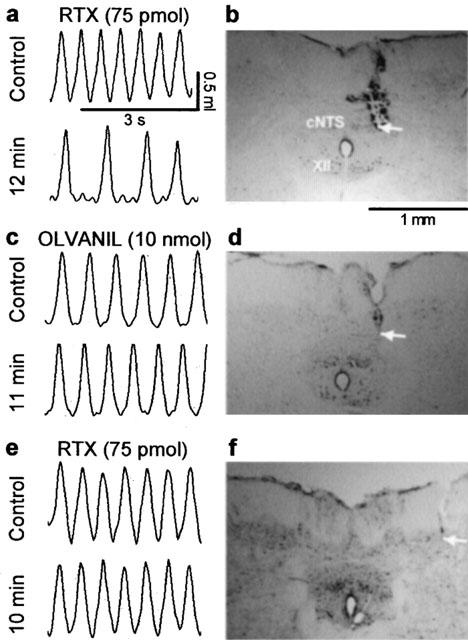
Representative chart tracings which show respiratory movements before and 10–12 min after microinjection of (a) resiniferatoxin (RTX, 75 pmol in 500 nl) or (c) olvanil (10 nmol) into the commissural nucleus of the solitary tract (cNTS) of urethane-anaesthetized, spontaneously breathing rats. The adjacent photomicrographs (b,d) display the location of the injection sites (arrows) in neutral red stained brain stem sections from these same animals. Note that despite similar placement of the probe in both experiments only RTX evoked respiratory slowing (see Figures 2 and 3 for mean data). Injection of RTX (75 pmol in 500 nl) outside the lateral border of the cNTS (e,f) had no effect on respiration suggesting that the observed responses were evoked from within the cNTS. XII, hypoglossal nucleus.
Injection of agents
The dura mater was cut and retracted. A glass micropipette (tip OD 20–30 μm) was attached to a microprocessor-controlled pump (UltraMicroPump II, WPI) and mounted in a micromanipulator. Using obex as a reference point (see Paxinos & Watson, 1986), injections were made unilaterally into the cNTS: 0.1 to 0.5 mm caudal; 0 to 0.3 mm lateral to obex and 0.4–0.6 mm into the dorsal surface of the brain stem. The poor aqueous solubility of vanilloids necessitated using a large (500 nl over 30 s) injection volume (as previously described by Mazzone & Geraghty, 1999).
Initial experiments were designed to assess respiratory responses to a variety of pungent and non-pungent vanilloids following microinjection into the cNTS. To avoid the potentially confounding desensitizing effects resulting from the administration of increasing doses of a vanilloid receptor agonist into a single animal, dose-response curves to capsaicin (0.2–2 nmol), RTX (0.05–300 pmol, PPAHV (0.1 and 1 nmol), olvanil (0.1–10 nmol) and AEA (1.45 and 14.4 nmol) were constructed in a non-cumulative fashion. A minimum of three and a maximum of ten animals were used for each point on a dose-response curve. A single dose of vanilloid (or vehicle) was injected into the right cNTS and respiratory movements were continuously recorded for 60 min (when all evoked respiratory responses had returned to baseline). In control studies we injected RTX (75 pmol) outside the lateral boundaries of the cNTS at the same rostrocaudal and dorsoventral level (i.e. 0.5 mm caudal; 0.4 mm ventral and 0.7 and 1.0 mm lateral) to assess the anatomical specificity of any respiratory responses evoked by these agents.
In another series of experiments, we assessed the ability of each vanilloid receptor agonist to desensitize the respiratory responses evoked by a subsequent injection of RTX. In these studies, a single dose of either RTX (0.05–75 pmol; n=3–5 per dose), PPAHV (0.1 and 1 nmol; n=4 per dose), olanvil (0.1–10 nmol; n=3–4 per dose) or AEA (1.45 and 14.5 nmol; n=3–4 per dose) was injected into the right cNTS. Animals were then allowed 60 min to recover from the respiratory effects evoked by this first injection. Following the 60 min recovery period RTX (75 pmol) was injected into same cNTS location using the identical stereotaxic co-ordinates employed for the first injection. Respiration was monitored continuously throughout both injection periods. In some experiments the second injection was delayed for up to 3 h to assess the duration of the desensitization. In other experiments, a third injection (75 pmol RTX) was made 60 min after the second injection into the contralateral (left) cNTS. This injection was used to confirm that desensitization was confined unilaterally to the site of injection. Control animals received an initial injection of vehicle (35% EtOH or soya oil/water emulsion, see drugs and materials list) prior to a subsequent injection of 75 pmol RTX (n=3–4).
Data analysis
Using Chart4 software (ADInstruments, Sydney, Australia), the magnitude (equivalent to tidal volume, VT) and frequency of respiratory movements were averaged over a 10 s time interval every 1–5 min. VT and frequency data were expressed as per cent (pre-injection) control and change from control, respectively. Dose-effect data were analysed using PRISM (GraphPad, San Diego, CA, U.S.A.) to estimate ED50s (the dose of an agonist required to evoke a 50% response) for each agent studied. Statistical differences between means were determined using analysis of variance (ANOVA) followed by Dunnett's post-hoc test. A P value of less than 0.05 was considered statistically significant.
Drugs and materials
Capsaicin, resiniferatoxin (RTX), arachydonyl ethanolamine (AEA, anandamide) and olvanil (N-(3-methoxy-4-hydroxybenzyl)oleamide) were purchased from Tocris (Bristol, U.K.) whereas phorbol 12-phenylacetete 13-acetate 20-homovanillate (PPAHV) was obtained from the Alexis Corporation (San Diego, CA, U.S.A.). AEA was provided in a water-soluble emulsion and subsequently diluted in 0.9% normal saline for injection. All other agents were dissolved in 25–35% ethanol in saline and stored in frozen aliquots and diluted in normal saline for injection. All other reagents were of analytical grade.
Results
Respiratory actions of vanilloid receptor agonistsin the cNTS
As previously reported (Mazzone et al., 1998; Mazzone & Geraghty, 1999; 2000a, 2000b) baseline (pre-injection) respiratory patterns were similar in all animals studied. Resting tidal volumes ranged between approximately 1.1 and 1.3 ml in all experiments and rarely changed with any manipulation performed (see below). Baseline respiratory frequencies (breaths min−1) prior to microinjection of EtOH/saline vehicle (108±3, n=8), RTX (104±2, n=32), capsaicin (102±2, n=29), PPAHV (100±3, n=11), olvanil (107±3, n=9), AEA emulsion (109±4, n=8) or AEA (104±4, n=7) were very similar.
Microinjection of the naturally-occurring vanilloids, RTX and capsaicin, into the cNTS produced a decrease in respiratory frequency without significantly affecting VT (Figures 1a and 2b). The maximum respiratory responses evoked by equieffective doses of RTX (75 pmol) and capsaicin (1 nmol), (−42±8 and −48±10 breaths min−1, respectively) were observed at similar time points (7.8±0.6 and 6.8±0.8 min, respectively; n=10; Figure 2a,b). Furthermore, respiratory frequency returned to pre-injection values 30 min after injection of either vanilloid. Doses of RTX between 100 and 200 pmol produced unusual respiratory patterns. For example, periods of apnoea (lasting between 5 and 10 s) followed by 5–10 s of deep (albeit slow) breathing (data not shown). Higher doses of RTX (>250 pmol) invariably caused terminal apnoea (death). The phorboid derivative of RTX, PPAHV, produced qualitatively similar effects to RTX on respiration (Figures 2c,d and 3). However, the respiratory frequency nadir (−40±6 breaths min−1) was attained significantly (P<0.01) faster (3.0±0.8 min after injection; n=4) than with equieffective doses of RTX or capsaicin.
Figure 2.
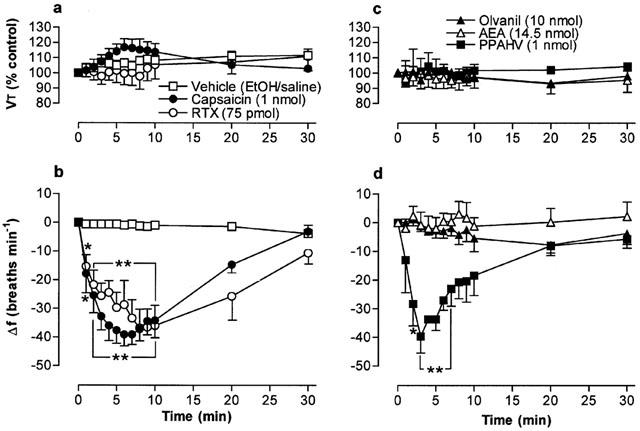
Tidal volume (VT) and respiratory frequency (f) evoked by vanilloid receptor agonists following microinjection into the cNTS of urethane-anaesthetized, spontaneously breathing rats. (a) and (b) show responses evoked by 1 nmol capsaicin (n=10), 75 pmol resiniferatoxin (RTX; n=7) and vehicle (35% EtOH in saline; n=8). (c) and (d) show responses evoked by 1 nmol phorbol 12-phenylacetete 13-acetate 20-homovanillate (PPAHV; n=5), 14.5 nmol arachidonyl ethanolamide (AEA; n=4) and 10 nmol olvanil (n=3). VT data are expressed as a percentage of the pre-injection control value and frequency data as change from pre-injection control value. The vehicle for AEA injection (soya oil-water emulsion) had no effect on respiration (not shown). Data are the mean and vertical bars represent s.e.mean. *P<0.05 and **P<0.01, significantly different from vehicle (ANOVA followed by Dunnett's test).
Figure 3.
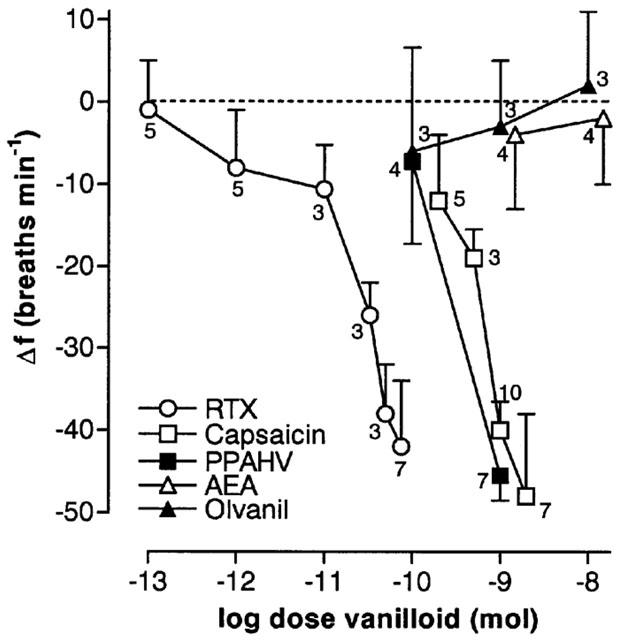
Log dose-response curves for vanilloids microinjected into the cNTS of spontaneously breathing, urethane-anaesthetized rats. Each point of a given dose-response curve represents the mean minimum respiratory frequency observed after agent injection (n values are shown adjacent to each point). The vertical bars represent the s.e.mean. Data for capsaicin are a combination of new and previously published data (Mazzone & Geraghty, 1999a). PPAHV, phorbol 12-phenylacetete 13-acetate 20-homovanillate; RTX, resinferatoxin, AEA, arachidonyl ethanolamide.
In contrast to RTX, PPAHV and capsaicin, the non-pungent capsaicin derivative, olvanil (up to 10 nmol), had negligible direct effects on respiratory frequency or VT (Figures 1c and 2c,d). Similarly, microinjection of AEA (up to 14.5 nmol, had no effect on respiration (Figure 2c,d). In parallel experiments, microinjection of the appropriate vehicle (see drugs and materials) was also devoid of any significant respiratory action (for example, see Figure 2). Furthermore, injection of 75 pmol RTX into sites near and outside the lateral boundaries of the cNTS (0.5 caudal; 0.4 mm ventral and 0.7 and 1.0 mm lateral to obex) failed to alter breathing frequency (mean change in frequency, 2±5 and −3±2 breaths min−1, respectively; n=3), confirming that these agents most likely produce their effects on respiration via a specific action in the cNTS (Figure 1e,f).
By excluding the doses of capsaicin, PPAHV and RTX that consistently induced irregular breathing patterns or apnoea, it was possible to construct partial dose-response curves for the three agents (Figure 3). Estimated ED50s (dose required to reduce respiratory frequency by 50%) were 120±11 pmol, 2.2±0.2 nmol and ∼1 nmol for RTX, capsaicin and PPAHV, respectively.
Desensitization of vanilloid receptors in the cNTS
Although microinjection of RTX (75 pmol) produced a reproducible slowing of respiration (−42±8 breaths min−1, n=8), reinjection of 75 pmol RTX into the same site 60 min later produced a markedly reduced response (−7±9 breaths min−1; P<0.05), indicative of desensitization (Figure 4). If the second RTX injection was delayed for 3 h, the normal response to RTX was still substantially reduced (−10 breaths min−1; n=2) suggesting that a prolonged period of desensitization proceeds the respiratory responses evoked by RTX injection into the cNTS. Prior injection of lower doses of RTX, including doses that did not significantly slow respiration (e.g., 0.1 and 1.0 pmol), also attenuated (if not abolished) the respiratory response to subsequent injection of 75 pmol RTX (P<0.05; Figure 4). The apparent ED50 of RTX for desensitization was less than 0.1 pmol (i.e., at least 1000 fold lower than the ED50 for slowing respiration). Injection of a third dose of RTX 60 min after the second injection (this time into the contralateral cNTS) evoked respiratory responses comparable to the first injection suggesting that the desensitization was confined to the ipsilateral side (data not shown).
Figure 4.

(a) Tidal volume (VT) and (b) respiratory frequency (f) in rats following an initial microinjection of resiniferatoxin (RTX, 75 pmol) and 60 min after microinjection of increasing doses of RTX into the commissural nucleus of the solitary tract (cNTS) of urethane-anaesthetized, spontaneously breathing rats. VT data are expressed as a percentage of the pre-injection control value and frequency data as change from pre-injection control value. (c) Maximum change in respiratory frequency (Δfmax) induced by microinjection of 75 pmol RTX (Control) and 60 min after injection of increasing doses of RTX into the cNTS. Data are the mean of 3–6 animals (individual n values are shown in parentheses) and vertical bars represent s.e.mean. *P<0.05 and **P<0.01, significantly different from the control response (ANOVA followed by Dunnett's test).
Microinjection of PPAHV, olvanil and AEA also reduced the response to a subsequent injection of RTX (75 pmol) 60 min later in to the cNTS (Figures 5, 6, 7. Since the lowest dose of PPAHV employed (0.1 nmol) reduced the response to RTX by 81%, the ED50 of PPAHV for desensitizing the RTX response was presumed to be significantly less than 0.1 nmol (Figure 5). At the lowest doses tested (0.1 and 1.45 nmol, respectively), olvanil and AEA reduced the respiratory response to RTX by 38 and 36%, respectively (Figures 6 and 7). Thus, the ED50s of olvanil and AEA for desensitizing the RTX response were estimated to be 0.2 and 2 nmol, respectively. Injection of either the vehicle for RTX, PPAHV and olvanil (35% EtOH) or the vehicle for AEA (soya oil/water emulsion) failed to reduce the subsequent respiratory response evoked by injection of 75 pmol RTX in to the same site 60 min later (−62±8 and −34±7 breaths min−1, respectively; n=3–5), confirming the specificity of the desensitizing effect.
Figure 5.
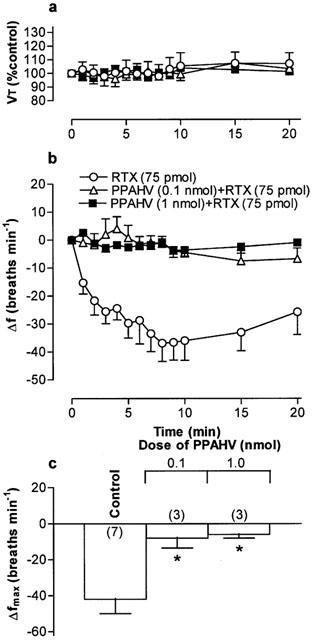
(a) Tidal volume (VT) and (b) respiratory frequency (f) in rats following an initial microinjection of resiniferatoxin (RTX, 75 pmol) and 60 min after microinjection of phorbol 12-phenylacetete 13-acetate 20-homovanillate (PPAHV) into the commissural nucleus of the solitary trace (cNTS) of urethane-anaesthetized, spontaneously breathing rats. VT data are expressed as a percentage of the pre-injection control value and frequency data as change from pre-injection control values. (c) Maximum change in respiratory frequency (Δfmax) induced by microinjection of 75 pmol RTX (Control) and 60 min after injection of increasing doses of PPAHV into the cNTS. Data are the mean of 3–6 animals (individual n values are shown in parentheses) and vertical bars represent s.e.mean. *P<0.05, significantly different from the control response (ANOVA followed by Dunnett's test).
Figure 6.
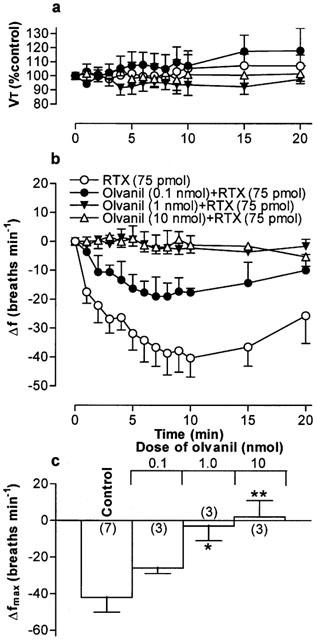
(a) Tidal volume (VT) and (b) respiratory frequency (f) in rats following an initial microinjection of resiniferatoxin (RTX, 75 pmol) and 60 min after microinjection of increasing doses of olvanil into the commissural nucleus of the solitary tract (cNTS) of urethane-anaesthetized, spontaneously breathing rats. VT data are expressed as a percentage of the pre-injection control value and frequency data as change from pre-injection control value. (c) Maximum change in respiratory frequency (Δfmax) induced by microinjection of 75 pmol RTX (Control) and 60 min after injection of increasing doses of olvanil into the cNTS. Data are the mean of 3–6 animals (individual n values are shown in parentheses) and vertical bars represent s.e.mean. *P<0.05 and **P<0.01, significantly different from the control response (ANOVA followed by Dunnett's test).
Figure 7.
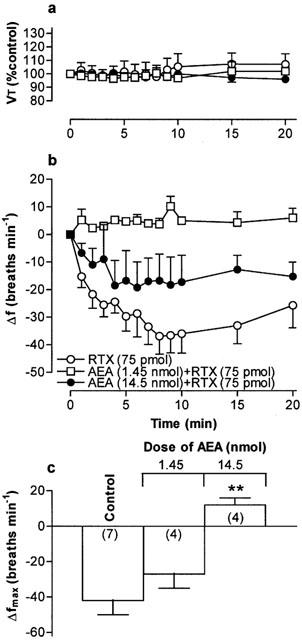
(a) Tidal volume (VT) and (b) respiratory frequency (f) in rats following an initial microinjection of resiniferatoxin (RTX, 75 pmol) and 60 min after microinjection of increasing doses of arachidonyl ethanolamide (AEA) into the commissural nucleus of the solitary tract (cNTS) or urethane-anaesthetized, spontaneously breathing rats. VT data are expressed as a percentage of the pre-injection control value and frequency data as change from pre-injection control value. (c) Maximum change in respiratory frequency (Δfmax) induced by microinjection of 75 pmol RTX (Control) and 60 min after injection of increasing doses of AEA into the cNTS. Data are the mean of 3–7 animals (individual n values are shown in parentheses) and vertical bars represent s.e.mean. **P<0.01, significantly different from the control response (ANOVA followed by Dunnett's test).
Discussion
We have previously shown that microinjection of capsaicin into the cNTS of urethane-anaesthetized rats produces dose-dependent respiratory slowing (Mazzone & Geraghty, 1999). The present study reports several additional findings regarding the pharmacology of vanilloid receptors in the cNTS. Firstly, the pungent vanilloid, RTX, is approximately 20 fold more potent than capsaicin at activating vanilloid receptors in the cNTS (i.e., slowing respiration). Moreover, RTX attenuates the respiratory response to subsequent RTX injection in to the cNTS at doses which are at least 1000 fold lower than those required to slow respiration. Secondly, the non-pungent RTX derivative, PPAHV, slows respiration with potency similar to capsaicin but attenuates the response to RTX at approximately 10 fold lower doses. Finally, the capsaicin derivative, olvanil, and putative endogenous vanilloid receptor agonist, AEA, do not appear to activate vanilloid receptors (minimal respiratory slowing, even at exceedingly high doses), but are moderately potent at reducing the response to RTX.
Respiratory actions of RTX and PPAHV in the cNTS
The respiratory actions of capsaicin in the cNTS appear to be mediated by vanilloid receptors, since they are blocked by the competitive vanilloid receptor antagonist capsazepine (Mazzone & Geraghty, 1999). In the present study, the effects of RTX on respiration were qualitatively similar to those of capsaicin, viz, dose-dependent respiratory slowing and, at progressive higher doses, dyspnoea and (finally) terminal apnoea (Mazzone & Geraghty, 1999). RTX was approximately 20 fold more potent than capsaicin at producing respiratory slowing (∼ED50s, 120 pmol and 2.2 nmol, respectively) and approximately 10 fold more potent at producing irreversible apnoea (doses>250 pmol and 3 nmol, respectively). Previous functional studies in other systems have yielded similar results. For example, compared with capsaicin RTX is 18 fold more potent at inducing inward currents in Xenopus oocytes expresing vanilloid receptors (Caterina et al., 1997), and 255 fold more potent at inducing Ca2+ uptake into cultured rat dorsal root ganglion (DRG) cells (ács et al., 1997). Thus, RTX and capsaicin are undoubtedly interacting with the same population of vanilloid receptors in the NTS.
PPAHV, a prototypic phorboid RTX derivative, was originally reported to desensitize against neurogenic inflammation with little or no irritation (Appendino et al., 1996). In the present studies, PPAHV was slightly more potent than capsaicin (∼ED50, 1 nmol) but significantly less potent than RTX at reducing respiratory frequency. We have previously reported a similar order to potency for vanilloids (viz, RTX>>PPAHV>capsaicin) at inducing vasoconstriction and inhibition of oxygen consumption in the rat hindlimb (Griffiths et al., 2000). In contrast, PPAHV was significantly (7 fold) less potent than capsaicin at inducing Ca2+ influx into vanilloid receptor-transfected HEK293 cells (Jerman et al., 2000). Liu et al. (1998) suggested that trigeminal ganglion neurons might possess vanilloid receptor subtypes based on pharmacological and electrophysiological experiments with the RTX analogue, PPAHV. Again, although such findings may reflect receptor subtypes, differences in the kinetics of channel opening in different cell types may similarly explain the observed results.
In the present study, as in our previous study (Mazzone & Geraghty, 1999), it was necessary to inject relatively large volumes (500 nl) of agents into the cNTS due to the poor solubility of the test compounds in aqueous solutions. Thus, although the highest concentration of agonist is likely to exist in the cNTS, it is possible that these agents may have diffused out of the cNTS, resulting in some uncertainty as to the exact site of action. It is conceivable that the respiratory responses were evoked by stimulating vanilloid receptors on neurons in other regions of the NTS, area postrema and dorsal motor nucleus of the vagus nerve. Nevertheless, these vanilloid receptor-expressing structures are likely to represent the terminals of primary visceral afferents (Szallasi et al., 1995; Guo et al., 1999). It may also be reasonable to suggest that the site of action is even further away, perhaps directly in respiratory structures of the ventrolateral medulla (VLM). However, this latter assertion seems unlikely since microinjection of 500 nl of 75 pmol RTX into the brain stem outside the lateral border of the cNTS failed to produce any respiratory effects. Furthermore, immunohistochemical studies have failed to identify vanilloid receptor-expressing neurons in the VLM (Guo et al., 1999), and microinjections of much smaller volumes of neurokinin receptor agonists (particularly NK3 agonists) into the cNTS produce identical respiratory effects (Mazzone & Geraghty, 2000a). Thus, we are confident that the actions of vanilloid receptor agonists in the present study are due to activation of vanilloid receptors in, or closely associated with, the cNTS.
Desensitizing effects of RTX and PPAHV in the cNTS
A hallmark of vanilloid action is desensitization and/or tachyphylaxis (a reduced response to continued or repeated exposure to agonists). In the present study, microinjection of 75 pmol RTX into the cNTS induced a profound fall in respiratory frequency. Microinjection of the same dose of RTX 1 h later produced only minimal respiratory slowing, indicative of desensitization. Interestingly, the dose of RTX required to desensitize respiratory responses was much lower than the dose required to evoke a fall in breathing frequency. For example, microinjection of 1 pmol RTX reduced respiratory frequency by only 7% but attenuated the response to subsequent injection of 75 pmol RTX by 80%. Indeed, we estimate that RTX is 1000 fold more potent at desensitizing than stimulating vanilloid receptors in the cNTS. Similar results were obtained with PPAHV. Thus PPAHV, like its parent compound, RTX, appeared to be more potent at desensitizing than stimulating vanilloid receptors (e.g., 0.1 nmol PPAHV reduced respiratory rate by ∼7% but abolished the response to subsequent exposure to RTX).
The attenuated response to RTX following either RTX or PPAHV pretreatment is indicative of desensitization. However, the hypo-responsiveness may result from tissue damage due to multiple injections or simply be an artefact reflecting the experimental design (for example, animals may be deeper into their anaesthetized state 60 min into an experiment and hence less likely to respond to neuronal stimulation). It seems unlikely that the attenuated response is due to tissue damage in the cNTS since prior injections of vehicle failed to attenuate the response to RTX. Furthermore, we have previously shown that multiple (up to four) microinjections into the cNTS do not adversely affect the respiratory response to a variety of agents (Mazzone & Geraghty, 1999; 2000a, 2000b). Similarly, baseline respiratory frequency is generally unaltered after 120 min and injection of 75 pmol RTX into the contralateral cNTS at this time point (or later) evokes profound respiratory responses (data not shown) suggesting that the attenuated response to is not an artefact of the experimental design.
Although there are limited reports on PPAHV in the literature, the superior potency of RTX for desensitization, compared with stimulation, of vanilloid receptor-expressing neurons is well documented (see Szallasi & Blumberg, 1999). Despite this, a stimulation-to-desensitization dose ratio as large as that observed in the present study, has yet to be reported. For example, ács et al. (1997) showed that RTX desensitized Ca2+ uptake in DRG neurons at only 15 fold lower concentrations than those required to stimulate Ca2+ uptake and the ratio for stimulation-to-desensitization by RTX of Ca2+ uptake by mast cells approaches unity (Bíró et al., 1998). Clearly, RTX desensitizes vanilloid receptors on neurons in the cNTS more readily than on DRG neurons and mast cells. The mechanism underlying the desensitization observed in the present study is unknown. We have previously shown that the respiratory depressant action of capsaicin in the NTS is mediated by tachykinin release, since prior microinjection of tachykinin NK2 and NK3 (but not NK1) receptor antagonists markedly reduces the effect of capsaicin (Mazzone & Geraghty, 1999). Thus, one would presume that RTX and PPAHV, likewise release tachykinins that subsequently act on post-synaptic neurokinin receptors to ultimately slow respiration. Since neurotransmitter release is dependent upon an influx of extracellular Ca2+, it is possible that desensitization of vanilloid receptors in the cNTS is due (at least in part) to impaired Ca2+ influx into the central terminals of tachykinin-containing neurons following exposure to RTX and related compounds. Nevertheless, Ca2+ independent mechanisms of VR1 desensitization have also been reported (Caterina et al., 1997).
Actions of olvanil and AEA in the cNTS – desensitization without stimulation
Olvanil (NE-19550), the long fatty acyl chain derivative of capsaicin, is an orally-active analgesic and anti-inflammatory agent which lacks the pungency of capsaicin (Brand et al., 1987). Consistent with this, in the present study olvanil failed to alter respiratory frequency at doses up to 10 nmol. With the exceptions of the rat vas deferens, where olvanil appears to be inactive (Wardle et al., 1996), and rat spinal cord, where olvanil is a partial agonist (Wardle et al., 1997), this compound behaves as a full vanilloid receptor agonist in all other systems tested. Although the affinity (or potency) of olvanil is generally comparable to that of capsaicin in vanilloid receptor binding assays and functional studies (Liu et al., 1997; Szallasi et al., 1999a; Jerman et al., 2000; Ralevic et al., 2001; Wahl et al., 2001), we cannot rule out the possibility that olvanil would reduce breathing frequency in the cNTS at higher doses.
The arachadonic acid derivative, AEA, was originally classified as the endogenous cannabinoid receptor agonist (Devane et al., 1992). Subsequent studies showed that AEA also activated vanilloid receptors in a variety of preparations, including those expressed on the peripheral terminals of somatic and visceral capsaicin-sensitive afferent nerves (Zygmunt et al., 1999; Ralevic et al., 2001; Sprague et al., 2001; Tucker et al., 2001). In contrast, our studies suggest that AEA does not activate vanilloid receptors in the cNTS, since microinjection of up to 14.5 nmol has no effect on respiration. However, again we cannot exclude the possibility that AEA might have effects at higher doses (>14.5 nmol). In support of this assertion, AEA has been shown to be a weak vanilloid receptor agonist in other systems. For example, the EC50s of capsaicin and AEA for inducing Ca2+ influx in HEK cells transfected with vanilloid receptors are 11 nM and 2 μM, respectively (Ralevic et al., 2001). Furthermore, Lin & Lee (2002) recently showed that AEA is approximately 1000 fold less potent than capsaicin at activating vanilloid receptors on the peripheral terminals of airway C-fibres in rats.
The lack of direct respiratory slowing in response to microinjection of olvanil and AEA suggests that these agents do not release tachykinins (or other neurotransmitters) from capsaicin-sensitive afferent terminals in the cNTS. This in itself is unusual since olvanil and AEA apparently stimulate neuropeptide (substance P and calcitonin gene-related peptide) release from both the central (spinal cord) and peripheral terminals of other capsaicin-sensitive afferents (Dickenson et al., 1990; Hughes et al., 1992; Wardle et al., 1997; Zygmunt et al., 1999; Tucker et al., 2001). However our studies suggest that olvanil and AEA are not devoid entirely of any interaction with vanilloid receptors in the cNTS, since both agents attenuated the respiratory response to subsequent injections of RTX in to this site. Thus, our data would suggest that olvanil and AEA both interact with the vanilloid receptor protein in the cNTS in a manner that does not activate the receptor, but is sufficient to desensitize or (less likely) block responses to other vanilloid receptor agonists. Although the mechanisms underlying the desensitizing effects of olvanil and AEA are unknown, these results suggest that these agents act as modulators of central vanilloid receptor activity.
In conclusion, these data show that like capsaicin, RTX and the phorboid RTX derivative, PPAHV, activate vanilloid receptors in the cNTS (which slows respiration) and that this population of vanilloid receptors is desensitized by a single exposure to low doses of either compound. In contrast, olvanil and AEA, are devoid of direct centrally-mediated respiratory depressant actions, although both agents are capable of desensitizing vanilloid receptors to RTX.
Acknowledgments
This study was supported by grants from the National Health and Medical Research Council of Australia and University of Tasmania. We thank Candace Carter for expert technical support.
Abbreviations
- AEA
arachidonyl ethanolamide
- cNTS
commissural nucleus of the solitary trace
- f
respiratory frequency
- PPAHV
phorbol 12-phenylacetete 13-acetate 20-homovanillate
- RTX
resinferatoxin
- VT
tidal volume
References
- ÁCS G., BÍRÓ T., ÁCS P., MODARRES A., BLUMBERG P.M. Differential activation and desensitisation of sensory neurons by resiniferatoxin. J. Neurosci. 1997;17:5622–5628. doi: 10.1523/JNEUROSCI.17-14-05622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APPENDINO G., CRAVATTO G., PALMISANO G., ANNUNZIATA R., SZALLASI A. Synthesis and evaluation of phorboid 20-homovanillates: discovery of a class of ligands binding to the vanilloid (capsaicin) receptor with different degrees of cooperativity. J. Med. Chem. 1996;39:3123–3131. doi: 10.1021/jm960063l. [DOI] [PubMed] [Google Scholar]
- APPENDINO G., SZALLASI A. Minireview. Euphorbium: modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997;60:681–696. doi: 10.1016/s0024-3205(96)00567-x. [DOI] [PubMed] [Google Scholar]
- BÍRÓ T., MAURER M., MODARRES S., LEWIN N.E., BRODIE C., ÁCS G., ÁCS P., PAUS R., BLUMBERG P.M. Characterisation of functional vanilloid receptors expressed by mast cells. Blood. 1998;91:1332–1340. [PubMed] [Google Scholar]
- BLUMBERG P.M., SZALLASI A., ÁCS G.Resiniferatoxin - an ultrapotent capsaicin analogue Capsaicin in the Study of Pai 1993London: Academic Press; 45–82.ed. Wood, N.N. pp [Google Scholar]
- BRAND L., BERMAN E., SCHWEN R., LOOMANS M., JANUSZ J., BOHNE R., MADDIN C., GARDNER J., LAHANN T., FARMER R., JONES L., CHIABRANDO C., FANELLI R. NE-19550: a novel, orally active anti-inflammatory analgesic. Drugs Exp. Clin. Res. 1987;13:259–265. [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER, MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DICKENSON A.H.C., HUGHES C., RUEFF A., DRAY A. A spinal mechanism of action is involved in the antinociception produced by the capsaicin analogue NE 19550 (olvanil) Pain. 1990;43:353–362. doi: 10.1016/0304-3959(90)90032-9. [DOI] [PubMed] [Google Scholar]
- DRAY A., URBAN L. New pharmacological strategies for pain relief. Ann. Rev. Pharmacol. Toxicol. 1996;36:253–280. doi: 10.1146/annurev.pa.36.040196.001345. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS C.D., VINCENT M.A., SZALLASI A., COLQUHOUN E.Q., GERAGHTY D.P. Functional and desensitising effects of the novel synthetic vanilloid-like agent 12-phenylactetae 13-acetate 20-homovanillate (PPAHV) in the perfused rat hindlimb. Br. J. Pharmacol. 2000;131:1408–1412. doi: 10.1038/sj.bjp.0703702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO A., VULCHANOVA L., WANG J., LI X., ELDE R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinceptor and IB4. Eur. J. Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- HO C.Y., GU Q., LIN Y.S., LEE L.Y. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir. Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- HUGHES S.R., BUCKLEY T.L., BRAIN S.D. Olvanil: more potent than capsaicin at stimulating the efferent function of sensory nerves. Eur. J. Pharmacol. 1992;219:481–484. doi: 10.1016/0014-2999(92)90494-o. [DOI] [PubMed] [Google Scholar]
- JERMAN J.C., BROUGH S.J., PRINJHA R., HARRIES M.H., DAVIS J.B., SMART D. Characterization using FLIPR of rat vanilloid receptor (rVR1) pharmacology. Br. J. Pharmacol. 2000;130:916–922. doi: 10.1038/sj.bjp.0703390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN D., SPYER K.M.Brainstem integration of cardiovascular and pulmonary afferent activity Progress in Brain Research 1986Vol. 67New York: Elsevier Science Publishers; 295–314.ed. Cervero, F. & Morrison, J.F.B. pp [DOI] [PubMed] [Google Scholar]
- KALIA M., MESULAM M.M. Brain stem projections of sensory and motor components of the vagus complex in the cat. II. Laryngeal, tracheobronchial, pulmonary, cardiac and gastrointestinal branches. J. Comp. Neurol. 1980;191:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- LEE L.Y., LUNDBERG J.M. Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anesthetized rats. J. Appl. Physiol. 1994;76:1848–1855. doi: 10.1152/jappl.1994.76.5.1848. [DOI] [PubMed] [Google Scholar]
- LIN Y.S., LEE L.Y. Stimulation of pulmonary vagal C-fibres by anandamide in anaesthetized rats: role of vanilloid type 1 receptors. J. Physiol. 2002;539:947–955. doi: 10.1113/jphysiol.2001.013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L., LO Y.-C., CHEN I.-J., SIMON S.A. The responses of rat trigeminal ganglion neurons to capsaicin and to nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. J. Neurosci. 1997;17:4101–4111. doi: 10.1523/JNEUROSCI.17-11-04101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L., SZALLASI A., SIMON S.A. A non-pungent resiniferatoxin analogue, phorbol 12-phenylacetate 13-actetate 20-homovanillate, reveals vanilloid receptor subtypes on rat trigemminal ganglion neurons. Neuroscience. 1998;84:569–581. doi: 10.1016/s0306-4522(97)00523-x. [DOI] [PubMed] [Google Scholar]
- LUKOVIC L., DEJONG W., DE WIED D. Cardiovascular effects of substance P and capsaicin microinjected into the nucleus tractus solitarii of the rat. Brain Res. 1987;422:312–318. doi: 10.1016/0006-8993(87)90938-3. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO S., TAKEDA M., SAIKI C., TAKAHASHI T., OJIMA K. Effects of vagal and carotid chemoreceptor afferents on the frequency and pattern of spontaneous augmented breaths in rabbits. Lung. 1997;175:175–186. doi: 10.1007/pl00007565. [DOI] [PubMed] [Google Scholar]
- MAZZONE S.B., GERAGHTY D.P. Respiratory action of capsaicin microinjected into the nucleus of the solitary tract: involvement of vanilloid and tachykinin receptors. Br. J. Pharmacol. 1999;127:473–478. doi: 10.1038/sj.bjp.0702522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZONE S.B., GERAGHTY D.P. Respiratory actions of tachykinins in the nucleus of the solitary tract: characterisation of receptors using selective agonists and antagonists. Br. J. Pharmacol. 2000a;129:1121–1131. doi: 10.1038/sj.bjp.0703172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZONE S.B., GERAGHTY D.P. Respiratory actions of tachykinins in the nucleus of the solitary tract: effect of neonatal capsaicin pretreatment. Br. J. Pharmacol. 2000b;129:1132–1139. doi: 10.1038/sj.bjp.0703173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZONE S.B., HINRICHSEN C.F., GERAGHTY D.P. Hypoxia attenuates the respiratory response to microinjection of substance P into the nucleus of the solitary tract of the rat. Neurosci. Lett. 1998;256:9–12. doi: 10.1016/s0304-3940(98)00743-5. [DOI] [PubMed] [Google Scholar]
- MEZEY E., TOTH Z.E., CORTRIGHT D.N., ARZUBI M.K., KRAUSE J.E., ELDE R., GUO A., BLUMBERG P.M., SZALLASI A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The rat brain in stereotaxic coordinates (2nd edition) Sydney: Academic Press Inc; 1986. [Google Scholar]
- RALEVIC V., KENDALL D.A., JERMAN J.C., MIDDLEMISS D.N., SMART D. Cannabinoid activation of recombinant and endogenous vanilloid receptors. Eur. J. Pharmacol. 2001;424:211–219. doi: 10.1016/s0014-2999(01)01153-0. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J., HARRISON C., ROWBOTTOM D.J., SMART D., LAMBERT D.G. Temperature-dependent activation of recombinant rat vanilloid receptor VR1 receptors expressed in HEK293 cells by capsaicin and anandamide. Eur. J. Pharmacol. 2001;423:121–125. doi: 10.1016/s0014-2999(01)01123-2. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid receptor: new insights enhance potential as a therapeutic target. Pain. 1996;68:195–208. doi: 10.1016/s0304-3959(96)03202-2. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M., ANNICELLI L.L., KRAUSE J.E., CORTRIGHT D.N. The cloned rat vanilloid receptor VR1 mediates both R-type binding and C-type calcium response in dorsal root ganglion neurons. Mol. Pharmacol. 1999a;56:581–587. doi: 10.1124/mol.56.3.581. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., NILSSON S., FARKAS-SZALLASI T., BLUMBERG P.M., HOKFELT T., LUNDBERG J.M. Vanilloid receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995;703:175–183. doi: 10.1016/0006-8993(95)01094-7. [DOI] [PubMed] [Google Scholar]
- SZOLCSÁNYI J.Actions of capsaicin on sensory receptors Capsiacin in the Study of Pain 1993London: Academic Press; 1–27.ed. Wood, J.N. pp [Google Scholar]
- TUCKER R.C., KAGAYA M., PAGE C.P., SPINA D. The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. Br. J. Pharmacol. 2001;132:1127–1135. doi: 10.1038/sj.bjp.0703906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHL P., FOGED C., TULLIN S., THOMSEN C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol. Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- WARDLE K.A., RANSON J., SANGER G.J. Pharmacological characterization of the vanilloid receptor in the rat dorsal spinal cord. Br. J. Pharmacol. 1997;121:1012–1016. doi: 10.1038/sj.bjp.0701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLE K.A., RANSON J., SANGER G.J. Pharmacological characterization of the vanilloid receptor in the rat isolated vas deferens. J. Pharm. Pharmacol. 1996;48:285–291. doi: 10.1111/j.2042-7158.1996.tb05918.x. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


