Abstract
This study was designed to investigate the mechanisms for the contractions induced by tachykinins (substance P (SP), neurokinin A (NKA) and neurokinin B (NKB)) in the rabbit corpus cavernosum strips, using fura-PE3 fluorimetry and α-toxin permeabilization.
Tachykinins induced contractions in the rabbit corpus cavernosum in a concentration-dependent manner. The potency order was SP>NKA>NKB.
The tachykinin-induced contractions were enhanced by phosphoramidon (PPAD), an endopeptidase inhibitor, but not by Nω-nitro-L-arginine methylester (L-NAME).
The NK1 receptor selective antagonist, SR 140333 significantly inhibited the tachykinin-induced contractions. Although the NK2 receptor selective antagonist, SR 48968 alone did not influence the effects of tachykinins, it potentiated the inhibitory effect of SR 140333. The NK3 receptor selective antagonist, SR142801 had no effect.
In the rabbit corpus cavernosum, tachykinins induced sustained increases in [Ca2+]i and tension in normal PSS, while only small transient increases in [Ca2+]i and tension were observed in Ca2+-free solution.
In α-toxin permeabilized preparations, tachykinins induced an additional force development at a constant [Ca2+]i.
These results indicated that in the rabbit corpus cavernosum: (1) Tachykinins induced contractions by increasing both the [Ca2+]i and myofilament Ca2+ sensitivity; (2) The tachykinin-induced [Ca2+]i elevations were mainly due to the Ca2+ influx; (3) Tachykinin-induced contractions were mainly mediated through the activation of NK1 receptor expressed in the rabbit corpus cavernosum smooth muscle, and affected by the endopeptidase activity and (4) Tachykinins may thus play a role in controlling the corpus cavernosum tone.
Keywords: Corpus cavernosum, tachykinin, fura-PE3, intracellular Ca2+ concentration, skinned fibre, Ca2+ sensitivity
Introduction
Substance P (SP), neurokinin A (NKA) and neurokinin B (NKB) belong to a family of neuropeptides named tachykinins. They share a common C-terminal sequence Phe-X-Gly-Leu-Met-NH2 which characterizes the tachykinin family of peptides (Erspamer, 1981). SP and NKA are products of the same gene and are usually co-localized, while NKB is derived from a different gene and its distribution is usually different from that of SP and NKA. These tachykinins are widely distributed in the mammalian central and peripheral nervous system, where they produce a wide variety of biological effects, such as pain transmission, memory processing, cardiovascular functions and smooth muscle contraction and relaxation. The biological effects of tachykinins are mediated by receptors coupled with guanosine 5′-triphosphate binding protein (G-protein) to transduce the signal to effector proteins. Recently, three subtypes of tachykinin receptors have been determined and named NK1, NK2 and NK3 receptors (Otsuka & Yoshioka, 1993).
In smooth muscle, tachykinins have been reported to induce contraction of selected tissues including gastrointestinal tract, respiratory system and genitourinary tract (Otsuka & Yoshioka, 1993; Maggi, 1995). Regarding vascular tissues, although SP generally induces endothelium-dependent relaxation (Otsuka & Yoshioka, 1993), tachykinin-induced contractions have also been described in the rabbit pulmonary artery (D'orleans-Juste et al., 1985; Shirahase et al., 1995), rat portal vein (Mastrangelo et al., 1986) and rabbit mesenteric vein (Couture & Regoli, 1982). However, little is known about the effects of tachykinins on the corpus cavernosum smooth muscle. Previous studies have shown that SP immunoreactive nerves were present in the human corpus cavernosum (Gu et al., 1983) and SP induced contraction at resting tension (Hedlund & Andersson, 1985) and small relaxations in an endothelium dependent manner (Azadzoi et al., 1992; Patacchini et al., 2002). However, the underlying mechanisms and mediated receptor subtypes for the SP-induced contraction have not been reported before. The effects of NKB, which is another member of tachykinins, on the smooth muscle tone of the corpus cavernosum are not yet known.
The smooth muscle tone of the corpus cavernosum regulates penile flaccidity and erection. The contraction of the corpus cavernosum smooth muscle plays an important role in keeping the penis in a flaccid state, while its relaxation induces penile erection. The contraction of the corpus cavernosum smooth muscle is thought to be mainly maintained by the release of norepinephrine acting on α-adrenoceptors. However, it has also been reported that several other factors such as myogenic activity, prostanoids (thromboxane A2, prostaglandin F2α), endothelins and angiotensins directly contribute to the regulation of corpus cavernosum contractility (Andersson & Holmquist, 1994; Andersson, 2001). It is thus important to elucidate other receptors and potential pathways for mediating corpus cavernosum smooth muscle (Moreland et al., 2001).
In the present study, we examined the effect of tachykinins (SP, NKA and NKB) on the rabbit corpus cavernosum. We found that all three tachykinins induced contractions of the rabbit corpus cavernosum smooth muscle and specified the type of tachykinin receptors involved in the tachykinin-induced contractions by examining the effect of tachykinin receptor antagonists on the corpus cavernosum contraction. The mechanisms underlying the tachykinin-induced contractions were further elucidated by the simultaneous measurements of the intracellular Ca2+ concentration ([Ca2+]i) and tension of intact fura-PE3 loaded strips and by tension measurement under the clamped [Ca2+]i level using α-toxin permeabilized strips.
Methods
Tissue preparation
The study protocol was approved by the Animal Care and Committee of Research Institute of Angiocardiology, Faculty of Medicine, Kyushu University. Male Japanese white rabbits (2–3 kg) were anaesthetized with an overdose of sodium pentobarbitone and sacrificed. The penises were excised while being careful not to damage or overstretch the tissue. The connective tissue, urethra and surrounding tunica albuginea were removed and the corpus cavernosum preparation so obtained was cut into strips measuring approximately 0.5×1.0×3.0 mm in a longitudinal direction under a binocular microscope. The strips were placed in a modified physiological saline solution (PSS) consisting of the following compositions (in mM): NaCl 123, KCl 4.7, CaCl2 1.25, MgCl2 1.2, KH2PO4 1.2, NaHCO3 15.5, D-glucose 11.5, gassed with 95% O2 and 5% CO2.
Endothelium disruption
Endothelium disruption, when necessary, was performed by detergent treatment using the protocol of Kim et al. (1991) with minor modifications. The different points are as follows: (1) The concentration of CHAPS and the clamping time after infusion of CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propane-sulphonate) were 0.3% and 120 s in our methods, which were 0.5% and 20 s in an original protocol, respectively and (2) Just before organ bath experiments, we immersed the strips in 0.3% CHAPS for about 120 s.
Tension measurement
The rabbit corpus cavernosum strips were connected to a force transducer (TB-612T, Nihon Koden, Japan) and mounted vertically. The preparation was then immersed in 6 ml baths containing modified PSS solution and 1 μM tetrodotoxin (TTX) (maintained at 37°C and aerated with 5% CO2, 95% O2 to attain pH 7.4). In the presence of 1 μM TTX, electrical field stimulation-induced relaxation was completely abolished in the strip precontracted with phenylephrine (Takagi et al., 2001). This modified PSS solution with TTX was used throughout subsequent experiments. The strips were stimulated with 10 μM phenylephrine every 15 min with a stepwise increase in a resting load until the maximal response was obtained. When the difference of the amplitude of the contraction was within 10% of the previous contraction, that tension was considered optimal for isometric contraction.
Fura-PE3 loading and measurement of cytosolic Ca2+ concentration [Ca2+]i
The corpus cavernosum strips without endothelium were incubated in Dulbecco's modified Eagle medium (DMEM) gassed with 5% CO2 and 95% O2 containing 50 μM fura-PE3 in the form of acetoxymethyl ester (fura-PE3/AM) and 5% foetal bovine serum for 7 h at 37°C. The strips were equilibrated in normal PSS for at least 1 h before the measurements. The changes in the fluorescence ratio and tension development were simultaneously monitored with a front-surface fluorimeter CAM-OF-1 (JASCO, Tokyo, Japan). The fluorescence (500 nm) intensities at alternating (400 Hz) excitation (340 and 380 nm) and the ratio (F340/F380) were continuously measured. The data were stored in a Macintosh computer using a data acquisition system (MacLab: Analog Digital Instruments, Australia). The fluorescence ratio, which indicates [Ca2+]i, was expressed as a percentage, assigning the resting state and 10 μM phenylephrine induced contractions to be 0 and 100%, respectively. All simultaneous measurements of [Ca2+]i and force were performed at 37°C.
Tension measurement of α-toxin permeabilized corpus cavernosum strips
The permeabilization of the smooth muscle cell membrane in the rabbit corpus cavernosum was carried out using α-toxin according to the methods described by Nishimura et al. (1988) with minor modifications. The small strips (about 0.5 mm in width and 1.2 mm in length) of the rabbit corpus cavernosum were mounted between two tungsten wires, one of which was fixed and the other one was attached to a force transducer (UL2; Minebea Co., Japan). The strips were permeabilized in a relaxing solution (mM): potassium methansulphonate 100, Na2ATP 2.2, MgCl2 3.38, ethyleneglycol-bis (β-aminoethylether)-N′,N′,N′,N′-tetra acetic acid (EGTA) 10, creatine phosphate 10, Tris-maleate 20 (pH=6.8) containing 5000 units ml−1 staphylococcus aureus α-toxin for 45 min. The activating solution containing the indicated concentrations of free Ca2+ was made by adding an appropriate amount of CaCl2 to the relaxing solution, using the Ca2+-EGTA binding constant of 106/M (Saida & Nonomura, 1978). The tension measurements of the permeabilized tissues were all performed at room temperature. The tension in the relaxing solution and maximal tension induced by 10−5 M Ca2+ were taken as 0 and 100%, respectively.
Drugs and solutions
The composition of the normal physiological saline solution (normal PSS) has been described above. High- K+ PSS was made by an equimolar substitution of KCl for NaCl. The Ca2+-free solution (Ca2+-free PSS) containing 0.3 mM EGTA instead of 1.25 mM CaCl2 was produced by excluding of CaCl2 from the normal PSS. Each solution mentioned above was gassed with a mixture of 5% CO2 and 95% O2 (pH 7.4 at 37°C). The composition of the solution for the permeabilized preparations was described above. Neurokinin A and Neurokinin B were purchased from the Peptide Institute (Osaka, Japan). Fura PE3/AM was purchased from Texas Fluorescence Laboratory (Austin, TX, U.S.A.). Substance P, phenylephrine hydrochloride, senktide (succinyl-[Asp6,N-Me-Phe8]-SP6–11), carbachol (carbamylcholine chloride), L-NAME (L-Nω-nitro-arginine-methylester), phosphoramidon (N-(α-L-rhamnopyranosyl-oxyhydroxy-phosphinyl)-L-leucyl-L-tryptophan), CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propane-sulphonate), CPA (cyclopiazonic acid) and α-toxin were purchased from the Sigma Chemical Company (St. Louis, MO, U.S.A.). Tetrodotoxin (TTX) was purchased from Wako (Osaka, Japan). GTP (guanosine5′-triphosphate) was purchased from Calbiochem (La Jolla, CA, U.S.A.). The nonpeptide antagonists SR 140333 [(S)-(+)-1-(2-(3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl)piperidin-3-yl)-ethyl)-4-phenyl-1-azoniabicyclo (2,2,2) octane, chloride], SR48968 [(S)-(−)-N-methyl-N-(4-(4-acetylamino-4-phenyl)piperidono-2-(3,4-dichlorophenyl)-butyl)-benzamide, hydrochloride] and SR142801 [(R)-N-(1-(3-(1-benzol-3-(3,4-dichlorophenyl)-piperidin-3-yl)propyl)-4-phenylpiperidin-4-yl)-N-methylacetamide, hydrochloride] were donated by Sanofi Recherche (Montpellier, France). All other chemicals were of the highest grade commercially available.
Statistical analysis
All data were expressed as the mean±standard errors mean (s.e.m.) along with the number of observations (=n). One strip obtained from one animal was used for each experiment, therefore the number of experiments (n value) also indicates the number of animals. Student's t-test was used to determine any statistical differences between the two mean values. P<0.05 was considered to be significant. The four parameter logistic model was used to fit the sigmoidal curve to the concentration response of each drug (de lean et al., 1978). All data were collected using a computerized data acquisition system (MacLab; Analog Digital Instruments, Australia, Macintosh; Apple Computer, U.S.A.).
Results
Effect of SP, NKA and NKB on the contractility of the rabbit corpus cavernosum
Figure 1 shows the concentration-response relationships of the contractions induced by various concentrations of tachykinins (1 pM–30 μM) determined in the strips of the rabbit corpus cavernosum with an endothelium. In this plot, the values obtained with 10 μM phenylephrine-induced contractions were designated to be 100%, because the phenylephrine-induced contraction in the rabbit corpus cavernosum strips was most stable and reproducible. The maximal levels of contractions induced by 30 μM SP, NKA and NKB were almost similar to those induced by 10 μM phenylephrine (SP: 102.34±6.71%; n=5, NKA: 99.89±8.06%; n=5, NKB: 95.34±6.09%; n=6). However, a significant difference was observed in the EC50 values among SP-, NKA- and NKB-induced contractions. The rank order of potency of these tachykinins was SP (EC50=84.5±47.7 nM; n=5)>NKA (EC50=149±38 nM; n=5)>NKB (EC50=408±72 nM; n=6).
Figure 1.
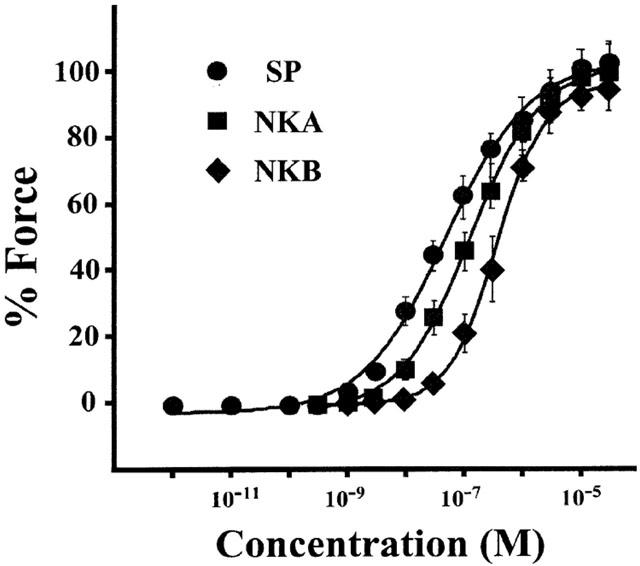
Concentration-response relationship of three tachykinin-induced contractions in rabbit corpus cavernosum strips with an endothelium. Various concentrations of tachykinins (1 pM–30 μM) were cumulatively applied in the normal PSS. For comparison purposes, SP-, NKA- and NKB-induced contractions were plotted by assigning the 10 μM phenylephrine-induced contraction to be 100%. Data are the mean±s.e.mean (n=5–6).
Effect of L-NAME and phosphoramidon on the tachykinin-induced contractions
Figure 2 shows the effects of L-NAME, an NO synthase inhibitor, and phosphoramidon (PPAD), an endopeptidase inhibitor, on the 1 μM tachykinin-induced contractions of the corpus cavernosum with an intact endothelium. When the strips were treated with 100 μM L-NAME for 15 min, the baseline tension was gradually increased (26.82±3.19% of the 10 μM phenylephrine-induced contraction; n=15) and reached a new steady state level. However, the developed tension induced by SP, NKA or NKB was not augmented by the treatment with L-NAME. The mean values of the SP-, NKA- and NKB-induced responses relative to that induced by 10 μM phenylephrine in the control and the L-NAME-treated strips were 82.23±2.34% (n=5) and 80.10±2.49% (n=5) for SP, 74.80±1.85% (n=5) and 69.07±4.55% (n=5) for NKA and 65.60±4.72% (n=5) and 63.80±4.34% (n=5) for NKB, respectively. When the strips were treated with 1 μM PPAD for 15 min, the resting tension gradually increased in a manner similar to that observed in L-NAME treatment (18.70±1.88% of the 10 μM phenylephrine-induced contraction; n=15). The subsequent applications of SP, NKA or NKB induced an enhanced contraction from 82.23±2.34% (n=5) to 95.17±5.80% (n=5) for SP, from 74.80±1.85% (n=5) to 98.80±3.99% (n=5) for NKA and from 65.6±4.72% (n=5) to 84.82±6.11% (n=5) for NKB. As shown in Figure 2e, 1 μM carbachol, a standard relaxing agent in the corpus cavernosum, induced a prompt relaxation in our preparation. However, SP (1 pM–1 μM) did not induce relaxation in strips precontracted by 10 μM phenylephrine (Figure 2d).
Figure 2.
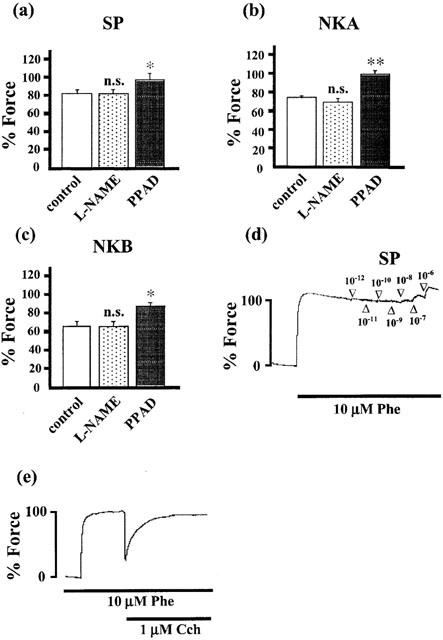
The effect of L-NAME and phosphoramidon (PPAD) on the tachykinin-induced contractions (a–c), and the effect of SP and carbachol on the contraction induced by phenylephrine (d, e) in the rabbit corpus cavernosum strips with an endothelium. In a–c, the tissues were treated with 100 μM L-NAME (an NO synthase inhibitor) or 1 μM PPAD (an endopeptidase inhibitor) 15 min before and during the application of 1 μM tachykinins. The tension developments were evaluated at sustained levels after the application of tachykinins and then were expressed as a percentage, assigning the 10 μM phenylephrine-induced contraction to be 100%. Data are the mean±s.e.mean (n=5) *P<0.05, **P<0.01, as compared with the control. n.s.; not significant. In (d) and (e), SP (1 pM–1 μM) and carbachol (1 μM) were applied during the sustained contraction induced by 10 μM phenylephrine, respectively.
Effect of subtype-selective tachykinin receptor antagonists on the tachykinin-induced contractions
To determine the tachykinin receptor subtypes responsible for the tachykinin-induced contractions, we examined the effects of SR140333 (an NK1 receptor selective antagonist), SR48968 (an NK2 receptor selective antagonist) and SR142801 (an NK3 receptor selective antagonist) on the tachykinin-induced contractions in the strips with an endothelium. As shown in Figure 3, 100 nM SR 140333 depressed the 30 μM SP-, NKA- and NKB-induced maximum contractions by 62.19±7.23% (n=5), 51.98±8.17% (n=5) and 55.72±9.23% (n=5) and increased the EC50 values to 9.1±4.0 μM (n=5), 276±45 nM (n=5) and 831±41 nM (n=5), respectively. SR 48968 alone did not influence the contractile response to tachykinins. However, the combined treatment with SR 48968 and SR 140333 potentiated the inhibitory effect of SR 140333 alone and almost completely inhibited tachykinin-induced contractions. SR 142801 had no effect on the tachykinin-induced contractions. In addition, the NK3 receptor selective agonist, senktide (0.1 nM–1 μM) did not induce any contraction (data not shown).
Figure 3.
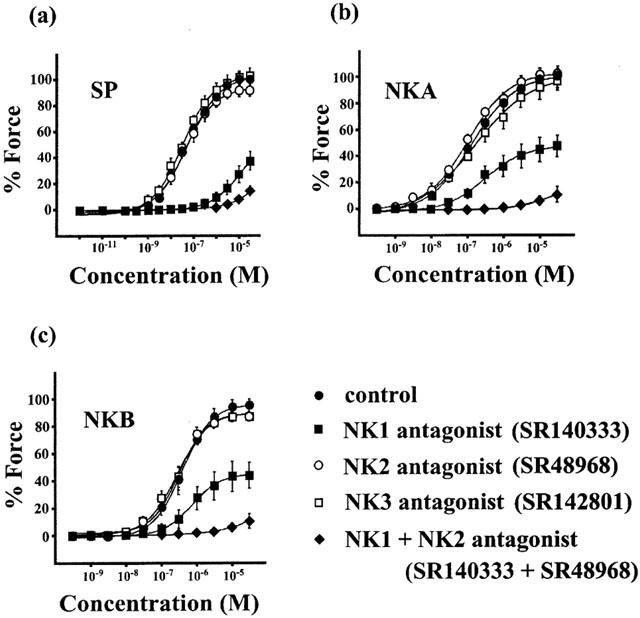
Effect of subtype-selective tachykinin receptor antagonists on the tachykinin-induced contractions in the rabbit corpus cavernosum strips with an endothelium. The tissues were treated with SR 140333 (100 nM), SR 48968 (100 nM) or SR 142801 (100 nM) alone or in combination with SR 140333 and SR 48968 for 15 min before and during the cumulative application of tachykinins. The tension developments were expressed as a percentage, assigning the 10 μM phenylephrine-induced contraction to be 100%. Data are the mean±s.e.mean (n=5).
Effect of tachykinins on the [Ca2+]i and tension of the rabbit corpus cavernosum
Figure 4a,c,e shows the representative recordings of the changes in [Ca2+]i and tension induced by 1 μM SP, NKA and NKB, respectively, in normal PSS in the strips without endothelium. When measuring the [Ca2+]i, we used strips without endothelium in order to avoid the fura-PE3 signal aroused from endothelial cells. SP-, NKA- and NKB-induced contractions were accompanied by the sustained elevation of [Ca2+]i. To examine the effect of tachykinins on the Ca2+ release from the intracellular store, we measured SP-, NKA- and NKB-induced changes in [Ca2+]i and tension in Ca2+-free PSS containing 0.3 mM EGTA. When the strips were exposed to the Ca2+-free PSS containing 0.3 mM EGTA for 3 min [Ca2+]i decreased to −46.22±3.88% (n=17) without affecting the resting tension. At this point depolarization with 118 mM K+ did not induce an increase in either [Ca2+]i or tension, thus indicating extracellular Ca2+ to be completely chelated by EGTA and no Ca2+-influx (data not shown). In a Ca2+-free PSS containing 0.3 mM EGTA, SP, NKA and NKB induced a small transient increase in [Ca2+]i accompanied by a small transient contraction (Figure 4b,d,f). Figure 5 summarizes the Ca2+ responses induced by tachykinins in the absence or presence of the extracellular Ca2+ as a function of time. These results indicated that tachykinins are able to induce Ca2+ release, but the elevation of [Ca2+]i and tension induced by tachykinins mainly depends on the Ca2+ influx from the extracellular space. In addition, we examined whether NK2 receptor is involved in the tachykinin-induced contractions in the absence of extracellular Ca2+. However, the treatment of SR 48968 (100 nM, NK2 receptor antagonist) for 15 min before and during the application of 0.3 mM EGTA did not attenuate the transient contractions induced by tachykinins in the absence of extracellular Ca2+. The mean values of the SP-, NKA- and NKB-induced contractions relative to that induced by 10 μM phenylephrine in the absence and presence of SR 48968 were 14.33±3.19% (n=5) and 14.97±2.20% (n=5) for SP, 18.60±6.33% (n=5) and 21.40±4.31% (n=5) for NKA and 15.00±6.04% (n=5) and 19.58±2.79% (n=5) for NKB, respectively.
Figure 4.
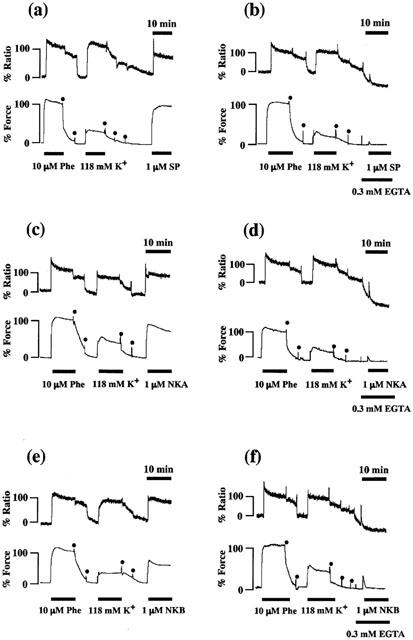
Effect of SP, NKA and NKB on the [Ca2+]i and tension in the rabbit corpus cavernosum strips without an endothelium. Representative recordings showing the effect of 1 μM SP, NKA and NKB on the [Ca2+]i (upper trace) and tension (lower trace) of the rabbit corpus cavernosum in normal PSS (a, c, e) and in Ca2+-free PSS containing 0.3 mM EGTA (b, d, f). Ca2+-free PSS with 0.3 mM EGTA was applied 3 min before and during the addition of SP, NKA and NKB. The developed tension and [Ca2+]i were expressed as a percentage, assigning the 10 μM phenylephrine-induced contraction to be 100%. The dots represent the wash out with PSS.
Figure 5.
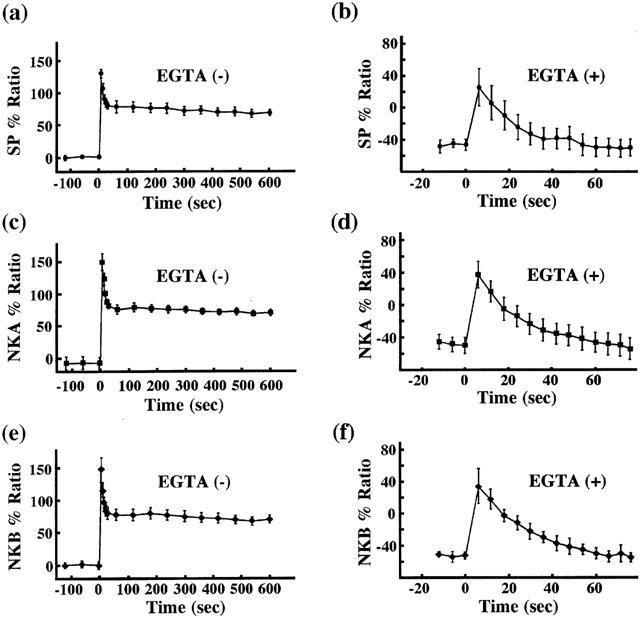
Summary of the effects of tachykinins on the [Ca2+]i in the presence or absence of extracellular Ca2+. All measurements were performed as shown in Figure 4 in normal PSS or in Ca2+-free PSS containing 0.3 mM EGTA. The time course of the change in [Ca2+]i induced by tachykinins in the absence or presence of EGTA was presented. As shown in Figure 4, the levels of [Ca2+]i at rest and phenylephrine-induced sustained contraction were designated as 0 and 100%, respectively. Data are the mean±s.e.mean (n=5–6).
In each panel of Figure 4, the increases in [Ca2+]i and tension induced by 10 μM phenylephrine and 118 mM K+ depolarization are also shown. Although the sustained increase in [Ca2+]i induced by SP, NKA and NKB were smaller than or almost the same as that induced by 118 mM K+ depolarization, the tension induced by SP, NKA and NKB were much greater than that induced by 118 mM K+ depolarization (Figure 4a,c,e). In addition, phenylephrine also induced greater contractions than expected from the [Ca2+]i increase, compared with increases in [Ca2+]i and tension induced by 118 mM K+ depolarization (Figure 4a–f). These results indicated that tachykinin as well as phenylephrine induced an increase in the Ca2+ sensitivity of the contractile apparatus.
Effect of tachykinins on the Ca2+ sensitivity of the contractile apparatus in the α-toxin permeabilized corpus cavernosum
To further confirm the effect of tachykinins on the Ca2+ sensitivity of the contractile apparatus in the rabbit corpus cavernosum, we measured the tension development induced by three tachykinins using the α-toxin permeabilized rabbit corpus cavernosum strips. In order to rule out the effect of Ca2+ release, we treated the strips with 10 μM CPA (a sarcoplasmic reticulum Ca2+-ATPase inhibitor) for 10 min before and during the protocol. As shown in Figure 6a, the application of 1 μM SP, NKA and NKB in the presence of 10 μM GTP had no contractile effect in the nominally Ca2+ free 10 mM EGTA solution. Figure 6b–d shows representative recordings of the effect of 1 μM SP, NKA and NKB on tension developed by 300 nM Ca2+ and 10 μM GTP in α-toxin permeabilized rabbit corpus cavernosum. The application of SP, NKA and NKB during steady state contractions by the mixture of 300 nM Ca2+ and 10 μM GTP induced additional force development at a constant [Ca2+]i and CPA had no effect.
Figure 6.
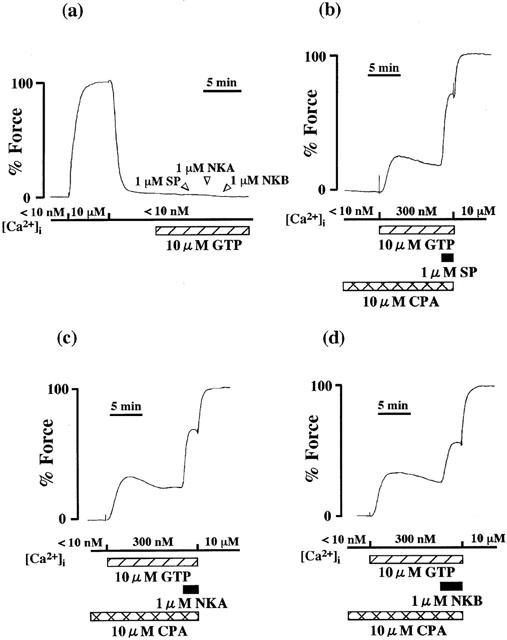
Effect of tachykinins on the Ca2+-induced contractions in the α-toxin permeabilized corpus cavernosum strips. In (a), 1 μM SP, NKA and NKB were applied in the presence of 10 μM GTP after the treatment with 10 mM EGTA relaxing solution for 10 min. In (b–d), the corpus cavernosum strips were precontracted by 300 nM Ca2+ and 10 μM GTP. The tissue was treated with 10 μM CPA for 10 min before and during the application of this solution. When the tension reached a steady state, 1 μM SP, NKA and NKB were applied. After the application of tachykinins, maximal tensions were induced by 10 μM Ca2+. The developed tension was expressed as a percentage, assigning the values in the relaxing solution ([Ca2+]i<10 nM) and in the activating solution ([Ca2+]i=10 μM; maximal tension) to be 0 and 100%, respectively.
Discussion
In the present study, we investigated the mechanisms underlying the tachykinin-induced contractions of the smooth muscle in the rabbit corpus cavernosum. The major findings are as follows: Tachykinins induced contractions in the rabbit corpus cavernosum through the activation of NK1 receptor, with a minor cooperative contribution of NK2 receptor. Contractile mechanisms involve both an increase in [Ca2+]i, and an increase in the Ca2+ sensitivity of the contractile apparatus. Tachykinin-induced increases in [Ca2+]i are mainly due to the Ca2+ influx from the extracellular space. Tachykinin-induced contractions are affected by the endopeptidase activity. To our knowledge, this is the first study which systematically describes the mechanisms for the Ca2+ regulation of the contraction in the corpus cavernosum smooth muscle, using simultaneous measurements of [Ca2+]i and tension as well as a receptor coupled permeabilized preparation.
The major function of SP and NKA in the blood vessels is suggested to be an induction of endothelium dependent relaxation (Otsuka & Yoshioka, 1993). However, we could not detect a significant SP-induced endothelium dependent relaxation in the present study (Figure 2d). In the previous studies, the SP-induced endothelium dependent relaxations were reported in the corpus spongiosum urethrae (Andersson et al., 1983; Patacchini et al., 2002). Regarding the corpus cavernosum, the findings remain controversial. A small (about 10–25% of the precontraction; Azadzoi et al., 1992; Patacchini et al., 2002) or no (Okamura et al., 1999) SP-induced endothelium dependent relaxations have been detected in the corpus cavernosum. These discrepancies could be explained by the experimental condition. The former two groups used a PSS containing 2.5 mM CaCl2, while the latter and we used a PSS containing 2.2 mM or 1.25 mM CaCl2, respectively. Indeed, we could detect a significant SP-induced endothelium dependent relaxation in one out of five tissues, when the CaCl2 concentration was raised to 2.5 mM (data not shown). We thus concluded that SP can induce an endothelium-dependent relaxation in the corpus cavernosum, the extent of which is markedly affected by the experimental conditions.
In contrast to the endothelium-dependent relaxation, Hedlund & Andersson (1985) reported that SP induced a contraction when added at a resting state. NKA, other member of tachykinins, also has been reported to induce contraction in the corpus cavernosum (Patacchini et al., 2002). The present results clearly showed that SP, NKA and NKB induce contractions of the rabbit corpus cavernosum (Figure 1). It should be noted that the contractions induced by tachykinins were as great as those induced by 10 μM phenylephrine and that the former may become even greater than the latter when the endopeptidase was inhibited by PPAD (Figure 2). We thus speculated that tachykinins and the endopeptidase activity could potentially be regulators of the corpus cavernosum tone, in addition to the stimulation of α-adrenoceptors by norepinephrine. These results indicated that the major effect of tachykinins on the corpus cavernosum is a contraction rather than an endothelium-dependent relaxation.
The biological actions of the tachykinins are mediated through three subtypes of receptors belonging to the G-protein-linked receptor family, named NK1, NK2 and NK3 receptors (Otsuka & Yoshioka, 1993). The affinities of agonists for NK1 receptors are SP>NKA>NKB, while those for NK2 receptors are NKA>NKB>SP and those for NK3 receptors are NKB>NKA>SP (Lecci et al., 2000). Because the potency order of three tachykinin-induced contractions were SP>NKA>NKB (Figure 1), it was suggested that the tachykinin-induced contractions of the corpus cavernosum smooth muscle may be mainly mediated by NK1 receptors. The results obtained by the use of the subtype selective receptor antagonists (Figure 3) indicated that the tachykinin-induced contractions in the rabbit corpus cavernosum are mediated mainly by NK1 receptor and partly by NK2 receptor. The effect of SR 140333 (an NK1 receptor selective antagonist) was apparently non-competitive, because this antagonist reduced the tachykinin-induced maximum contractions. The non-competitive nature of the inhibition by SR 140333 toward smooth muscle contraction induced by tachykinins is in good agreement with the previous studies (Emonds-Alt et al., 1993; Goldhill et al., 1999). Although SR 48968 (an NK2 receptor selective antagonist) alone did not influence the contractile responses to tachykinins, it potentiated the inhibitory effects of SR 140333 on the tachykinin-induced contractions (Figure 3). A similar synergetic effect of these two compounds has been observed in the previous studies (Charette et al., 1994; Girard et al., 1997), using guinea-pig trachea expressing NK1 and NK2 receptors. To explain this phenomenon, Girard et al. (1997) speculated some form of cross-talk between NK1 and NK2 receptors. However, the minute mechanism for this phenomenon is still unclear. In addition, the involvement of NK3 receptors in the tachykinin-induced contractions could be ruled out, because the treatment with SR 142801 (an NK3 receptor selective antagonist) had no effect on the tachykinin-induced contractions (Figure 3) and because senktide (an NK3 selective agonist) did not induce any contractions.
The contraction of the corpus cavernosum smooth muscle was believed to be regulated by the level of [Ca2+]i (Stief et al., 1997), similar to other smooth muscles. Recently, the direct evidence for this was reported (Hashitani et al., 2002). They reported that the contraction of the corpus cavernosum smooth muscle was also accompanied by the sustained increase in [Ca2+]i. The contractions induced by tachykinins as well as those induced by phenylephrine and high K+ depolarization were accompanied by the increases in [Ca2+]i in the present study (Figure 4). Tachykinins induced the sustained increases in [Ca2+]i in the presence of extracellular Ca2+. These sustained increases in [Ca2+]i were thought to be mainly due to the Ca2+ influx from the extracellular space, because tachykinins induced small transient increases in the [Ca2+]i in the absence of extracellular Ca2+ (Figures 4b,d,f and 5). We thus concluded that: (1) Tachykinins induce contractions of the rabbit corpus cavernosum by increasing [Ca2+]i mainly through the Ca2+ influx from the extracellular space and (2) Tachykinins are able to release Ca2+ from intracellular store, but this is not enough to induce a sustained increase in [Ca2+]i. In addition, the previous study has described that in the human colon, which expresses both NK1 and NK2 receptors, NK2 receptor is involved in Ca2+ release (Cao et al., 2000). Therefore, we speculated that the residual contractions induced by tachykinins in the absence of extracellular Ca2+ in our preparations might be an NK2 receptor mediated component. However, as described in results, NK2 receptor antagonist had no effect on these contractions. Therefore in our preparation, the contractions induced by tachykinins in the absence of extracellular Ca2+ are solely mediated by NK1 receptor.
It is now generally accepted that, although smooth muscle contraction is primarily regulated by [Ca2+]i, the modulation of Ca2+ sensitivity also plays an important role in smooth muscle contraction (Nishimura et al., 1988; Somlyo & Somlyo, 1994; Kanaide, 1999). However, the involvement of this mechanism in the contraction of the corpus cavernosum smooth muscle has not yet been examined before, probably due to the lack of application of the receptor coupled permeabilization method for this tissue. The results obtained in Figures 4 and 6 demonstrated that the increase in Ca2+ sensitivity is involved in tachykinin-induced contractions. The possibility that the tachykinin-induced additional force developments might be due to the effect of Ca2+ release would be excluded by the following reasons: (1) In α-toxin permeabilized preparations, tachykinins did not induce any contractions in the nominally Ca2+ free 10 mM EGTA solution (Figure 6a) and (2) the tachykinin-induced additional contractions in the 300 nM Ca2+ solution could not be abolished by the treatment with 10 μM CPA (Figure 6b–d). It is thus concluded that three tachykinins induced contractions of the rabbit corpus cavernosum not only by increasing [Ca2+]i but also by increasing the Ca2+ sensitivity of the contractile apparatus, but the tachykinin receptor involved in this effect remains to be determined.
The clinical implications of the present results might be related to the pathogenesis of erectile dysfunction. The pathophysiology of erectile dysfunction is attributed to a metabolic imbalance between contractile and relaxatory factors in the corpus cavernosum. In the case of erectile dysfunction, contractile factors predominate, or relaxatory factors are inhibited and, as a result, the trabecular smooth muscle remains contracted and the penis remains flaccid (Moreland et al., 2001). It is thus speculated that the increased responsiveness to tachykinins or the decreased endopeptidase activity in the corpus cavernosum might be involved in the pathophysiology of erectile dysfunction. Because tachykinin receptor antagonists have been reported to possibly be clinically effective for the treatment of several human diseases such as irritable bowel syndrome, asthma and in hyperactive bladder (Lecci et al., 2000), such an approach could also potentially be useful as a treatment for erectile dysfunction.
Acknowledgments
We thank Mr Brian Quinn for comments and help with the manuscript. This study was supported in part by Grants-in-Aid for Scientific Research (No. 13557067, 13470149, 13832006, 13670723, 13671591, 14657174, 14570675) and for Scientific Research on Priority Area (No. 14026038) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, by the Research Grant for Cardiovascular Diseases (12C-2, 13C-4) from the Ministry of Health, Labour and Welfare, Japan, and by grants from the Japan Space Forum, Kanehara Ichiro Memorial Foundation and Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- DMEM
Dulbecco's modified Eagle medium
- EGTA
ethyleneglycol-bis (β-aminoethyl-ether)-N,N,N′,N′,-tetraacetic acid
- fura-PE3/AM
an acetoxymethylester form of fura-PE3
- L-NAME
L-Nω-nitro-arginine methylester
- NKA
neurokinin A
- NKB
neurokinin B
- PPAD
phosphoramidon
- PSS
physiological saline solution
- SP
substance P
References
- ANDERSSON K.E., HEDLUND H., MATTIASSON A., SJOGREN C., SUNDLER F. Relaxation of isolated human corpus cavernosum induced by vasoactive intestinal polypeptide, substance P, carbachol and electrical field stimulation. World J. Urol. 1983;1:203–208. [Google Scholar]
- ANDERSSON K.E., HOLMQUIST F. Regulation of tone in penile cavernous smooth muscle. World J. Urol. 1994;12:249–261. doi: 10.1007/BF00191204. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E. Pharmacology of penile erection. Pharmacol. Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- AZADZOI K.M., KIM N., BROWN M.L., GOLDSTEIN I., COHEN R.A., SAENZ DE TEJADA I. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J. Urol. 1992;147:220–225. doi: 10.1016/s0022-5347(17)37201-4. [DOI] [PubMed] [Google Scholar]
- CAO W., PRICOLO V.E., ZHANG L., BEHAR J., BIANCANI P., KIRBER M.T. Gq-linked NK2 receptors mediate neurally induced contraction of human sigmoid circular smooth muscle. Gastroenterology. 2000;119:51–61. doi: 10.1053/gast.2000.8552. [DOI] [PubMed] [Google Scholar]
- CHARETTE L., FOULON D., RODGER I.W., JONES T.R. Neurokinin (NK2) receptors mediate nonadrenergic noncholinergic contractile responses to electrical stimulation and resiniferatoxin in guinea pig trachea. Can. J. Physiol. Pharmacol. 1994;72:182–188. doi: 10.1139/y94-028. [DOI] [PubMed] [Google Scholar]
- COUTURE R., REGOLI D. Mini review: smooth muscle pharmacology of substance P. Pharmacology. 1982;24:1–25. doi: 10.1159/000137572. [DOI] [PubMed] [Google Scholar]
- DE LEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am. J. Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- D'ORLEANS-JUSTE P., DION S., DRAPEAU G., REGOLI D. Different receptors are involved in the endothelium-mediated relaxation and the smooth muscle contraction of the rabbit pulmonary artery in response to substance P and related neurokinins. Eur. J. Pharmacol. 1985;125:37–44. doi: 10.1016/0014-2999(86)90081-6. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., DOUTREMEPUICH J.D., HEAULME M., NELIAT G., SANTUCCI V., STEINBERG R., VILAIN P., BICHON D., DUCOUX J.P., PROIETTO E., VAN BROECK D., SOUBRIE P., LE FUR G., BRELIERE J.C. In vitro and in vivo biological activities of SR 140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- ERSPAMER V. The tachykinin peptide family. Trends Neurosci. 1981;4:267–269. [Google Scholar]
- GIRARD V., FELETOU M., ADVENIER C., CANET E. Effects of tachykinins and capsaicin on the mechanical and electrical activity of the guinea-pig isolated trachea. Br. J. Pharmacol. 1997;122:841–848. doi: 10.1038/sj.bjp.0701459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDHILL J., PORQUET M.F., SELVE N. Antisecretory and relaxatory effects of tachykinin antagonists in the guinea-pig intestinal tract. J. Pharm. Pharmacol. 1999;51:1041–1048. doi: 10.1211/0022357991773375. [DOI] [PubMed] [Google Scholar]
- GU J., POLAK J.M., PROBERT L., ISLAM K.N., MARANGOS P.J., MINA S., ADRIAN T.E., MCGREGOR G.P., O'SHAUGHNESSY D.J., BLOOM S.R. Peptidergic innervation of the human male genital tract. J. Urol. 1983;130:386–391. doi: 10.1016/s0022-5347(17)51174-x. [DOI] [PubMed] [Google Scholar]
- HASHITANI H., FUKUTA H., DICKENS E.J., SUZUKI H. Cellular mechanisms of nitric oxide-induced relaxation of corporeal smooth muscle in the guinea-pig. J. Physiol. 2002;538.2:573–581. doi: 10.1113/jphysiol.2001.013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDLUND H., ANDERSSON K.E. Effects of some peptides on isolated human penile erectile tissue and cavernous artery. Acta Physiol. Scand. 1985;124:413–419. doi: 10.1111/j.1748-1716.1985.tb07677.x. [DOI] [PubMed] [Google Scholar]
- KANAIDE H.Measurement of [Ca2+]i in smooth muscle strips using front-surface fluorimetry Methods in Molecular Biology, Vol 114: Calcium Signaling Protocols 1999Totowa, NJ: Humana Press Inc; ed(ed) Lambert, D.G [DOI] [PubMed] [Google Scholar]
- KIM N., AZADZOI K.M., GOLDSTEIN I., SAENZ DE TEJADA I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J. Clin. Invest. 1991;88:112–118. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., TRAMONTANA M., CARINI F., MAGGI C.A. Peripheral actions of tachykinins. Neuropeptides. 2000;34:303–313. doi: 10.1054/npep.2000.0825. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Progress Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- MASTRANGELO D., MATHISON R., HUGGEL H.J., DION S., D'ORLEANS-JUSTE P., RHALEB N.E., DRAPEAU G., ROBERO P., REGOLI D. The rat isolated portal vein: a preparation sensitive to neurokinins, particularly neurokinin B. Eur. J. Pharmacol. 1986;134:321–326. doi: 10.1016/0014-2999(87)90363-3. [DOI] [PubMed] [Google Scholar]
- MORELAND R.B., HSIEH G., NAKANE M., BRIONI J.D. The biochemical and neurologic basis for the treatment of male erectile dysfunction. J. Pharmacol. Exp. Ther. 2001;296:225–234. [PubMed] [Google Scholar]
- NISHIMURA J., KOLBER M., VAN BREEMEN C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem. Biophys. Res. Commun. 1988;157:677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- OKAMURA T., AYAJIKI K., FUJIOKA H., TODA M., FUJIMIYA M., TODA N. Effects of endothelial impairment by saponin on the responses to vasodilators and nitrergic nerve stimulation in isolated canine corpus cavernosum. Br. J. Pharmacol. 1999;127:802–808. doi: 10.1038/sj.bjp.0702623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUKA N., YOSHIOKA K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., BARBAGLI G., PALMINTERI E., LAZZERI M., TURINI D., MAGGI C.A. Tachykinin NK1 and NK2 receptors mediate inhibitory vs excitatory motor responses in human isolated corpus cavernosum and spongiosum. Br. J. Pharmacol. 2002;135:1351–1354. doi: 10.1038/sj.bjp.0704650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIDA K., NONOMURA Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J. Gen. Physiol. 1978;481:677–688. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRAHASE H., KANDA M., KURAHASHI K., NAKAMURA S., USHI H., SHIMIZU Y. Endothelium-dependent contraction in intrapulmonary arteries: mediation by endothelial NK1 receptors and TXA2. Br. J. Pharmacol. 1995;115:1215–1220. doi: 10.1111/j.1476-5381.1995.tb15028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- STIEF C.G., NOACK T., ANDERSSON K.E. Signal transduction in cavernous smooth muscle. World J. Urol. 1997;15:27–31. doi: 10.1007/BF01275153. [DOI] [PubMed] [Google Scholar]
- TAKAGI M., MOCHIDA H., NOTO T., YANO K., INOUE H., IKEO T., KIKKAWA K. Pharmacological profile of T-1032, a novel specific phosphodiesterase type 5 inhibitor, in isolated rat aorta and rabbit corpus cavernosum. Eur. J. Pharmacol. 2001;411:161–168. doi: 10.1016/s0014-2999(00)00907-9. [DOI] [PubMed] [Google Scholar]


