Abstract
The present study was aimed to investigate intracellular pathways involved in acetylcholine (ACh)-induced contraction in cat detrusor muscle cells
Contraction was expressed as per cent shortening of length of individually isolated smooth muscle cells obtained by enzymatic digestion. Dispersed intact and permeabilized cells were prepared for the treatment of drugs and antibody to enzymes, respectively. Using Western blot, we confirmed the presence of related proteins.
The maximal contraction to ACh was generated at 10−11 M. This response was preferentially antagonized by M3 muscarinic receptor antagonist ρ-fluoro-hexahydrosiladifenidol (ρF-HSD) but not by the M1 antagonist pirenzepine and the M2 muscarinic receptor antagonist methoctramine. We identified G-proteins Gq/11, Gs, G0, Gi1, Gi2 and Gi3 in the bladder detrusor muscle. ACh-induced contraction was selectively inhibited by Gq/11 antibody but not to other G subunit.
The phosphatidylinositol-specific phospholipase C (PI-PLC) inhibitor neomycin reduced ACh-induced contraction. However, the inhibitors of the phospholipase D, the phospholipase A2 and protein kinase C did not attenuate the ACh-induced contraction. ACh-induced contraction was inhibited by antibody to PLC-β1 but not PLC-β3 and PLC-γ. Thapsigargin or strontium, which depletes or blocks intracellular calcium release, inhibited ACh-induced contraction. Inositol 1,4,5-triphosphate (IP3) receptor inhibitor heparin reduced ACh-induced contraction.
These results suggest that in cat detrusor muscle contraction induced by ACh is mediated via M3 muscarinic receptor-dependent activation of Gq/11 and PLC-β1 and IP3-dependent Ca2+ release.
Keywords: cat bladder, smooth muscle, detrusor, acetylcholine, muscarinic receptor, G-protein, phospholipase C, calcium
Introduction
Acetylcholine (ACh), through its action at muscarinic receptors on smooth muscle cells, is the primary neurotransmitter controlling bladder voiding. Muscarinic receptor density and the sensitivity of bladder smooth muscle to muscarinic stimulation are the greatest in the dome and the lowest in the base, allowing efficient bladder emptying (Ferguson & Christopher, 1996).
Pharmacological, biochemical and genetic data provide ample evidence that muscarinic receptors are heterogeneous in nature. One approach has been to clone of a family of five (m1–m5) genes that code for muscarinic receptor subtypes, to transfect clonal cell lines with cDNA coding for individual muscarinic receptor subtypes and then to establish their biochemical properties (Buckley et al., 1989). It is well established that the ‘odd-numbered' muscarinic receptors (M1, M3, and M5) typically couple via the α subunits of the Gq/11 family, whereas the ‘even-numbered' members (M2, M4) couple via the α subunits of the Gi and G0 and share the same proposed overall structure and a large degree of protein sequence homology (Bonner et al., 1987). This preferential coupling resides at the molecular level mainly in the postulated membrane-proximal regions of the i2 and i3 loops of the different receptors, which are notably different between the ‘odd' and ‘even' receptor groups, and similar within each of the two groups. The coupling selectivity at the G-protein level is reflected generally, but not exclusively, observed downstream second-messenger pathways activated by the two groups of muscarinic receptors; PLC-β is activated by the ‘odd' receptors, that is M1, M3 and M5, whereas adenylyl cylcase is inhibited by the ‘even' receptors, that is M2 and M4. At least three subtypes of muscarinic receptors (M1, M2, and M3) are pharmacologically distinguishable. The M1 muscarinic receptor has high affinity for pirenzepine but low affinity for AF-DX 116, whereas the M2 muscarinic receptor has low affinity for pirenzepine but high affinity for AF-DX 116 (Hammer & Giachetti, 1982). A third type of muscarinic receptor is characterized by low affinity for both pirenzepine and AF-DX 116 and by high affinity for 4-DAMP.
In functional studies, stimulation of bladder muscarinic receptors causes smooth muscle contraction. Because bladder appears to contain more than one muscarinic receptor subtype, it is possible that one muscarinic receptor subtype is responsible for bladder detrusor smooth muscle contraction whereas the others are coupled to another parasympathetic associated function, such as influencing neuronal acetylcholine release. It remains to be discovered exactly what factors determine how the specificity of this receptor-effector coupling occurs. It could occur at the level of the coupling of one receptor subtype, through multiple G proteins, to several responses in bladder (Yang et al., 2000).
There are evidences on the study of the detrusor muscle that is characterized principally with M2 and M3 muscarinic receptors; M3 muscarinic receptors, coupled to stimulation of phosphoinositide turnover, resulting in the production of IP3, mediate the direct contractile effects of ACh in the detrusor, wheareas M2 muscarinic receptors, via inhibition of adenylyl cyclase, cause contraction indirectly (Andersson et al., 1991; Barras et al., 1999; Harriss et al., 1995; Hegde et al., 1997; Hegde & Eglen, 1999; Sellers et al., 2000; Sellers et al., 2000; Yamanishi et al., 2000). However it was not well studied about intracellular pathways including (a) certain G protein(s) and PLC isozymes in the mediation of muscarinic contraction in detrusor muscle.
In this study, we investigated the characterization of muscarinic receptor subtypes and clarified G protein subtypes or PLC isozymes that were involved in ACh-induced muscle contraction of cat detrusor muscle cells.
Methods
Preparation of bladder detrusor muscle tissue squares
All experimental methods were approved by the Institutional Animal Care and Use Committee of the Chung Ang University in Seoul, Korea. Adult cats of randomized either sex, weighing 2.5–3 kg were used in this study, since ACh-induced maximal contractions of these cells in male (21.3±0.2% shortening, n=45) were not different when compared to that in female (21.7±0.3% shortening, n=21). Animals were killed by an overdose of 25% urethane (Aldrich, St. Louis, MO, U.S.A.). After the midline was opened, and the entire urinary bladder was removed, the bladder dome was isolated from the trigone region, the surrounding connective tissue and epithelium were carefully removed from the trigone region. The detrusor smooth muscle was divided into horizontal strips and cut into 0.5 mm thick slices with a Stadie Riggs tissue slicer (Thomas Scientific Apparatus, Philadelphia, PA, U.S.A.). The last slices containing serosa were discarded. The slices of smooth muscle layers were placed flat on a wax surface, and tissue squares were made by cutting twice with a 2 mm blade block, the second cut at right angles to the first.
Preparation of dispersed intact and permeabilized smooth muscle cells
Isolated smooth muscle cells were obtained by enzymatic digestion (Sohn et al., 1993). The bladder tissue squares were digested in the Krebs solution, containing 10 mM HEPES, 0.09 mg ml−1 soybean trypsin inhibitor, 10% BSA, and collagenase 50 unit ml−1 and equilibrated with 95% O2–5% CO2 to maintain pH 7.45±0.05 at 31°C. The Krebs solution contained (in mM): NaCl 118, KCl 4.8, MgSO4 1.2, NaHCO3 24, KH2PO4 1.2, Glucose 11 and CaCl2 2.5. The solution was gently gassed with 95% O2–5% CO2. At the end of digestion period, the tissue was placed over a 350 μm Nitex mesh, rinsed in collagenase-free Krebs solution enough to remove any trace of collagenase, and then incubated in collagenase-free Krebs solution at 31°C and gassed with 95% O2–5% CO2. The cells were allowed to dissociate freely in this Krebs solution for 10–20 min. Throughout the entire procedure, care was taken not to agitate the fluid to avoid cell contraction in response to mechanical stress.
Cells were permeabilized, when required, to allow the use of agents such as G protein antibodies or PLC isozyme antibodies or heparin, which do not diffuse across the intact cell membrane (Cao et al., 2001; Shim et al., 2002; Yang et al., 2000). The cells' length after permeabilization was not changed (75.6+0.3 μm n=37 versus control 75.8+0.4 μm, n=46), and contractile properties was also same. After completion of the enzymatic phase of the digestion process, the partly digested muscle tissue was washed with an enzyme-free cytosolic buffer of the following composition (in mM): NaCl 20; KCl 100; MgSO4 5.0; NaH2PO4 0.96; EGTA 1.0; and CaCl2 0.48 and 2% BSA. The cytosolic buffer was equilibrated with 95% O2–5% CO2 to maintain a pH of 7.2 at 31°C. Muscle cells dispersed spontaneously in this medium. The cytosolic buffer contained 0.48 mM CaCl2 and 1 mM EGTA, yielding 0.18 μM free Ca2+, as calculated according to Fabiato & Fabiato (1979). After dispersion, the cells were permeabilized by incubation for 5 min in cytosolic buffer that contained saponin (0.1 μg ml−1). After exposure to saponin, the cell suspension was spun at 350×g and the resulting pellet was washed with saponin-free modified cytosolic buffer that contained antimycin A (10 μM), ATP (1.5 mM) and an ATP-regenerating system that consisted of creatine phosphate (5 mM) and creatine phosphokinase (10 units ml−1). After the cells were washed free of saponin, they were resuspended in modified cytosolic buffer.
Agonist-induced contraction of isolated muscle cells
Medium containing the test agents was added to an aliquot of cell suspension and then the cells were contracted by exposure for 30 s to ACh. When muscarinic antagonists were used, the cells were incubated in appropriate concentrations of the antagonists for 1 min before the addition of ACh. The pretreatment of G proteins antibodies or PLC isozymes antibodies was respectively incubated for 1 h before the addition of ACh in modified cytosolic buffer after permeabilization (Murthy & Makhlouf, 1991; Sohn et al., 1993; 1995). After exposure to ACh, the cells suspension was fixed in formalin at a final 0.8% concentration. A drop of the cells suspension including agonist added in test tube was placed on a glass slide and covered by a cover slip and the edges were sealed with clear nail enamel to prevent evaporation.
The length of 30 consecutive intact cells, encountered at random in each slide, were measured with a phase-contrast microscope (model ULWCD 0.30, Olympus, Tokyo, Japan), and a digital closed-circuit video camera (CCD colour camera, Toshiba, Tokyo, Japan) connected to a Macintosh computer (Apple, Cupertino, CA, U.S.A.) with a software program, Image 1.57 (National Institutes of Heath, Bethesda, MD, U.S.A.). Contraction was expressed as the per cent shortening of individual cells compared with the control cell length.
Determination of muscarinic receptor antagonist potencies
Concentration-response curves were constructed for ACh alone and in the presence of four concentrations of M1 (pirenzepine), M2 (methoctramine), or M3 (pF-HSD) antagonists. The EC50 value for ACh alone and in the presence of each antagonist was calculated (Result 1), and Schild plots were then generated to calculate pA2 and Schild slope values (Figure 1C) according to the method of Arunlakshana & Schild (1959) using a software program (PHARM/PCS Ver. 4) for pharmacological calculations. In all cases slopes did not differ significantly from unity.
Figure 1.
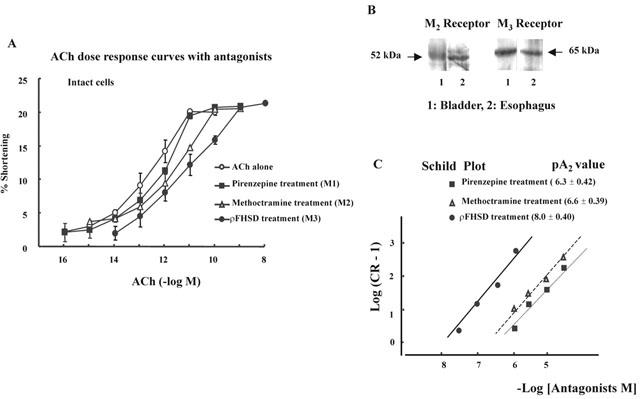
(A) The concentration-response curve of ACh in presence of antagonists (10−6 M). Intact bladder detrusor muscle cells were contracted with the increased concentration of ACh alone or after a 1 min pretreatment with 10−6 M of the M1, M2 or M3 muscarinic antagonists, pirenzepine, methoctramine or pF-HSD, respectively. The M3 antagonist pF-HSD produced a significant shift to the right in the ACh concentration-response curve. The values are means±s.e.mean (n=4). (B) Identification of M2 and M3 receptors of cat bladder. The bands of M2 and M3 receptors of cat esophagus were presented as control. M2 and M3 receptors were all presented in cat bladder smooth muscle. (C) Comparison of antagonistic effects (pA2) of muscarinic antagonists, pirenzepine (M1), methoctramine (M2) and pF-HSD (M3), in ACh-induced contraction of cat detrusor muscle cells. Each value represents the mean of five experiments. The data points plotted by the method of Arunlakshana & Schild (1959) were fitted by linear regression to obtain the pA2. All slopes were not significantly different from unity. Each antagonist was used in four different concentrations.
Identification of M2, M3 muscarinic receptors, PLC isozymes, and G protein subtypes by Western blot
Muscle layers were isolated from bladder detrusor and homogenized in homogenizing buffer containing (mM): Tris HCl 20 (pH 7.5), EGTA 0.5, EDTA 0.5, β-mercaptoethanol 10, leupeptin 10 μg ml−1, and aprotinin 10 μg ml−1. When identification of M2, M3 muscarinic receptors, PLC isozyme or G protein is needed, homogenized muscle cells are respectively centrifuged at 14,000×g for 15 min at 4°C (Murthy & Makhlouf, 2000; Sohn et al., 1993; 1995; Wang et al., 2000; Yang et al., 2000). The supernant was subjected to sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis (M2 & M3 muscarinic receptors: 7.5%, PLC isozymes: 5%, G protein subtypes: 12.5% gradient gel). Prestained molecular mass markers were run in an adjacent lane to permit molecular mass determination. The separated proteins were electrophoretically transferred to nitrocellulose (NC) membranes at 80 V in 25 mM Tris (pH 8.3), 192 mM glycine, and 20% methanol. Transfer of proteins to the NC membrane was confirmed with Ponseau S staining reagent. The blots were incubated for 1 h at room temperature in phosphate-buffered saline (PBS) containing 5% nonfat dry milk to block non-specific antibody binding. After three or four washes in PBS, the blots were incubated for 1 h at 4°C with isozyme-specific monoclonal antibodies (PLC: 0.5 μg ml−1, G protein: 20 μg ml−1) to PLC isozyme or G protein with shaking, were removed by washing in PBS containing 0.1% BSA and 0.05% Tween 20, followed by washing three times in PBS containing 0.05% Tween 20 and incubated the blots with horseradish peroxidase-conjugated goat anti-rabbit antibody (1 mg ml−1). Enhanced chemiluminescence reagents detected the G protein bands.
Materials
Methoctramine HCl and ρF-HSD were purchased from Research Biochemical (Natick, MA, U.S.A.); PLC-β antibodies (PLC-β1, PLC-β2, PLC-β3, PLC-β4, PLC-α1, α2, PLC-δ1, and PLC-δ2) and G protein antibodies (Gq–G11, Gi1, Gi2, Gi3, G0, and Gs) from Santa Cruz Biotechnology (California, CA, U.S.A.); chelerythrine chloride from LC Laboratories (Woburn, MA, U.S.A.); chemilumiscence agents from NENTM Life Science (Boston, MA, U.S.A.); PBS from Boehringer Mannheim (Indianapolis, IN, U.S.A.); collagenase from Worthington Biochemicals (Freehold, NJ, U.S.A.); rainbow prestained-molecular weight marker from Amersham (Arlington Heights, IL, U.S.A.); SDS sample buffer and nitrocellulose membrane from BioRad (Richmond, CA, U.S.A.). Horseradish peroxidase-conjugated goat anti-rabbit antibody were obtained from Pierce (Rockford, IL, U.S.A.); Anti-muscarinic M2 and M3 receptor serum from Research & Diagnostic Antibodies (Berkeley, CA, U.S.A.); strontium choloride, ρCMB, neomycin, saponin, EGTA, EDTA, HEPES, soybean trypsin inhibitor, BSA, pirenzepine, Ponseau S, leupeptin, aprotinin, and β-mercaptoethanol from Sigma (St. Louis, MO, U.S.A.); thapsigargin and DEDA from Calbiochem (La Jolla, CA, U.S.A.). All other chemicals were of the highest purity or molecular biology grade available from commercial sources.
Data analysis
Data are expressed as the means±standard errors mean (s.e.mean). Statistical differences between groups were determined by Student's t-test. Differences between multiple groups were tested using ANOVA for repeated measures and checked for significance using Scheff's F test.
Results
Characterization of muscarinic receptors mediating ACh-induced contraction
The mean length of detrusor muscle cell (n=4) was 75.6±0.70 μm. ACh produced concentration-dependent contraction of the bladder detrusor muscle cell of cat (Figure 1A). The contraction in response to ACh was not significantly affected by the M1 antagonist pirenzepine (10−6 M) or M2 antagonist methoctramine (10−6 M). In contrast, the M3 antagonists ρF-HSD (10−6 M) caused a significant parallel shift to the right in the concentration-response curve to ACh without alteration of the maximum response. In the presence of ρF-HSD, the negative logarithm value of the EC50 of ACh ((11.3±0.26), n=4) was significantly greater than that of the control (13.1±0.14), pirenzepine (12.3±0.29), or methoctramine (12.3±0.17), respectively. Esophagus was used as control when investigating the presence of M2 or M3 muscarinic receptors. As shown in Figure 1B, M2 and M3 muscarinic receptors were presented in cat bladder smooth muscle. (Figure 1B). In addition, it was further shown that, zusing four different concentrations of each antagonists, the pA2 value of M3 antagonist was greater than that of M1 or M2 antagonist, which suggests that the contraction of detrusor muscle cells may be primarily mediated by the activation of muscarinic M3 receptors (Figure 1C). The slope was not different from the unity, indicating that each antagonist is competitive.
G protein involved in contractile response to ACh
Identification of G protein subtypes by Western blot
Solubilized tissue samples were loaded into the 12.5% SDS polyacrylamide gel electrophoresis system, transferred to NC membrane and incubated with each G protein antibody at a 1 : 1000 concentration for 1 h with continuous shaking. Bands corresponding to Gq/11 (42 kDa), GS (46 kDa), G0 (40 kDa), Gi1, Gi2, and Gi3 (40 kDa) protein were identified (Figure 2).
Figure 2.
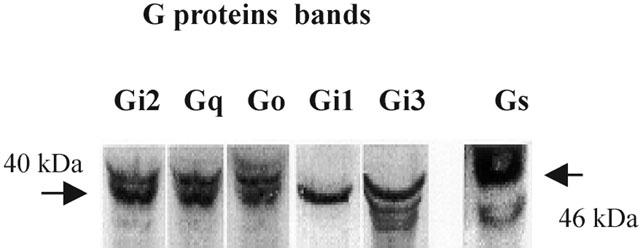
Identification of G-protein in detrusor muscle. Plasma membranes isolated from dispersed bladder muscle cells were homogenized as described in Methods. Membrane proteins were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, electrophoretically transferred to nitrocellulose membranes, and then probed with G protein-specific antibodies and anti-rabbit IgG conjugated to horseradish perosicase. Enhanced chemiluminescence reagents identified the G protein bands. Bands corresponding to Gs (46 kDa), Gi group (40 kDa), G0 (40 kDa) and Gq group (42 kDa) were detected.
Clarification of G protein subtypes by use of G protein antibodies
To identify the specific G proteins involved in bladder detrusor contraction, we used G protein antibodies developed by Spiegel (Goldsmith et al., 1987; Shenker et al., 1991) and Sternweis (Gutowski et al., 1991). These antibodies block receptor-induced activation of G protein by binding to the terminal peptide region of the G protein that interacts with the receptor. The cells were permeabilized with saponin to allow diffusion of the antibody into the cytosolic region of the cell membrane (1 : 200). ACh-induced contraction in permeabilized muscle cells was significantly inhibited by antibody to Gαq/111 (Figure 3B, *P<0.05), but not by antibodies Gαil–3, Gα5 or G0 antibody. These data suggest that ACh-induced contraction of detrusor occurs via PTX-insensitive Gq/11 protein.
Figure 3.
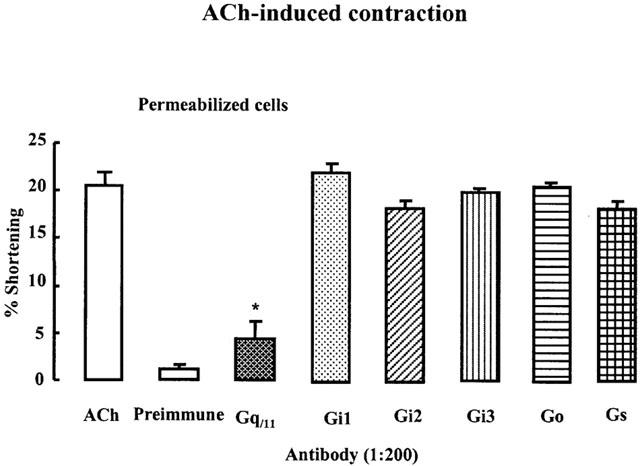
Role of G-protein in ACh-induced contraction. Muscle cells were permeabilized by brief exposure to saponin to allow diffusion of antibodies into the cytosolic side of the cell membrane. Preimmune antiserum did not have any effects on cells. ACh-induced contraction of bladder detrusor muscle cells was inhibited after a 60 min preincubation in cytosolic medium that contained Gq/11 antibody but not any other G-protein antibodies. Each point is the mean±s.e.mean with 30 cells counted for each (n=4). These data suggest ACh-induced contraction occurs via Gq/11 protein. *P<0.01 by ANOVA.
Involvement of phospholipase in ACh-induced contraction
Effect of phospholipase inhibitors on ACh-induced contraction
In search of ACh-activated phospholipase, we examined the effects of phospholipase A2 inhibitor DEDA (Sohn et al., 1994b), PI-PLC inhibitor neomycin (DeLegge et al., 1993), and phosphatidylcholine-specific PLD inhibitor rCMB (Sohn et al., 1993). ACh-induced contraction of the bladder was reduced by the 10 min preincubation of neomycin (10−6, 10−5 or 10 −4 M, *P<0.05, **P<0.01 in Figure 4). However, pretreatment for 1 min with different concentrations (10−8, 10 −7 , 10−6, or 10−5 M) of ρCMB or DEDA did not affect the ACh-induced contraction. These data suggest that detrusor muscle cells contraction is mediated by PI-PLC.
Figure 4.
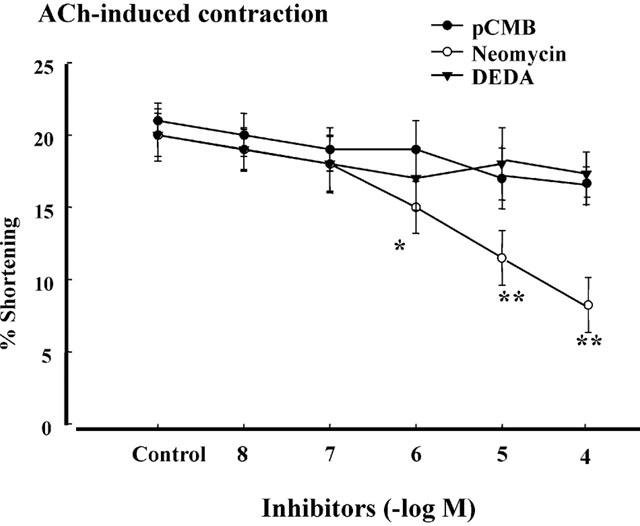
Effects of phospholipases inhibitors on ACh-induced contraction. Intact detrusor muscle cells were contracted with the maximally effective concentration of ACh (10−10 M) alone (control) or after 1 min preincubation with PLD inhibitor pCMB (10−8–10−5 M) and with PLA2 inhibitor DEDA (10−8–10−5 M). PLC inhibitor neomycin (10−6, 10−5 or 10−4 M) reduced the ACh-induced contraction. The values are the means±s.e.mean (n=5). *P<0.05 , **P<0.01 by ANOVA.
Identification of PLC isozymes by Western blot
Western blot analysis of homogenates obtained separately from dispersed circular muscle using monoclonal antibodies to the main PLC types demonstrated the presence of immunoreactive protein bands corresponding to 150 kDa with PLC-β1 and PLC-β3 antibody, and 145 kDa with PLC-γ1 antibody (Figure 5A).
Figure 5.
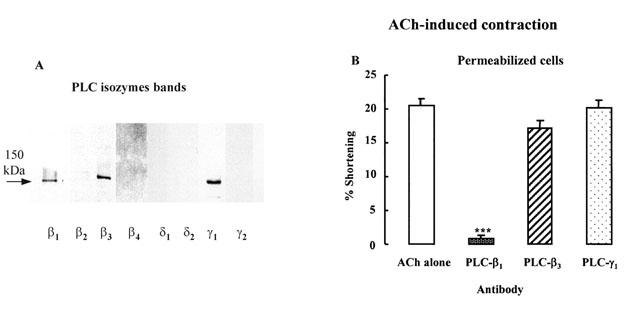
Detection and involvement of PLC isozyme in ACh-induced contraction in detrusor muscle cells. (A) Immunoreactivity of PLC isozymes in homogenates obtained separately from bladder detrusor muscle. After transfer to nitrocellulose membranes, immunoreactive protein bands corresponding to PLC-β1 and β3 (150 kDa) and PLC-γ1 (145 kDa) are identified. (B) The permeabilized bladder muscle cells by brief exposure to saponin were pretreated for 1 h by PLC-β isozymes antibodies and then contracted by ACh (10−10 M). These results show that inhibition to ACh-induced contraction by PLC-β1 antibody was significant (***P<0.001 by ANOVA). Values are the means±s.e.mean of four experiments. Consequently, ACh-induced contraction depends on PLC-β1 activation.
Clarification of PLC isozymes by use of PLC antibodies
To identify the specific PLC isozyme that was involved in ACh-induced detrusor contraction, we used PLC isozyme antibodies in permeabilized cells. When antibodies were used, the permeabilized cells incubated with the antibody at a 1 : 200 dilution for 1 h before addition of ACh (Bitar et al., 1992). The results showed that ACh-induced permeabilized cell contraction was significantly reduced by a 1 h preincubation with PLC-β1 antibody, but not by PLC-β3 or PLC-γ1 antibody (Figure 5B, ***P<0.001).
PKC or IP3 receptor involvement in ACh-induced contraction
Figure 6A shows that ACh (10−10 M)-induced contraction of bladder detrusor muscle cells is not affected by the PKC inhibitor chelerythrine (10−5 M), suggesting that ACh-induced detrusor muscle contraction is independent with PKC activation.
Figure 6.
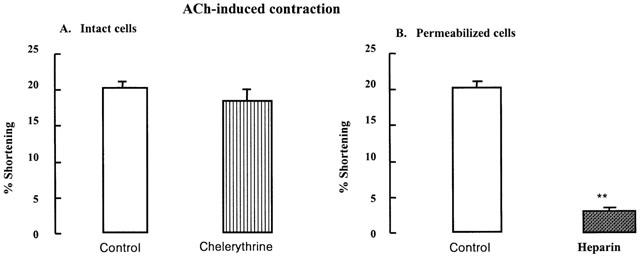
Effect of the preincubation of chelerythrine or heparin on ACh-induced contraction. (A) Contraction of intact bladder muscle cells in response to a maximally effective concentration of ACh (10−10 M) alone (control) was not blocked by PKC inhibitor chelerythrine (10−5 M) (n=4). (B) The permeabilized muscle cells by brief exposure to saponin were contracted with ACh (10−10 M) alone (control). IP3 receptor antagonist heparin (10 mg ml−1, 1 min) significantly reduced the ACh-induced contraction (n=4). These data suggest that bladder detrusor contraction in response to ACh depends on IP3 formation but not PKC activation. The values are the mean±s.e.mean. **P<0.01 by Student's t-test.
Incubation with IP3 receptor antagonist heparin (10 μg ml−1) for 1 min, inhibited ACh-induced contraction in permeabilized muscle cells (Figure 6B, **P<0.01). These data shows that ACh-induced bladder contraction occurs via IP3-dependent Ca2+ release from intracellular stores receptor activation but not via PKC-dependent mechanisms.
Effect of intracellular Ca2+ on contraction of detrusor muscle cells
We examined the dependence of ACh-induced contraction on the presence of extracellular Ca2+ or intracellular Caa2+. When Ca2+ is replaced by 4 mM Sr2+, which blocks contraction mediated by Ca2+ release from intracellular stores, bladder detrusor muscle contraction in response to ACh (10−10 M) is significantly reduced (Figure 7A, **P<0.01). When intact cells were incubated in Ca2+-free medium containing 2 mM EGTA, ACh-induced contraction was not affected. These data suggest that detrusor muscle contraction in response to ACh depends on intracellular Ca2+ release. This hypothesis was further confirmed in Figure 7B, in which cell contraction is significantly reduced by prolonged incubation in thapsigargin (2 μM, 30 min preincubation) which depletes IP3-sensitive and IP3-insensitive Ca2+ stores (*P<0.05).
Figure 7.
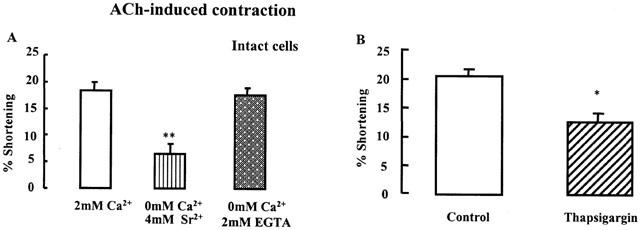
Effect of Ca2+ on ACh-induced bladder contraction. (A) The contraction in response to maximally effective ACh (10−10 M) was significantly reduced when intact detrusor muscle cells were incubated in Ca2+-free medium containing 4 mM Sr2+. Conversely, Ca2+-free medium containing 2 mM EGTA had no effect (n=4, **P<0.01 by ANOVA). (B) The cells were contracted with the maximally effective concentration of ACh (10−10 M) alone (control) in 2 mM Ca2+ medium. ACh-induced contraction of bladder muscle cells was blocked by a 30 min preincubation of thapsigargin (2 mM). ACh-induced contraction mainly uses intracellular Ca2+. The values are the means±s.e.mean (n=4). *P<0.05 by Student's t-test.
Discussion
Characterization of muscarinic receptors mediating ACh-induced contraction
ACh acts through muscarinic receptors on the cell membrane, which are known to be linked to GTP binding proteins (Bonner, 1992; Casey & Gilman, 1988; Hosey, 1992). The pharmacological classification is based on different receptor affinities for selective antagonists such as pirenzepine (M1<M2=M3) (Doods et al., 1987; Hammer et al., 1980; Hammer & Giachetti, 1982), methoctramine and AF-DX 116 (M2<M1<M3) (Melchiorre et al., 1987), hexa-hydro-sila-difenidol (M1=M3<M2) (Giraldo et al., 1988b) and ρF-HSD (M3<M1<M2). Subsequently, five distinct ACh muscarinic receptors were cloned. Three (m1, m2, m3) of the five subtypes of muscarinic receptors were recognized through radioligand binding and functional data (Hosey, 1992). A comparison of muscarinic receptors subtypes showed that the antagonist binding properties of the m1, m2 and m3 cloned receptors and their pattern of expression in various tissues corresponded closely to those of the pharmacologically defined M1, M2 and M3 receptors, respectively (Buckley et al., 1989; Hosey, 1992). It has been suggested that human esophageal smooth muscle cells express muscarinic receptor subtypes M1 through M5 (Wang et al., 2000). The M3 receptor is known as the major muscarinic subtype in the animal bladder responsible for detrusor contraction (Tong & Cheng, 2002). The M2 receptor is quantitatively the dominant muscarinic subtype in animal bladders (Tong et al., 1999). The presence of the M2 and M3 receptor in bladder or parotid of human, rabbit, guinea-pig, and rat was confirmed (Wang et al., 1995; 2000). In this study, we found the presence of M2 and M3 receptors in cat bladder smooth muscle.
Methoctramine is the prototype of the polymethylene tetramine class of muscarinic receptor antagonists and is reported to display selectivity toward cardiac M2 muscarinic receptors (Giraldo et al., 1988a; Melchiorre et al., 1987). The drug ρF-HSD is a hexahydro-sila-difenidol analogue that has recently been shown to possess considerable selectivity for the smooth muscle M3 muscarinic receptor in guinea-pig ileum.
In this study, there was no difference of the maximal response between male and female. In bladder strips from females were more sensitive to carbachol than those from males, but there were no differences in sensitivity to electrical field stimulation, as well as no differences in pA2 values of muscarinic antagonists between bladder strips from male and female rats (Longhurst & Levendusky, 2000). The reason for the different sensitivity may be the use of different species, maximum dose, muscle types (tissue strips and isolated cells). Maximal contraction in isolated cells was produced around 0.1–10 nM range, similar to esophagus, gall bladder and lower esophageal sphincter's maximal contraction, in other isolated cells of other tissues, the potency of the isolated cells was similar about 3–4 log units higher than that of muscle strips at μM ranges (Biancani et al., 1994; Sohn et al., 1997; 2001; Yu et al., 1998). The bladder detrusor muscle cells contraction to ACh in our experiments is significantly reduced by pretreatment of M3 receptor antagonist, ρF-HSD. However, M1 receptor antagonist pirenzepine or M2 receptor antagonist methoctramine did not reduce contraction but rather shifted the ACh concentration-response curve. It was confirmed by pA2 values; the value of M3 antagonist was greater than that of M1 antagonist or M2 antagonist. It is possible that detrusor muscle contraction is mainly mediated by the activation of muscarinic M3 receptor. This is consistent with the view that M3 receptors appear to mediate direct contraction, whereas M2 receptors act only after selective M3 inactivation or act indirectly reserving sympathetically (i.e. β-adrenoceptor)-mediated relaxation (Hegde et al., 1997; Hegde & Eglen, 1999).
G protein characterization of receptor mediating contractile response to ACh
Western blot analysis of solubilized membrane fractions derived from dispersed bladder detrusor muscle cells demonstrated the presence of a full complement of G proteins: Gq/11, Gs, G0, Gi1, Gi2, and Gi3. Additionally, after the muscle cells were permeabilized by brief exposure to saponin, contraction to ACh was significantly reduced by a 1 h preincubation of Gq/11 protein antibody but not G5, Gi, or G0 protein antibody. Therefore, our data suggest that bladder detrusor M3 receptors may be linked to a G protein of the Gq class. These results were consistent with those that reported the involvement of two members of the Gq class of G α subunits, Gq and Gα11, in PTX-resistant coupling to PLC activation. It appears that the contraction of detrusor muscle depends on the activation of M3 muscarinic receptors, linked to Gq/11 type of G proteins.
Role of phospholipase C in ACh-induced contraction
The binding of various hormones, neurotransmitters, which include ACh, and peptide growth factors to their cell surface receptors initiates signalling cascades that results in hydrolysis of phosphatidylinositol by phosphoinositide-specific phospholipase C (PI-PLC). As a result of hydrolysis of PLC, IP3, a Ca2+-mobilizing messenger (Murthy et al., 1992; Murthy & Makhlouf, 1991) and diacylglycerol, which activate PKC and results in the phosphorylation of intracellular proteins, are generated (Cockcroft & Thomas, 1992; Nishizuka, 1986; 1992).
Neomycin was reported to inhibit IP3-dependent Ca2+ release in skinned muscle strips of rabbit main pulmonary artery (Kobayashi et al., 1989), in gastric circular muscle cells (DeLegge et al., 1993), and inhibit PI-PLC in esophageal circular cells (Shim et al., 2002). Our data suggest that ACh-induced contraction of detrusor muscle results from PI- PLC activation because ACh-induced contraction of detrusor muscle cells was inhibited by 65% inhibition by neomycin high concentration (10−4 M). However, we cannot exclude the possibility that other mechanism may be involved, since the remaining inhibition portion (35%) is still ineffective by this inhibitor. Another PI-PLC inhibitor U73122 remains to be tested in this contraction. Muscarinic M3-mediated contraction of detrusor muscle is not antagonized by the phosphatidylcholine-specific PLD antagonist ρCMB, which was reported to reduce phosphatidic acid production by directly inhibiting phosphatidylcholine-specific PLD. In addition, PLA2 was also not involved in ACh-induced contraction of this detrusor mucle, but other PLA2 inhibtors except DEDA may be needed to be clarified. This results of detrusor muscle was consistent with other findings that muscarinic agonist increase in IPs production by inositol phospholipid hydrolysis via PLC by M3 receptor activation (Barras et al., 1999; Mimata et al., 1995).
Several subtypes of PLC-β (PLC-β1, PLC-β2, PLC-β3, and PLC-β4) have now been identified that differ in their mode of activation by G proteins (Boyer et al., 1992; Lee et al., 1992; Smrcka et al., 1991). PLC-β4 is activated by the a-subunits of all five members of the Gq family of G proteins (Gq, G11, G14, G15, and G16). PLC-β1 and PLC-β3 are preferentially activated by the homologous α-subunits of Gq and G11 (Lee et al., 1992; Smrcka et al., 1991), whereas PLC-β2 is preferentially activated by the α-subunit of G16. PLC-β isoforms are also activated by βγ-dimers, with PLC-β2 and PLC-β3 being more responsive than PLC-β1 (Boyer et al., 1992). PLC-β isoforms activated by βγ-dimers derived from interaction of ligands with receptors coupled to G0 or Gi exhibit PTX sensitivity (Simon et al., 1991).
Western blot of PLC isozymes in bladder detrusor muscle showed that PLC-β1, PLC-β3 and PLC-γ1 are present. Additionally, contraction in response to ACh in detrusor muscle cells was significantly abolished by PLC-β1 antibody but not PLC-β3 or PLC-γ1 antibody. These results suggest as new finding, not studied yet, in detrusor muscle cells ACh-induced bladder contraction is mediated by M3 receptor-dependent activation of Gq/11 and PLC-β1.
PKC or IP3 receptor relationship in ACh-induced contraction
Chelerythrine, a benzophenathridine alkaloid is a potent, selective antagonist of the Ca2+/phospholipid-dependent PKC from the rat brain. Half-maximal inhibition (IC50) of PKC occurs at 0.66 μM. Chelerythrine interacted with the catalytic domain of PKC, was a competitive inhibitor with respect to the phosphate acceptor (histone IIIS, Ki=0.7 μM) and a non-competitive inhibitor with respect to ATP. This effect was further evidenced by the fact that chelerythrine inhibited native PKC and its catalytic fragment identically and did not affect [3H]-phorbol-12, 13-dibutyrate binding to PKC. Chelerythrine is >100 times more potent against PKC than against tyrosine protein kinase, cAMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase (Herbert et al., 1990; Hidaka et al., 1981). ACh-induced contraction of bladder smooth muscle cells is not significantly affected by chelerythrine (Herbert et al., 1990), suggesting that contraction of bladder muscle may be independent on activation of PKC.
The role of PLC/IP3 in ACh-induced contraction of the bladder is further supported by the finding that, in permeabilized cells, ACh-induced contraction of bladder was inhibited by the IP3 receptor antagonist heparin. Heparin acts by blocking the binding of IP3 to its receptor, thereby preventing the release of IP3-sensitive intracellular Ca2+ (Ghosh et al., 1988; Kobayashi et al., 1989).
Effect of blockade of intracellular Ca2+ release on contraction of detrusor muscle cells
To test the possibility that some Ca2+ may be released from intracellular storage sites in response to ACh, we examined the effect of thapsigargin on ACh-induced contraction of intact cells. Thapsigargin enhances the release and/or prevents uptake of IP3-sensitive or -insensitive Ca2+, resulting in depletion of these stores. After 30 min incubation in thapsigargin, ACh-induced contraction of bladder muscle is significantly reduced, suggesting that in this time complete depletion of bladder Ca2+ stores may occur (Bian et al., 1991; Sohn et al., 1994a). The theory that ACh-induced contraction of bladder muscle is dependent on intracellular Ca2+ release, is also supported by the finding that the contraction is affected by substituting Ca2+ with Sr2+. ACh-Induced contraction of bladder muscle depends on release of Ca2+ from intracellular Ca2+ stores since it is significantly reduced by incubation in 4 mM Sr2+ medium instead of Ca2+ but not in Ca2+-free medium containing 2 mM EGTA. Blockade of intracellular Ca2+ release was obtained by manipulating intracellular Ca2+ stores with strontium. Strontium is thought to displace and replace Ca2+ at storage sites in smooth muscle, but is not readily released from these sites by stimulatory agents. Sr2+ mimics the influx of extracellular Ca2+, supports a K+-induced response, which depends on extracellular Ca2+ and does not support equally well the response to stimulatory agents (e.g., norepinephrine), which are resistant to blockade of extracellular Ca2+ influx (Yasuda & Sakai, 1984). Thus Sr2+ may substitute for extracellular Ca2+ and support muscle contractions that utilize influx of extracellular Ca2+, while inhibit contractions that depend on release of intracellular Ca2+. These findings are consistent with the hypothesis that detrusor muscle utilizes intracellular Ca2+. Thus Sr2+ blocks actions mediated by Ca2+ release while it maintains effects mediated by extracellular Ca2+ influx (Biancani et al., 1987; Hillemeier et al., 1991). This result may support the finding that agonists, including carbachol, histamine and ATP, also activated repetitive increases of Ca2+ released from Ca2+ store (Chambers et al., 1996; Masters et al., 1999). These data support the view that contraction of detrusor muscle is dependent on PI- PLC and IP3 and requires the release of intracellular Ca2+.
In conclusion, we have found in this study that in detrusor muscle cell signalling, detrusor muscle contraction to ACh in cat is mediated via muscarinic M3 receptors – Gq/11 protein coupling and involves the activation of PLC-β1 and intracellular Ca2+ mobilization via IP3 receptor on Ca2+ stores.
Abbreviations
- ACh
acetylcholine
- AF-DX 116
(11,11-[[2-(diethyl-amino)methyl]-1-piperidinyl]acetyl)-5,11-di-hydro-H-pyrido[2,3-6][1,4]benzodiazepine-6-)one
- BSA
Bovine serum albumin
- ρCMB
para-choloromercuribenzoic acid
- 4-DAMP
4-diphenylacetoxy-N-methylpiperidine methiodide
- DEDA
dimethyleicosa-dienoic acid
- EDTA
ethylenediamine tetraacetic acid
- EGTA
ethylene glycol-bis (β-aminoehtyl ether)-N,N,N′,N′-tetraacetic acid
- ρF-HSD
para-fluoro-hexahydrosila-difenidol
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethane sulphonic acid
- IP3
inositol 1,4,5-triphosphate
- PI
polyphosphoinositide
- PLA2
phospholipase A2
- PLC
phospholipase C
- PLD
phospholipase D
- SDS
sodium dodecyl sulphate
- Tris
2-aminio-2-hydroxymethyl-1,3-propanediol
References
- ANDERSSON K.E., HOLMQUIST F., FOVAEUS M., HEDLUND H., SUNDLER R. Muscarinic receptor stimulation of phosphoinositide hydrolysis in the human isolated urinary bladder. J. Urol. 1991;146:1156–1159. doi: 10.1016/s0022-5347(17)38030-8. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRAS M., COSTE A., EON M.T., GUILLOT E. Pharmacological characterization of muscarinic receptors implicated in rabbit detrusor muscle contraction and activation of inositol phospholipid hydrolysis in rabbit detrusor and parotid gland. Fundam. Clin. Pharmacol. 1999;13:562–570. doi: 10.1111/j.1472-8206.1999.tb00362.x. [DOI] [PubMed] [Google Scholar]
- BIAN J.H., GHOSH T.K., WANG J.C., GILL D.L. Identification of intracellular calcium pools. Selective modification by thapsigargin. J. Biol. Chem. 1991;266:8801–8806. [PubMed] [Google Scholar]
- BIANCANI P., HARNETT K.M., SOHN U.D., RHIM B.Y., BEHAR J., HILLEMEIER C., BITAR K.N. Differential signal transduction pathways in cat lower esophageal sphincter tone and response to ACh. Am. J. Physiol. 1994;266:G767–G774. doi: 10.1152/ajpgi.1994.266.5.G767. [DOI] [PubMed] [Google Scholar]
- BIANCANI P., HILLEMEIER C., BITAR K.N., MAKHLOUF G.M. Contraction mediated by Ca2+ influx in esophageal muscle and by Ca2+ release in the LES. Am. J. Physiol. 1987;253:G760–G766. doi: 10.1152/ajpgi.1987.253.6.G760. [DOI] [PubMed] [Google Scholar]
- BITAR K.N., STEIN S., OMANN G.M. Specific G proteins mediate endothelin induced contraction. Life Sci. 1992;50:2119–2124. doi: 10.1016/0024-3205(92)90578-d. [DOI] [PubMed] [Google Scholar]
- BONNER T.I. Domains of muscarinic acetylcholine receptors that confer specificity of G protein coupling. Trends. Pharmacol. Sci. 1992;13:48–50. doi: 10.1016/0165-6147(92)90021-w. [DOI] [PubMed] [Google Scholar]
- BONNER T.I., BUCKLEY N.J., YOUNG A.C., BRANN M.R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- BOYER J.L., WALDO G.L., HARDEN T.K. Beta gamma-subunit activation of G-protein-regulated phospholipase C. J. Biol. Chem. 1992;267:25451–25456. [PubMed] [Google Scholar]
- BUCKLEY N.J., BONNER T.I., BUCKLEY C.M., BRANN M.R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol. Pharmacol. 1989;35:469–476. [PubMed] [Google Scholar]
- CAO W., CHEN Q., SOHN U.D., KIM N., KIRBER M.T., HARNETT K.M., BEHAR J., BIANCANI P. Ca2+-induced contraction of cat esophageal circular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2001;280:C980–C992. doi: 10.1152/ajpcell.2001.280.4.C980. [DOI] [PubMed] [Google Scholar]
- CASEY P.J., GILMAN A.G. G protein involvement in receptor-effector coupling. J. Biol. Chem. 1988;263:2577–2580. [PubMed] [Google Scholar]
- CHAMBERS P., NEAL D.E., GILLESPIE J.I. Ca2+ signalling in cultured smooth muscle cells from human bladder. Exp. Physiol. 1996;81:553–564. doi: 10.1113/expphysiol.1996.sp003958. [DOI] [PubMed] [Google Scholar]
- COCKCROFT S., THOMAS G.M. Inositol-lipid-specific phospholipase C isoenzymes and their differential regulation by receptors. Biochem. J. 1992;288:1–14. doi: 10.1042/bj2880001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELEGGE M., MURTHY K.S., GRIDER J.R., MAKHLOUF G.M. Characterization of distinct receptors for the peptidyl leukotrienes LTC4 and LTD4/LTE4 coupled to the same signaling pathway in isolated gastric muscle cells. J. Pharmacol. Exp. Ther. 1993;266:857–863. [PubMed] [Google Scholar]
- DOODS H.N., MATHY M.J., DAVIDESKO D., VAN CHARLDORP K.J., DE JONGE A., VAN ZWIETEN P.A. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J. Pharmacol. Exp. Ther. 1987;242:257–262. [PubMed] [Google Scholar]
- FABIATO A., FABIATO F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. Paris. 1979;75:463–505. [PubMed] [Google Scholar]
- FERGUSON D., CHRISTOPHER N. Urinary bladder function and drug development. Trends Pharmacol. Sci. 1996;17:161–165. doi: 10.1016/0165-6147(96)81593-8. [DOI] [PubMed] [Google Scholar]
- GHOSH T.K., EIS P.S., MULLANEY J.M., EBERT C.L., GILL D.L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J. Biol. Chem. 1988;263:11075–11079. [PubMed] [Google Scholar]
- GIRALDO E., MICHELETTI R., MONTAGNA E., GIACHETTI A., VIGANO M.A., LADINSKY H., MELCHIORRE C. Binding and functional characterization of the cardioselective muscarinic antagonist methoctramine. J. Pharmacol. Exp. Ther. 1988a;244:1016–1020. [PubMed] [Google Scholar]
- GIRALDO E., VIGANO M.A., HAMMER R., LADINSKY H. Characterization of muscarinic receptors in guinea pig ileum longitudinal smooth muscle. Mol. Pharmacol. 1988b;33:617–625. [PubMed] [Google Scholar]
- GOLDSMITH P., GIERSCHIK P., MILLIGAN G., UNSON C.G., VINITSKY R., MALECH H.L., SPIEGEL A.M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J. Biol. Chem. 1987;262:14683–14688. [PubMed] [Google Scholar]
- GUTOWSKI S., SMRCKA A., NOWAK L., WU D.G., SIMON M., STERNWEIS P.C. Antibodies to the alpha q subfamily of guanine nucleotide-binding regulatory protein alpha subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J. Biol. Chem. 1991;266:20519–20524. [PubMed] [Google Scholar]
- HAMMER R., BERRIE C.P., BIRDSALL N.J., BURGEN A.S., HULME E.C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980;283:90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- HAMMER R., GIACHETTI A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982;31:2991–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- HARRISS D.R., MARSH K.A., BIRMINGHAM A.T., HILL S.J. Expression of muscarinic M3-receptors coupled to inositol phospholipid hydrolysis in human detrusor cultured smooth muscle cells. J. Urol. 1995;154:1241–1245. [PubMed] [Google Scholar]
- HEGDE S.S., CHOPPIN A., BONHAUS D., BRIAUD S., LOEB M., MOY T.M., LOURY D., EGLEN R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGDE S.S., EGLEN R.M. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999;64:419–428. doi: 10.1016/s0024-3205(98)00581-5. [DOI] [PubMed] [Google Scholar]
- HERBERT J.M., AUGEREAU J.M., GLEYE J., MAFFRAND J.P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- HIDAKA H., ASANO M., TANAKA T. Activity-structure relationship of calmodulin antagonists, Naphthalenesulfonamide derivatives. Mol. Pharmacol. 1981;20:571–578. [PubMed] [Google Scholar]
- HILLEMEIER C., BITAR K.N., MARSHALL J.M., BIANCANI P. Intracellular pathways for contraction in gastroesophageal smooth muscle cells. Am. J. Physiol. 1991;260:G770–G775. doi: 10.1152/ajpgi.1991.260.5.G770. [DOI] [PubMed] [Google Scholar]
- HOSEY M.M. Diversity of structure, signaling and regulation within the family of muscarinic cholinergic receptors. FASEB J. 1992;6:845–852. [PubMed] [Google Scholar]
- KOBAYASHI S., KITAZAWA T., SOMLYO A.V., SOMLYO A.P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J. Biol. Chem. 1989;264:17997–18004. [PubMed] [Google Scholar]
- LEE C.H., PARK D., WU D., RHEE S.G., SIMON M.I. Members of the Gq alpha subunit gene family activate phospholipase C beta isozymes. J. Biol. Chem. 1992;267:16044–16047. [PubMed] [Google Scholar]
- LONGHURST P.A., LEVENDUSKY M. Influence of gender and the oestrous cycle on in vitro contractile responses of the rat urinary bladder to cholinergic stimulation. Br. J. Pharmacol. 2000;131:177–184. doi: 10.1038/sj.bjp.0703551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTERS J.G., NEAL D.E., GILLESPIE J.I. The contribution of intracellular Ca2+ release to contraction in human bladder smooth muscle. Br. J. Pharmacol. 1999;127:996–1002. doi: 10.1038/sj.bjp.0702640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELCHIORRE C., ANGELI P., LAMBRECHT G., MUTSCHLER E., PICCHIO M.T., WESS J. Antimuscarinic action of methoctramine, a new cardioselective M-2 muscarinic receptor antagonist, alone and in combination with atropine and gallamine. Eur. J. Pharmacol. 1987;144:117–124. doi: 10.1016/0014-2999(87)90509-7. [DOI] [PubMed] [Google Scholar]
- MIMATA H., WHEELER M.A., FUKUMOTO Y., TAKIGAWA H., NISHIMOTO T., WEISS R.M., LATIFPOUR J. Enhancement of muscarinic receptor-coupled phosphatidylinositol hydrolysis in diabetic bladder. Mol. Cell Biochem. 1995;152:71–76. doi: 10.1007/BF01076465. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., GRIDER J.R., MAKHLOUF G.M. Receptor-coupled G proteins mediate contraction and Ca++ mobilization in isolated intestinal muscle cells. J. Pharmacol. Exp. Ther. 1992;260:90–97. [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Phosphoinositide metabolism in intestinal smooth muscle: preferential production of Ins(1,4,5)P3 in circular muscle cells. Am. J. Physiol. 1991;261:G945–G951. doi: 10.1152/ajpgi.1991.261.6.G945. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Heterologous desensitization mediated by G protein-specific binding to caveolin. J. Biol. Chem. 2000;275:30211–30219. doi: 10.1074/jbc.M002194200. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- SELLERS D.J., YAMANISHI T., CHAPPLE C.R., COULDWELL C., YASUDA K., CHESS-WILLIAMS R. M3 muscarinic receptors but not M2 mediate contraction of the porcine detrusor muscle in vitro. J. Auton. Pharmacol. 2000;20:171–176. doi: 10.1046/j.1365-2680.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- SHENKER A., GOLDSMITH P., UNSON C.G., SPIEGEL A.M. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J. Biol. Chem. 1991;266:9309–9313. [PubMed] [Google Scholar]
- SHIM J.O., SHIN C.Y., LEE T.S., YANG S.J., AN J.Y., SONG H.J., KIM T.H., HUH I.H., SOHN U.D. Signal transduction mechanism via adenosine A1 receptor in the cat esophageal smooth muscle cells. Cell. Signal. 2002;14:365–372. doi: 10.1016/s0898-6568(01)00270-4. [DOI] [PubMed] [Google Scholar]
- SIMON M.I., STRATHMANN M.P., GAUTAM N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- SMRCKA A.V., HEPLER J.R., BROWN K.O., STERNWEIS P.C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- SOHN U.D., CAO W., TANG D.C., STULL J.T., HAEBERLE J.R., WANG C.L., HARNETT K.M., BEHAR J., BIANCANI P. Myosin light chain kinase- and PKC-dependent contraction of LES and esophageal smooth muscle. Am. J. Physiol. 2001;281:G467–G478. doi: 10.1152/ajpgi.2001.281.2.G467. [DOI] [PubMed] [Google Scholar]
- SOHN U.D., CHIU T.T., BITAR K.N., HILLEMEIER C., BEHAR J., BIANCANI P. Calcium requirements for acetylcholine-induced contraction of cat esophageal circular muscle cells. Am. J. Physiol. 1994a;266:G330–G338. doi: 10.1152/ajpgi.1994.266.2.G330. [DOI] [PubMed] [Google Scholar]
- SOHN U.D., HAN B., TASHJIAN A.H., BEHAR J., BIANCANI P. Agonist-independent, muscle-type-specific signal transduction pathways in cat esophageal and lower esophageal sphincter circular smooth muscle. J. Pharmacol. Exp. Ther. 1995;273:482–491. [PubMed] [Google Scholar]
- SOHN U.D., HARNETT K.M., CAO W., RICH H., KIM N., BEHAR J., BIANCANI P. Acute experimental esophagitis activates a second signal transduction pathway in cat smooth muscle from the lower esophageal sphincter. J. Pharmacol. Exp. Ther. 1997;283:1293–1304. [PubMed] [Google Scholar]
- SOHN U.D., HARNETT K.M., DE PETRIS G., BEHAR J., BIANCANI P. Distinct muscarinic receptors, G proteins and phospholipases in esophageal and lower esophageal sphincter circular muscle. J. Pharmacol. Exp. Ther. 1993;267:1205–1214. [PubMed] [Google Scholar]
- SOHN U.D., KIM D.K., BONVENTRE J.V., BEHAR J., BIANCANI P. Role of 100-kDa cytosolic PLA2 in ACh-induced contraction of cat esophageal circular muscle. Am. J. Physiol. 1994b;267:G433–G441. doi: 10.1152/ajpgi.1994.267.3.G433. [DOI] [PubMed] [Google Scholar]
- TONG Y.C., CHENG J.T. Alteration of M(3) subtype muscarinic receptors in the diabetic rat urinary bladder. Pharmacology. 2002;64:148–151. doi: 10.1159/000056164. [DOI] [PubMed] [Google Scholar]
- TONG Y.C., CHIN W.T., CHENG J.T. Alterations in urinary bladder M2-muscarinic receptor protein and mRNA in 2-week streptozotocin-induced diabetic rats. Neurosci. Lett. 1999;277:173–176. doi: 10.1016/s0304-3940(99)00871-x. [DOI] [PubMed] [Google Scholar]
- WANG J., KRYSIAK P.S., LAURIER L.G., SIMS S.M., PREIKSAITIS H.G. Human esophageal smooth muscle cells express muscarinic receptor subtypes M(1) through M(5) Am. J. Physiol. Gastrointest. Liver. Physiol. 2000;279:G1059–G1069. doi: 10.1152/ajpgi.2000.279.5.G1059. [DOI] [PubMed] [Google Scholar]
- WANG P., LUTHIN G.R., RUGGIERI M.R. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- YAMANISHI T., CHAPPLE C.R., YASUDA K., CHESS-WILLIAMS R. The role of M(2)-muscarinic receptors in mediating contraction of the pig urinary bladder in vitro. Br. J. Pharmacol. 2000;131:1482–1488. doi: 10.1038/sj.bjp.0703719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG S.J., AN J.Y., SHIM J.O., PARK C.H., HUH I.H., SOHN U.D. The mechanism of contraction by 2-chloroadenosine in cat detrusor muscle cells. J. Urol. 2000;163:652–658. [PubMed] [Google Scholar]
- YASUDA N., SAKAI Y. A possible explanation for effects of Sr2+ on contraction-relaxation cycle in canine stomach. Comp. Biochem. Physiol. A. Physiol. 1984;78:35–41. doi: 10.1016/0300-9629(84)90088-4. [DOI] [PubMed] [Google Scholar]
- YU P., CHEN Q., XIAO Z., HARNETT K., BIANCANI P., BEHAR J. Signal transduction pathways mediating CCK-induced gallbladder muscle contraction. Am. J. Physiol. 1998;275:G203–G211. doi: 10.1152/ajpgi.1998.275.2.G203. [DOI] [PubMed] [Google Scholar]


