Abstract
Endogenous neuronal lipid mediator anandamide, which can be synthesized in the lung, is a ligand of both cannabinoid (CB) and vanilloid receptors (VR). The tussigenic effect of anandamide has not been studied. The current study was designed to test the direct tussigenic effect of anandamide in conscious guinea-pigs, and its effect on VR1 receptor function in isolated primary guinea-pig nodose ganglia neurons.
Anandamide (0.3–3 mg·ml−1), when given by aerosol, induced cough in conscious guinea-pigs in a concentration dependent manner. When guinea-pigs were pretreated with capsazepine, a VR1 antagonist, the anandamide-induced cough was significantly inhibited. Pretreatment with CB1 (SR 141716A) and CB2 (SR 144528) antagonists had no effect on anandamide-induced cough. These results indicate that anandamide-induced cough is mediated through the activation of VR1 receptors.
Anandamide (10–100 μM) increased intracellular Ca2+ concentration estimated by Fluo-4 fluorescence change in isolated guinea-pig nodose ganglia cells. The anandamide-induced Ca2+ response was inhibited by two different VR1 antagonists: capsazepine (1 μM) and iodo-resiniferatoxin (I-RTX, 0.1 μM), indicating that anandamide-induced Ca2+ response was through VR1 channel activation. In contrast, the CB1 (SR 141716A, 1 μM) and CB2 (SR 144528, 0.1 μM) receptor antagonists had no effect on Ca2+ response to anandamide.
In conclusion, these results provide evidence that anandamide activates native vanilloid receptors in isolated guinea-pig nodose ganglia cells and induces cough through activation of VR1 receptors.
Keywords: Anandamide, vanilloid receptor, cough, intracellular calcium, nodose ganglia, cannabinoid receptors, capsazepine
Introduction
Capsaicin-sensitive vanilloid receptor is expressed mainly in sensory nerves including those emanating from dorsal root ganglion (DRG) and afferent fibers that innervate the airway wall emanating from the vagal ganglia. In vagal nodose ganglion, 82% neurons express VR1 mRNA, which is higher than in DRG cells (47%, Michael & Priestley, 1999). VR1 receptor has also been detected in guinea-pig and human airways by receptor binding assay (Szallasi et al., 1995). VR1 is a cation channel with preference for Ca2+. Activation of VR1 in sensory nerves by vanilloids such as capsaicin, induces Ca2+ influx, leading to release of neurotransmitters from both peripheral and central endings, resulting in neurogenic inflammation, bronchoconstriction, cough, and nociception.
Cough is a major manifestation of respiratory diseases. Vagal sensory nerves (i.e. afferent fibers) are involved in the cough reflex. Inhaled VR1 agonist capsaicin, an active ingredient of red pepper, induces cough in normal and diseased human subjects (Higenbottam & Lowry, 1990; Hathaway et al., 1993) as well as in animals such as guinea-pigs (McLeod et al., 2001). However, potential primary endogenous agonists of the VR1 receptors have not been identified. Furthermore, it is not known whether endogenously occurring anandamide play a role in the cough reflex.
Anandamide, an endogenous neuronal lipid mediator, is the ethanolamine amide of arachidomic acid first isolated from porcine brain (Devane et al., 1992). Anandamide is synthesized in the nervous system (di marzo et al., 1994) as well as in peripheral tissues including lungs (Calignano et al., 2000). It has been reported recently that anandamide activates human and rat VR1 receptors in VR1 transfected cells and in rat dorsal root ganglia cells (Zygmunt et al., 1999; Smart et al., 2000; Ralevic et al., 2001). Therefore, anandamide may be a possible endogenous agonist of VR1 receptors. In the airways, anandamide has been reported to induce airway smooth muscle contraction through the activation of VR1 receptors (Tucker et al., 2001). However, the tussigenic effect of anandamide has not been studied. As anandamide is also a known ligand of cannabinoid receptors (Felder et al., 1993), the tussigenic effect of anandamide may be dependent on its relative ability to act on both CB1 and VR1 receptors, and thus may not be predictive of the activity of other select VR1 agonists such as capsaicin. In the current studies, we tested the direct tussigenic effect of anandamide in conscious guinea-pigs. The present findings show that anandamide induces cough through the activation of VR1 receptors.
Methods
Cough measurement in conscious guinea-pigs
Cough in conscious guinea-pigs was measured as described previously (McLeod et al., 2001). Briefly, fasted male Hartley guinea-pigs were placed in a 12′′×14′′ transparent chamber. The animals were exposed to aerosolized anandamide (0.3–3 mg·ml−1) for 7 min, or capsaicin (0.3 mM) for 4 min produced by a jet nebulizer. The number of coughs in response to anandamide or capsaicin were detected by a microphone placed in the chamber and verified by a trained observer. The signal from the microphone was relayed to a polygraph, which provided a record of the number of coughs. Either capsazepine or vehicle were given by aerosol for 4 min prior to challenge with aerosolized anandamide. Anandamide or vehicle were given by aerosol for 7 min prior to challenge with aerosolized capsaicin.
Nodose ganglia cell isolation
Male Hartley guinea-pigs (400–700 g, Charles River, Bloomington, MA, U.S.A.) were euthanized with CO2. Nodose ganglia were removed under aseptic conditions and enzyme digested as previously described (Jia et al., 2002). Briefly, individual ganglia were collected, washed in Hank's Balanced Salt Solution (HBSS) and then transferred to HBSS containing collagenase (type IA, 1 mg·ml−1) for 45 min at 37°C in a water bath. The enzyme solution was aspirated from the tissues, after which they were rinsed with HBSS and then incubated in HBSS contgaining DNAse IV (0.1 mg·ml−1) for 15 min at 37°C in a water bath. Tissues were washed with HBSS and subjected to gentle trituration using a Pasteur pipette. The resulting cell suspension was filtered though a sterile nylon mesh (Becton Dickinson Labware, MA, U.S.A.), plated into poly-lysine coated black walled clear-based 96-well plates (∼10,000 cells/well, Becton Dickinson Labware, MA, U.S.A.). Cells were incubated for 3 h at 37°C prior to the intracellular Ca2+ measurements.
Measurements of intracellular Ca2+ concentration using fluorometric imaging plate reader (FLIPR)
Intracellular Ca2+ (Ca2+]i ) in nodose ganglia cells was measured using FLIPR (Molecular Devices Corp., CA, U.S.A.) technique (Jia et al., 2002). Briefly, cells were incubated with a calcium sensitive fluorescence dye, Fluo-4-AM (5 μg ml−1, Molecular Probes, OR, U.S.A.), in HBSS containing 0.4% bovine serum albumin (BSA) for 45 min at 37°C. The dye-loading solution was removed and the cells were washed three times with HBSS containing 0.4% BSA. The cells were pre-incubated with various antagonists and the plates were then placed into a FLIPR. Fluorescence change due to the change of [Ca2+]i was measured by FLIPR. Anandamide responses were elicited by direct additions to an individual culture well during real-time recording (10 s after commencing recording).
Data analysis
All results are expressed as means±standard error of the mean (s.e.mean). An analysis of variance was performed on the different treatment groups to determine significant effects of the treatments. Post-hoc analysis between the different groups was performed with a Dunnett's t-test. A value of P<0.05 was accepted as the level of statistical significance.
Materials
Capsaicin, capsazepine, DNAse IV, collagenase (type IA), were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.), Anandamide and iodo-resiniferatoxin (I-RTX) from Tocris (Avonmouth, Bristol, U.K.). HU210 was purchased from Cayman Chemical (Ann Arbor, MI, U.S.A.) HBSS was obtained from Gibco (NY, U.S.A.). Indomethacin, SR 144528 and SR 141716A were synthesized by Schering-Plough Corp. (Kenilworth, NJ, U.S.A.).
Results
Anandamide induces cough in conscious guinea-pigs
Effect of anandamide on cough was tested in conscious guinea-pigs. Anandamide (0.3–3 mg·ml−1), when given by aerosol, induces cough in a concentration-dependent manner (Figure 1A). The number of coughs induced by anandamide (3 mg·ml−1) is significantly higher than that in the vehicle inhaled group (Figure 1A, P<0.05). When guinea-pigs were pretreated with capsazepine (0.3 mM, aerosol for 4 min), a VR1 antagonist, anandamide-induced cough was significantly inhibited. In contrast, the CB1/CB2 receptor antagonists, SR 141716A (0.5 mg·kg−1, i.v.) and SR 144528 (0.3 mg·kg−1, i.v.), had no effect on anandamide-induced cough (Figure 1A), thereby excluding the involvement of CB receptors in anandamide-induced cough in guinea-pigs. We also tested the effect of anandamide on capsaicin-induced cough. Guinea-pigs were exposed to aerosolized anandamide (3 mg·ml−1) or vehicle for 7 min. The number of coughs induced by aerosolized capsaicin (0.3 mM) was recorded for 4 min after the anandamide treatment. Anandamide had no effect on capsaicin-induced cough (Figure 1B).
Figure 1.
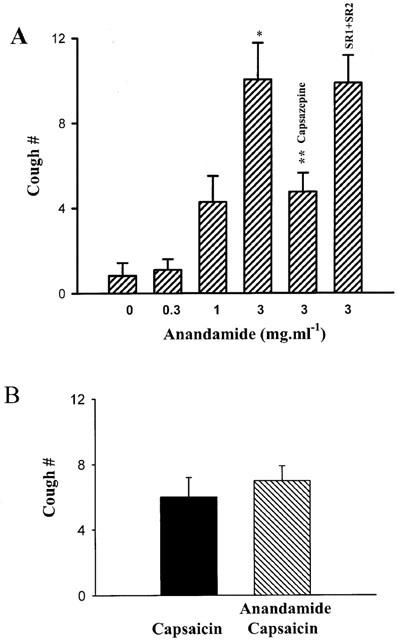
(A) Tussigenic effect of anandamide in conscious guinea pig. Anandamide or vehicle were given by aerosol for seven minutes. Capsazepine (0.3 mM) was given by aerosol for 4 min before the application of anandamide. SR 141716A (SR1, 0.5 mg·kg−1) and SR 144528 (SR2, 0.3 mg·kg−1) was given by i.v. (B) Effect of anandamide on capsaicin-induced cough. Guinea-pigs were aerosolized with anandamide (3 mg·kg−1) for 7 min. Cough number was recorded in response to capsaicin (0.3 mM, aerosol for 4 min). The results are means±s.e.mean. *P<0.005, compared with vehicle control group. **P<0.05, compared with anandamide (3 mg·kg−1) group in the absence of capsazepine.
Anandamide activates VR1 receptors in isolated primary guinea-pig nodose ganglia cells
VR1 function on isolated guinea-pig nodose ganglia cells was evaluated by measuring intracellular Ca2+ concentration determined by Fluo-4 fluorescence change. Anandamide (3–100 μM) increased [Ca2+]i in nodose ganglia cells in a concentration-dependent manner as shown in Figure 2. The anandamide-induced Ca2+ response was inhibited when cells were pretreated with two different VR1 antagonists capsazepine (10 μM) or iodo-resiniferatoxin (0.1 μM, Wahl et al., 2001, Figure 2). Capsazepine and iodo-resiniferatoxin alone had no effect on intracellular Ca2+ level (data not shown). Capsazepine inhibited the anandamide-induced Ca2+ response by about 70%, while the CB1 receptor antagonist SR 141716A (1 μM) and CB2 receptor antagonist SR 144528 (0.1 μM) had no effect on anandamide-induced Ca2+ response (Figure 3B). In addition, HU 210 (1 μM) alone, a CB1/CB2 agonist, did not affect intracellular Ca2+ level in nodose ganglia cells (Figure 3A). These results exclude the involvement of CB1 and CB2 receptors in the anandamide-induced Ca2+ response and suggest that anandamide-induced Ca2+-response is mediated through VR1 channels.
Figure 2.
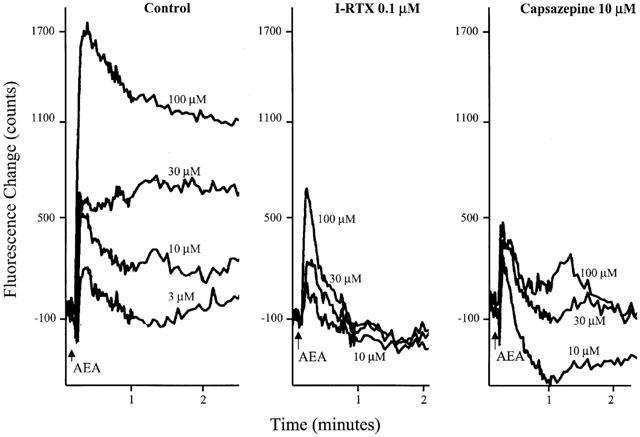
Effect of anandamide on intracellular Ca2+ in isolated guinea pig nodose ganglia cells. [Ca2+]i was determined by Flou-4 fluorescence change and measured using FLIPR. The figure shows representative traces of the Ca2+ response to anandamide (AEA) in nodose ganglia cells, in the absence and presence of iodo-resiniferatoxin (I-RTX) and capsazepine. Cells were pretreated with capsazepine (10 μM) or I-RTX (0.1 μM) for 10 min. Anandamide was added 10 s after commencing recording in all the experiments.
Figure 3.
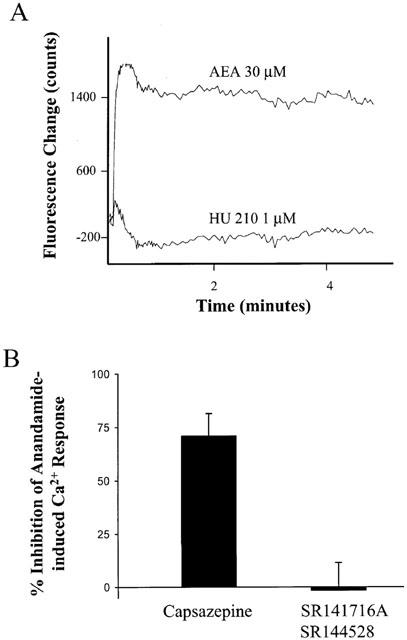
Anandamide-induced Ca2+ response in guinea-pig nodose ganglia cells is through VR1 channels. (A) Representative traces of Ca2+ response to anandamide (AEA, 30 μM) and HU 210 (1 μM). (B) Effect of capsazepine (10 μM) and SR 141716A (1 μM)/SR 144528 (0.1 μM) on anandamide induced Ca2+ response in isolated guinea-pig nodose ganglia cells. The results are means±s.e.mean and are expressed as % inhibition of anandamide-induced Ca2+ response.
Discussion
Capsaicin induces cough in many species including human (Doherty et al., 2000) and guinea-pigs (Bolser et al., 1991; McLeod et al., 2001), suggesting the involvement of VR1 receptors in the cough reflex arc. However, endogenous agonists for the receptor have not been elucidated. Anandamide, an endogenous lipid ligand known to occur in the lung (Calignano et al., 2000), has been identified as a potential endogenous agonist for both CB1 (Felder et al., 1993) and VR1 receptors (Zygmunt et al., 1999; Smart et al., 2000; Ralevic et al., 2001). It has been reported that both VR1 and CB1 may be involved in the regulation of experimental cough. In guinea-pigs, capsaicin-induced cough is likely mediated through VR1 activation (Bolser et al., 1991; McLeod et al., 2001). Calignano's study shows that capsaicin-induced cough is inhibited through CB1 receptors (Calignano et al., 2000). The direct tussigenic effect of anandamide has not been tested before. In the current study, we found that aerosolized anandamide induced cough in a concentration dependent manner in conscious guinea-pigs. To elucidate the mechanism of tussigenic effect of anandamide, we tested the effect of enandamide on guinea-pigs pretreated with VR1 or CB1 receptor antagonists. Capsazepine, a selective VR1 antagonist, significantly inhibited anandamide-induced cough, indicating the involvement of VR1 receptors. To exclude the involvement of CB receptors in anandamide-induced cough, we tested the effect of SR 141716A, a CB1 antagonist, and SR 144528, a CB2 antagonist, on anandamide-induced cough. The dose of SR 141716A (0.5 mg·kg−1 i.v.) and SR 144528 (0.3 mg·kg−1 i.v.) used in this study has been shown to inhibit CB receptors effectively in guinea-pig airways (Calignano et al., 2000). SR 141716A and SR 144528 had no effect on anandamide-induced cough, excluding the involvement of CB receptors. Taken together, these findings indicate that the tussigenic effect of anandamide is mediated exclusively through the activation of VR1 receptors.
Calignano et al. (2000) reported that pretreatment of guinea-pigs by inhaled anandamide (10 mg·ml−1, pretreatment by aerosol for 15 min) inhibited capsaicin-induced cough through CB1 receptors. In our studies, anandamide-induced cough was not increased by inhibition of CB receptors, suggesting that CB receptors are not activated by anandamide in our experimental conditions. The concentration of anandamide and treatment time was different between Calignano's group and our group. We used 0.3–3 mg·ml−1 anandamide and recorded cough during the exposure period (0–7 min) while Calignano's group used 10 mg·kg−1 anandamide and pretreated the animals for 15 min before recording cough. Calignano's study treated the animals with a higher concentration of anandamide and for a longer time. It is less likely that the higher concentration of anandamide accounts for the activation of airway CB1 receptors because the concentration to activate CB1 receptors is much lower than the concentration required for activation of VR1 receptors. On the other hand, the different treatment time may explain the observed differences. To experimentally address this issue, we tested the effect of anandamide (7 min aerosol) on capsaicin-induced cough. We found that 7 min treatment with anandamide did not inhibit capsaicin-induced cough (Figure 1B). Given Calignano's findings that 15 min treatment by anandamide activates CB1 receptors in the airways and inhibits capsaicin-induced cough, the duration of anandamide treatment may be important for the activation of CB1 receptors in the airways. In addition, the size of aerosol particles generated in different labs may vary and thus the extent of drug deposition at different depths of the airway may also be significantly different. CB1 receptors are detected on 63% of sympathetic nerves (Calignano et al., 2000) which display a differential distribution of innervation in the upper airway and lower airway (reviewed by Barnes, 1990). This may also contribute to the differential effect of aerosolized anandamide on CB1 receptors observed in different labs.
It seems that anandamide has bidirectional effects on cough. Inhaled anandamide acutely induces cough by activation of VR1 and inhibits cough through CB1 receptors when airways are pretreated with the ligand for a longer time in experimental conditions. Whether endogenously synthesized anandamide acts as a VR1 or CB1 receptor agonist may be determined by the physiological and pathophysiological conditions. For example, PKC mediated phosphorylation of VR1 leads to a strong (10–15 fold) enhancement of anandamide action on VR-gated currents (Primkumar & Ahern, 2000; Vellani et al., 2001). On the contrary, CB1 activity is down regulated by PKC phosphorylation (Garcia et al., 1998). PKA phosphorylation also lowers by approximately 5 fold the concentration of anandamide necessary for the activation of VR1 (de petrocellis et al., 2001). Furthermore, VR1 activation can also be potentiated by acidic environment (McLatchie & Bevan, 2001) which is present in airway inflammatory diseases such as asthma (Hunt et al., 2000). Under these conditions, anandamide may favor the stimulation of VR1 and induce cough. Furthermore, the effect of anandamide on VR1 or CB1 may also depend on the distribution of the receptors. VR1 is known to be expressed mainly on sensory nerves while CB1 receptor is detected on sympathetic nerves (Calignano et al., 2000). Therefore, the endogenous synthesized anandamide may activate different receptors when synthesized in different cells.
Airway afferent fibers emanating from the vagal nodose ganglia are believed to be involved in the cough reflex. In the current study, we tested the direct effect of anandamide on VR1 channel function in isolated primary guinea-pig nodose ganglia neurons. VR1 is a cation channel with preference for Ca2+. VR1 channel function was tested by measuring intracellular Ca2+ level using FLIPR technique. We found that anandamide increased intracellular Ca2+ level in guinea-pig nodose ganglia cells. Anandamide-induced Ca2+ response was inhibited by two different VR1 antagonists indicating that the Ca2+ influx was through VR1 channel. CB1 or CB2 receptors were not involved in anandamide-induced Ca2+ response because CB1/CB2 antagonists had no effect on the response. In addition, HU210, a known CB1/CB2 receptor agonist, had no effect on intracellular Ca2+ concentration when added to the cells alone. This observation suggests that anandamide activates native VR1 receptors in guinea-pig nodose ganglia cells. This is consistent with the finding that anandamide activates human and rat VR1 receptors in transfected cells and in rat DRG cells (Zygmunt et al., 1999; Smart et al., 2000; Ralevic et al., 2001) and that anandamide activates rat pulmonary vagal c-fiber through the activation of VR1 but not CB1 receptors (Lin & Lee, 2002). This finding and the finding by Lin & Lee (2002) provide a mechanism for the current observation that anandamide induces cough and the previous observation that anandamide induces airway contraction (Tucker et al., 2001) in guinea-pigs.
Anandamide is an endogenous neuronal messenger formed through phosphodiesterase-mediated cleavage of a phospholipid precursor in central neurons (di marzo et al., 1994). Recent studies indicate that anandamide can also be synthesized in lung tissue as a result of Ca2+ stimulation (Calignano et al., 2000), suggesting that locally generated anandamide may be involved in the intrinsic control of respiratory functions in normal and/or diseased lung. In the current studies, anandamide activates VR1 receptors in nodose ganglia cells and induces cough in conscious guinea-pigs through VR1 receptors, consistent with this hypothesis. In fact, the respiratory response to VR1 activation is enhanced in some disease conditions, and it has been reported that the cough response to capsaicin is increased in pulmonary diseases such as asthma and chronic obstructive pulmonary disease (Doherty et al., 2000). In addition, neuropeptides in nodose ganglia cells are upregulated in animal models of allergic airway inflammation (Fischer et al., 1996) and respiratory viral infection (Carr et al., 2002). In the current studies, the high concentrations of anandamide (3–100 μM) are required to activate VR1 receptors in nodose ganglia cells. This potentially argues against anandamide being a primary endogenous VR1 agonist. The apparent low potency of anandamide however, may be due to poor distribution into cells. Anandamide has been shown, using displacement and binding studies, to bind to the same site on VR1 as capsaicin (Ross et al., 2001), which is in the intracellular region of cell membrane (Jung et al., 1999). Presently, anandamide was applied to the extracellular side of the cell. The actual anandamide concentration inside the cell may be lower because membrane permeability of anandamide is low and is limited by an endocannabinoid transport system (de petrocellis et al., 2001). This may account for the weak potency on VR1 activation in nodose ganglia cells and tussigenic effect in guinea-pigs in the present studies. It is known that endogenous anandamide is synthesized inside the cell, 80% of which remains in the cells (di marzo et al., 1994). Thus it is possible that a lower concentration of anandamide is required for the activation of VR1 when made available from an intracellular source.
In summary, anandamide is an agonist of native VR1 receptors on guinea-pig nodose ganglia cells and induces cough through the activation of VR1. If endogenous anandamide (or other endogenous mediators) activates VR1 in the airway, and thus plays a role in airway physiology and inflammatory lung diseases (e.g. cough, asthma and chronic obstructive pulmonary disease), VR1 inhibition may represent a potential treatment of these diseases.
Abbreviations
- AEA
anandamide
- Capz
capsazepine
- [Ca2+]i
intracellular Ca2+
- CB
cannabinoid
- DRG
dorsal root ganglion
- FLIPR
fluorometric imaging plate reader
- HBSS
Hank's Balanced Salt Solution, I-RTX, iodo-resiniferatoxin
- VR1
type 1 vanilloid receptor
References
- BARNES P. Neural control of airway function: new perspectives. Molec. Aspects. Med. 1990;11:351–423. doi: 10.1016/0098-2997(90)90003-k. [DOI] [PubMed] [Google Scholar]
- BOLSER D.C., AZIZ S.M., CHAPMAN R.W. Ruthenium red decreases capsaicin and citric acid-induced cough in guinea-pigs. Neurosci. Lett. 1991;126:131–133. doi: 10.1016/0304-3940(91)90536-3. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., KATONA I., DESARNAUD F., GIUFFRIDA A., LA RANA G., MACKIE K., FREUND T.F., PIOMELLI D. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408:96–101. doi: 10.1038/35040576. [DOI] [PubMed] [Google Scholar]
- CARR M.J., HUNTER D.D., JACOBY D.B., UNDEM B.J. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am. J. Respir. Crit. Care Med. 2002;165:1071–1075. doi: 10.1164/ajrccm.165.8.2108065. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., MACCARRONE M., DAVIS J.B., FINAZZI-AGRO A., DI MARZO V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid recepor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., FONTANA A., CADAS H., SCHINELLI S., CIMINO G., SCHWARTZ J.C., PIOMELLI D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;237:686–689. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- DOHERTY M.J., MISTER R., PEARSON M.G., CALVERLEY P.M. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax. 2000;55:643–649. doi: 10.1136/thorax.55.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDER C.C., BRILEY E.M., AXELROD J., SIMPSON J.T., MACKIE K., DEVANE W.A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHER A., MCGREGOR G.P., SARIA A., PHILIPPIN B., KUMMER W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J. Clin. Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA D.E., BROWN S., HILLE B., MACKIE K. Protein kinase C disrupts cannabinoid actions by phosphorylation of CB1 receptor. J. Neurosci. 1998;18:2834–2841. doi: 10.1523/JNEUROSCI.18-08-02834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATHAWAY T.J., HIGENBOTTAM T.W., MORRISON J.F.J., CLELLAND C.A., WALLWORK J. Effects of inhaled capsaicin in heart-lung transplant patients and asthmatic subjects. Am. Rev. Respir. Dis. 1993;148:1233–1237. doi: 10.1164/ajrccm/148.5.1233. [DOI] [PubMed] [Google Scholar]
- HIGENBOTTAM T., LOWRY R. Adaptation and cross-adaptation of the cough reflex in response to distilled water. Capsaicin and prostaglandin E2 aerosols in man. J. Physiol. 1990;422:32p. [Google Scholar]
- HUNT J.F., FANG K., MALID R., SNYDER A., MALHOTRA N., PLATTS-MILLS R.A.E., GASTON B. Endogenous airway acidification. Am. J. Respir. Crit. Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- JIA Y., WANG X., APONTE S.A., RIVELLI M.A., YANG R., RIZZO C.A., CORBOZ M.R., PRIESTLEY T., HEY J.A. Nociceptin/orphanin FQ inhibits capsaicin-induced guinea pig airway contraction through an inward-rectifier potassium channel. Br. J. Pharmacol. 2002;135:764–770. doi: 10.1038/sj.bjp.0704515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG J., HWANG S.W., KWAK J., LEE S.Y., KANG C.J., KIM W.B., KIM D., OH U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN Y.S., LEE L.Y. Stimulation of pulmonary vagal C-fibres by anandamide in anaesthetized rats: role of vanilloid type 1 receptors. J. Physiol. 2002;539:947–955. doi: 10.1113/jphysiol.2001.013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLATCHIE L.M., BEVAN S. The effects of pH on the interaction between capsaicin and the vanilloid receptor in rat dorsal root ganglia neurons. Br. J. Pharmacol. 2001;132:899–908. doi: 10.1038/sj.bjp.0703900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEOD R.L., PARRA L.E., MUTTER J.C., EROCLSPM C.H., CAREY G.J., TULSHIAN D.B., FAWZI A.B., SMITH-TORHAM A., EGAN R.W., FRANCIS M.C., HEY J.A. N/OFQ inhibits cough in the guinea-pig by activation of ORL1 receptors. Br. J. Pharmacol. 2001;132:1175–1178. doi: 10.1038/sj.bjp.0703954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAEL G.J., PRIESTLEY J.V. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J. Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIMKUMAR L.S., AHERN G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., DENDALL D.A., JERMAN J.C., MIDDLEMISS D.N., SMART D. Cannabinoid activation of recombinant and endogenous vanilloid receptors. Eur. J. Pharmacol. 2001;424:211–219. doi: 10.1016/s0014-2999(01)01153-0. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., BROCKIE H.C., LESLIE M., PASHMI G., CRAIB S.J., MARZO V.D., PERTWEE R.G. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., GUNTHORP M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZALLASI A., GOSO C., MANZINI S. Resiniferatoxin binding to vanilloid receptors in guinea pig and human airways. Am. J. Respir. Crit. Care Med. 1995;152:59–63. doi: 10.1164/ajrccm.152.1.7599863. [DOI] [PubMed] [Google Scholar]
- TUCKER R.C., KAGAYA M., PAGE C.P., SPINA D. The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. Br. J. Pharmacol. 2001;132:1127–1135. doi: 10.1038/sj.bjp.0703906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELLANI V., MAPPLEBECK S., MORIONDO A., DAVIS J.B., MCNAUGHTON P.A. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J. Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHL P., FORED C., TULLIN S., THOMSEN C. Indo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol. Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H.-H., SORGARD M., DIMARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


