Abstract
The role of central 5-HT1A receptors in the control of the bradycardia and changes in central respiratory drive, renal nerve activity and blood pressure evoked by stimulating cardiopulmonary afferents with phenylbiguanide, baroreceptors by electrical stimulation of the aortic nerve and chemoreceptors by injections of sodium cyanide (NaCN) in atenolol-pretreated anaesthetized rabbits were studied.
Buspirone (100 μg kg−1; i.c.) potentiated the bradycardia (increase in R-R interval) and the changes in blood pressure and renal nerve activity evoked by all three reflexes. These effects could be attenuated by pretreatment with the 5-HT1A receptor antagonist WAY-100635 (100 μg kg−1); i.v.), which alone had no effect on these reflex-evoked changes. However, WAY-100635 (100 μg kg−1; i.c.) did attenuate these reflex-evoked responses produced by activation of cardiopulmonary and aortic baroreceptors but not that caused by stimulation of chemoreceptors. When given i.v., buspirone was less effective in modulating the responses evoked by these three reflexes.
The present data are consistent with the view that central 5-HT1A receptors play a role in the reflex activation of cardiac preganglionic vagal motoneurones. However, although antagonists of 5-HT1A receptors affected the responses evoked by cardiopulmonary and aortic nerve afferents, they were not effective on chemoreceptor reflex-evoked changes. This suggests that 5-HT1A receptors play a different role in chemoreceptor pathways compared to that for the other reflexes. This may relate to the fact that the chemoreceptor afferents travel in the IXth (glossopharyngeal) nerve whilst the other afferents travel in the Xth (vagus) nerve and thus may use different central circuitry and neurotransmitters.
Keywords: 5-HT1A receptors, heart rate, baroreceptors, chemoreceptors, cardiopulmonary receptors, buspirone, WAY-100635, rabbits, sympathetic outflow
Introduction
Central applications of 5-HT1A receptor agonists have been shown to increase vagal drive to the heart in anaesthetized cats (Shepheard et al., 1994), rats (Sporton et al., 1991) and rabbits (Dando et al., 1998) and this was associated with hypotension and sympathoinhibition. These vagal effects on the heart may be due to actions at the level of the nucleus tractus solitarius (NTS) where cardiovascular afferents terminate (see Jordan & Spyer, 1986; Lawrence & Jarrott, 1996) and/or at the dorsal vagal motor nucleus (DVN) and/or nucleus ambiguus (NA), the location of cardiac vagal preganglionic neurones (Nosaka et al., 1979; 1982; Jordan et al., 1982). Indeed, microinjections of 5-HT1A receptor agonists into the DVN (Sporton et al., 1991) or NA (Izzo et al., 1988; Chitravanshi & Calaresu, 1992) evoke vagal bradycardia. This is consistent with the presence of binding sites for 5-HT1A receptors in these regions in rats (Pazos & Palacios, 1985; Thor et al., 1992), cats (Dashwood et al., 1988) and humans (Pazos et al., 1987) and the demonstration that 5-HT immunoreactive fibres innervate these areas (Steinbusch, 1981), where they make synaptic contact with cardiac vagal preganglionic neurones (Izzo et al., 1988; 1993). Finally, ionophoretic applications of 5-HT1A receptor agonists have been shown to have both excitatory and inhibitory actions on neurones within the dorsal vagal nucleus (Wang et al., 1995), nucleus ambiguus (Wang & Ramage, 2001) and nucleus tractus solitarius (Wang et al., 1997).
In addition to baseline cardiovascular variables, cardio-respiratory reflexes also appear to be modified by 5-HT1A receptor activation. The reflex fall in heart rate produced by stimulating cardiopulmonary afferents in rats was attenuated by central administration of 5-HT1A receptor antagonists (Bogle et al., 1990) suggesting a facilitatory role for these receptors. However, in anaesthetized rabbits, the archetypal 5-HT1A receptor agonist 8-OH-DPAT attenuated, rather than potentiated, the vagal bradycardia evoked by stimulating upper airway, whereas the partial agonist buspirone (Schoeffter & Hoyer, 1988) potentiated the bradycardia (Futuro-Neto et al., 1993). In a recent detailed study, which re-examined the modulatory effects on this reflex this contradiction was explained (Dando et al., 1998). The potentiating action of buspirone, but not the attenuating actions of 8-OH-DPAT were antagonized by the 5-HT1A receptor antagonist WAY-100635. Thus in rabbits, buspirone appears to act primarily on 5-HT1A receptors, whereas the overriding effect of 8-OH-DPAT is on 5-HT1B/1D receptors, although from binding data it has low affinity for these receptors (Hoyer et al., 1994). Interestingly, in those experiments, buspirone potentiated the fall in heart rate, but not the pressor response, renal sympatho-excitation or apnoea evoked by stimulating upper airway receptors. This might suggest that the agonist acts at the level of the motor outflow rather than at the NTS where all components of the reflex might be expected to be affected. If this were true, it might be expected that all reflex-evoked changes in heart rate mediated by these preganglionic neurones would be similarly modified by buspirone. The present experiments tested this hypothesis by comparing the reflex falls in heart rate evoked by stimulating aortic nerve (primarily baroreceptor) afferents, cardiopulmonary and arterial chemoreceptor afferents before and following administration of buspirone and the 5-HT1A receptor antagonist WAY-100635 (Forster et al., 1995). Preliminary accounts of some of this work have been published (Skinner et al., 1997a,1997b,1997c; 1998).
Methods
The experiments were carried out under the Animals (Scientific Procedures) Act 1986. After completion of experiments, animals were killed by an overdose of pentobarbitone sodium (i.v.).
General preparation
Experiments were performed on 25 male New Zealand White rabbits (2.3–3.6 kg body weight) anaesthetized with urethane (1.5 g kg−1 given via an ear vein) and supplemented with 0.15 g kg−1 i.v. when necessary. The level of anaesthesia was assessed by regular monitoring of the stability of arterial blood pressure, heart rate, phrenic nerve discharge, and the absence of limb withdrawal in response to paw pinch. The animal preparation was similar to that described by Dando et al. (1998). A tracheotomy was performed low in the neck to allow the lungs to be ventilated with O2-enriched room air if necessary. The right and left axillary arteries were cannulated for measurement of arterial blood pressure using a pressure transducer (Gould Statham P23) and to allow arterial blood samples to be taken. Arterial blood gases were measured (Corning 238 pH/blood gas analyser) and kept within the ranges PO2 100–140 mmHg, PCO2 25–40 mmHg, [HCO3−] 12–20 mMol l−1 and pH 7.30–7.45. The left and right axillary veins were cannulated for administration of drugs and an infusion respectively. The infusion solution (6 ml kg−1 h−1), which maintained blood volume and prevented non-respiratory acidosis, consisted of 50 ml each of distilled water and gelofusin containing 0.84% (w v−1) sodium bicarbonate and 0.2% glucose. In all experiments rabbits were pretreated with the β1-adrenoceptor antagonist atenolol (1 mg kg−1) to block sympathetic drive to the heart (see Bogle et al., 1990). Any subsequent changes in heart rate could be presumed to be due to changes in vagal drive to the heart. The bladder was also cannulated to prevent overfilling during the course of the experiments. In experiments in which cardiopulmonary receptors were stimulated, a cannula was inserted via the jugular vein so that its tip lay in the right atrium. In addition, in some animals a small polyethylene cannula (0.28 mm i.d.) was inserted into the lingual artery to allow access to the carotid body chemoreceptors. Lead II E.C.G. was recorded for measurement of R-R intervals. Following placement in a stereotaxic head frame, the atlanto-occipital membrane was exposed and a needle connected to a 100 μl Hamilton syringe used to gain access to the cisternal space (see Dando et al., 1998). The phrenic nerve was isolated low in the neck by a lateral approach, crushed peripherally, and placed on bipolar silver wire recording electrodes. Activity was amplified (Neurolog NL104) and filtered (NL125, 0.2–10 kHz). The left renal nerve was isolated by a retroperitoneal approach, placed on bipolar silver wire recording electrodes and isolated from the surrounding medium with President light body dental polyvinylsiloxane (Coltene). Renal nerve activity (RNA) was amplified (NL104), filtered (NL125, 100–1500 Hz) and integrated (NL703, 20 ms time constant). In some experiments the left aortic nerve was isolated from the vago-sympathetic trunk and placed on bipolar silver wire stimulating electrodes and was electrically stimulated (1 ms, 5–7.5 V for 5 s) at six frequencies (5, 10, 20, 40, 80 and 160 Hz) at 2 min intervals. In other experiments, stimulation was performed only at the two highest frequencies. Since in the rabbit the aortic nerve contains mainly baroreceptor afferent fibres (Neil et al., 1949), this was taken to represent stimulation of arterial baroreceptors. Carotid arterial chemoreceptors were stimulated by bolus injections (8–189 μg kg−1) of 0.075–0.5% sodium cyanide, through the catheter inserted into the lingual artery. Cardiopulmonary afferents were stimulated by injections of phenylbiguanide (PBG; 7–40 μg kg−1) into the right atrium (Dawes & Comroe, 1954; Coleridge & Coleridge, 1984). PBG acts on 5-HT3 receptors to activate these vagal afferents (Kay & Armstrong, 1990). For stimulation of carotid chemoreceptors and cardiopulmonary afferents doses of sodium cyanide and phenylbiguanide were chosen that evoked submaximal changes in heart rate.
Protocols
To compare directly the effects of ligands on the different reflex responses, in each animal two different stimuli were given alternately. A schematic diagram of the protcols used in these experiments is shown in Figure 1. Control stimuli were repeated until consistent heart rate responses of similar magnitude were attained. Five minutes following a stimulus the selectiveβ1-adrenoceptor antagonist atenolol (1 mg kg−1; i.v.) was administered. After injection of the atenolol the stimuli were resumed until consecutive responses of similar magnitude were produced. Buspirone, saline vehicle (Figure 1a,b) or WAY-100635 (Figure 1c) were then administered either i.c. in a volume of 20 μl or i.v. in a volume of 1 ml given over 20 s and the alternating stimuli resumed. In some experiments the buspirone injection was preceded by intravenous administration of WAY-100635 (Figure 1a and b–shaded).
Figure 1.
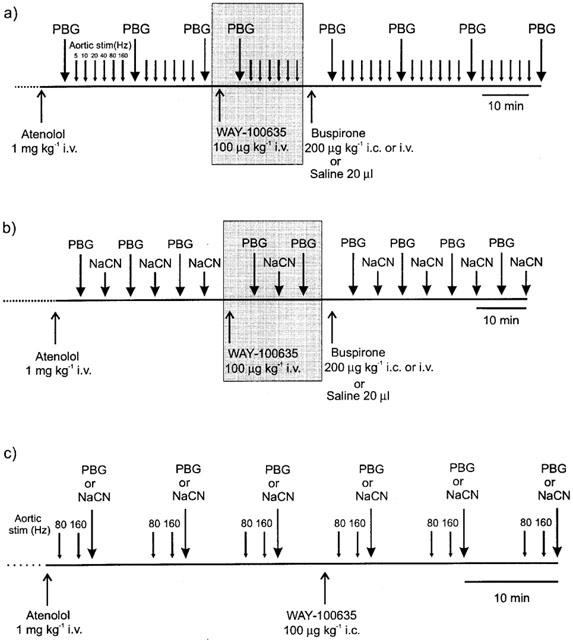
Diagrams illustrating the experimental protocols used to investigate: (a) the effects of drug or vehicle applied i.c. or i.v. on the cardiorespiratory responses evoked alternately by stimulating cardiopulmonary afferents with PBG and electrical stimulation of the aortic nerve afferents at increasing frequencies. In some experiments (shaded) the effects of buspirone were tested following intravenous pre-treatment with the 5-HT1A receptor antagonist WAY-100635. (b) the effects of drug or vehicle applied i.c. or i.v. on the cardiorespiratory responses evoked alternately by stimulating cardiopulmonary afferents with PBG and the carotid chemoreceptors with sodium cyanide (NaCN). In some experiments (shaded) the effects of buspirone were tested following pre-treatment with the 5-HT1A receptor antagonist WAY-100635 i.v. (c) the effects of the 5-HT1A receptor antagonist WAY-100635 applied i.c. on the cardiorespiratory responses evoked alternately by electrical stimulation of aortic nerve afferents and cardiopulmonary afferents with PBG or carotid chemoreceptors with sodium cyanide (NaCN).
Protocol 1
Investigation of the effect of buspirone on responses evoked by stimulation of cardiopulmonary afferents (PBG) and aortic nerve stimulation. Five minutes after administration of atenolol, alternate PBG and aortic nerve stimuli at six different frequencies (5, 10, 20, 40, 80 and 160 Hz) at 2 min intervals were made. PBG was given first, followed 2.5 min later by the first aortic nerve stimulation. Another PBG stimulus was given 2.5 min after the last aortic stimulation. After a control period of three PBG stimuli and two sets of aortic nerve stimulation, saline or buspirone were given i.c. 2.5 min after the last PBG injection. Five minutes after the i.c. injection of the test solution the cycle (length 15 min) of PBG and aortic nerve stimuli were repeated for a period of 45 min (see Figure 1a). To test the involvement of 5-HT1A receptors in the effects of buspirone on the reflexes, in some experiments (Figure 1a, shaded) WAY-100635 was given i.v. 2.5 min after the third PBG stimulus. After 5 min a cycle of PBG and aortic nerve stimuli were performed. Buspirone was injected i.c. 2.5 min after the last aortic stimulation and 5 min after this i.c. injection the cycles of PBG and aortic stimuli were repeated.
Protocol 2
Investigation of the effect of buspirone on responses evoked by stimulation of carotid chemoreceptor and cardiopulmonary (PBG) afferents. Five minutes after administration of atenolol, alternate PBG (acting as a positive control) and NaCN stimuli were performed at 5 min intervals. Five minutes after the third NaCN injection buspirone or saline were administered i.c. followed 5 min later by PBG and then 5 min later NaCN and so on (see Figure 1b). To test the involvement of 5-HT1A receptors in the effects of buspirone on these reflexes, in some experiments (Figure 1b, shaded) WAY-100635 was given i.v. 5 min after the third NaCN injection and 5 min later PBG was injected followed by NaCN and then PBG. Five minutes after this injection of PBG buspirone was administered i.c. and 5 min later the alternating stimuli recommenced.
Protocol 3
Investigation the effect of WAY-100635 on the responses evoked by stimulation of carotid chemoreceptor, cardiopulmonary and aortic nerve afferents. One and a half min after atenolol administration the aortic nerve was stimulated at 80 Hz and then 2 min later at 160 Hz. After a further 1.5 min a NaCN or PBG injection was carried out. This pattern was then repeated twice at 10 min intervals. WAY-100635 was given i.c. 5 min after the last of these NaCN or PBG injections and 1.5 min later the 80 Hz stimulation was performed and the cycles resumed (see Figure 1c).
Analysis of data
Arterial blood pressure, ECG, phrenic nerve activity, raw and integrated renal nerve activity were recorded onto a computer hard disk and optical disk (Panasonic LF7010) using a CED 1401+interface and Spike2 data collection software. Heart rate was derived electronically from the blood pressure signal using a tachometer (Grass Instruments 7P4). The R-wave of the ECG was discriminated using an amplitude discriminator (Neurolog NL201, Digitimer) and R-R intervals calculated from the output using Spike2 data analysis software. R-R intervals were measured immediately before a reflex stimulus and at the maximal R-R interval attained. From these two measurements, the reflex change in R-R interval was obtained (max R-R interval-baseline R-R interval). Mean arterial pressure (MAP), calculated as diastolic pressure+(systolic pressure-diastolic pressure)/3, and was derived immediately before a stimulus (baseline) and at the peak response. Changes in MAP (peak response MAP-baseline MAP) were calculated for each response. Renal nerve activity was rectified and integrated with a 20 ms time constant using an EMG integrator (Neurolog NL 703; Digitimer Ltd.). The mean level of integrated RNA (IRNA) for the 30 s periods prior to (baseline) and following a reflex stimulus was derived using a Spike2 data analysis script, and the reflex change calculated (reflex IRNA-baseline IRNA). Due to the shorter duration of the responses, in the case of the baroreceptor reflexes, renal nerve activities were derived over 10 s periods. Since the absolute values of RNA vary substantially between animals, the mean control values of the baseline and reflex changes in IRNA were normalized to 100%. Changes evoked by the drug were then compared to these pre-drug values. Raw phrenic nerve activity (PNA) was displayed using Spike2 data analysis package. The mean rates of phrenic bursts over the 30 s period prior to and immediately following each stimulus were calculated. For all measured variables statistical analyses were performed using ANOVA (2-way for chemoreceptor and cardiopulmonary reflexes, 3-way for baroreceptor reflexes) and least significant difference test to allow time-matched comparison of drug-induced changes with those of vehicle alone. Changes in baseline or response size were calculated by subtracting the mean control value before the drug from the absolute value at any given time after treatment. The changes in these values were compared with the relevant values obtained at the same time points in the vehicle treatment group. All values are expressed as mean±s.e.mean. Differences in mean were taken as significant when P<0.05.
Drugs and solutions
Drugs and chemicals were obtained from the following sources: urethane, atenolol, buspirone (8-(4-(4-(2-pyrimidinyl)-1-piperazinyl)butyl)-8-azaspirol(4,5)decane-7,9-dione hydrochloride) and WAY-100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide trichloride) from Sigma Aldrich Chemical Co., Poole, Dorset, U.K.; sodium cyanide from Merck, Poole, Dorset, U.K. and gelofusin from Braun Medical Ltd, Aylesbury, Bucks. U.K. All drugs were dissolved in 0.9% saline.
Results
Effect of 5-HT1A receptor ligands on baseline variables
The resting baseline values of the cardio-respiratory variables measured in each of the experimental groups of animals are given in Table 1. For each experimental drug regime, the change in these baseline variables at 5 and 20 min following drug or vehicle administration are also given. There were no significant differences between baseline variables in any of the groups. The effects of buspirone and WAY-100635 on these baseline values are similar to those reported previously (Dando et al., 1998) and thus will only be summarized briefly (see Table 1). Administration of the saline vehicle had no significant effect on any of the measured variables at any of the time points. Central administration of buspirone (200 μg kg−1, i.c.) produced significant increases in R-R interval (fall in heart rate) and phrenic burst rate as described previously (Dando et al., 1998). Within 5 min renal nerve activity increased by 70±38% and this was associated with a small and significant increase in arterial blood pressure (6±7 mmHg). However, renal nerve activity soon returned to control levels and this was associated with a significant decline in blood pressure (−17±4 mmHg from baseline). The buspirone-evoked increases in baseline R-R interval and phrenic rate were not observed when the same dose of buspirone was given intravenously.
Table 1.
Resting (baseline) mean arterial blood pressure (MAP; mmHg), R-R interval (ms) and phrenic nerve activity (burst min−1) before vehicle or drug and changes (Δ) in these baseline variables including per cent change in renal nerve activity (RNA; %) 5 and 20 min following saline vehicle or drug challenge in atenolol (1 mg kg−1) pretreated spontaneously breathing anaesthetized rabbits
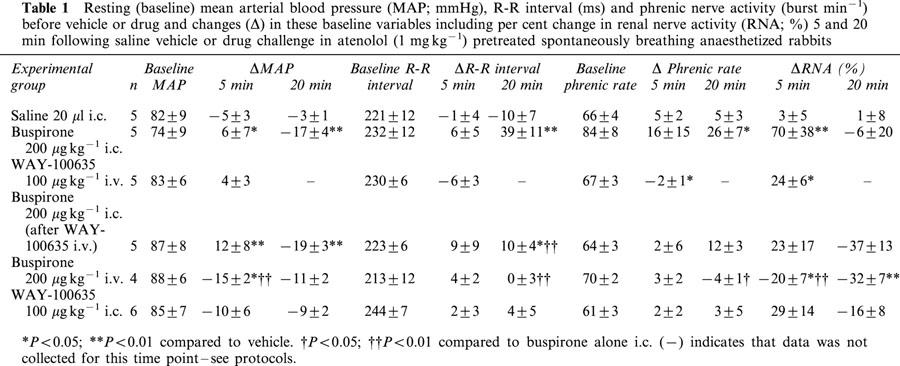
Central administration of the 5-HT1A receptor antagonist WAY-100635 (100 μg kg−1; i.c.) had no significant effects on any of the cardiorespiratory variables measured. However, 5 min after i.v. administration, renal nerve activity had significantly increased by 24±6% and phrenic burst rate had significantly declined by 2±1 burst min−1. Further, i.v. WAY-100635 only significantly attenuated the i.c. buspirone-evoked bradycardia, although the renal sympatho-excitation was no longer significant when compared to vehicle.
Effect of 5-HT1A receptor ligands on cardiopulmonary afferent-evoked changes caused by right atrial injection of phenylbiguanide
Right atrial bolus administration of phenylbiguanide (PBG, 7–40 μg kg−1; n=30) evoked an increase in R-R interval of 39±3 ms from a control of 240±4 ms and a fall in renal nerve activity of 46±3%. Mean arterial blood pressure was reduced by 30±1 mmHg from a control of 81±2 mmHg and phrenic burst rate increased by 31±2 from 71±2 bursts min−1. These reflex changes usually occurred within 2 s of the PBG stimulus and returned to baseline within 30 s, except for the changes in phrenic rate, which sometimes took up to 2 min to return to baseline (see Figure 2). The size of the reflex responses evoked in the individual experimental groups of animals are shown in Figure 3 along with the effect on the size of the reflex response at 5 and 20 min following each drug or vehicle administration.
Figure 2.
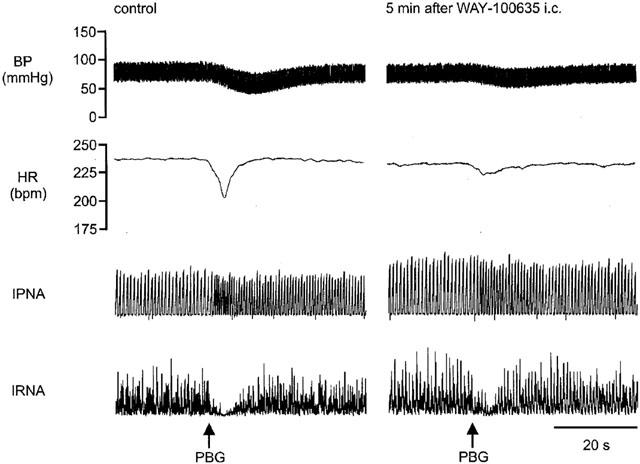
Traces from an anaesthetized spontaneously breathing rabbit pretreated with atenolol (1 mg kg−1; i.v.) showing the effects on arterial blood pressure (BP), heart rate (HR), integrated phrenic nerve activity (IPNA) and integrated renal sympathetic nerve activity (IRNA), produced by right atrial bolus injection of phenylbiguanide (PBG; 10 μg kg−1) before and 5 min after administration of WAY-100635 (100 μg kg−1; i.c.).
Figure 3.
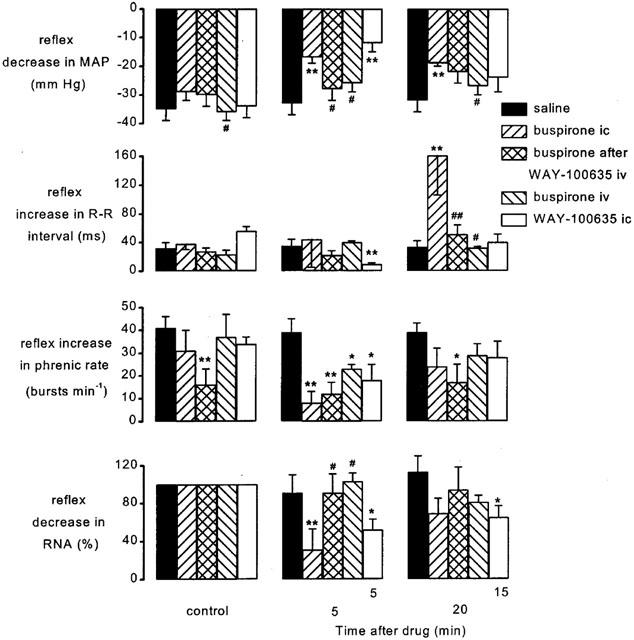
Atenolol (1 mg kg−1; i.v.) pretreated spontaneously breathing anaesthetized rabbits: histograms showing the size of the PBG-evoked decrease in mean arterial pressure (MAP), increase in R-R interval, increase in phrenic burst rate and decrease in renal sympathetic nerve activity (RNA) before (control, left columns), at 5 (middle columns) and 20 min (right columns) following administration of saline (20 μl, i.c.; n=5), buspirone (200 μg kg−1; i.c.; n=5), buspirone (200 μg kg−1; i.c.) following pretreatment with WAY-100635 (100 μg kg−1; i.v.; n=5), buspirone (200 μg kg−1; i.v.; n=4) and WAY-100635 (100 μg kg−1; i.c.; n=6). It should be noted that WAY-100635 i.c. study has been carried out using a different protocol to the rest of the data and thus time after drugs is different (5 and 15 min). Each column shows the mean change in the size of the reflex and bars show s.e.mean. Changes caused by the treatment have been compared with time-matched controls. *P<0.05, **P<0.01 compared to vehicle (saline); #P<0.05, ##P<0.01 compared to buspirone i.c. alone.
After administration of saline (20 μl i.c.; n=5) the reflex responses evoked by PBG were not significantly different from control stimuli. However, intracisternal (i.c.) administration of buspirone (200 μg kg−1; n=5) significantly potentiated the reflex increase in R-R interval by 124±56 ms from 38±8 ms after 20 min (Figure 3) compared with saline. Within 5 min buspirone had also attenuated the reflex hypotension by 12±4 mmHg, the renal sympathoinhibition by 69±22%, and the increase in phrenic rate by 23±7 bursts min−1. Pretreatment with WAY-100635 (100 μg kg−1 i.v.) had no significant effect on the cardiopulmonary reflex responses but it significantly reduced the effects of buspirone on these responses. Buspirone still attenuated the reflex increase in phrenic rate but did not attenuate the reflex hypotension, renal sympathoinhibition or potentiate the increase in R-R interval. Buspirone given intravenously was without effect on the reflex bradycardia hypotension or renal sympathoinhibition. However, it did produce a small attenuation of the tachypnoea (Figure 3). Central administration of WAY-100635 attenuated all the effects of cardiopulmonary afferent stimulation on the variables recorded (Figure 3). Within 5 min the reflex increase in R-R interval (56±6 ms) was significantly reduced by 46±6 ms and the reflex hypotension, tachypnoea and renal sympatho inhibition were reduced by 22±4 mmHg, 16±8 bursts min−1 and 48±11%, respectively.
Effect of 5-HT1A receptor ligands on aortic nerve-evoked responses
Control electrical stimulation of the left aortic nerve (5–7.5 V, 1 ms, 5–160 Hz) evoked reflex increases in R-R interval, inhibition of renal sympathetic nerve activity and hypotension, which were frequency dependent (Figures 4 and 5). Maximal responses were obtained at 80 Hz, those evoked by stimulation at 160 Hz were not significantly greater. Stimulation of the aortic nerve at 20 Hz and above evoked a small but not statistically significant increase in phrenic burst rate, and this did not increase further on increasing frequency of stimulation (Figure 5).
Figure 4.
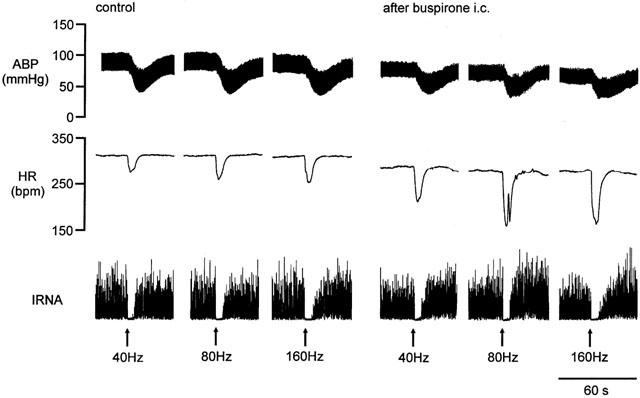
Traces from an anaesthetized spontaneously breathing rabbit pretreated with atenolol (1 mg kg−1; i.v.) showing the effects on arterial blood pressure (BP), heart rate (HR), and integrated renal sympathetic nerve activity (IRNA) produced by electrical stimulation of the left aortic nerve at 40, 80 and 160 Hz during the control period and during the first set of stimuli following administration of buspirone (200 μg kg−1; i.c.).
Figure 5.
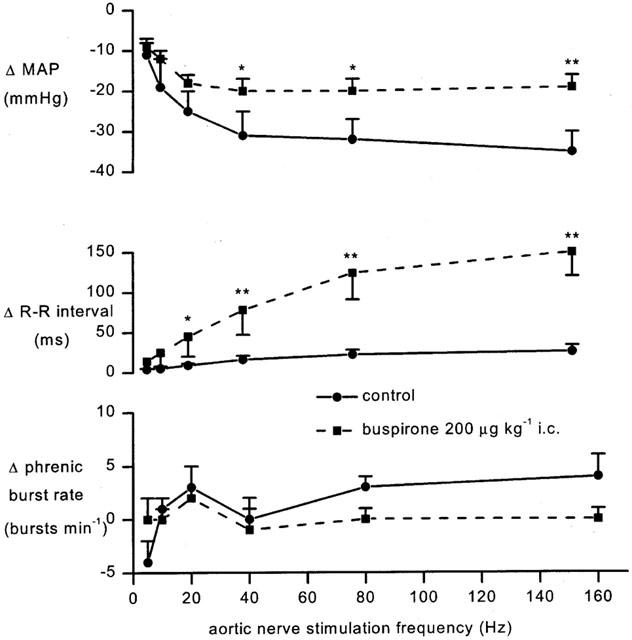
Anaesthetized spontaneously breathing rabbit pretreated with atenolol (1 mg kg−1; i.v.) showing the changes (δ) from baseline in mean arterial blood pressure (MAP), R-R interval and phrenic burst rate evoked by aortic nerve stimulation (5–7.5 v, 1 ms) at increasing frequencies 7.5 min after saline (20 μl; i.c.) or buspirone (200 μg kg−1; i.c.). Each point represents the mean value and the error bars show s.e.mean (n=5), *P<0.05, **P<0.01 compared to saline i.c.
The effects of buspirone and WAY-100635 on these responses were investigated using the protocols shown in Figure 1a,c. Although the effects of ligands were tested on all frequencies of stimulation, this description will concentrate on those evoked by 80 Hz stimuli as representative of the group. The size of the reflex responses evoked in the individual experimental groups of animals and the effect on the size of the reflex response to 80 Hz stimulation 7.5 and 22.5 min following each drug or vehicle administration are shown in Figure 6. Since the aortic nerve stimuli evoked only small changes in phrenic burst rate and none of the ligands had any effect on these responses, this will not be considered further (see Figure 5). Within 7.5 min of its application, buspirone (200 μg kg−1; i.c.) potentiated the aortic nerve stimulation-evoked increases in R-R interval (Figure 5). The response to 80 Hz stimulation was significantly increased from 34±8 ms to 124±33 ms, an increase of 89±29 and this was still apparent after 22.5 min. Despite the larger falls in heart rate there were small but significant reductions in the hypotensive responses over the same timescales, although renal evoked sympathoinhibition was unaffected. Administration of the same dose of buspirone i.v. failed to cause any significant change in the reflex decreases in R-R interval or blood pressure at the 40 Hz stimulation frequency but it did significantly potentiate the 80 Hz evoked-bradycardia at 7.5 min though this was significantly smaller than that observed for the i.c. route of administration (Figure 6). WAY-100635 (100 μg kg−1) given i.v. was without effect on any of the reflex responses. Nevertheless this dose of WAY-100635 significantly attenuated the ability of buspirone to potentiate the reflex bradycardia and to reduce the reflex hypotension, although this only occurred at the 80 Hz frequency (Figure 6). A full data set for 160 Hz aortic stimulation has not been included for the sake of clarity as it did not differ from that of 80 Hz. Using another experimental protocol (Figure 1c) within 1.5 min of i.c. administration, WAY-100635 (100 μg kg−1) significantly reduced the reflex bradycardia and hypotension evoked by 80 or 160 Hz stimulation (Figures 6 and 7). Interestingly, the renal sympathoinhibition evoked by aortic nerve stimulation at 160 Hz was also significantly attenuated by 73±14% (Figure 7).
Figure 6.
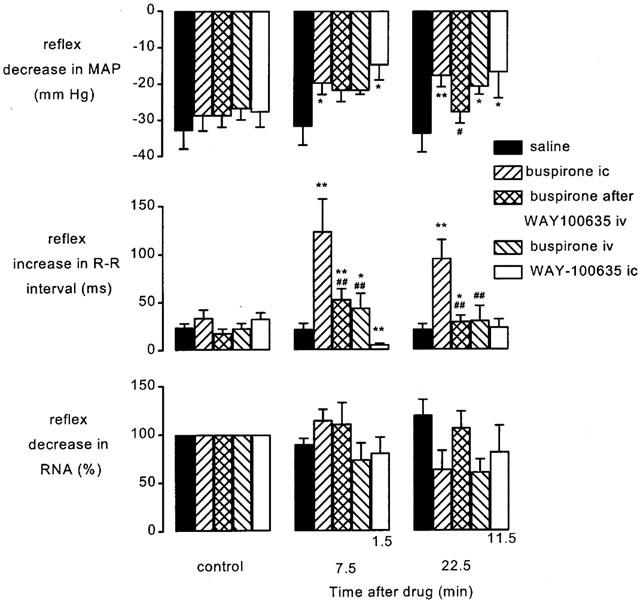
Atenolol (1 mg kg−1; i.v.) pretreated spontaneously breathing anaesthetized rabbits: histograms showing the changes in the size of the aortic nerve-evoked (80 Hz decrease in mean arterial pressure (MAP), increase in R-R interval and decrease in renal sympathetic nerve activity (RNA) before (control, left columns), at 7.5 (middle columns) and 22.5 min (right columns) following administration of saline (20 μl, i.c.; n=5), buspirone (200 μg kg−1; i.c.; n=5), buspirone (200 μg kg−1, i.c.) following pretreatment with WAY-100635 (100 μg kg−1; i.v.; n=5), buspirone (200 μg kg−1; i.v.; n=4) and WAY-100635 (100 μg kg−1; i.c.; n=6). It should be noted that WAY-100635 i.c. study has been carried out using a different protocol to the rest of the data and thus time after drugs is different (1.5 and 11.5 min). Each column shows the mean change in the size of the reflex and bars show s.e.mean. Changes caused by the treatment have been compared with time-matched controls. *P<0.05, **P<0.01 compared to saline #P<0.05, ##P<0.01 compared to buspirone i.c. alone.
Figure 7.
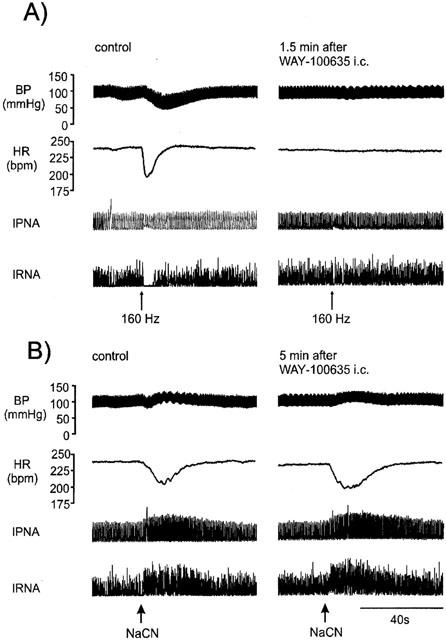
Traces from an anaesthetized spontaneously breathing rabbit pretreated with atenolol (1 mg kg−1; i.v.) showing the effects on arterial blood pressure (BP), heart rate (HR), integrated phrenic nerve activity (IPNA) and integrated renal nerve sympathetic activity (IRNA) produced by (A) aortic nerve stimulation (160 Hz) and (B) injection of sodium cyanide (NaCN) via lingual artery before and after administration of WAY-100635 (100 μg kg−1; i.c.).
Effect of 5-HT1A receptor ligands on carotid chemoreceptor-evoked responses
Carotid arterial chemoreceptors were stimulated by bolus injections of NaCN (8–189 μg kg−1) into the carotid artery. As a group (n=25) chemoreceptor stimuli increased R-R interval by 29±3 ms, renal nerve activity by 74±14%, phrenic burst rate by 14±1 bursts min−1 and mean arterial blood pressure by 8±1 mmHg. These reflex changes occurred within 2 s of the injection of NaCN, were short lasting, and returned to normal values within 30 s. The effects of buspirone and WAY-100635 on these responses were investigated using the protocols shown in Figure 1b,c. The size of the reflex responses evoked in the individual experimental groups of animals and the effect on the size of this reflex response 10 and 20 min following each drug or vehicle administration are shown in Figure 8. Saline i.c. had no significant effect on any of the reflex responses. In contrast, within 10 min of administering buspirone i.c. the reflex increase in R-R interval was significantly potentiated from a control of 31±7 ms to 86±24 ms (Figure 8). The reflex increase in renal sympathetic nerve activity was also potentiated and this became significant at 20 min (926±304%). Associated with this increased renal sympathoexcitation there was a non-significant increase in the reflex pressor response from a control of 5±2 mmHg to 12±1 mmHg. Buspirone had no significant effects on the reflex tachypnoea.
Figure 8.
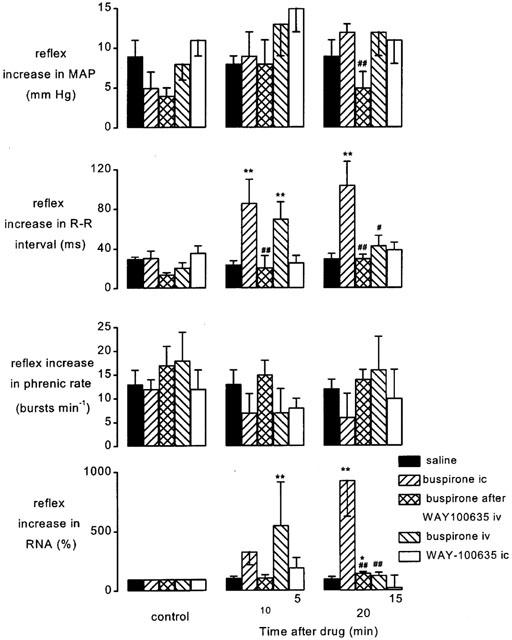
Atenolol (1 mg kg−1; i.v.) pretreated spontaneously breathing anaesthetized rabbits: histograms showing the changes in the size of the carotid chemoreceptor-evoked increases in mean arterial pressure (MAP), R-R interval, phrenic burst rate and renal sympathetic nerve activity (RNA) before (control, left columns), at 10 (middle columns) and 20 min (right columns) following administration of saline (20 μl, i.c.; n=5), buspirone (200 μg kg−1; i.c.; n=5), buspirone (200 μg kg−1; i.c.) following pretreatment with WAY-100635 (100 μg kg−1; i.v.; n=5), buspirone (200 μg kg−1; i.v.; n=4) and WAY-100635 (100 μg kg−1; i.c.; n=6). It should be noted that WAY-100635 i.c. study has been carried out using a different protocol to the rest of the data and thus time after drugs is different (5 and 15 min). Each column shows the mean change in the size of the reflex and bars show s.e.mean. Changes caused by the treatment have been compared with time-matched controls. *P<0.05, **P<0.01 compared to saline. #P<0.05, ##P<0.01 compared to buspirone i.c. alone.
Pretreatment with WAY-100635 i.v. had no significant effects on the chemoreceptor-evoked responses. Nevertheless, such pre-treatment attenuated the potentiating effects of buspirone on the chemoreceptor reflex (Figure 8). The increase in the magnitude of the bradycardia seen at 10 and 20 min following buspirone were both abolished and at 20 min the increased pressor response and sympatho-excitation were also returned to control levels (Figure 8). Following intravenous administration of buspirone there was significant potentiation of both the reflex bradycardia and renal sympathoinhibition at the 10 min time point (Figure 8). Using another protocol (see Figure 1c) i.c. administration of WAY-100635 was demonstrated to have no significant effect on any of the chemoreceptor reflex responses measured (Figure 7), though it did significantly modulate those evoked by aortic nerve stimulation (Figure 6) and cardiopulmonary afferent activation (Figure 3).
Discussion
Reflex bradycardia
The present experiments are consistent with the observations of Futuro-Neto et al. (1993) and Dando et al. (1998) in demonstrating in anaesthetized rabbits that buspirone can potentiate reflex evoked bradycardias. This effect of buspirone is almost certainly due to activation of 5-HT1A receptors since it is attenuated by the selective 5-HT1A receptor antagonist WAY-100635 (Forster et al., 1995) at a dose which did not affect the reflex per se though there are reports that buspirone can antagonize dopamine receptors (McMillen et al., 1983). The ability of i.v. WAY-100635 to attenuate the effect of the exogenous agonist, buspirone, but not that of the endogenous transmitter, 5-HT, is most likely to be simply one of concentration, i.e. when given i.v. the brainstem concentration of WAY-100635 is high enough to block the effects of buspirone but not synaptic transmission involved in this reflex pathway. This is supported by the observation that the same dose of WAY-100635 given i.c. did attenuate the cardiopulmonary afferent and aortic nerve evoked falls in heart rate. Similar differences between the effects of i.v. and i.c. application of WAY-100635 has already been reported for the upper-airway evoked bradycardia in anaesthetized rabbits (Dando et al., 1998) and the reflex-evoked bronchoconstriction in anaesthetized cats and guinea-pigs (Bootle et al.,1996; 1998). Thus, 5-HT1A receptors appear to play a physiological role in the reflex activation of cardiac vagal preganglionic neurones by cardiopulmonary afferents in rats (Bogle et al., 1990), cats (Wang & Ramage, 2001) and rabbits (present study; Dando et al., 1998). In addition, in rabbits they appear to have a similar role in the reflex bradycardias evoked by upper-airway (Dando et al., 1998) and aortic baroreceptor (present study) but not chemoreceptor afferents (present study). However, as with the other reflexes, direct activation of 5-HT1A receptors does potentiate the chemoreceptor reflex-evoked bradycardia, but the reason these 5-HT1A receptors are not utilized as part of the reflex pathway remains to be determined. One difference between the chemoreceptor reflex pathways and the other reflexes that have been tested is that the chemoreceptor afferents travel in the glossopharyngeal (IXth) nerve, whilst the others are vagal (Xth) nerve afferents. Thus, it would be interesting to compare the effect of WAY-100635 on aortic nerve response with those of carotid baroreceptor stimulation. Interestingly, the micturition reflex which involves activation of sacral parasympathetic outflow also involves the central activation of 5-HT1A receptors (Testa et al., 1999; Conley et al., 2001). Thus, failure to demonstrate the involvement of central 5-HT1A receptors in the control of the chemoreceptor reflex is intriguing.
Reflex and baseline changes in blood pressure and renal sympathetic outflow
Activation of all three reflexes evoked changes in blood pressure and renal nerve activity. Cardiopulmonary and aortic nerve stimulation evoked decreases in both variables. The depressor responses were attenuated by buspirone and at least in the case of the aortic nerve reflex this would seem to be central action as the effect was greater when buspirone was administered i.c. This action of buspirone did not show any overt relationship between the evoked changes in resting blood pressure caused by buspirone i.c. or i.v. Chemoreceptor stimulation evoked increases in both blood pressure and renal nerve activity but buspirone had no effect on the pressor response. Interestingly, buspirone i.c. has previously been shown to have no effect on the pressor response to upper-airway receptor activation (Dando et al., 1998). These effects of buspirone on the reflex changes in blood pressure could be attenuated by i.v. WAY-100635 indicating at least part of these effects on evoked changes in blood pressure is mediated by 5-HT1A receptors. However, i.c. administration of WAY-100635 alone, which had no effect on baseline variables, also attenuated the depressor responses evoked by aortic nerve and cardiopulmonary afferent stimulation. Furthermore, as with the bradycardic response, WAY-100635 i.c. was without effect on the chemoreceptor evoked pressor response, though in the same experiment it attenuated the aortic nerve evoked depressor response (Figure 7) and it has previously been shown to attenuate the pressor response to upper-airway receptor activation (Dando et al., 1998). Thus the reflex evoked changes in blood pressure, with the exception of the chemoreceptor response, involves activation of 5-HT1A receptors. The surprising observation that the antagonist (WAY-100635) and agonist (buspirone) had similar effects is likely related to the partial agonist action of buspirone at 5-HT1A receptors (see Hoyer et al., 1994).
Although the present experiments do not provide absolute proof as to the central or peripheral location of the 5-HT1A receptors involved in regulating blood pressure, previously published data on 5-HT1A receptors in cardiovascular control would favour a central site (see Ramage, 2000). Similar falls in baseline blood pressure were evoked whether buspirone was administered i.c. or i.v. and surprisingly, (although only the i.c. route was tested), these were unaffected by i.v. administration of WAY-100635, suggesting that buspirone must be acting at other receptors to produce these effects. One possibility is the fact that buspirone has some affinity for αi-adrenoceptor subtypes (Eltze et al., 1999) and hence may be acting partially through these receptors.
The reflex changes in blood pressure described above are probably mediated by changes in sympathetic outflow. Indeed, the observed effects of buspirone and WAY-100635 on renal nerve activity would support this. Buspirone attenuated the reflex sympathoinhibition but only for the cardiopulmonary reflex did this reach significance and this effect was blocked by i.v. WAY-100635. As with the blood pressure response, WAY-100635 given i.c. also attenuated the sympathoinhibition.
Changes in central inspiratory drive, i.e. phrenic nerve activity
Activation of the three reflexes increased phrenic burst rate though the aortic nerve response was very small. In the case of cardiopulmonary and aortic nerve afferent responses it is difficult to determine how much of the increase is due to activation of the afferents themselves and how much to the concomitant fall in blood pressure which would be expected to increase phrenic nerve activity by activating arterial chemoreceptors (see Daly, 1997). Buspirone i.c. attenuated the increase in phrenic nerve rate caused by cardiopulmonary afferents and this could be reduced by pre-treatment with i.v. WAY-100635. However, such pre-treatment with WAY-100635 also significantly reduced the attenuation of the hypotension produced by buspirone i.c. WAY-100635 i.c. also inhibited the cardiopulmonary evoked increase in phrenic nerve burst rate but again the evoked depressor response was also attenuated. Thus it is difficult to determine whether activation of 5-HT1A receptors can affect the increase in phrenic burst rate. The increase in phrenic burst rate caused by chemoreceptor afferents activation was only significantly attenuated by buspirone administered via the i.v. route. Thus, the data is equivocal for a role of 5-HT1A receptors in these reflex evoked increases in phrenic nerve burst activity caused by activation of these afferents. Previously, the apnoea produced by activation of upper airway afferents was shown to be unaffected by buspirone or WAY-100635 (Dando et al., 1998). On the other hand, i.c. buspirone does cause an increase in baseline phrenic burst rate which is blocked by i.v. WAY-100635. This would be consistent with other data showing that activation of central 5-HT1A receptors can increase respiratory rate (Gillis et al., 1989; Shepheard et al., 1990; Sporton et al., 1991; Anderson et al., 1992; Lalley et al., 1994).
Site of action
The possible sites of action of the ligands applied by the current protocols were discussed in detail in a previous publication (see Dando et al., 1998). Recent experiments in anaesthetized cats demonstrated that ionophoretic application of WAY-100635 to the vicinity of cardiac vagal preganglionic neurones in the nucleus ambiguus can attenuate their activation by cardiopulmonary afferents (Wang & Ramage, 2001). However, activation of 5-HT1A receptors can also modulate activity of other vagal preganglionic neurones in the dorsal vagal nucleus (Wang et al., 1995), neurones in the nucleus tractus solitarius, the site of termination of these visceral afferents (Wang et al., 1997) and also pre-sympathetic vasomotor neurones in the rostral ventrolateral medulla (Wang & Lovick, 1992). Although the above data favours location of the parasympathetic preganglionic neurones for the observed modulation of the evoked bradycardias, the other sites may be important for the modulation of the sympathetic component of the reflex and baseline changes observed. All these areas receive serotonergic innervation (Steinbusch, 1981). There are several possible sites of origin of these 5-HT containing pathways. Anatomical studies have localized serotonin-containing neurones projecting to the NTS in the midline raphé nuclei (magnus, obscurus and pallidus) and in the ventral medulla, lateral to the pyramidal tract (Thor & Helke, 1987). Similar groups of 5-HT containing neurones in raphé obscurus, pallidus, magnus and adjacent parapyramidal nucleus also innervate preganglionic neurones in the nucleus ambiguus (Haxhiu et al., 1993).
In conclusion, the present data confirms the importance of central 5-HT1A receptors in the control of the vagal bradycardia evoked by activation cardiopulmonary afferents and extends this to that produced by stimulation of aortic nerve afferents. The present data also demonstrates that chemoreceptor evoked vagal bradycardias can also be potentiated by activation of 5-HT1A receptors. However, unlike the other reflexes studied, chemoreceptor stimulation does not appear to recruit 5-HT1A receptors. A possible explanation for this difference may lie in the fact that those reflex-evoked bradycardias that have been demonstrated to involve the activation of 5-HT1A receptors involve Xth nerve afferents, whilst those for the chemoreceptor reflex travel in the IXth nerve and thus there may be a difference in the circuitry and transmitters. In addition, the present data also demonstrates, at least for cardiopulmonary and the aortic baroreceptor reflexes, the involvement of 5-HT1A receptors extends to the sympathetic mediated changes observed. Thus the combined data indicates important roles for central 5-HT containing neurones in the reflex evoked changes in parasympathetic and sympathetic outflows.
Acknowledgments
This work was supported by the British Heart Foundation. M.R. Skinner was a British Heart Foundation PhD student.
Abbreviations
- DVN
dorsal vagal motor nucleus
- E.C.G
electrocardiogram
- i.c.
intra-cisternal
- i.d.
internal diameter
- IRNA
integrated renal nerve activity
- MAP
mean arterial pressure
- NA
nucleus ambiguus
- NTS
nucleus tractus solitarius
- PNA
phrenic nerve activity
- RNA
renal sympathetic nerve activity
References
- ANDERSON I.K., MARTIN G.R., RAMAGE A.G. Evidence that i.c.v. administration of 5-HT causes sympathoexcitation through activation of 5-HT1A receptors and vasopressin release through activation of 5-HT2/1C receptors in anaesthetized rats. Br. J. Pharmacol. 1992;107:1020–1028. doi: 10.1111/j.1476-5381.1992.tb13401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGLE R.G., PIRES J.G.P., RAMAGE A.G. Evidence that central 5-HT1A receptors play a role in the von Bezold-Jarisch reflex in the rat. Br. J. Pharmacol. 1990;100:757–760. doi: 10.1111/j.1476-5381.1990.tb14088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTLE D.J., ADCOCK J.J., RAMAGE A.G. Involvement of central 5-HT1A receptors in the reflex activation of pulmonary vagal motoneurones by inhaled capsaicin in anaesthetized cats. Br. J. Pharmacol. 1996;117:724–728. doi: 10.1111/j.1476-5381.1996.tb15250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTLE D.J., ADCOCK J.J., RAMAGE A.G. The role of central 5-HT receptors in the bronchoconstriction evoked by inhaled capsaicin in anaesthetized guinea-pigs. Neuropharmacol. 1998;37:243–250. doi: 10.1016/s0028-3908(98)00019-7. [DOI] [PubMed] [Google Scholar]
- CHITRAVANSHI V.C., CALARESU F.R. Additive effects of dopamine and 8-OH-DPAT microinjected into the nucleus ambiguus in eliciting vagal bradycardia in rats. J. Auton. Nerv. Syst. 1992;41:121–128. doi: 10.1016/0165-1838(92)90134-3. [DOI] [PubMed] [Google Scholar]
- COLERIDGE J.C.G., COLERIDGE H.M. Afferent vagal C-fibre innervation of the lungs and airways and its functional significance. Rev. Physiol. Biochem. Pharmacol. 1984;99:1–109. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- CONLEY R.K., WILLIAMS T.J., FORD A.P.D.W., RAMAGE A.G. The role of α1-adrenoceptors and 5-HT1A receptors in the control of the micturition reflex in male anaesthetized rats. Br. J. Pharmacol. 2001;133:61–72. doi: 10.1038/sj.bjp.0704043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALY M DE B. Peripheral arterial chemoreceptors and respiratory-cardiovascular integration. Oxford: Clarendon Press; Monographs of Physiology Society. 1997;46 [Google Scholar]
- DANDO S.B., SKINNER M.R., JORDAN D., RAMAGE A.G. Modulation of the vagal bradycardia evoked by stimulation of upper airway receptors by central 5-HT1 receptors in anaesthetized rabbits. Br. J. Pharmacol. 1998;125:409–417. doi: 10.1038/sj.bjp.0702085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DASHWOOD M.R., GILBEY M.P., JORDAN D., RAMAGE A.G. Autoradiographic localisation of 5-HT1A binding sites in the brainstem of the cat. Br. J. Pharmacol. 1988;94:386P. [Google Scholar]
- DAWES G.S., COMROE J.H. Chemoreflexes from the heart and lungs. Physiol. Rev. 1954;34:167–201. doi: 10.1152/physrev.1954.34.2.167. [DOI] [PubMed] [Google Scholar]
- ELTZE M., KONG H., ULRICH B., GREBE T. Buspirone functionally discriminates tissues endowed with α1-adrenoceptor subtypes A, B, D and L. Eur. J. Pharmacol. 1999;378:69–83. doi: 10.1016/s0014-2999(99)00426-4. [DOI] [PubMed] [Google Scholar]
- FORSTER E.A., CLIFFE I.A., BILL D.J., DOVER G.M., JONES D., REILLY Y., FLETCHER A. A pharmacological profile of the selective 5-HT1A receptor antagonist WAY-100635. Eur. J. Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- FUTURO-NETO H., PIRES J.G.P., GILBEY M.P., RAMAGE A.G. Evidence for the ability of central 5-HT1A receptors to modulate the vagal bradycardia induced by stimulating the upper airways in anaesthetized rabbits with smoke. Brain Res. 1993;629:349–354. doi: 10.1016/0006-8993(93)91345-s. [DOI] [PubMed] [Google Scholar]
- GILLIS R.A., HILL K.J., KIRBY J.S., QUEST J.A., HAMOSH P., NORMAN W.P., KELLAR K.J. Effect of activation of central nervous system serotonin 1A receptors on cardiorespiratory function. J. Pharmacol. Exp. Ther. 1989;248:851–857. [PubMed] [Google Scholar]
- HAXHIU M.A., JANSEN A.S.P., CHERNIACK N.S., LOEWY A.D. CNS innervation of airway-related parasympathetic preganglionic neurons: A transneuronal labelling study using psueodorabies virus. Brain Res. 1993;618:115–134. doi: 10.1016/0006-8993(93)90435-p. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. VII. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- IZZO P.N., DEUCHARS J., SPYER K.M. Localization of cardiac vagal preganglionic motoneurones in the rat: Immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. J. Comp. Neurol. 1993;327:572–583. doi: 10.1002/cne.903270408. [DOI] [PubMed] [Google Scholar]
- IZZO P.N., JORDAN D., RAMAGE A.G. Anatomical and pharmacological evidence supporting the involvement of serotonin in the central control of cardiac vagal motoneurones in the anaesthetized cat. J. Physiol. 1988;406:19P. [Google Scholar]
- JORDAN D., KHALID M.E.M., SCHNEIDERMAN N., SPYER K.M. The location and properties of preganglionic vagal cardiomotor neurones in the rabbit. Plügers Arch. 1982;395:244–250. doi: 10.1007/BF00584817. [DOI] [PubMed] [Google Scholar]
- JORDAN D., SPYER K.M. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog. Brain Res. 1986;67:295–314. doi: 10.1016/s0079-6123(08)62769-7. [DOI] [PubMed] [Google Scholar]
- KAY I.S., ARMSTRONG D.J. Phenylbiguanide not phenyldiguanide is used to evoke the pulmonary chemoreflex in anaesthetized rabbits. Experimental Physiol. 1990;75:383–389. doi: 10.1113/expphysiol.1990.sp003413. [DOI] [PubMed] [Google Scholar]
- LALLEY P.M., BISCHOFF A.M., RICHTER D.W. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J. Physiol. 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- LAWRENCE A.J., JARROTT B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog. Neurobiol. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- MCMILLEN B.A., MATTHEWS R.T., SANGHERA M.K., SHEPARD P.D. Dopamine receptor antagonism by the novel antianxiety drug, buspirone. J. Neurosci. 1983;3:733–738. doi: 10.1523/JNEUROSCI.03-04-00733.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIL E., REDWOOD C.R.M., SCHWEITZER A. Effects of electrical stimulation of the arotic nerve on blood pressure and respiration in cats and rabbits under chloralose and nembutal anaesthesia. J. Physiol. 1949;109:392–401. doi: 10.1113/jphysiol.1949.sp004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSAKA S., YAMAMOTO T., YASUNAGA K. Localisation of vagal cardioinhibitory preganglionic neurones within the rat brainstem. J. Comp. Neurol. 1979;186:79–92. doi: 10.1002/cne.901860106. [DOI] [PubMed] [Google Scholar]
- NOSAKA S., YASUNAGA K., TAMAI S. Vagal preganglionic neurones; distribution, sell types and reflex discharges. Am. J. Physiol. 1982;243:92–98. doi: 10.1152/ajpregu.1982.243.1.R92. [DOI] [PubMed] [Google Scholar]
- PAZOS A., PALACIOS J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- PAZOS A., PROBST A., PALACIOS J.M. Serotonin receptors in the human brain. III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience. 1987;21:97–122. doi: 10.1016/0306-4522(87)90326-5. [DOI] [PubMed] [Google Scholar]
- RAMAGE A.G. Central 5-HT1A receptors and vagal tone to the airways. TiPS. 2000;21:201–202. doi: 10.1016/s0165-6147(00)01481-4. [DOI] [PubMed] [Google Scholar]
- SHEPHEARD S.L., JORDAN D., RAMAGE A.G. Actions of 8-OH-DPAT on sympathetic respiratory drives, blood pressure and heart rate in the rabbit. Eur. J. Pharmacol. 1990;186:267–272. doi: 10.1016/0014-2999(90)90442-9. [DOI] [PubMed] [Google Scholar]
- SHEPHEARD S.L., JORDAN D., RAMAGE A.G. Comparison of the effects of IVth ventricular administration of some tryptamine analogues with those of 8-OH-DPAT on autonomic outflow in the anaesthetized cat. Br. J. Pharmacol. 1994;111:616–624. doi: 10.1111/j.1476-5381.1994.tb14781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOEFFTER P., HOYER D. Centrally acting hypotensive agents with affinity for 5-HT1A binding sites inhibit forskolin-stimualted adenylate cyclase activity in calf hippocampus. Br. J. Pharmacol. 1988;95:975–985. doi: 10.1111/j.1476-5381.1988.tb11728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKINNER M.R., RAMAGE A.G., JORDAN D. Evidence that central 5-HT1A receptors are involved in the vagal bradycardia evoked by aortic nerve stimulation in anaesthetized rabbits. Br. J. Pharmacol. 1997a;120:25P. [Google Scholar]
- SKINNER M.R., RAMAGE A.G., JORDAN D. Evidence that central 5-HT1A receptors are involved in the vagal bradycardia evoked by carotid chemoreceptor stimulation in anaesthetized rabbits. J. Physiol. 1997b;501:72P. [Google Scholar]
- SKINNER M.R., RAMAGE A.G., JORDAN D. Further evidence that central 5-HT1A receptors are involved in the reflex activation of cardiac vagal preganglionic neurones in anaesthetized rabbits. J. Physiol. 1997c;504:197P. [Google Scholar]
- SKINNER M.R., RAMAGE A.G., JORDAN D. Effect of antagonism of central 5-HT1A receptors on baroreceptor and cardiopulmonary receptor reflexes in anaesthetized rabbits. J. Physiol. 1998;513:85P. [Google Scholar]
- SPORTON S.C.E., SHEPHEARD S.L., JORDAN D., RAMAGE A.G. Microinjections of 5-HT1A agonists into the dorsal motor vagal nucleus produce a bradycardia in the atenolol-pretreated anaesthetized rat. Br. J. Pharmacol. 1991;104:466–470. doi: 10.1111/j.1476-5381.1991.tb12452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBUSCH H.W.M. Distribution of serotonin-immunoreactivity in the central nervous system of the rat - cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- TESTA R., GUARNERI L., POGGESI E., ANGELICO P., VELASCO C., IBBA M., CILIA A., MOTTA G., RIVA C., LEONARDI A. Effect of several 5-hydroxytryptamine1A receptor ligands on the micturition reflex in rats: comparison with WAY 100635. J. Pharmacol. Exp. Ther. 1999;290:1258–1269. [PubMed] [Google Scholar]
- THOR K.B., BLITZ-SIEBERT A., HELKE C.J. Autoradiographic localization of 5HT1 binding sites in autonomic areas of the rat dorsomedial medulla oblongata. Synapse. 1992;10:217–227. doi: 10.1002/syn.890100305. [DOI] [PubMed] [Google Scholar]
- THOR K.B., HELKE C.J. Serotonin- and Substance P-containing projections to the nucleus tractus solitarri of the rat. J. Comp. Neurol. 1987;265:275–293. doi: 10.1002/cne.902650210. [DOI] [PubMed] [Google Scholar]
- WANG Y., JONES J.F.X., RAMAGE A.G., JORDAN D. Effects of 5-HT and 5-HT1A receptor agonists and anataonigst on dorsal vagal preganglionic neurones in anaesthetized rats: an ionophoretic study. Br. J. Pharmacol. 1995;116:2291–2297. doi: 10.1111/j.1476-5381.1995.tb15067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG W.H., LOVICK T.A. Inhibitory serotonergic effects on rostral ventrolateral medullary neurons. Pflügers Arch. 1992;422:93–97. doi: 10.1007/BF00370407. [DOI] [PubMed] [Google Scholar]
- WANG Y., RAMAGE A.G. The role of central 5-HT1A receptors in the control of B-fibre cardiac and bronchoconstrictor vagal preganglionic neurones in anaesthetized cats. J. Physiol. 2001;536:753–767. doi: 10.1111/j.1469-7793.2001.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., RAMAGE A.G., JORDAN D. In vivo effects of 5-hydroxytryptamine receptor activation on rat nucleus tractus solitarius neurones excited by vagal C-fibre afferents. Neuropharmacol. 1997;36:489–498. doi: 10.1016/s0028-3908(97)00063-4. [DOI] [PubMed] [Google Scholar]


