Abstract
We investigated the role of voltage-operated calcium channels in sympathetic transmission and depolarization-induced contractions in the rat mesenteric artery. In particular, we investigated the role of the T-type voltage-operated calcium channels (T-channels) in mediating excitatory junction potentials (EJPs).
EJPs were evoked by electrical field stimulation (trains of five stimuli at 0.9 Hz) in small mesenteric arteries. The average resting membrane potential was −59.8±0.5 mV (n=65). Trains of stimuli evoked individual EJPs with the peak EJP of 6±0.2 mV (n=34) occurring with the second stimulus. Trains of EJPs were inhibited 90% by tetrodotoxin (0.1 μM) or by ω-conotoxin GVIA (GVIA, 10 nM) indicating their neural origin.
The EJPs were not inhibited by the L-type calcium channel blocker nicardipine at 0.1 μM, a concentration sufficient to abolish the contraction to potassium depolarization. However, mibefradil (3 μM), considered a relatively selective T-channel antagonist, inhibited the EJPs by about 50%. This concentration of mibefradil did not inhibit GVIA-sensitive electrically-evoked twitches of the rat vas deferens. Thus the action of mibefradil in reducing EJPs is unlikely to be due to either inhibition of L- or N-type channels but is probably due to inhibition of T-channels.
The finding that Ni2+ (300 μM), an inhibitor of T-type calcium channels, also reduced EJP amplitude by about 80% but did not block electrically-evoked twitches in the rat vas deferens, further supports an important role of T-channels in mediating small depolarizations associated with the EJPs evoked by sympathetic nerve stimulation.
Keywords: Mibefradil, excitatory junction potentials, T-type voltage-operated calcium channels, mesenteric artery, nicardipine, nickel, vas deferens
Introduction
The entry of calcium into neurones and smooth muscle cells can be regulated by voltage-operated calcium channels (VOCCs) which can be classified as high voltage-activated or low voltage-activated channels (Hermsmeyer, 1991; Cribbs, 2001). A role for the high voltage activated channels such as the L- and N-type VOCCs in control of vascular function has been extensively investigated. L-type VOCCs (L channels) located on smooth muscle cells play an important role in smooth muscle contraction in response to a variety of agonists and potassium-induced depolarizations (Sarsero et al., 1998; van der Lee et al., 1999). N-type VOCCs (N-channels) located in the peripheral sympathetic nerve terminals open in response to arrival of the action potential and control the amount of transmitter released to activate the smooth muscle cells (Olivera et al., 1994; Brock et al., 1989). In contrast, a role for low voltage activated T-type VOCCs (T-channels) in vascular function remains to be determined (Cribbs, 2001).
Transmitter release from sympathetic nerves is able to elicit depolarization of smooth muscle, measured as excitatory junction potentials (EJPs). L-channel antagonists have been shown not to affect EJP amplitude in the guinea-pig vas deferens (Blakeley et al., 1981). It is now well established that the EJPs are mediated by activation of P2X1 receptors by the sympathetic co-transmitter ATP (Kennedy, 1996; Mulryan et al., 2000) and that P2X receptors are ligand-gated ion channels that are permeant to calcium (Benham, 1992). However, this does not preclude a role for VOCCs in EJP generation. Thus, EJPs in response to a single pulse of field stimulation in the rat mesenteric artery measure 5∼10 mV from a resting membrane potential of around −60 mV (Angus et al., 1988). This is within the voltage range for the opening of T-channels (Carbone & Lux, 1984; Hermsmeyer, 1991) and there have been suggestions that T-channels may provide the link between a small depolarization and the influx of extracellular calcium (Schrier et al., 2001). Thus, we hypothesize that T-channels may be involved in the generation of EJPs in rat mesenteric artery.
At present, three T-channel subunits (α1G, α1H, and α1I) have been cloned (Cribbs et al., 1998; Lee et al., 1999a; Perez-Reyes et al., 1998; 1999). All three subunits are expressed in the rat brain (Craig et al., 1999; Talley et al., 1999), while only α1G and α1H are expressed in human heart (Cribbs et al., 1998; 2000) and in the rat mesenteric (Gustafsson et al., 2001) and renal arteries (Hansen et al., 2001). The lack of a highly selective antagonist for T-channels has limited studies on their functional role in the vasculature. However, there are some chemicals identified as partially selective T-channel blockers, which may be of use. These include, nickel (Lee et al., 1999b), mibefradil (Martin et al., 2000), and kurtoxin (Chuang et al., 1998). The selectivity of nickel and mibefradil for T-channels were considered most suitable for the present study. Mibefradil can block T-channels with a 15∼30 fold selectivity over L-channels (Martin et al., 2000; Mishra & Hermsmeyer, 1994). Similarly, nickel can effectively block the three T-channel subunits (IC50=12±2 μM, 250±22 μM and 216±9 μM at α1H, α1G, α1I subunits, respectively), ahead of other calcium channel subunits (Lee et al., 1999b; Zamponi et al., 1996).
We have used nickel and mibefradil as probes to investigate the role of T-channels in the EJPs and contractions of rat mesenteric arteries. In order to account for the possible involvement of L-channel block we have compared the effects of these compounds with nicardipine, a selective L-type calcium channel antagonist. We have found that both mibefradil and nickel blocked EJPs but nicardipine did not, suggesting that T-channels may contribute to the generation of EJPs in the rat mesenteric artery.
Methods
Male Sprague–Dawley rats (300∼350 g) were killed by gassing with 80% CO2 in O2 and exsanguinated. Tissues (intestines or vasa deferentia) were bathed during dissection, mounting, and the experiment in a Krebs physiological salt solution (PSS). The composition of the PSS was (in mM): NaCl 119; KCl 4.69; MgSO4 1.17; KH2PO4 1.18; NaHCO3 25; CaCl2 2.5; Glucose 5.5 (11 for rat vas deferens) and EDTA 0.026.
Rat mesenteric artery preparation
A loop of intestine with mesenteric arteries attached was removed and a third order branch of mesenteric artery (i.d. 250∼350 μm) was dissected free of fat and connective tissue under a dissecting microscope. Segments of the artery were then used for EJP recording or contraction studies as described below.
EJP recording
A 15-mm segment of the rat mesenteric artery was pinned down in a Sylgard® (Dow Corning)-covered base of a 4 ml petri dish through two platinum ring electrodes. The petri dish was filled with the oxygenated PSS, which was suffused at rate of 4.4∼4.6 ml−1 min by Minipuls 3 pump (Gilson). The mesenteric arteries were equilibrated (bath temperature 32–33°C) for 30 min before any impalement was attempted.
With the reference electrode beside the mesenteric artery immersed in the petri dish, intracellular recordings were made from smooth muscle cells of the rat mesenteric artery with borosilicate glass micro-electrodes connected to a Axoclamp bridge amplifier (Axon Instruments, Inc., Foster City, CA, U.S.A.). Recordings were filtered (DC–3 kHz) with a Neurolog 125A Filter and stored on a computer using SCAN software (J. Dempster, Glasgow). Microelectrodes were fabricated from glass capillaries (GC100F-15, Clark Electromedical Instruments, Pangbourne, U.K.) by a Flaming/Brown micropipette puller (Model P-87, Sutter Instruments Co., Novato, CA, U.S.A.) and had resistances of 80–160 MΩ when filled with 0.5 M KCl. Impalements were made within 1–2 mm of the platinum ring stimulating electrode; criteria for accepting impalements were the same as those adopted by Cassell et al. (1988). Recordings were only made after the membrane potential had been stable for ⩾5 min. The EJPs were evoked by trains of five stimuli at 0.9 Hz, 25∼30 V, 0.25 ms duration and each train was separated by 90 s. The first four trains were regarded as the initial period. Drugs were added immediately following the initial period. The incubation of the test drugs lasted for 15 trains. The effects of drugs on EJPs were determined in single cell experiments in which both control and test recordings were made during the same impalement. At the end of each experiment, 0.1 μM tetrodotoxin (TTX) was present for at least five trains.
Contraction
Two separate vessels were mounted as 2 mm-long cylinders on 40 μm diameter stainless steel wires for isometric force recording in a dual channel myograph initially designed by Mulvany & Halpern (1977) (J.P. Trading, Denmark). One artery was used to examine the effect of drugs on either K+ (62 mM) or αβ-MeATP (1 μM) evoked contractions while the other served as a vehicle or time control. After warming the myograph to 37°C, each vessel was incrementally stretched radially to about 100% with three steps by moving one jaw via a micrometer. The increase in isometric force was made in steps to generate a length–tension relationship as previously described (Angus et al., 1988). From a computer fitted curve, we determined the parameters to finally stretch the vessel to L0.9, i.e. circumference (L) of 90% of that where the vessel would be passively distended at 100 mmHg, determined from the Laplace relationship where T=r·P (P is transmural pressure of 100 mmHg, T=wall tension, r=internal radius). The diameter at this equivalent transmural pressure of 100 mmHg (D100) was taken as the internal diameter of the vessels. Force was recorded on a dual flatbed chart recorder (Model 320, W&W Scientific Instruments, Basel, Switzerland).
Potassium-induced contractions
Thirty minutes after normalization and stabilization, the arteries were exposed to a depolarizing solution of K+ 124 mM (KPSS where Na+ 124 mM was replaced by K+) for 2 min. The chamber was replaced with PSS three times to rapidly return the contraction to KPSS back to baseline passive force. Following KPSS, the arteries were contracted twice with 50% KPSS (K+ 62 mM with Na+ 62 mM) for 2 min and washing with three exchanges of PSS. These two contractions served as controls before a concentration of calcium antagonist (3 μM mibefradil, 0.1 μM nicardipine, or 300 μM nickel) or vehicle (DMSO) was added to the bath to equilibrate with the arteries for 30 min prior to testing 50% KPSS (with the same concentration of calcium antagonist).
αβ-MeATP-induced contraction
Following normalization and exposure to KPSS, the small mesenteric arteries were contracted with αβ-MeATP (1 μM) at 30 min intervals. After the second control contraction to αβ-MeATP (1 μM), a concentration of calcium antagonist (mibefradil at 3 μM, nicardipine at 0.1 μM, or nickel 300 μM) or vehicle (DMSO) was added to the bath to equilibrate with the arteries for 30 min prior to next addition of αβ-MeATP (1 μM).
Rat isolated vasa deferentia
The upper end (epididymal) of the rat vas deferens was attached to an isometric force transducer (Grass FT03C, Quincy, MA, U.S.A.) and the lower end (prostatic) threaded through two platinum electrodes (2 mm apart, 5 mm long) and tied to a fixed support. Output from the transducer amplifier was recorded on a flat-bed recorder (Linearcorder WR3300, Graphtec, Tokyo, Japan). Tissues were initially stretched to 2 g force and allowed to equilibrate for 30 min before any treatments were commenced.
Potassium-induced contractions
Rat vasa deferentia were allowed to equilibrate for at least 30 min. Tissues were then contracted by a potassium depolarization solution (50% KPSS, K+ 62 mM with Na+ 62 mM) replacing the normal PSS. After the tissues were washed thoroughly over 30 min, another 50% KPSS exposure was made as the second control contraction. Drugs studied were GVIA (10 nM), mibefradil (3 μM) and nicardipine (0.1 μM).
Nerve stimulation
Tissues were equilibrated with benextramine (BNX, 10 μM) for 30 min to cause irreversible blockade of α1- and α2-adrenoceptors, leaving ATP as the functional transmitter from nerve stimulation. The tissues were contracted to nerve stimulation by applying electrical field stimulation of 0.05 Hz, 150 V, 0.5 ms duration settings (Grass S88 stimulator). Following the nerve stimulation and two washes with PSS (two controls), a concentration of calcium channel antagonist (GVIA 10 nM, mibefradil 3 μM, nicardipine 0.1 μM, or nickel 300 μM) or vehicle (DMSO) was added to the bath to equilibrate for 30 min prior to the next field stimlulation. TTX (0.1 μM) and αβ-MeATP (100 μM) were also tested.
Voltage-clamp recording
Oocytes were injected with cRNA encoding P2X1 receptors and two-electrode voltage clamp recordings were made 3–5 days later as described previously (Ennion et al., 2000). Briefly, two-electrode voltage clamp recordings were made using a GeneClamp 500B amplifier (Axon Instruments). Microelectrodes were filled with 3M KCl and the external solution consisted of ND96 buffer (mM: NaCl 96, KCl 2, MgCl2 1, sodium pyruvate 5, HEPES 5, pH 7.6) with 1.8 mM BaCl2 to prevent activation of calcium activated chloride channels. Membrane currents were recorded at a holding potential of −60 mV and were acquired using a Digidata 1200 analogue to digital converter with pClamp7 acquisition software (Axon Instruments). ATP (magnesium salt, Sigma) was applied from a nearby U-tube perfusion system. The antagonist mibefradil was bath-perfused for 5 min and was also present at the appropriate concentration in the U-tube perfusion of ATP. Repeated exposures at ATP were separated by at least 5 min to allow for recovery of any receptor desensitization (Ennion et al., 2000).
Drugs
Drugs used and suppliers were: ATP and αβ-MeATP, Sigma, St. Louis, MO, U.S.A.), benextramine tetrachloride (BNX, Sigma), ω-conotoxin GVIA (GVIA, synthesized by J.P. Flinn & R. Murphy, Department of Pharmacology, University of Melbourne, Australia); mibefradil (Roche, Australia); nicardipine (Nicardipine HCl) (Sigma); tetrodotoxin (TTX, Sigma). All other drugs were of analytical grade. αβ-MeATP, BNX, GVIA, mibefradil, and TTX were prepared in Milli-Q water. Nicardipine (100 μM) was prepared in DMSO and protected from light during all experiments. All subsequent dilutions were prepared daily.
Data analysis
For rat mesenteric arteries, in the EJP studies, the records were analysed by the computer programme SCAN (J. Dempster, Glasgow, U.K.). The peak amplitude of each EJP from the last three trains within each of the initial, test, and post TTX periods were measured and their means and standard errors calculated for each experiment. Values of all peak EJP amplitudes were grouped according to pulse number and treatment. In the contraction experiments, the increase in contraction force above passive (stretch) force in response to the second 50% KPSS (K+ 62 mM) at the end of 2 min or after αβ-MeATP 1.0 μM at the peak was measured and taken as 100% for the control. Subsequent contractions of 50% KPSS or αβ-MeATP 1.0 μM were calculated as a percentage of the control contraction.
For rat vas deferens, the effects of drugs on the responses of rat vasa deferentia to field stimulation were expressed as a percentage of the control twitch contraction measured just prior to the addition of the test drugs. In the contraction experiments, the effects of drugs on the contractions of rat vasa deferentia to exposure to potassium depolarization were expressed as a percentage of the control response to potassium depolarization in each tissue.
Data are presented as mean±1 standard error of the mean (s.e.m.). For each preparation, within and between drug treatment groups, responses to different stimuli were assessed by repeated measures of ANOVA, calculated by the statistical programme SuperANOVA 1.11 for Macintosh. Where appropriate, comparisons between two particular treatments were made with a post-hoc Tukey Kramer test. Values of P<0.05 were accepted as statistically significant.
Results
Rat mesenteric arteries
Effects of calcium antagonists on EJPs
In rat small mesenteric arteries, the average resting membrane potential was −59.8±0.5 mV (n=65). Figure 1 shows the characteristics of excitatory junctional potentials (EJPs) evoked by trains of five pulses of electrical field stimulation (EFS) at 0.9 Hz repeated every 90 s. During the initial period prior to any drug addition the peak EJP amplitude was 6±0.2 mV (n=34). EJPs were stable over time. Figure 2A shows that 15 min exposure to vehicle (DMSO) used for nicardipine did not significantly alter the amplitude of EJPs during the train of stimuli. The highly selective sodium channel blocker TTX (0.1 μM) significantly reduced the peak EJP amplitude from 5.5±0.3 mV to 0.9±0.3 mV (n=3, Figure 2B). A similar inhibitory effect on EJPs was also caused by ω-conotoxin GVIA (GVIA), a specific N-channel antagonist and subsequent addition of TTX did not further inhibit EJP amplitude (Figure 2C).
Figure 1.
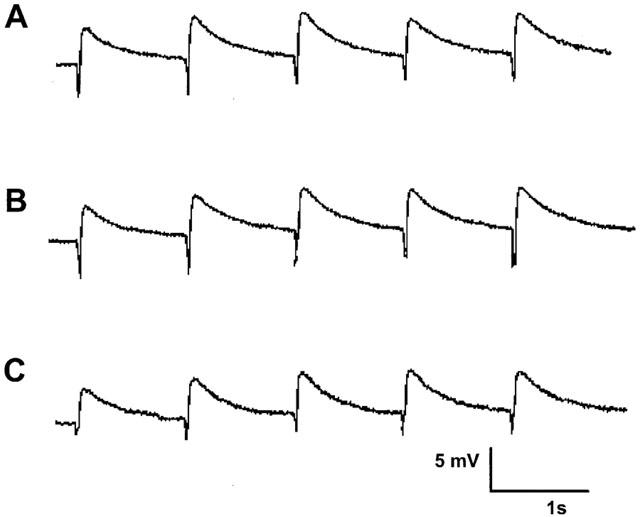
EJPs elicited by three trains of stimuli (five pulses at 0.9 Hz every 90 s) from a single impalement in a time control experiment. Record (A) corresponds to EJPs recorded in the initial period. Record (B) was taken 15 min later corresponding to drug treatment and record (C) a further 15 min later. The small downward deflections represent the stimulus artifact and have been digitally reduced.
Figure 2.
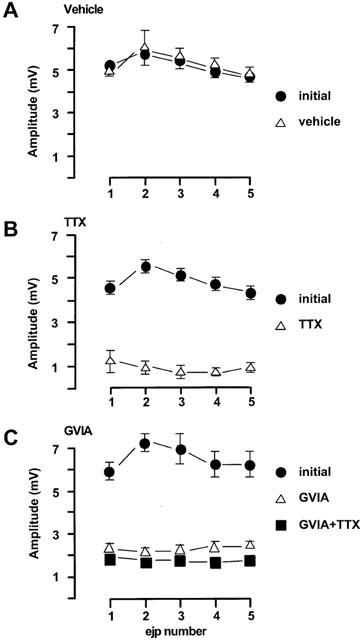
Characteristics of EJPs in the rat mesenteric arteries evoked by trains of five stimuli at 0.9 Hz every 90 s. (A) vehicle (n=4); (B) TTX (0.1 μM, n=3); (C) GVIA (0.1 μM, n=4). The first four trains of each protocol were regarded as initial period. Drugs or vehicle were added immediately following the initial period. The incubation of the test drugs was at least 30 min. For (C) at the end of each experiment, TTX (0.1 μM) was tested. All values were expressed as the mean±1 s.e.mean. For each experiment described, the n value was equal to the number of rats from which the arteries were obtained.
The relatively selective L-channel blocker, nicardipine (0.1 μM), did not alter the amplitude of EJPs (Figure 3A). In contrast mibefradil (3 μM), which can block both L- and T-channels, significantly inhibited EJPs by 40–50% (Figure 3B). The resting membrane potential was not altered by either nicardipine or mibefradil.
Figure 3.
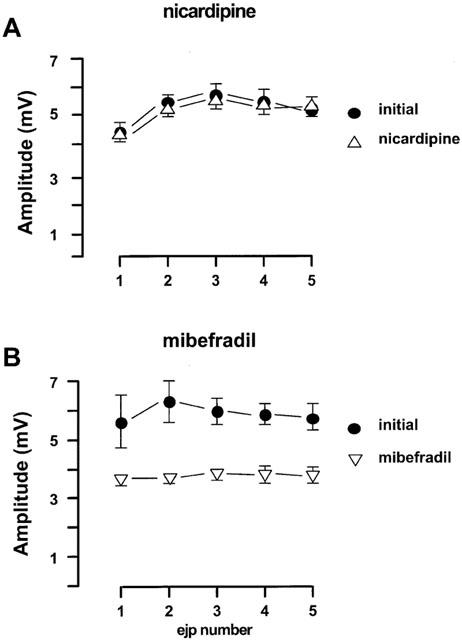
Effects of nicardipine and mibefradil on the EJPs in the rat mesenteric arteries evoked by trains of five stimuli at 0.9 Hz every 90 s. (A) nicardipine (0.1 μM, n=4); (B) mibefradil (3 μM, n=4). For both panels, the first four trains of each protocol were regarded as initial period. All values were expressed as the mean±1 s.e.mean. For each experiment, the n value was equal to the number of rats from which the arteries were obtained.
The effects of nickel chloride, which blocks T-channels, on EJP amplitude are shown in Figure 4. Interestingly nickel chloride at 30 μM hyperpolarized the smooth muscle cells from −57.6±0.9 mV (n=4) to −68±4.4 mV (n=4) and increased the EJPs by 37±13% (n=3) (Figure 4A). However, nickel chloride at 300 μM caused a transient (3–5 min) hyperpolarization, during which time EJPs were enhanced but after 15 min equilibration, the membrane potential returned to resting potential and the EJPs were blocked by 79±7% (n=4).
Figure 4.
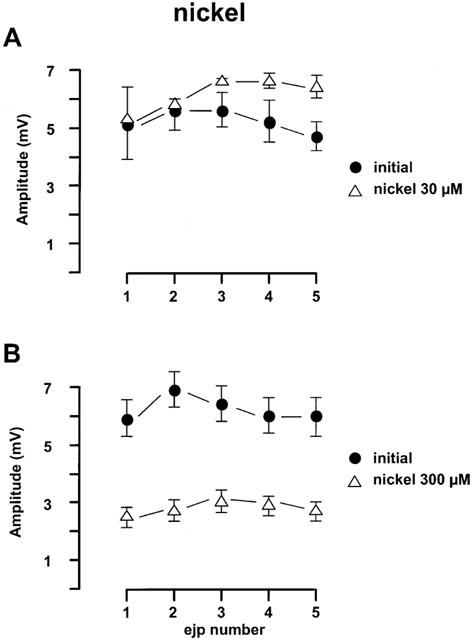
Effects of nickel chloride on the EJPs in the rat mesenteric arteries evoked by trains of five stimuli at 0.9 Hz every 90 s. (A) Nickel chloride (30 μM, n=4); (B) nickel chloride (300 μM, n=4). For both panels, the first four trains of each protocol were regarded as initial period. All values were expressed as the mean±1 s.e.mean. For each experiment, the n value was equal to the number of rats from which the arteries were obtained.
Effects of calcium antagonists on contractions
The mesenteric arteries contracted immediately to a potassium depolarization solution (50% KPSS) with an increase in equivalent transmural pressure of 17.2±1.0 kPa (n=28). These responses were reproducible when evoked every 30 min (data not shown) but were nearly abolished in the presence of either nicardipine (0.1 μM) or mibefradil (3 μM) but only slightly (20±6.25%) reduced by nickel up to 300 μM (n=6, Figure 5).
Figure 5.
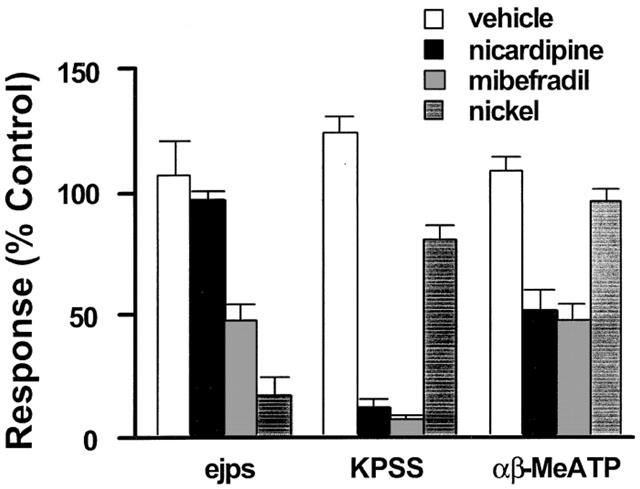
Effects of vehicle (open column), mibefradil (3 μM, striped filled column), nicardipine (0.1 μM, black filled column), and nickel (300 μM, filled column with parallel lines) on EJPs and the contractions to 50% KPSS (KPSS) or αβ-MeATP (1 μM) in rat mesenteric arteries. n=4–6. All values were expressed as the mean±1 s.e.mean. For each experiment, the n value was equal to the number of rats from which the arteries were obtained.
The smooth muscle P2X receptor agonist (αβ-MeATP, 1 μM) caused an increase in transmural pressure of 14.3±1.8 kPa (n=17). When applied for only brief periods to avoid desensitization of the P2X receptor, the responses were very reproducible in time control experiments (Figure 5). In this case, mibefradil (3 μM) and nicardipine (0.1 μM) again showed a similar inhibitory effect with contractions to αβ-MeATP reduced by 53±7% (n=6) and 49±8% (n=6) by mibefradil and nicardipine respectively. In contrast, nickel up to 300 μM had only a modest effect on the αβ-MeATP-elicited contraction (n=6, Figure 5).
For comparison, Figure 5 also summarizes the findings showing that mibefradil and nickel but not nicardipine inhibited the EJP amplitude.
Rat vas deferens
Potassium (K+ 62 mM) caused a contraction of 1.79±0.07 g (n=20) in the rat vas deferens preparation. These contractions were stable when repeated every 30 min and were not affected by GVIA (10 nM) (Figure 6A). However, as in the mesenteric artery, they were reduced to a similar extent by mibefradil (3 μM) and nicardipine (0.1 μM) (Figure 6A).
Figure 6.
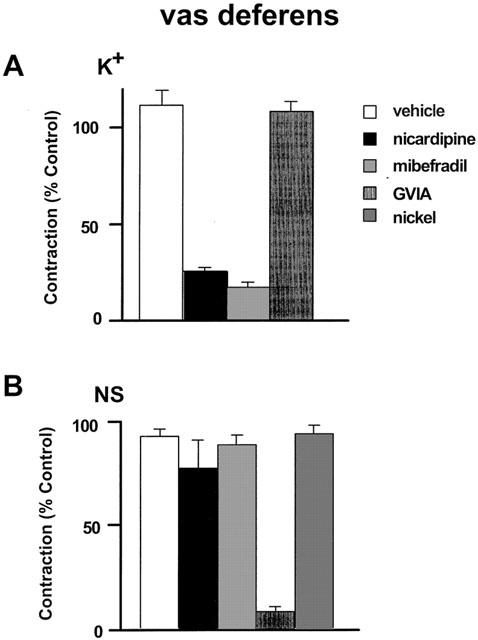
Effects of vehicle, mibrefradil (3 μM), nicardipine (0.1 μM), ω-conotoxin GVIA (GVIA, 10 nM), and nickel (300 μM) on the contractions to 50% KPSS (A: K+) in rat vas deferens and twitch like contraction to nerve stimulation (B: NS, 0.05 Hz, 150 V, 0.5 ms duration). n=4∼6. All values were expressed as the mean±1 s.e.mean. For each experiment, the n value was equal to the number of rats from which the arteries were obtained. Some data for this figure is re-plotted from a previously published paper from our laboratory (Xi et al., 2001)
After BNX pretreatment, sympathetic nerve stimulation at 0.05 Hz evoked stable twitch contractions of 2.39±0.23 g (n=15). TTX (0.1 μM) abolished the responses within 10–20 min and αβ-MeATP (100 μM) substantially reduced the contractions consistent with P2X-receptor desensitization (not shown). Figure 6B shows that these neurogenically evoked purinergic contractions were sensitive to inhibition by GVIA (10 nM) but were not altered by either mibefradil (3 μM), nicardipine (0.1 μM), or nickel (300 μM).
Oocytes expressing P2X1 receptors
ATP, at an EC50 concentration of 1 μM (Ennion et al., 2000), elicited reproducible currents in oocytes expressing P2X1 receptors. These currents were unaffected in the presence of 30 μM mibefradil (Figure 7). In four experiments the peak current evoked by ATP (1 μM) in the presence of mibefradil was 103±7.7% of the control ATP (1 μM) current.
Figure 7.
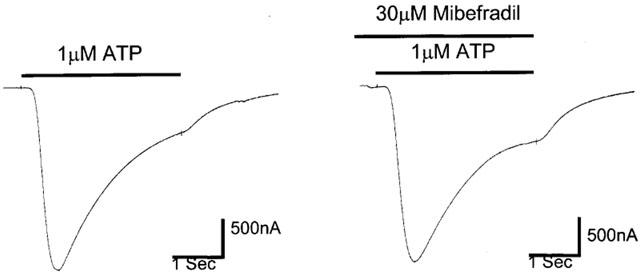
Effect of mibefradil (30 μM) on ATP responses in oocytes expressing P2X1-receptors.
Discussion
The aim of this study was to establish whether T-channels were involved in the EJPs elicited by sympathetic perivascular nerve stimulation in rat small mesenteric arteries. We found that mibefradil, which has a 15 fold selectivity for T-channels over L-channels, significantly reduced the EJPs evoked by stimulation of periarterial sympathetic nerves. Our findings suggest that the inhibitory effect of mibefradil on EJPs was most likely due to T-channel blocking activity given that (1) the L-channel inhibitor nicardipine did not inhibit EJPs but both nicardipine and mibefradil inhibited K+-induced contractions; (2) nickel chloride (300 μM), which can inhibit T-channels, also significantly reduced the EJPs in these experiments. Moreover, inhibition of neuronal N-channels can be excluded in the mechanisms of action of mibefradil or nickel since neither mibefradil nor nickel inhibited other neurogenically mediated responses such as the twitch responses in the rat vas deferens preparation. P2X1 receptors underlie the EJP in the mouse vas deferens (Mulryan et al., 2000). Mibefradil also failed to inhibit ATP evoked depolarizations in oocytes expressing P2X1 receptors. By inference therefore, we conclude that T-channels play a significant but not exclusive role in the EJP in the rat resistance arteries in response to sympathetic nerve stimulation.
There is evidence in the literature that mibefradil is an N-channel antagonist (Bezprozvanny & Tsien, 1995; Göthert & Molderings, 1997; Molderings et al., 2000; van der Lee et al., 2000) and that it can inhibit neuronal Na+-channels (Eller et al., 2000). However, in the present study, mibefradil 3 μM differentially affected neurogenic responses in that it did not inhibit twitch responses to nerve stimulation in the rat vas deferens preparation but did inhibit EJP amplitude in the rat mesenteric artery. In contrast both the Na+-channel blocker TTX (0.1 μM) and the N-channel blocker ω-conotoxin GVIA (10 nM) essentially abolished the EJPs. We have previously established that twitch responses in the rat vas deferens preparation can be used as a highly sensitive functional assay of N-channel function (Xi & Angus, 2001). Mibefradil in concentrations up to 30 μM did not show any inhibitory effects in the rat vas deferens preparation but ω-conotoxin GVIA (10 nM) and TTX (0.1 μM) caused full inhibition of the twitch responses (Xi & Angus, 2001). Thus, in our functional assays, we have no evidence to suggest a prejunctional action of mibefradil on either N-channels or the Na+-channels.
The present studies do not unequivocally exclude a prejunctional inhibitory mechanism for mibefradil, but the data does suggest a post junctional mechanism is more likely. Thus, in addition to reducing EJP amplitude, mibefradil inhibited the contractile responses to αβ-MeATP and K+ in the mesenteric arteries. It is now well established that the EJPs in sympathetically innervated tissues are mediated by P2X receptor activation following the N-channel dependent release of the co-transmitter ATP (Angus et al., 1988; Brock et al., 1989). Therefore a postjunctional inhibitory effect of mibefradil could involve blockade of L- or T-type channels or P2X receptors. The data in the oocytes expressing P2X1 receptors clearly demonstrate a lack of effect of mibefradil on P2X1 receptor mediated ATP responses. Therefore, any inhibitory effect of mibefradil on EJPs may arise due to the involvement of Ca2+ influx following the activation of P2X receptors. Thus, it has been shown that activation of P2X receptors can lead to influx of calcium through both VOCCs and receptor operated channels in rat mesenteric arteries (Lagaud et al., 1996). Therefore the EJP may involve a combination of direct entry of calcium through the P2X receptor and entry through VOCCs accounting for the 40–50% inhibition of EJP amplitude by mibefradil. Furthermore, the findings that nicardipine and mibefradil were both able to inhibit contractions to both K+ and αβ-MeATP in the mesenteric artery support the findings of Lagaud et al. (1996) and point to a postjunctional mechanism for the inhibition of EJPs by mibefradil.
L- or T-type calcium channels are found in vascular smooth muscle (Cribbs, 2001) and are the most likely targets through which mibefradil inhibits the EJP. Nicardipine, a classic L-channel antagonist, did not alter any characteristics of the EJPs at a concentration of 0.1 μM; a concentration that was sufficient to abolish the contractions to high K+ depolarization mediated by L-channels. These findings suggest that L-channels do not contribute to the induction of EJPs in the rat mesenteric arteries and are consistent with earlier studies where it has been reported that the L-channel blocker nifedipine inhibited smooth muscle action potentials and twitch responses to electrical stimulation without reducing the EJPs (Blakeley et al., 1981). Therefore, the 40–50% inhibition of neuronally evoked EJPs in rat mesenteric arteries by mibefradil 3 μM is unlikely to involve L-channel blockade and is most likely due to T-channel blockade.
The finding that nickel chloride at 300 μM, a non-selective T-channel blocker could also inhibit EJPs further supports this suggestion. The effect of nickel is not likely to be due to inhibition of P2X receptors (Chaïb et al., 1998) as at a concentration of 300 μM that inhibited the purinergic EJP, nickel did not significantly affect purinergic twitch responses in the rat vas deferens or the responses to αβ-meATP in the mesenteric artery. Nickel has also been suggested to inhibit N-type calcium channels with an IC50 of 300 μM and Na+ channels (Bleakman et al., 1995). However, the failure of nickel (300 μM) to inhibit the electrically evoked purinergic twitches in the vas deferens suggests that neither N-type calcium channels or Na+-channels are sensitive to nickel (300 μM) under the physiological conditions of our experiments. Furthermore, in experiments when benextramine was not added to the PSS nickel failed to alter either the purinergic or noradreneric components of the twitch response (unpublished observation) suggesting a prejunctional action for nickel is unlikely. Nickel 30 μM is selective for blocking the α1H subunit current (IC50=12±2 μM) and 300 μM will block the α1I subunit (IC50=216±9 μM) and the α1G subunit (IC50=250±22 μM) (Lee et al., 1999b; Zamponi et al., 1996). A recent RT–PCR study demonstrated that α1G and α1H subunits are expressed in rat mesenteric arteries but not α1I (Gustafsson et al., 2001). Therefore, given that an inhibitory effect of nickel on EJPs was observed at 300 μM, but not at 30 μM, we propose that the α1G subunit may be the T-channel subtype involved in the generation of EJPs in the mesenteric arteries.
In the mesenteric artery, in addition to inhibiting K+-induced contraction, both mibefradil and nicardipine inhibited αβ-MeATP-induced contractions suggesting involvement of L-channels in these responses, but only mibefradil inhibited EJP amplitude. However, in the vas deferens preparation, although both mibefradil and nicardipine inhibited K+-induced contraction, they each failed to inhibit the purinergically mediated contractions in response to nerve stimulation. It has previously been reported that purinergic contractions may or may not involve generation of smooth muscle action potentials which are sensitive to L-channel inhibitors (Blakeley et al., 1981; Amobi & Smith, 1986). Furthermore, different L-channel blockers have been shown to have different effects on purinergic contractile responses evoked by neuronal stimulation and application of exogeneous agonist in the rat vas deferens (Amobi & Smith, 1986). However, in mesenteric arteries, it has been reported that the L-channel blocker nifedipine has little or no effect on both the neuronally mediated contractions and responses to applied αβ-MeATP depending on vessel diameter (Gitterman & Evans, 2001). In this study we reported that the L-channel blocker nicardipine did reduce responses to αβ-MeATP by 40–50% in the mesenteric artery. This difference may relate to the different L-channel blockers used and further studies are required to identify the factors which contribute to L-channel recruitment in purinergic contractions in vascular and non-vascular smooth muscle. In contrast, as shown by Blakeley et al. (1981), and confirmed in this study, there is no involvement of L-channels in the generation of the EJP. In the mesenteric artery the amplitude of individual EJPs is up to 10 mV and the membrane potential can change from −60 to −50 mV. These conditions are favourable for T-channel opening (Carbone & Lux, 1984) and the findings with mibefradil and nickel suggest that T-channels probably contribute in some way to the EJPs in rat mesenteric arteries.
In conclusion, the principal new findings of this study is that mibefradil and nickel can markedly reduce EJPs in rat mesenteric arteries suggesting that T-channels may contribute to EJPs in rat mesenteric artery. Schrier et al. (2001) have suggested that the biophysical properties of the T-channel allow it to act as a sensor for coupling minimal changes in membrane potential to prolonged calcium influx. This effect of T-channels is sufficient to sustain secretion of aldosterone from the adrenal zona glomerulosa cells (Chen et al., 1999; Lotshaw, 2001) and may, therefore, be sufficient to sustain the EJPs in the rat mesenteric artery. We propose that the α1G subunit could be the subtype of T-channels involved in EJPs in rat mesenteric arteries and that this preparation could be useful to distinguish a T-channel blocking action of drugs considered to have mixed actions at both T- and L-type calcium channels.
Acknowledgments
The authors would like to thank Dr Michael Lew for discussion; Dr Arthur Christopoulos for critically reviewing this manuscript; Ms Lilly Quan, Mr Peter Coles and Mr Mark Ross-Smith as research assistants and Roche Australia Pty Ltd for kindly supplying mibefradil as a gift. Qi Xi is from China Medical University and has a postgraduate scholarship from the University of Melbourne. The use of animals in this study was approved by the University of Melbourne Animal Experimentation Ethics Sub-Committee (Register number 97091)
Abbreviations
- BNX
benextramine
- EFS
electrical field stimulation
- EJPs
excitatory junction potentials
- GVIA
ω-conotoxin GVIA
- T-channels
T-type voltage-operated calcium channels
- TTX
tetrodotoxin
- VOCCs
voltage-operated calcium channels
References
- AMOBI N.I.B., SMITH I.C.H. Differential effects of calcium channel blockers on the responses of the rat vas deferens to intramural nerve stimulation and exogenous drugs. Experentia. 1986;42:410–412. doi: 10.1007/BF02118633. [DOI] [PubMed] [Google Scholar]
- ANGUS J.A., BROUGHTON A., MULVANY M.J. Role of α-adrenoceptors in constrictor responses of rat, guinea-pig and rabbit small arteries to neural activation. J. Physiol. (Lond) 1988;403:495–510. doi: 10.1113/jphysiol.1988.sp017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENHAM C.D. Neurotransmitters. ATP joins the fast lane. Nature. 1992;359:144–147. doi: 10.1038/359103a0. [DOI] [PubMed] [Google Scholar]
- BEZPROZVANNY I., TSIEN R.W. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967) Mol. Pharmacol. 1995;48:540–549. [PubMed] [Google Scholar]
- BLAKELEY A.G., BROWN D.A., CUNNANE T.C., FRENCH A.M., MCGRATH J.C., SCOTT N.C. Effects of nifedipine on electrical and mechanical responses of rat and guinea pig vas deferens. Nature. 1981;294:759–761. doi: 10.1038/294759a0. [DOI] [PubMed] [Google Scholar]
- BLEAKMAN D., BOWMAN D., BATH C.P., BRUST P.F., JOHNSON E.C., DEAL C.R., MILLER R.J., ELLIS S.B., HARPOLD M.M., HANS M.Characteristics of a human N-type calcium channel expressed in HEK293 cells Neuropharmacology 199534753–765.et al [DOI] [PubMed] [Google Scholar]
- BROCK J.A., CUNNANE T.C., EVANS R.J., ZIOGAS J. Inhibition of transmitter release from sympathetic nerve endings by ω-conotoxin. Clin. Exp. Pharmacol. Physiol. 1989;16:333–339. doi: 10.1111/j.1440-1681.1989.tb01568.x. [DOI] [PubMed] [Google Scholar]
- CARBONE E., LUX H.D. A low voltage-activated, fully inactivating Ca2+ channel in vertebrate sensory neurones. Nature. 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- CASSELL J.F., MCLACHLAN E.M., SITTIRACHA T. The effect of temperature on neuromuscular transmission in the main caudal artery of the rat. J. Physiol. 1988;397:31–49. doi: 10.1113/jphysiol.1988.sp016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAÏB N., KABRE E., METIOUI M., ALZOLA E., DANTINNE C., MARINO A., DEHAYE J.P. Differential sensitivity to nickel and SK&F96365 of second messenger-operated and receptor-operated calcium channels in rat submandibular ductal cells. Cell Calcium. 1998;23:395–404. doi: 10.1016/s0143-4160(98)90096-3. [DOI] [PubMed] [Google Scholar]
- CHEN X.L., BAYLISS D.A., FERN R.J., BARRETT P.Q. A role for T-type Ca2+ channels in the synergistic control of aldosterone production by ANG II and K+ Am. J. Physiol. 1999;276:F674–F683. doi: 10.1152/ajprenal.1999.276.5.F674. [DOI] [PubMed] [Google Scholar]
- CHUANG R.S., JAFFE H., CRIBBS L., PEREZ-REYES E., SWARTZ K.J. Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat. Neurosci. 1998;1:668–674. doi: 10.1038/3669. [DOI] [PubMed] [Google Scholar]
- CRAIG P.J., BEATTIE R.E., FOLLY E.A., BANERJEE M.D., RREVES M.B., PRIESTLEY J.V., CARNEY S.L., SHER E., PEREZ-REYES E., VOLSEN S.G. Distribution of the voltage-dependent calcium channel α1G subunit mRNA and protein throughout the mature rat brain. Eur. J. Neurosci. 1999;11:2949–2964. doi: 10.1046/j.1460-9568.1999.00711.x. [DOI] [PubMed] [Google Scholar]
- CRIBBS L.L. Vascular smooth muscle calcium channels: Could ‘T' be a target. Circ. Res. 2001;89:560–562. [PubMed] [Google Scholar]
- CRIBBS L.L., GOMORA J.C., DAUD A.N., LEE J., PEREZ-REYES E. Molecular cloning and functional expression of Cav3.1c, a T-type calcium channel from human brain. FEBS Lett. 2000;466:54–58. doi: 10.1016/s0014-5793(99)01756-1. [DOI] [PubMed] [Google Scholar]
- CRIBBS L.L., LEE J.H., YANG J., SATIN J., ZHANG Y., DAUD A., BARCLAY J., WILLIAMSON M.P., FOX M., REES M., PEREZ-REYES E. Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ. Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- ELLER P., BERJUKOV S., WANNER S., HUBER I., HERING S., KNAUS H.G., TOTH G., KIMBALL S.D., STRIESSNIG J. High affinity interaction of mibefradil with voltage-gated calcium and sodium channels. Br. J. Pharmacol. 2000;130:669–677. doi: 10.1038/sj.bjp.0703352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNION S., HAGAN S., EVANS R.J. The role of positvely charged amino acids in ATP recognition by human P2X1 receptors. J. Biol. Chem. 2000;275:29361–29367. doi: 10.1074/jbc.M003637200. [DOI] [PubMed] [Google Scholar]
- GITTERMAN D.P., EVANS R.J. Nerve evoked P2X receptor contractions of rat mesenteric arteries; dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br. J. Pharmacol. 2001;132:1201–1208. doi: 10.1038/sj.bjp.0703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÖTHERT M., MOLDERINGS G.J. Mibefradil- and ω-conotoxin GVIA-induced inhibition of noradrenaline release from the sympathetic nerves of the human heart. Naunyn. Schmiedebergs Arch. Pharmacol. 1997;356:860–863. doi: 10.1007/pl00005130. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON F., ANDREASEN D., SALOMONSSON M., JENSEN B.L., HOLSTEIN-RATHLOU N. Conducted vasoconstriction in rat mesenteric arterioles: role for dihydropyridine-insensitive Ca2+ channels. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H582–H590. doi: 10.1152/ajpheart.2001.280.2.H582. [DOI] [PubMed] [Google Scholar]
- HANSEN P.B., JENSEN B.L., ANDREASEN D., SKOTT O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ. Res. 2001;89:630–638. doi: 10.1161/hh1901.097126. [DOI] [PubMed] [Google Scholar]
- HERMSMEYER K. Differences of calcium channels in vascular muscle in hypertension. Am. J. Hypertens. 1991;4:412S–415S. doi: 10.1093/ajh/4.7.412s. [DOI] [PubMed] [Google Scholar]
- KENNEDY C. ATP as a cotransmitter in perivascular sympathetic nerves. J. Auton. Pharmacol. 1996;16:337–340. doi: 10.1111/j.1474-8673.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- LAGAUD G.J., STOCLET J.C., ANDRIANTSITOHAINA R. Calcium handling and purinoceptor subtypes involved in ATP-induced contraction in rat small mesenteric arteries. J. Physiol. 1996;492:689–703. doi: 10.1113/jphysiol.1996.sp021338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE J.H., DAUD A.N., CRIBBS L.L., LACERDA A.E., PEREVERZEV A., KLOCKNER U., SCHNEIDER T., PEREZ-REYES E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J. Neurosci. 1999a;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE J.H., GOMORA J.C., CRIBBS L.L., PEREZ-REYES E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys. J. 1999b;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOTSHAW D.P. Role of membrane depolarization and T-type Ca2+ channels in angiotensin II and K+ stimulated aldosterone secretion. Mol. Cell. Endocrinol. 2001;175:157–171. doi: 10.1016/s0303-7207(01)00384-7. [DOI] [PubMed] [Google Scholar]
- MARTIN R.L., LEE J.H., CRIBBS L.L., PEREZ-REYES E., HANCK D.A. Mibefradil block of cloned T-type calcium channels. J. Pharmacol. Exp. Ther. 2000;295:302–308. [PubMed] [Google Scholar]
- MISHRA S.K., HERMSMEYER K. Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circ. Res. 1994;75:144–148. doi: 10.1161/01.res.75.1.144. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., LIKUNGU J., GÖTHERT M. N-type calcium channels control sympathetic neurotransmission in human heart atrium. Circulation. 2000;101:403–407. doi: 10.1161/01.cir.101.4.403. [DOI] [PubMed] [Google Scholar]
- MULRYAN K., GITTERMAN D.P., LEWIS C.J., VIAL C., LECKIE B.J., COBB A.L., BROWN J.E., CONLEY E.C., BUELL G., PRITCHARD C.A., EVANS R.J. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- OLIVERA B.M., MILJANICH G.P., RAMCHANDRAN J., ADAMS M.E. Calcium channel diversity and neurotransmitter release: the ω-conotoxins and ω-agatoxins. Ann. Rev. Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- PEREZ-REYES E., CRIBBS L.L., DAUD A., LACERDA A.E., BARCLAY J., WILLIAMSON M.P., FOX M., REES M., LEE J.H. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- PEREZ-REYES E., LEE J.H., CRIBBS L.L. Molecular characterization of two members of the T-type calcium channel family. Ann. NY Acad. Sci. 1999;868:131–143. doi: 10.1111/j.1749-6632.1999.tb11283.x. [DOI] [PubMed] [Google Scholar]
- SARSERO D., FUJIWARA T., MOLENAAR P., ANGUS J.A. Human vascular to cardiac tissue selectivity of L- and T-type calcium channel antagonists. Br. J. Pharmacol. 1998;125:109–119. doi: 10.1038/sj.bjp.0702045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRIER A.D., WANG H., TALLEY E.M., PEREZ-REYES E., BARRETT P.Q. α1H T-type Ca2+ channel is the predominant subtype expressed in bovine and rat zona glomerulosa. Am. J. Physiol. Cell. Physiol. 2001;280:C265–C272. doi: 10.1152/ajpcell.2001.280.2.C265. [DOI] [PubMed] [Google Scholar]
- TALLEY E.M., CRIBBS L.L., LEE J.H., DAUD A., PEREZ-REYES E., BAYLISS D.A. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J. Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER LEE R., PFAFFENDORF M., DE MEY J.G., VAN ZWIETEN P.A. Inhibitory effect of mibefradil on contractions induced by sympathetic neurotransmitter release in the rat tail artery. Naunyn Schmiedebergs Arch. Pharmacol. 2000;361:74–79. doi: 10.1007/s002109900155. [DOI] [PubMed] [Google Scholar]
- VAN DER LEE R., PFAFFENDORF M., VAN ZWIETEN P.A. Comparative effects of mibefradil and other calcium antagonists on resistance arteries of different end organs. Fundam. Clin. Pharmacol. 1999;13:198–203. doi: 10.1111/j.1472-8206.1999.tb00339.x. [DOI] [PubMed] [Google Scholar]
- XI Q., ANGUS J.A. Evidence against an action of mibefradil at N-type voltage-operated calcium channels. Naunyn-Schmiedebergs Arch. Pharmacol. 2001;364:430–436. doi: 10.1007/s002100100470. [DOI] [PubMed] [Google Scholar]
- ZAMPONI G.W., BOURINET E., SNUTCH T.P. Nickel block of a family of neuronal calcium channels: subtype- and subunit-dependent action at multiple sites. J. Membr. Biol. 1996;151:77–90. doi: 10.1007/s002329900059. [DOI] [PubMed] [Google Scholar]


