Abstract
Acute moderate hypoxia modifies the catalytic activity and expression of certain isoenzymes of hepatic cytochrome P450 (P450). The aim of this study was to document whether hypoxia affects hepatic P450 directly or through the release of serum mediators.
Rabbits were subjected to a FiO2 of 8% for 48 h, sacrificed, and serum and hepatocytes were isolated; hepatocytes from control and rabbits with hypoxia were incubated with serum from control and hypoxic rabbits for 4 and 24 h, and total P450 content, CYP1A1, 1A2 and 3A6 activities and expressions were assessed. Sera were fractionated by size exclusion chromatography and fractions tested for their ability to modify activity and amount of P450, and serum mediators were identified through neutralization experiments.
Total serum and fractions with proteins of 15–23 and 65–94 kDa of Mr reduced P450 content and expression of CYP1A1, 1A2 and 3A6, as well as CYP1A1, 1A2 and 3A6 mRNA. Total serum and the fraction with 32–44 kDa proteins increased CYP3A6 activity and protein and mRNA. The serum mediators implicated in the decrease in activity and expression of CYP1A1, 1A2 and 3A6 were interferon-γ (IFN-γ), interleukin-1β (IL-1β) and IL-2. Erythropoietin (Epo) was partly responsible for the increase in P450 content and CYP3A6 expression.
In conclusion, acute moderate hypoxia diminishes the activity and expression of CYP1A1, 1A2 and CYP1A1, 1A2 mRNA, and increases CYP3A6 protein, activity and CYP3A6 mRNA. Several mechanisms contribute to these changes in P450, among them the release of cytokines acting as serum mediators.
Keywords: Cytochrome P450, CYP1A1, CYP1A2, CYP3A6, hypoxia, inactivation, down-regulation, interferon-γ, interleukin-1β, interleukin-2, erythropoietin
Introduction
The clearance of theophylline can be decreased in patients with chronic obstructive lung disease, pulmonary oedema, pulmonary heart disease or congestive heart failure (Powell et al., 1978). Since patients with these disease states present episodes of acute hypoxia, the decrease in theophylline metabolic clearance has been ascribed to hypoxia (Richer & Lam, 1993). Early in vivo and in vitro studies supported that acute hypoxia reduces the activity of multiple biotransformation pathways (Jones, 1981). Modulation of cytochrome P450 (P450) by hypoxia was confirmed in vivo in animals with acute moderate hypoxia where the clearance of theophylline was reduced (Letarte & du Souich, 1984). Moreover, sub-chronic hypoxia also reduces the activity and expression of enzymes involved in the biotransformation of drugs (Shan et al., 1992). Supporting that in man hypoxia modulates P450, it has been shown that in patients with congestive heart failure, total hepatic P450 content is reduced, as well as the expression of CYP1A1 and 1A2 isoenzymes (Ng et al., 2000). Interestingly, in vivo, in rabbits subjected to acute hypoxia, the expression of CYP3A6 is enhanced (Kurdi et al., 1999).
In vivo, the effect of hypoxia on P450 resembles that elicited by an acute local inflammatory reaction, e.g. there is a decrease in activity and down-regulation of several P450 isoforms, with the exception that an inflammatory reaction also down-regulates CYP3A (Morgan, 1997). In rabbits with a turpentine-induced inflammatory reaction, interleukin-6 (IL-6) is the serum mediator responsible for the decrease in activity of P450 isoforms; IL-1β, tumour necrosis factor (TNF-α) and interferon-γ (IFN-γ) have a minor role (Bleau et al., 2000).
Hypoxia prompts the release of numerous cytokines, such as IL-1β, IL-2, IL-4, IL-5, IL-6, TNF-α and IFN-γ (Naldini et al., 1997), and erythropoietin (Epo) (Lacombe & Mayeux, 1999). We speculated that these cytokines might mediate the changes in hepatic P450 isoforms in animals subjected to acute moderate hypoxia. This hypothesis was supported by the fact that these cytokines can induce changes in the expression of P450 genes (Calleja et al., 1997; 1998).
The aims of the present study were to assess whether acute moderate hypoxia triggers the release of serum mediators eventually leading to changes in P450 content and activity. To document the presence of serum mediators, P450 content, activity and amount of CYP1A1, 1A2 and 3A6 apoproteins and genes were assessed following 4 and 24 h incubation periods of hepatocytes with sera from rabbits with acute moderate hypoxia. Mediators in sera were isolated by size-exclusion high performance liquid chromatography, and the potential effect of IL-2, IL-1β, IL-6, IFN-γ and Epo, on P450 isoforms was counterbalanced by direct neutralization with antibodies. In rabbits with moderate acute hypoxia, IFN-γ is the primary serum mediator responsible for the depression of CYP1A1 and 1A2 proteins and mRNA, and Epo is partly responsible for the up-regulation of CYP3A6 protein and gene.
Methods
Animals and hepatocyte collection
Male New Zealand White rabbits (1.8–2.2 Kg) were obtained from Ferme Charles Rivers (St-Constant, Quebec, Canada). Rabbits were maintained on Purina Laboratory Chow and water ad libitum for at least 7 days before any experimental work was undertaken. To induce the hypoxia, rabbits were introduced in a plexiglas chamber (0.75×1.20×1.25 m3) with a fractional concentration of inspired O2 (FiO2) of 8%, adjusted with an oxygen monitor (OM-15, Sensor Medics Corp., CA, U.S.A.) connected to an electrovalve (Asco Valves, Brantford, Ontario, Canada) that allowed the access of nitrogen into the chamber which displaced the air off. All the rabbits had access to Purina Laboratory Chow and water ad libitum for the 48 h that lasted the hypoxia. Control rabbits were also placed into the chamber for the experiments, but breathing room air (FiO2=21%). All the experiments were conducted according to the Canadian Council on Animal Care guidelines for use of laboratory animals.
Hepatocytes from rabbits with hypoxia (HHYPO) and from control rabbits (HCONT) were isolated 48 h after the induction of hypoxia or breathing room air, respectively, according to the two-step liver perfusion method of Seglen (1976) with minor modifications (El-Kadi et al., 1997). Briefly, rabbits were anaesthetized with sodium pentobarbitone (30 mg kg−1), a midline laparotomy was performed and the portal, the suprahepatic and inferior cava veins were cannulated. The liver was perfused via the portal vein with a solution containing (mM) NaCl 15, KCl 5, KH2PO4 1, HEPES 25, EGTA 0.5, glucose 5.5, and 56.8 mg ml−1 heparin with a peristaltic pump (Harvard Apparatus Co., Inc., U.S.A.), followed by a perfusion with a solution of 0.013% collagenase, 1 mM CaCl2 and 0.25 mM trypsin inhibitor. All solutions were maintained at 37°C and saturated with 100% O2. The liver was maintained wet with saline during the entire period of perfusion. Living cells were isolated on a 40% Percoll gradient. Viability was assessed by Trypan blue exclusion to ensure that it was greater than 90%; viability was not affected by hypoxia or any experimental condition. Cell concentration was adjusted to 4×106 ml−1 with William's medium E (WME) supplemented with 10% calf serum and 1 μM insulin. Aliquots of 2 ml of the hepatocytes in suspension were transferred into 6-well plastic culture plates (Falcon, Becton Dickinson Labware, Rutherford, NJ, U.S.A.) coated with type 1 rat tail collagen and incubated for 4 and 24 h at 37°C in an atmosphere of 95% O2 and 5% CO2. Cell cultures were always conducted under sterile conditions.
Rabbit serum preparation
Blood samples (10 ml) were withdrawn from the rabbits 48 h after the induction of hypoxia and from control rabbits in a sterile Vacutainer Brand SST (Becton Dickinson, Mississauga, ON, Canada). Blood samples were allowed to clot at room temperature for 2 h, thereafter were centrifuged at 2500 r.p.m. for 5 min, and the serum was decanted and stored frozen at −20°C in 1 ml aliquots until use. Preliminary studies have shown that when samples were handled as described, serum mediators conserved their activity for up to 12 months.
Fractionation of serum proteins
Serum proteins were separated by size exclusion high performance liquid chromatography (HPLC) on a Superose 12 HR column from Pharmacia Biotech (Baie d'Urfé, Quebec, Canada). Flow rate of the mobile phase was set at 0.3 ml min−1 and column pressure was maintained between 9–12 bar with a LKB 2150 HPLC pump (Bromma, Sweden). Absorbance was measured at 280 nm with a Waters 490E spectrophotometric detector (Millipore, Milford, MA, U.S.A.). The eluant buffer included (mM) NaCl 115, KCL 5, KH2PO4 1, HEPES 1, EGTA 25, and glucose 5.5; the pH of the solution was adjusted to 7.4 and filtered through a nylon mesh (pore size 0.22 μm). Serum aliquots of 300 μl were injected into the column and fractions of 1.2 ml were collected with a fraction collector (LKB 2211 Superrac). To calculate the relative molecular mass (Mr) of the serum proteins contained in each HPLC fraction, a calibration curve was established by injecting 300 μl of the buffer containing a mixture of six standard proteins (100 μg ml−1): L-glutamic dehydrogenase (55.6 kDa), aldolase (39.2 kDa), triosephosphate isomerase (26.6 kDa), trypsin inhibitor (26.6 kDa), cytochrome c (12.5 kDa) and aprotinin (6.5 kDa). The proteins isolated in the first fraction were assumed to have a Mr greater than 95 kDa, considering that the column sorts out proteins with a Mr lower than 95 kDa.
In order to increase inhibitory activity of the fractions collected, these were concentrated on Microsep 3K membranes (Pall Filtron, Northborough, MA, U.S.A.) which retain proteins greater than 3 kDa. Three millilitres of the fractions were added to the sample reservoir and centrifuged at 75,000×g to reduce the volume and hence concentrate serum fractions 1.25 times.
Experimental protocol
The efficiency of serum mediator(s) to modify hepatic P450 content and activity was characterized by incubating hepatocytes from control rabbits and from rabbits with hypoxia with 200 μl of total serum or 200 μl of the HPLC fractions for 4 and 24 h, and assessing total P450 content and activity. Controls included hepatocytes incubated for 4 and 24 h in absence of serum, and HCONT incubated for 24 h with HPLC fractions of serum from control rabbits (SCONT). Hepatic P450 content was measured spectrophotometrically in cell lysates as described by Omura & Sato (1964). The amount of proteins in hepatocytes was measured in cell lysates by the method of Lowry et al. (1951).
The effect of serum mediators on the activity of CYP1A1 and 1A2 was determined by measuring the ability of the hepatocytes to biotransform theophylline to 3-methylxanthine (3MX), 1-methyluric acid (1MU) and 1,3-dimethyluric acid (1,3DMU) (Kurdi et al., 1999). Theophylline was dissolved in serum-free WME, and 100 μl were added to each well and incubated for 4 and 24 h with the hepatocytes at a final concentration of 176 μM. At time zero, 350 μl of the supernatant were collected from each well (control sample). The remaining supernatant was collected following 4 and 24 h of incubation and frozen at −20°C until theophylline, 3MX, 1MU and 1,3DMU were assayed by HPLC (Kurdi et al., 1999). The effect of serum mediators on the activity of CYP3A6 was determined by measuring the ability of the hepatocytes to convert 3,4-difluorobenzyloxy)-5,5-dimethyl-4-(4-methylsulphonylphenyl)-(5H)-furan-2-one (DFB), a reported CYP3A4 probe in humans and rabbits to DFH, its fluorescent des-(difluoro)-benzyl metabolite (Chauret et al., 1999; unpublished observations). Incubations were performed according to a published procedure (Silva & Nicoll-Griffith, 2001). Briefly, 60 μM DFB was incubated with the hepatocytes for 15 min. An aliquot of the media was then transferred to a microtiter plate and quenched with an equal volume of acetonitrile containing 40% TRIS buffer (0.05 M). The fluorescence of the metabolite DFH was measured at excitation and emission wavelengths of 360 nm and 440 nm, respectively, using a fluorescent plate reader Wallac Victor2 1420 Multilabel Counter, and expressed in arbitrary units.
The information about the activity and expression of P450 isoenzymes in hepatocytes obtained after 4 h of incubation with saline, e.g. in absence of serum or its fractions, was assumed to reflect the repercussions of 48 h of in vivo hypoxia on hepatic P450.
Western blot analysis
Hepatocytes were washed, harvested in ice-cold PBS and centrifuged at 1500×g for 5 min. The pellet was resuspended in cold lysis buffer (mM) HEPES 10 pH 7.9, KCl 10, EDTA 0.1, EGTA 0.1, dithiothreitol 1, protease inhibitor mixture, and cells were allowed to swell on ice for 15 min, and vortexed for 30 s. For Western blot analysis, 50 μg of cell lysate were separated by SDS-polyacrylamide gel electrophoresis (7.5% polyacrylamide) (Smith, 1994). Separated proteins were electrophoretically transferred to a nitro-cellulose membrane using a semi-dry transfer process (Bio-Rad, Hercules, CA, U.S.A.). CYP1A1 and 1A2 were detected with a polyclonal anti-rabbit CYP1A1 (Oxford Biochemical Research, Oxford, MI, U.S.A.) diluted 1 : 100 in 5% nonfat milk in PBS/0.1% Tween 20 and visualized with an alkaline phosphatase conjugated secondary antibody using nitro blue tetrazolium as substrate (Kruger, 1994). CYP3A6 was detected with a monoclonal anti-rat CYP3A1 (Oxford Biochemical Research, Oxford, MI, U.S.A.) diluted 1 : 500 in 5% nonfat milk in PBS/0.1% Tween 20 using a secondary antibody conjugated with chemiluminescence reagent (horseradish peroxidase enzyme) and visualized by autoradiography (Thorpe et al., 1985). The assay was linear in the range of protein amounts assessed under the present experimental conditions. The intensities of the bands were measured with the software Un-Scan-It-Gel (Silk Scientific Inc., Orem, UT, U.S.A.) and are represented in arbitrary units.
Northern blot analysis of CYP1A1, 1A2 and 3A6 mRNAs
Following 4 or 24 h of incubation, liver cells were washed in 3 ml PBS and flash-frozen in liquid nitrogen. Samples were kept at −80°C until RNA extraction and quantification according to the method described by Leblond et al. (2001). Total RNA was isolated using 1 ml of TRIZOL Reagent (Life Technologies Inc.) per 5–10×106 cells. RNA concentration was measured spectrophotometrically at the absorbance of 260 nm (A260/280 ratio≈2). Total RNA samples were denatured by heating at 60°C for 10 min in buffer containing 30 mM 4-morpholinopropanesulphonic acid, 42% deionized formamide, and 8.5% formaldehyde. Thirty micrograms of RNA species were then separated by electrophoresis through a denaturing 1% agarose–1.7% formaldehyde gel submerged in 20 mM 4-morpholinopropanesulphonic acid, 8 mM sodium acetate and 1 mM EDTA buffer, pH 7.2. Isolated RNA was transferred to a nylon membrane (Qiabrane, Qiagen) by capillary blotting with a solution of 1.2 M NaCl and 0.15 M sodium citrate, pH 7.0. RNA was fixed to the membrane by exposure to UV light. Membranes were prehybridized for 2 h at 52°C with 250 μg ml−1 denatured salmon sperm DNA, 1% dextran sulphate, 1% bovine serum albumin, 1 mM EDTA, 7% SDS, and 0.5 M NaPO4 (pH 7.2). The cDNA probes (rabbit CYP1A1, 1A2, 3A6 and rat 18S) were labelled with [α-32P]dCTP (3000 Ci mmol−1; Amersham Pharmacia Biotech) using Klenow fragment according to the oligo-priming method of the Oligolabelling kit (Amersham Pharmacia Biotech). Hybridization was performed at 52°C for 24 h with the radiolabelled cDNA probe in the prehybridization buffer. Blots were washed at 65°C for 10 min with a solution containing 3 M NaCl, 0.2 M NaH2PO4.H2O, 0.02 M EDTA, pH 7.4 and 0.1% SDS. Membranes were exposed to an autoradiography film Biomax by means of Biomax TranScreen-HE intensifying screens (Kodak) at −80°C during 24 h. The intensities of the bands were measured with the software Un-Scan-It-Gel and are represented in arbitrary units. The assay is linear in the range of protein amounts assessed under the present experimental conditions.
There are two forms of CYP3A6 mRNA expressed in the liver, one with 1.85-kb and the other with 1.7-kb. Even if these forms differ by the length of their 3′ untranslated region, they originate the same protein (Dalet et al., 1988). Both forms can be separated and quantified simultaneously.
Immuno-neutralisation of cytokines
The selection of the antibodies used for the immuno-neutralization of cytokines was based (a) upon the Mr of the proteins incorporated in the HPLC fractions having the ability to change P450 content and activity, and (b) according to the kind of cytokines hypoxia releases (Naldini et al., 1997). To prevent the changes in P450 content and activity induced by the HPLC fractions, the following antibodies were used: a goat anti-rabbit IL-1β (anti-IL-1β) polyclonal antibody, and an anti-human IL-2 (anti-IL2), an anti-human IFN-γ (anti-IFN-γ), an anti-human IL-6 (anti-IL-6), and an anti-human Epo (anti-Epo) monoclonal antibodies. The antibodies against human proteins were used to neutralize the homologous rabbit proteins because of the known inter-species reactivity of these antibodies (Huang et al., 1997; Muscettola et al., 1995). An irrelevant monoclonal antibody (IgG to Pseudomona aeruginosa) served as control. Aliquots of 2 μg of each antibody were added individually to 200 μl of the HPLC fractions having the ability to change P450 content and activity, and were incubated at 37°C for 1 h. The antibody and the HPLC fractions were added to the hepatocytes at the beginning of the 4 and 24 h periods of incubations and activity and content of P450 was assessed. The amount of antibodies used, e.g. 2 μg, was selected because it was proven that 2 μg were effective to immuno-neutralize IL-1β, IL-6, and IFN-γ in the sera of rabbits and of humans with an inflammatory reaction (Bleau et al., 2000). In the present study, 2 and 4 μg of anti-IL-6 antibody were used.
Drugs and chemicals
Percoll gradient, William's medium E, calf serum, type I rat tail collagen, NaCl, KCl, KH2PO4, HEPES, EGTA, glucose, theophylline, 3MX, 1MU and 1,3DMU were purchased from Sigma Chemicals (Sigma, St. Louis, MO, U.S.A.). DFB and DFH were provided by Merck Frosst Canada (Kirkland, Québec, Canada). Insulin was acquired from Boehringer Mannheim Biochemica (Mannheim, Germany), and L-glutamic dehydrogenase, aldolase triosephosphate isomerase, trypsin inhibitor, cytochrome c, and aprotinin from Pharmacia Biotech (Baie d'Urfé, QC, Canada). The polyclonal anti-rabbit CYP1A1 and the monoclonal anti-rat CYP3A1 antibodies were purchased from Oxford Biochemical Research (Oxford, MI, U.S.A.), the anti-IL-1β antibody from Cedar Lane (Hornby, ON, Canada), the anti-IL-2, anti-IL-6, and anti-IFN-γ antibodies from R&D Systems (Minneapolis, MN, U.S.A.). Finally, anti-Epo antibody was purchased from Genzyme Diagnostics (Cambridge, MA, U.S.A.). Specific cDNA probes for rabbit CYP1A1, 1A2 and 3A6 were kindly provided by Prof P. Maurel (INSERM U128, Montpelier, France) and rat 18S by Dr V. Pichette (Hôpital Maisonneuve-Rosemont, Montréal, Canada).
Statistical analysis
All results are presented as mean±s.e.mean. The comparison of the results from the various experimental groups and their corresponding controls was carried out by a one-way analysis of variance (ANOVA), followed by the Newman-Keuls post hoc test. Differences were considered significant when P<0.05.
Results
Effect of hypoxia in vivo on hepatic P450 content, activity and amount of P450 isoforms
Exposure of rabbits (n=7) to an 8% FiO2 generated a stable hypoxemia, with an average PaO2 of 34.2±1.3 mmHg, without influencing the PaCO2 (≈20.7±1.0) and arterial pH (7.47±0.05). Compared with HCONT, 48 h of hypoxia in vivo reduced total P450 content by 45% and decreased 3MX, 1MU and 1,3DMU output by 58, 42 and 33% (P<0.05), respectively (Table 1).
Table 1.
Effect of 48 h hypoxia in vivo and of the incubation of control hepatocytes with serum of control and hypoxic rabbits for 4 and 24 h on P450 content and metabolism theophylline
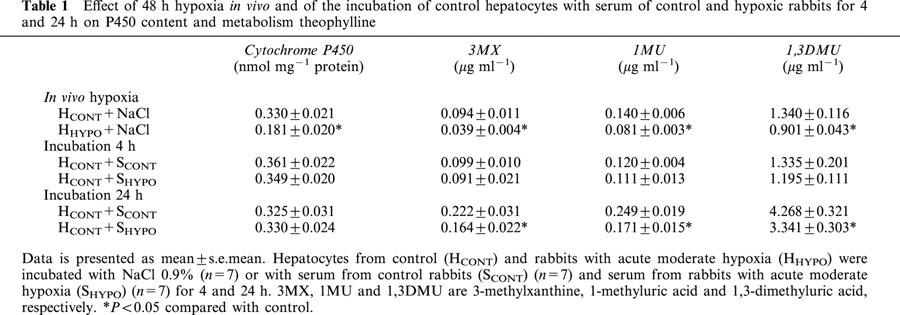
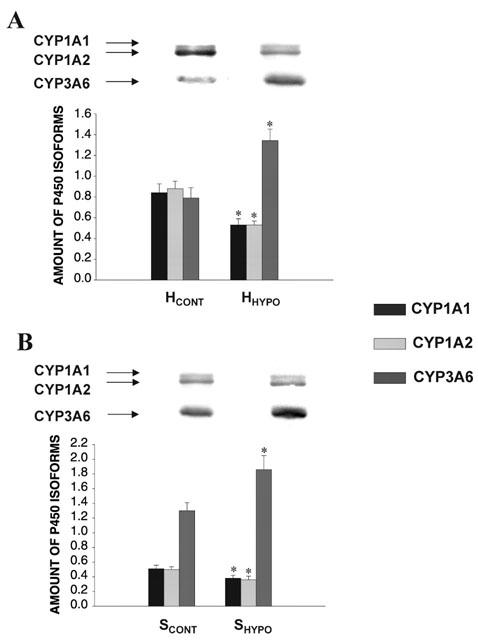
Compared with rabbits (n=7) breathing a 21% FiO2, e.g. room air, hypoxia reduced the amount of CYP1A and 1A2 proteins by 37 and 40%, respectively (Figure 1A). In parallel, CYP1A and 1A2 mRNAs were decreased in animals exposed to 8% FiO2 (Figure 2A). Forty-eight hours of hypoxia increased the amount of CYP3A6 by 70% (Figure 1A) as well as the two forms of CYP3A6 mRNA (Figure 2A). This increase in CYP3A6 expression resulted in the enhancement of CYP3A6 activity, that is the formation of the fluorescent metabolite DFH was ≈76% higher (P<0.05) in HHYPO than in HCONT, e.g. 11627±288 and 6606±245.
Figure 1.
Effect (A) of 48 h acute moderate hypoxia in vivo on the amount of CYP1A1, 1A2 and 3A6 apoproteins in hepatocytes (n=7), and (B) of serum from rabbits with acute moderate hypoxia (SHYPO) (n=7) on the amount of CYP1A1, 1A2 and 3A6 apoproteins incubated for 24 h with hepatocytes from rabbits with acute moderate hypoxia (HHYPO). Bands are representative Western blots of CYP1A1, 1A2 and 3A6. Data is presented in arbitrary units as mean±s.e.mean. *P<0.05 compared with control.
Figure 2.
Effect (A) of in vivo 48 h acute moderate hypoxia on the amount of CYP1A1, 1A2 and 3A6 mRNA in hepatocytes of control rabbits (HCONT) and rabbits subjected to hypoxia (HHYPO); (B) effect of serum from control rabbits (SCONT) and rabbits with acute moderate hypoxia (SHYPO) on the amount of CYP1A1, 1A2 and 3A6 mRNA incubated for 24 h with hepatocytes from control rabbits (HCONT); and (C) effect of SCONT and SHYPO on the amount of CYP1A1, 1A2 and 3A6 mRNA following 24 h incubation with hepatocytes from rabbits with hypoxia (HHYPO).
Effect of SCONT and serum of rabbits with hypoxia (SHYPO) on HCONT following 4 and 24 h of incubation
Incubation for 4 h of HCONT with SCONT (n=7) and SHYPO (n=7) did not modify P450 content, theophylline biotransformation (Table 1) or the amount of CYP1A1, 1A2 and 3A6 proteins (data not shown).
Compared with HCONT incubated with SCONT, 24 h of incubation of HCONT with SHYPO (n=7), reduced the output of 3MX, 1MU and 1,3DMU by 26, 31 and 21% (P<0.05), respectively, increased the amount of CYP3A6 by 28% (data not shown) and the formation of DFH by 29% (P<0.05), e.g. 4842±191 for SCONT and 6246±202 for SHYPO. No changes in P450 content (Table 1) or amount of CYP1A1, 1A2 proteins (data not shown) were observed. However, SHYPO diminished (P<0.05) the amount of CYP1A1 mRNA by 20%, e.g. 0.531±0.027 with SCONT and 0.425±0.014 with SHYPO, and that of CYP1A2 mRNA by 24%, e.g. 0.424±0.041 with SCONT and 0.322±0.014 with SHYPO. On the other hand, SHYPO increased the amount of CYP3A6 mRNA by 49%, e.g. 0.120±0.020 with SCONT and 0.179±0.006 with SHYPO (P<0.05, n=4; Figure 2B).
Effect of SCONT and SHYPO on HHYPO following 4 and 24 h of incubation
Compared with SCONT (n=18), 4 h of incubation of SHYPO (n=18) with HHYPO did not modify P450 content or amount of CYP1A1, 1A2 and 3A6 proteins. However, SHYPO reduced the output of 3MX, 1MU and 1,3DMU by 31, 29 and 37%, respectively (P<0.05) (Table 2).
Table 2.
Effect of incubation of hepatocytes from hypoxic rabbits with serum from control and hypoxic rabbits for 4 and 24 h on P450 content and metabolism of theophylline
Following 24 h incubation, SHYPO (n=18) did not affect P450 content in HHYPO, but reduced the output of 3MX, 1MU and 1,3DMU (P<0.05) (Table 2). In addition, SHYPO reduced the amount of CYP1A1 and 1A2 proteins by an average of 25 and 28%, respectively (P<0.05), and increased the expression of CYP3A6 by an average of 43% (P<0.05) (Figure 1B). However, SHYPO did not diminish the amounts of CYP1A1 and 1A2 mRNA, but increased the amount of CYP3A6 mRNA by 59%, e.g. 0.358±0.045 with SCONT and 0.570±0.016 with SHYPO (P<0.05, n=4; Figure 2C). The activity of CYP3A6 was increased by 30% (P<0.05) e.g. 8702±299 with SCONT and 11312±268 with SHYPO.
Effect of HPLC fractions of SHYPO on P450 content, activity and isoforms of HHYPO following 4 and 24 h of incubation
Serum fractions of SCONT when incubated for 4 h with HCONT did not modify the amount and activity of total P450 (data not shown). Incubation of the HPLC fractions of SHYPO (n=12) with HHYPO for 4 h did not modify P450 content. However, the amount of theophylline metabolites generated by HHYPO was decreased by the fractions containing proteins with a Mr of 15–23 kDa and of 65–94 kDa (Figure 3), despite that these two fractions did not affect the amount of CYP1A1, 1A2 and 3A6 proteins.
Figure 3.
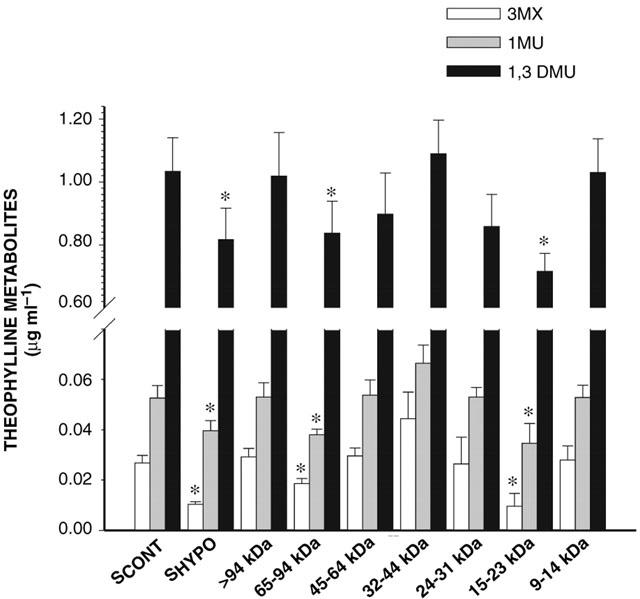
Effect of serum of control rabbits (SCONT) (n=10), serum from rabbits with acute moderate hypoxia (SHYPO) (n=12), and HPLC fractions of SHYPO (n=12) incubated for 4 h with hepatocytes from rabbits with acute moderate hypoxia on the formation of theophylline metabolites. Data is presented as mean±s.e.mean. *P<0.05 compared with SCONT.
The serum fractions of SCONT incubated for 24 h with HCONT did not modify the expression and activity of CYP1A1, 1A2 and 3A6 (data not shown). Incubation of the HPLC fractions of SHYPO (n=12) with HHYPO for 24 h shows that the 15–23 kDa fraction decreased P450 content by 38% (P<0.05), while the 32–44 kDa fraction increased P450 content by 33% (P<0.05) (Figure 4). The 15–23 kDa and 65–94 kDa HPLC fractions reduced the output of 3MX, 1MU and 1,3DMU (P<0.05) (Figure 5). In addition, the 15–23 kDa fraction (n=7) reduced the amount of CYP1A1 and 1A2 proteins by 42 and 30%, respectively (P<0.05), and the amount of CYP3A6 by 21% (Figure 6). On the other hand, the amount of CYP3A6 protein was increased by 51% (P<0.05) by the 32–44 kDa fraction (n=7) (Figure 6).
Figure 4.
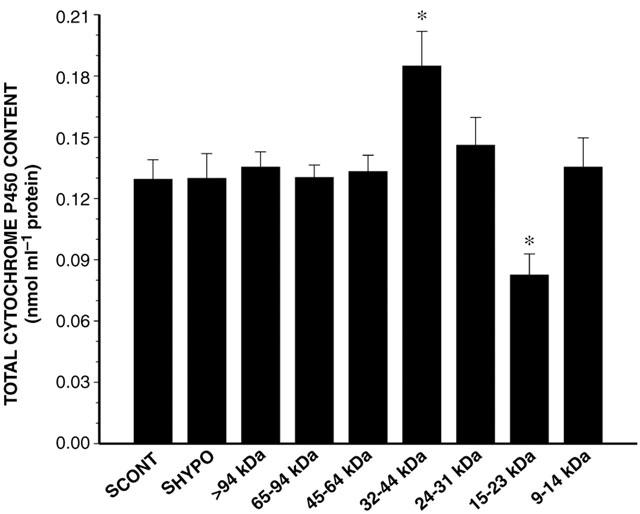
Effect of serum of control rabbits (SCONT) (n=10), serum from rabbits with acute moderate hypoxia (SHYPO) (n=12), and HPLC fractions of SHYPO (n=12) incubated for 24 h with hepatocytes from rabbits with acute moderate hypoxia on cytochrome P450 content. Data is presented as mean±s.e.mean. *P<0.05 compared with SCONT.
Figure 5.
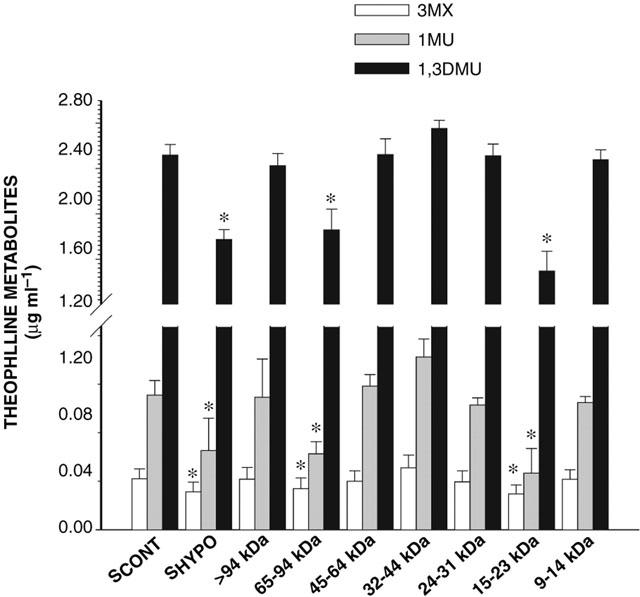
Effect of serum of control rabbits (SCONT) (n=10), serum from rabbits with acute moderate hypoxia (SHYPO) (n=7), and HPLC fractions of SHYPO (n=12) incubated for 24 h with hepatocytes from rabbits with acute moderate hypoxia on the formation of theophylline metabolites. Data is presented as mean±s.e.mean. *P<0.05 compared with SCONT.
Figure 6.
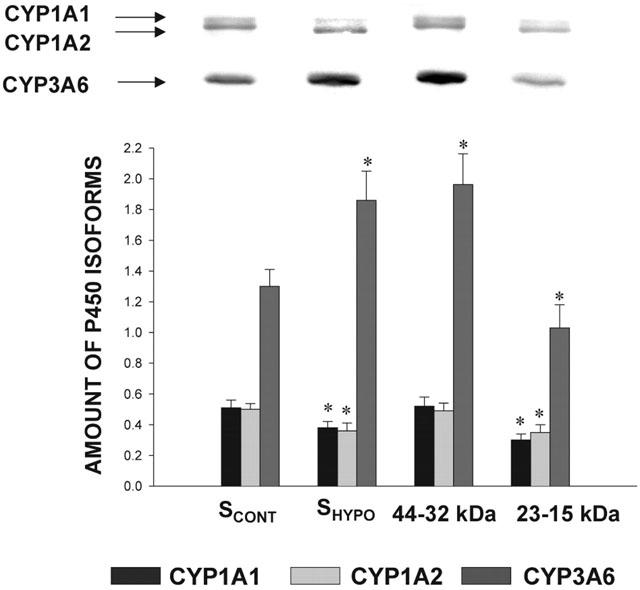
Effect of serum of control rabbits (SCONT) (n=10), serum from rabbits with acute moderate hypoxia (SHYPO) (n=7), and HPLC fractions of SHYPO (n=7) on the amount of CYP1A1, 1A2 and 3A6 apoproteins in hepatocytes from rabbits with acute moderate hypoxia (HHYPO) following 24 h incubation. Bands are representative Western blots of CYP1A1, 1A2 and 3A6. Data is presented in arbitrary units as mean±s.e.mean. *P<0.05 compared with control.
Identification of the mediators in SHYPO responsible for the changes in the amount and activity of P450
Compared with the biotransformation of theophylline by HHYPO in presence of SCONT, incubation of HHYPO for 4 h with the 15–23 kDa and 65–94 kDa fractions of SHYPO in presence of anti-IFN-γ antibody (n=6), completely abrogated the decrease of theophylline metabolism elicited by these fractions (Figure 7). On the other hand, anti-IL-1β and anti-IL-2 antibodies (n=6) only partially reverted the decrease in theophylline metabolism elicited by the 15–23 kDa and 65–94 kDa fractions. The presence of anti-IL-6 antibody (n=6) did not prevent the effect of these two HPLC fractions on the output of 3MX, 1MU and 1,3DMU (Figure 7).
Figure 7.
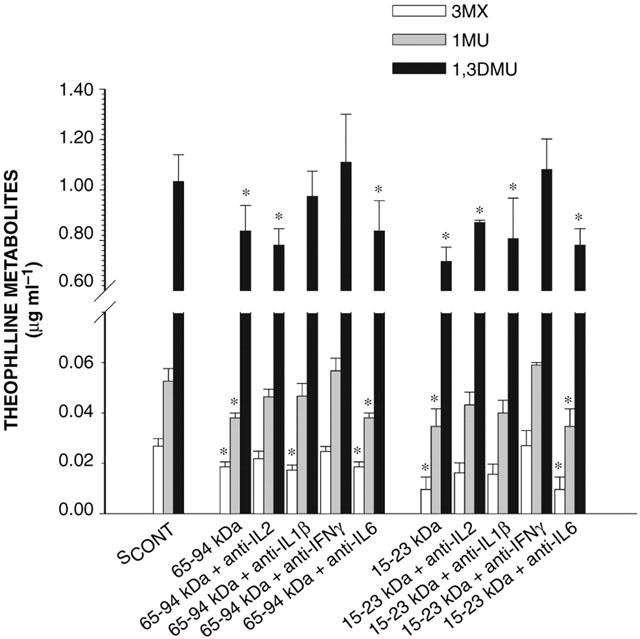
Effect of anti-cytokine antibodies on the ability of the 65–94 kDa and 15–23 kDa serum fractions to modify the formation of theophylline metabolites incubated for 4 h with hepatocytes from rabbits with acute moderate hypoxia. SCONT is serum from control rabbits; IL-2, IL-1β, IL-6 and IFN-γ are interleukin-2, -1β and interferon-γ, respectively. Data is presented as mean±s.e.mean. *P<0.05 compared with control.
Following 24 h incubation of HHYPO with the 15–23 kDa fraction of SHYPO, P450 content was reduced by 38% (n=12), and the addition of anti-IL-2, anti-IL-1β and anti-IFN-γ antibodies (n=6) to the 15–23 kDa fraction incremented P450 content to 84, 80 and 92% of control values, respectively (P<0.05) (Figure 8). Anti-IFN-γ, -IL-1β, -IL-2 and -IL-6 antibodies added to the 15–23 kDa and 65–94 kDa fractions elicited a variable effect on the output of 3MX, 1MU and 1,3DMU (Figure 9). Anti-IFN-γ antibody (n=6) prevented the effect of the two HPLC fractions, whereas anti-IL-2 (n=6) and anti-IL-1β antibodies (n=6) only partially, and anti-IL-6 antibody (n=6) did not elicit any effect, despite that the amount of antibody was increased to 4 μg. The addition of anti-IL-2, anti-IL-1β and anti-IFN-γ antibodies (n=6) to the 15–23 kDa fraction augmented the amount of CYP1A1 protein to 84, 80 and 90% of control values; only anti-IFN-γ antibody (n=6) increased CYP1A2 protein to 89% of control values (Figure 10).
Figure 8.
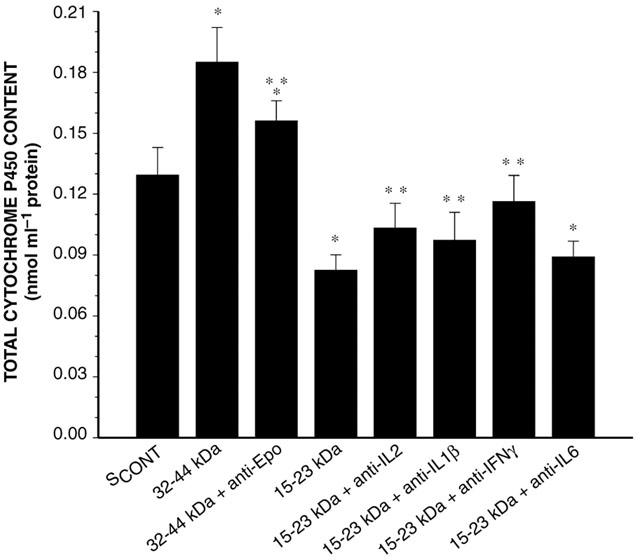
Effect of anti-cytokine antibodies on the ability of the 32–44 kDa and 15–23 kDa serum fractions to modify P450 content incubated for 24 h with hepatocytes from rabbits with acute moderate hypoxia. SCONT is serum from control rabbits; Epo is erythropoietin; IL-2, IL-1β, IL-6 and IFN-γ are interleukin-2, -1β, -6 and interferon-γ, respectively. Data is presented as mean±s.e.mean. *P<0.05 compared with control; **P<0.05 compared with the serum fraction without the antibody.
Figure 9.
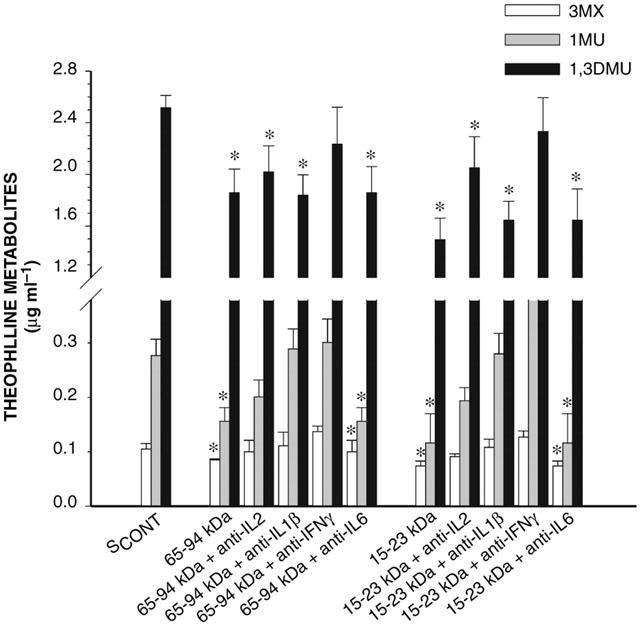
Effect of anti-cytokine antibodies on the ability of the 65–94 kDa and 15–23 kDa serum fractions to modify the formation of theophylline metabolites incubated for 24 h with hepatocytes from rabbits with acute moderate hypoxia. SCONT is serum from control rabbits; IL-2, IL-1β, IL-6 and IFN-γ are interleukin-2, -1β, -6 and interferon-γ, respectively. Data is presented as mean±s.e.mean. *P<0.05 compared with control.
Figure 10.
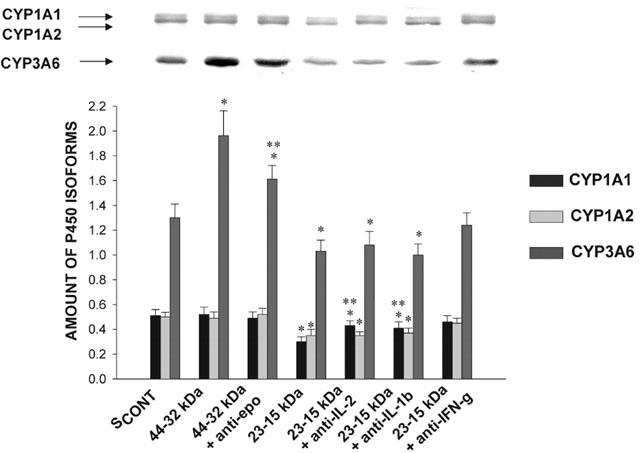
Effect of anti-cytokine antibodies on the ability of 32–44 kDa and 15–23 kDa serum fractions to modify the amount of CYP1A1, 1A2 and 3A6 apoproteins incubated for 24 h with hepatocytes from rabbits with acute moderate hypoxia. SCONT is serum from control rabbits; Epo is erythropoietin; IL-2, IL-1β, IL-6 and IFN-γ are interleukin-2, -1β, -6 and interferon-γ, respectively. Bands are representative Western blots of CYP1A1, 1A2 and 3A6. Data is presented in arbitrary units as mean±s.e.mean. *P<0.05 compared with control; **P<0.05 compared with the serum fraction without the antibody.
The 32–44 kDa fraction of SHYPO (n=12) increased P450 content by 33% (P<0.05) after 24 h of incubation with HHYPO. The addition of anti-Epo antibody (n=6) to the 32–44 kDa fraction reduced the increase in P450 content (P<0.05), however, was unable to normalize it, e.g. it was still 14% greater than control (P<0.05) (Figure 8). On the other hand, compared to the effect of the 32–44 kDa fraction on CYP3A6 (50% increase), the addition of anti-Epo antibody (n=6) to the 32–44 kDa fraction reduced the increase in amount of CYP3A6 protein to 25% (Figure 10).
Discussion
In the liver, cytochrome P450 is located primarily in zone 3 or perivenous zone, where under control conditions the concentration of oxygen is the lowest (Oinonen & Lindros, 1998; Jungermann & Kietzmann, 2000). From zone 1 or periportal to the perivenous zone, the concentration of the signal oxygen falls from about 13 (arterial) to nine (mixed periportal) and then to four (hepatic venous) volume per cent gas atmosphere (Jungermann & Kietzmann, 1997). Acute moderate hypoxia depresses enzymatic activity in both periportal, such as phosphoenolpyruvate carboxykinase (Jungermann & Kietzmann, 2000) and glutathione peroxidase (Proulx & du Souich, 1995), and perivenous zone, such as CYP1A1 and 1A2 and glucuronoconjugation (Jones, 1981). This is consistent with the fact that acute anoxia produces injuries which can be more extensive in the periportal zone than in the perivenous zone (Brass et al., 1992). Cultured hepatocytes harvested from the entire liver without taking into account zonation should not distort the observations but could dilute the effect of hypoxia on CYP1A1, 1A2 and 3A6.
The present study demonstrates that in vivo, acute moderate hypoxia reduces total P450 content and activity, as well as the expression of CYP1A1 and CYP1A2, and up-regulates the expression and activity of CYP3A6. Incubation of SHYPO with HHYPO for 4 h diminishes the formation of theophylline metabolites without affecting total P450 content or amount of CYP1A1, 1A2 and 3A6 proteins. Moreover, incubation of SHYPO with HHYPO for 24 h reduces theophylline metabolites output, as well as the expression of CYP1A1 and 1A2, but increases that of CYP3A6 and its activity. On the other hand, incubation of SHYPO with HCONT for 4 h does not affect any of the parameters estimated, but after 24 h, reduces the output of theophylline metabolites and increases the activity of CYP3A6. These results indicate that (1) incubation of SHYPO with HHYPO for 24 h portrays the effect of hypoxia in vivo, supporting that SHYPO does contain serum mediators; (2) 4 h incubations demonstrate that the down-regulation of CYP1A1 and 1A2 is preceded by a decrease in their activity; and (3) the effect of hypoxia in vivo is not recreated by incubating SHYPO with HCONT, suggesting that in vivo the effect of hypoxia results from an increase in circulating serum mediators and from changes in the hepatocyte.
The HPLC fractions with proteins of 15–23 kDa and 65–94 kDa Mr down-regulate CYP1A1, 1A2 and 3A6; although, in both fractions, the mediators contributing to the decrease in the expression of these isoforms are IFN-γ, IL-1β and IL-2. The efficiency and specificity of these cytokines to down-regulate CYP1A1, 1A2 and 3A6 differ. IFN-γ is not only the most potent, but also the only to down-regulate CYP1A2 and CYP3A6, whereas CYP1A1 is down-regulated by all three cytokines. These differences in efficiency and specificity explain why anti-IFN-γ antibody prevents the decrease in theophylline biotransformation, since theophylline is primarily metabolized by CYP1A2. IL-1β and IL-2 diminish theophylline metabolism because they reduce the expression of CYP1A1. Actually, individual antibodies do not prevent totally the down-regulation of CYP1A1, 1A2 and 3A6, suggesting that the effect of the serum mediators results from concerted action and/or cross-talk of several cytokines. Further supporting a concerted action of several cytokines is the fact that in vitro experiments where individual cytokines are incubated with hepatocytes from normal animals or humans, show that IL-1β has the greatest ability to depress CYP1A1 and 1A2 isoforms (Abdel-Razzak et al., 1993), and that IL-2 and IL-1β depress CYP3A isoforms (Calleja et al., 1998). These results differ from ours and may be explained by the presence of several mediators in the serum, by differences in the concentrations of the mediators, and by differences in the experimental model, e.g. control hepatocytes or hepatocytes from animals subjected to the hypoxic stress.
The fact that anti-IFN-γ, -IL-1β and -IL-2 antibodies prevented the effect of the 65–94 kDa fraction on P450 may be explained by the binding of these cytokines to plasma proteins. Effectively, IFN-γ binds to soluble receptors with Mr ranging from 45 to 67 kDa (Bello et al., 1998); IL-1β circulates in plasma bound to the IL-1 receptor accessory protein with a Mr of approximately 66 kDa (Greenfeder et al., 1995); and IL-2 can bind to a soluble receptor with a Mr of 67 kDa (Jacques et al., 1990). Moreover, in the case of IFN-γ, selected activities are elicited by the tetramer of 72 kDa (Langer et al., 1994).
Several mechanisms may have contributed to down-regulate CYP1A1 and 1A2 under hypoxic conditions. On one hand, the fact that the expression of CYP1A1/1A2 apoproteins and mRNAs decreased, supports the possibility that the effect of IFN-γ, IL-1β and IL-2 implicates a pre-translational mechanism (Morgan, 1997). On the other hand, hypoxia modifies the expression of multiple genes to improve blood delivery and cellular metabolism (Bunn & Poyton, 1996), effect in part regulated by hypoxia-inducible factor 1 transcriptional activator (HIF-1) (Semenza, 1999). HIF-1 is a basic heterodimer, with a HIF-1α subunit, unique to HIF-1, and a HIF-1β or aryl hydrocarbon receptor nuclear translocator (Arnt) subunit. HIF-1β/Arnt dimerizes also with the aryl hydrocarbon receptor (AhR) (Park, 1999). Under hypoxic conditions, HIF-1β dimerizes preferentially with HIF-1α, in such a way that less HIF-1β is available for recruitment with AhR as a heterodimeric partner. Since in adult rabbits, AhR is constitutively activated, and CYP1A1 and CYP1A2 genes are expressed constitutively (Strom et al., 1992), we may speculate that activation of HIF-1 by hypoxia and by SHYPO reduces AhR/Arnt heterodimer and CYP1A expression (Park, 1999). This explanation may apply to HCONT where SHYPO decreased CYP1A1 and 1A2 mRNA. However, SHYPO did not reduce CYP1A1 and 1A2 mRNA further in HHYPO, suggesting that the decrease in CYP1A1 and 1A2 proteins is secondary to a post-transcriptional effect. The reason why SHYPO reduced CYP1A1 and 1A2 mRNA in HCONT but not in HHYPO remains unknown.
It is not clear how SHYPO reduces the activity of CYP1A1/1A2 without affecting their amount in HCONT after 4 and 24 h of incubation, and in HHYPO after 4 h of incubation. We may postulate that the decrease in activity is associated with the presence of reactive oxygen intermediates (ROI). Hypoxia induces a time-dependent decrease in reduced glutathione and other cellular antioxidants, and an increase in lipid peroxidation in the liver (Proulx & du Souich, 1995), probably as a result of the formation hydrogen peroxide (H2O2) (Weissmann et al., 2000) and nitric oxide (NO•) (Gess et al., 1997). There is evidence that H2O2 and NO• can inactivate P450 apoproteins (Karuzina & Archakov, 1994; Takemura et al., 1999). Indirect evidence supporting a role for ROI in P450 decrease in activity, is the fact that L-NAME, N-acetylcysteine and dimethylthiourea dose-dependently prevent the decrease in CYP1A1/1A2 activity induced by IL-6, INF-γ and IL-1β (El-Kadi et al., 2000).
Hypoxia in vivo and incubation of SHYPO with HHYPO in vitro increase the expression of CYP3A6 and CYP3A6 mRNA. The expression of CYP3A4 is modulated by nuclear receptors, such as constitutive androstane receptor (CAR), pregnane X receptor (PXR), retinoid X receptor (RXR), hepatocyte nuclear factor 4 (HNF-4), and glucocorticoid receptor (GR) (El-Sankary et al., 2000). Assuming that CYP3A6 is modulated as CYP3A4, since hypoxia activates HIF-1 in cooperation with HNF-4, RXR, and GR (Bunn et al., 1998; Machein et al., 1999; Kambe et al., 2000), we may speculate that these proteins are implicated in the increase in CYP3A6 gene and CYP3A6 apoprotein. The increase in expression of CYP3A6 by the 32–44 kDa fraction is partially reversed by anti-Epo antibodies. There is no data supporting that Epo modulates directly the expression of CYP3A. On the other hand, Epo binding to its membrane receptor activates phosphatidylinositol 3-kinase (PI3-K) (Gaffen et al., 1999; Lacombe & Mayeux, 1999), which is required for HIF-1 stabilization and translocation (Mazure et al., 1997). We postulate that Epo facilitates HIF-1 cooperation with nuclear receptors to enhance CYP3A6 expression.
The increase in CYP3A6 induced by SHYPO was greater in HHYPO than in HCONT, suggesting that serum mediators elicit a greater effect in primed hepatocytes. Several mechanisms, not mutually exclusive, are plausible to prime the hepatocyte: (a) hypoxia increases the expression of the nuclear factors RXR, HNF-4, and GR (Machein et al., 1999; Kambe et al., 2000); (b) hypoxia modulates protein tyrosine kinase (Uchiyama et al., 2000), AMP-activated protein kinase Blazquez et al., 1999), and protein kinase C (Hsu & Huang, 1998) activities, known to regulate the expression of CYP3A (Brown et al., 1997); (c) hypoxia activates PI-3K required for HIF-1 stabilization and translocation (Mazure et al., 1997); and (d) hypoxia increases plasma levels of cytokines which enhance the density of cytokines surface receptors (Simms & D'amico, 1996; Takabatake et al., 2000). Any of these mechanisms could contribute to increase responsiveness of HHYPO by comparison with HCONT.
In conclusion, the present study demonstrates that acute moderate hypoxia modifies the expression of hepatic P450 isoforms by several mechanisms, including serum mediators and intracellular adaptation to hypoxia. Hypoxia induces the release of serum mediators, among them IFN-γ, IL-1β and IL-2, that reduce initially the activity and thereafter decrease the expression of CYP1A1 and 1A2 and of CYP1A1 and 1A2 genes. In addition, acute moderate hypoxia increases the expression of CYP3A6 protein and CYP3A6 gene, effect partially mediated by circulating Epo. The pathophysiological role of CYP3A6 induction is unknown. Intracellular adaptation to hypoxia is evidenced by the fact that the combined effect of all these mediators reproduce the effects observed in vivo when they are incubated with HHYPO but not with HCONT.
Acknowledgments
Supported by a grant from the Canadian Institutes of Health Research (MOP-43925). The technical assistance of Mrs Lucie Héroux is gratefully acknowledged. We are grateful to Dr François Leblond for his assistance in the measure of CYP mRNA.
Abbreviations
- AhR
aryl hydrocarbon receptor
- Arnt
aryl hydrocarbon receptor nuclear translocator
- 1,3DMU
1,3-dimethyluric acid
- DFB
(3,4-difluorobenzyloxy)-5,5-dimethyl-4-(4-methylsulphonylphenyl)-(5H)-furan-2-one
- DFH
3-hydroxy-5,5-dimethyl-4-(4-methylsulphonylphenyl)-(5H)-furan-2-one
- Epo
erythropoietin
- FiO2
fractional concentration of inspired O2
- HCONT
hepatocytes from control rabbits
- 13-HETE
13-hydroxyeicosatrienoic acid
- HHYPO
hepatocytes from rabbits with hypoxia
- HIF-1
hypoxia-inducible factor 1 transcriptional activator
- H2O2
hydrogen peroxide
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- Mr
relative molecular mass
- 1MU
1-methyluric acid
- 3MX
3-methylxanthine
- NO˙
nitric oxide
- P450
cytochrome P450
- PI3-K
phosphatidylinositol 3-kinase
- ROI
reactive oxygen intermediates
- SCONT
serum from control rabbits
- SHYPO
serum from rabbits with hypoxia
- TNF-α
tumour necrosis factor
- WME
William's medium E
References
- ABDEL-RAZZAK Z., LOYER P., FAUTREL A., GAUTIER J.C., CORCOS L., TURLIN B., BEAUNE P., GUILLOUZO A. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol. Pharmacol. 1993;44:707–715. [PubMed] [Google Scholar]
- BELLO I., PEREZ A., TORRES A.M., HERNANDEZ M.V., LOPEZ SAURA P. High levels of soluble IFN gamma receptor alpha chain in the plasma of rheumatoid arthritis patients. Biotherapy. 1998;11:53–57. doi: 10.1023/a:1008087100315. [DOI] [PubMed] [Google Scholar]
- BLAZQUEZ C., WOODS A., DE CEBALLOS M.L., CARLING D., GUZMAN M. The AMP-activated protein kinase is involved in the regulation of ketone body production by astrocytes. J. Neurochem. 1999;73:1674–1682. doi: 10.1046/j.1471-4159.1999.731674.x. [DOI] [PubMed] [Google Scholar]
- BLEAU A.M., LEVITCHI M., MAURICE H., DU SOUICH P. Different cytokines mediate in vivo the human viral- and the rabbit turpentine-induced inflammation inactivation of hepatic cytochrome P450. Br. J. Pharmacol. 2000;130:1777–1784. doi: 10.1038/sj.bjp.0703486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRASS C.A., CRAWFORD J.M., NARCISO J., GOLLAN J.L. Hypoxic liver injury and the ameliorating effects of fructose: the ‘glucose paradox' revisited. Am. J. Physiol. 1992;263:G293–300. doi: 10.1152/ajpgi.1992.263.3.G293. [DOI] [PubMed] [Google Scholar]
- BROWN S.E., QUATTROCHI L.C., GUZELIAN P.S. Characterization of a pretranscriptional pathway for induction by phenobarbital of cytochrome P450 3A23 in primary cultures of adult rat hepatocytes. Arch. Biochem. Biophys. 1997;342:134–142. doi: 10.1006/abbi.1997.0112. [DOI] [PubMed] [Google Scholar]
- BUNN H.F., GU J., HUANG L.E., PARK J.W., ZHU H. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J. Exp. Biol. 1998;201:1197–1201. doi: 10.1242/jeb.201.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUNN H.F., POYTON R.O. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- CALLEJA C., EECKHOUTTE C., DACASTO M., LARRIEU G., DUPUY J., PINEAU T., GALTIER P. Comparative effects of cytokines on constitutive and inducible expression of the gene encoding for the cytochrome P450 3A6 isoenzyme in cultures rabbit hepatocytes: consequences on progesterone 6beta-hydroxylation. Biochem. Pharmacol. 1998;56:1279–1285. doi: 10.1016/s0006-2952(98)00178-6. [DOI] [PubMed] [Google Scholar]
- CALLEJA C., EECKHOUTTE C., LARRIEU G., DUPUY J., PINEAU T., GALTIER P. Differential effects of interleukin-1 beta, interleukin-2, and interferon-gamma on the inducible expression of CYP1A1 and 1A2 in cultured rabbit hepatocytes. Biochem. Biophys. Res. Commun. 1997;239:273–278. doi: 10.1006/bbrc.1997.7468. [DOI] [PubMed] [Google Scholar]
- CHAURET N., TREMBLAY N., LACKMAN R.L., GAUTHIER J.Y., SILVA J.S., MAROIS J., YERGEY J.A., NICOLL-GRIFFITH D.A. Description of a 96-well plate assay to measure P4503A inhibition in human liver microsomes using a selective fluorescent probe. Analytic. Biochemistry. 1999;276:215–225. doi: 10.1006/abio.1999.4348. [DOI] [PubMed] [Google Scholar]
- DALET C., CLAIR P., DAUJAT M., FORT P., BLANCHARD J.M., MAUREL P. Complete sequence of cytochrome P450 3c cDNA and presence of two mRNA species with 3′ untranslated regions of different lengths. DNA. 1988;7:39–46. doi: 10.1089/dna.1988.7.39. [DOI] [PubMed] [Google Scholar]
- EL-KADI A.O., BLEAU A.M., DUMONT I., MAURICE H., DU SOUICH P. Role of reactive oxygen intermediates in the decrease of hepatic cytochrome P450 activity by serum of humans and rabbits with an acute inflammatory reaction. Drug Metab. Dispos. 2000;28:1112–1120. [PubMed] [Google Scholar]
- EL-KADI A.O.S., MAURICE H., ONG H., DU SOUICH P. Down regulation of the hepatic cytochrome P450 by an acute inflammatory reaction: implication of human and animal serum, and intrahepatic mediators. Br. J. Pharmacol. 1997;121:1164–1170. doi: 10.1038/sj.bjp.0701232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-SANKARY W., PLANT N.J., GIBSON G.G., MOORE D.J. Regulation of the CYP3A4 gene by hydrocortisone and xenobiotics: role of the glucocorticoid and pregnane X receptors. Drug. Metab. Dispos. 2000;28:493–496. [PubMed] [Google Scholar]
- GAFFEN S.L., LAI S.Y., LONGMORE G.D., LIU K.D., GOLDSMITH M.A. Genetic evidence for an additional factor required for erythropoietin-induced signal transduction. Blood. 1999;94:74–86. [PubMed] [Google Scholar]
- GESS B., SCHRICKER K., PFEIFER M., KURTZ A. Acute hypoxia upregulates NOS gene expression in rats. Am. J. Physiol. 1997;273:R905–R910. doi: 10.1152/ajpregu.1997.273.3.R905. [DOI] [PubMed] [Google Scholar]
- GREENFEDER S.A., NUNES P., KWEE L., LABOW M., CHIZZONITE R.A., JU G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J. Biol. Chem. 1995;270:13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- HSU K.S., HUANG C.C. Protein kinase C inhibitors block generation of anoxia-induced long-term potentiation. Neuroreport. 1998;9:3525–3529. doi: 10.1097/00001756-199810260-00035. [DOI] [PubMed] [Google Scholar]
- HUANG W.T., LIN M.T., WONG S.J. Staphylococcal enteroxin A-induced fever is associated with increased circulating levels of cytokines in rabbits. Inf. Immunol. 1997;65:2656–2662. doi: 10.1128/iai.65.7.2656-2662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACQUES Y., LE MAUFF B., GODARD A., NAULET J., CONCINO M., MARSH H., IP S., SOULILLOU J.P. Biochemical study of a recombinant soluble interleukin-2 receptor. Evidence for a homodimeric structure. J. Biol. Chem. 1990;265:20252–20258. [PubMed] [Google Scholar]
- JONES D.P. Hypoxia and drug metabolism. Biochem. Pharmacol. 1981;30:1019–1023. doi: 10.1016/0006-2952(81)90436-6. [DOI] [PubMed] [Google Scholar]
- JUNGERMANN K., KIETZMANN T. Role of oxygen in the zonation of carbohydrate metabolism and gene expression in liver. Kidney Int. 1997;51:402–412. doi: 10.1038/ki.1997.53. [DOI] [PubMed] [Google Scholar]
- JUNGERMANN K., KIETZMANN T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- KAMBE T., TADA-KAMBE J., KUGE Y., YAMAGUCHI-IWAI Y., NAGAO M., SASAKI R. Retinoic acid stimulates erythropoietin gene transcription in embryonal carcinoma cells through the direct repeat of a steroid/thyroid hormone receptor response element half-site in the hypoxia-response enhancer. Blood. 2000;96:3265–3271. [PubMed] [Google Scholar]
- KARUZINA I.I., ARCHAKOV A.I. The oxidative inactivation of cytochrome P450 in mooxygenase reaction. Free. Rad. Biol. Med. 1994;16:73–97. doi: 10.1016/0891-5849(94)90245-3. [DOI] [PubMed] [Google Scholar]
- KRUGER N.J.Detection of polypeptides on immunoblots using secondary antibodies or protein A. Methods of Molecular Biology 1994Vol. 32Totowa, N.J.: Humana Press; 215–226.ed. Walker, J.M. pp [DOI] [PubMed] [Google Scholar]
- KURDI J., MAURICE H., EL-KADI A.O.S., ONG H., DALKARA S., BÉLANGER P.M., DU SOUICH P. Effect of hypoxia alone or combined with inflammation and 3-methylcholanthrene on hepatic cytochrome P450 in conscious rabbits. Br. J. Pharmacol. 1999;128:365–373. doi: 10.1038/sj.bjp.0702795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACOMBE C., MAYEUX P. The molecular biology of erythropoietin. Nephrol. Dial. Transplant. 1999;14 Suppl. 2:S22–S28. doi: 10.1093/ndt/14.suppl_2.22. [DOI] [PubMed] [Google Scholar]
- LANGER J.A., RASHIDBAIGI A., GAROTTA G., KEMPNER E. Radiation inactivation of human gamma-interferon: cellular activation requires two dimers. Proc. Natl. Acad. Sci. U.S.A. 1994;91:5818–5822. doi: 10.1073/pnas.91.13.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBLOND F., GUEVIN C., DEMERS C., PELLERIN I., GASCON-BARRE M., PICHETTE V. Down-regulation of hepatic cytochrome P450 in chronic renal failure. J. Am. Soc. Nephrol. 2001;12:326–332. doi: 10.1681/ASN.V122326. [DOI] [PubMed] [Google Scholar]
- LETARTE L., DU SOUICH P. Influence of hypercapnia and/or hypoxemia and metabolic acidosis on theophylline kinetics in the conscious rabbit. Am. Rev. Respir. Dis. 1984;129:762–766. doi: 10.1164/arrd.1984.129.5.762. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MACHEIN M.R., KULLMER J., RONICKE V., MACHEIN U., KRIEG M., DAMERT A., BREIER G., RISAU W., PLATE K.H. Differential downregulation of vascular endothelial growth factor by dexamethasone in normoxic and hypoxic rat glioma cells. Neuropathol. Appl. Neurobiol. 1999;25:104–112. doi: 10.1046/j.1365-2990.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- MAZURE N.M., CHEN E.Y., LADEROUTE K.R., GIACCIA A.J. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- MORGAN E.T. Regulation of cytochromes P450 during inflammation and infection. Drug Metab. Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- MUSCETTOLA M., GIROLAMI L., TANGANELLI C., FONTANI G., LUPO C. Immune and endocrine aspects of behaviour in male rabbits. Neuroimmunomodulation. 1995;2:155–160. doi: 10.1159/000096886. [DOI] [PubMed] [Google Scholar]
- NALDINI A., CARRARO F., SILVESTRI S., BOCCI V. Hypoxia affects cytokines production and proliferative responses by human peripheral mononuclear cells. J. Cell. Physiol. 1997;173:335–342. doi: 10.1002/(SICI)1097-4652(199712)173:3<335::AID-JCP5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- NG C.Y., GHABRIAL H., MORGAN D.J., CHING M.S., SMALLWOOD R.A., ANGUS P.W. Impaired elimination of propranolol due to right heart failure: drug clearance in the isolated liver and its relationship to intrinsic metabolic capacity. Drug Metab. Dispos. 2000;28:1217–1221. [PubMed] [Google Scholar]
- OMURA T., SATO R. The carbon monoxide-binding pigment of liver microsomes. 1. Evidence for its hemoprotein nature. J. Biol. Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- OINONEN T., LINDROS K.O. Zonation of hepatic cytochrome P-450 expression and regulation. Biochem. J. 1998;329:17–35. doi: 10.1042/bj3290017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK H. Aromatic hydrocarbon nuclear translocator as a common component for the hypoxi- and dioxin-induced gene expression. Mol. Cells. 1999;9:172–178. [PubMed] [Google Scholar]
- POWELL J.R., VOZEH S., HOPEWELL P., COSTELLO J., SCHEINER L.B., RIEGELMAN S. Theophylline disposition in acutely ill hospitalized patients. The effect of smoking, heart failure, severe airway obstruction and pneumonia. Am. Rev. Respir. Dis. 1978;118:229–238. doi: 10.1164/arrd.1978.118.2.229. [DOI] [PubMed] [Google Scholar]
- PROULX M., DU SOUICH P. Acute moderate hypoxia in conscious rabbits: effect on hepatic cytochrome P450 and on reactive species. J. Pharm. Pharmacol. 1995;47:392–397. doi: 10.1111/j.2042-7158.1995.tb05817.x. [DOI] [PubMed] [Google Scholar]
- RICHER M., LAM Y.W. Hypoxia, arterial pH and theophylline disposition. Clin. Pharmacokinet. 1993;25:283–299. doi: 10.2165/00003088-199325040-00004. [DOI] [PubMed] [Google Scholar]
- SEGLEN P.O. Preparation of isolated rat liver cells. Methods Cell. Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- SEMENZA G.L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell. Dev. Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- SHAN X., AW T.Y., SMITH E.R., INGELMAN-SUNDBERG M., MANNERVIK B., IYANAGI T., JONES D.P. Effect of chronic hypoxia on detoxication enzymes in rat liver. Biochem. Pharmacol. 1992;43:2421–2426. doi: 10.1016/0006-2952(92)90322-a. [DOI] [PubMed] [Google Scholar]
- SILVA J.M., NICOLL-GRIFFITH D.A.In vitro models for studying induction of cytochromes P450 enzymes Drug–drug Interactions 2001New York: Marcel Dekker; 199–201.ed. Rodrigues, A.D. pp [Google Scholar]
- SIMMS H., D'AMICO R. Regulation of polymorphonuclear leukocyte cytokine receptor expression: the role of altered oxygen tensions and matrix proteins. J. Immunol. 1996;157:3605–3616. [PubMed] [Google Scholar]
- SMITH B.J.SDS polyacrylamide gel electrophoresis of proteins Methods of Molecular Biology 1994Vol. 32Totowa, N.J.: Humana Press; 23–34.ed. Walker, J.M. pp [DOI] [PubMed] [Google Scholar]
- STROM D.K., POSTLIND H., TUKEY R.H. Characterization of the rabbit CYP1A1 and CYP1A2 genes: developmental and dioxin-inducible expression of rabbit liver P4501A1 and P4501A2. Arch. Biochem. Biophys. 1992;294:707–716. doi: 10.1016/0003-9861(92)90745-i. [DOI] [PubMed] [Google Scholar]
- TAKABATAKE N., NAKAMURA H., ABE S., INOUE S., HINO T., SAITO H., YUKI H., KATO S., TOMOIKE H. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000;161:1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- TAKEMURA S., MINAMIYAMA Y., IMAOKA S., FUNAE Y., HIROHASHI K., INOUE M., KINOSHITA H. Hepatic cytochrome P450 is directly inactivated by nitric oxide, not by inflammatory cytokines, in the early phase of endotoxemia. J. Hepatol. 1999;30:1035–1044. doi: 10.1016/s0168-8278(99)80257-8. [DOI] [PubMed] [Google Scholar]
- THORPE G.H.G., KRICKA L.J., MOSELEY S.B., WHITEHEAD T.P. Phenols as enhancers of chemiluminescent horseradish peroxidase-luminol-hydrogen peroxide reaction: application in luminescence-monitored enzyme immunoassays. Clin. Chem. 1985;31:1335–1341. [PubMed] [Google Scholar]
- UCHIYAMA T., KURABAYASHI M., OHYAMA Y., UTSUGI T., AKUZAWA N., SATO M., TOMONO S., KAWAZU S., NAGAI R. Hypoxia induces transcription of the plasminogen activator inhibitor-1 gene through genistein-sensitive tyrosine kinase pathways in vascular endothelial cells. Arterioscler. Thromb. Vasc. Bio. 2000;20:1155–1161. doi: 10.1161/01.atv.20.4.1155. [DOI] [PubMed] [Google Scholar]
- WEISSMANN N., TADIC A., HANZE J., ROSE F., WINTERHALDER S., NOLLEN M., SCHERMULY R.T., GHOFRANI H.A., SEEGER W., GRIMMINGER F. Hypoxic vasoconstriction in intact lungs: a role for NADPH oxidase-derived H(2)O(2) Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L683–L690. doi: 10.1152/ajplung.2000.279.4.L683. [DOI] [PubMed] [Google Scholar]


