Abstract
Since the role of prostanoid receptors in intestinal peristalsis is largely unknown, the peristaltic motor effects of some prostaglandin (DP, EP, IP), thromboxane (TP) and leukotriene (LT) receptor agonists and antagonists were investigated.
Propulsive peristalsis in fluid-perfused segments from the guinea-pig small intestine was triggered by a rise of the intraluminal pressure and recorded via the intraluminal pressure changes associated with the peristaltic waves. Alterations of distension sensitivity were deduced from alterations of the peristaltic pressure threshold and modifications of peristaltic performance were reflected by modifications of the amplitude, maximal acceleration and residual baseline pressure of the peristaltic waves.
Four categories of peristaltic motor effects became apparent: a decrease in distension sensitivity and peristaltic performance as induced by the EP1/EP3 receptor agonist sulprostone and the TP receptor agonist U-46,619 (1–1000 nM); a decrease in distension sensitivity without a major change in peristaltic performance as induced by PGD2 (3–300 nM) and LTD4 (10–100 nM); a decrease in peristaltic performance without a major change in distension sensitivity as induced by PGE1, PGE2 (1–1000 nM) and the EP1/IP receptor agonist iloprost (1–100 nM); and a decrease in peristaltic performance associated with an increase in distension sensitivity as induced by the EP2 receptor agonist butaprost (1–1000 nM). The DP receptor agonist BW-245C (1–1000 nM) was without effect.
The peristaltic motor action of sulprostone remained unchanged by the EP1 receptor antagonist SC-51,089 (1 μM) and the DP/EP1/EP2 receptor antagonist AH-6809 (30 μM), whereas that of U-46,619 and LTD4 was prevented by the TP receptor antagonist SQ-29,548 (10 μM) and the cysteinyl-leukotriene1 (cysLT1) receptor antagonist tomelukast (10 μM), respectively.
These observations and their pharmacological analysis indicate that activation of EP2, EP3, IP, TP and cysLT1 receptors, but not DP receptors, modulate intestinal peristalsis in a receptor-selective manner, whereas activation of EP1 seems to be without influence on propulsive peristalsis. In a wider perspective it appears as if the effect of prostanoid receptor agonists to induce diarrhoea is due to their prosecretory but not peristaltic motor action.
Keywords: Intestinal peristalsis, prostaglandins, thromboxanes, leukotrienes, prostaglandin receptors, thromboxane receptors, leukotriene receptors, peristaltic pressure threshold, distension sensitivity, peristaltic performance
Introduction
Prostaglandins (PGs) and thromboxanes are known to modify gastrointestinal motility, but the cell surface receptors mediating these prostanoid actions have not been fully identified. PGE receptors of the EP2 type, but also of the EP1, EP3 and EP4 type, are expressed in the gut of several species including man (An et al., 1993; Bastien et al., 1994; Morimoto et al., 1997; Northey et al., 2000). Cellular localization studies indicate that the external muscle layers bear EP1, EP2 and EP3 receptors and that some EP3 receptors are also associated with enteric neurons of the rat and mouse intestine (Morimoto et al., 1997; Northey et al., 2000), while pharmacological investigations have shown that circular muscle cells of the guinea-pig ileum exhibit EP1 and EP3 receptors which on activation by agonists mediate contraction, whereas EP2 receptors bring about relaxation (Botella et al., 1993). From receptor knockout studies it would appear that activation of receptors for PGD (DP), PGF, PGE (EP1, EP3, EP4), PGI (IP) and thromboxane (TP) alters the motor activity of the mouse ileum, either by inducing contraction or relaxation (Okada et al., 2000). In the longitudinal muscle of the guinea-pig ileum, PGE causes contraction most likely via activation of EP1 and EP3 receptors on muscle cells and EP2 receptors on myenteric neurons (Lawrence et al., 1992). IP receptor agonists may lead both to a nerve-mediated contraction and, through a direct action on myocytes, to a relaxation of the muscle (Lawrence et al., 1992).
Consistent with these effects is the ability of PGs to influence intestinal peristalsis, the clinically most relevant motor pattern of the gut. Thus, PGE1, PGE2, PGF1α and PGF2α modify motility in the guinea-pig isolated ileum and colon in a manner that has been interpreted as peristaltic motor stimulation (Bennett et al., 1976; Eley et al., 1977; Fontaine et al., 1977; Sanger & Watt, 1978). In addition, PGE1, PGE2 and PGF2α can induce peristalsis-like contractions of the circular muscle in the guinea-pig intestine (Ishizawa & Miyazaki, 1975; Grbovic & Radmanovic, 1987) and reverse the antiperistaltic action of indomethacin and acetylsalicylic acid (Bennett et al., 1976), although the effect of nonsteroidal anti-inflammatory drugs to disturb peristalsis (Bennett et al., 1976; Fontaine et al., 1977; Bruch et al., 1978) is not necessarily related to cyclo-oxygenase inhibition (Shahbazian et al., 2001). However, PGE1 has also been reported to inhibit peristalsis-like motility in the guinea-pig ileum via activation of sympathetic neurons (Radmanovic, 1972), and the PGE2 analogue enprostil has been found to impair antroduodenal motility in man (Hausken et al., 1991).
From these divergent reports it is difficult to deduce how activation of distinct prostanoid receptors modifies propulsive peristalsis in the gut. Since this nerve-coordinated pattern of intestinal motility involves both excitatory and inhibitory motor reflexes (Costa et al., 2000), it is likewise impossible to envisage which impact the contractile and relaxant motor effects of prostanoid receptor activation have on propulsive motility. Thus, the first aim of this study was to characterize the peristaltic motor actions of PGA2, PGD2, PGE1, PGE2, the PGI2 analogue iloprost, the EP1/EP3 receptor agonist sulprostone, the EP2 receptor agonist butaprost and the DP receptor agonist BW-245C in fluid-perfused segments from the guinea-pig small intestine. By analysis of four aspects of propulsive peristalsis and by use of some prostanoid receptor antagonists, we sought to distinguish between specific patterns of peristaltic motor changes caused by DP, EP2, EP3 and IP receptor activation. The second aim was to extend this analysis to thromboxane A2 and leukotriene (LT) D4, because cysteinyl-LTs (cysLTs) such as LTD4 are able to contract intestinal smooth muscle (Gardiner et al., 1990; Jonsson, 1998), whereas thromboxane A2 is rather ineffective in altering muscle activity in the guinea-pig intestine (Sanger & Bennett, 1980; Sametz et al., 2000). Therefore, we investigated whether the TP receptor agonist U-46,619 and LTD4 influence intestinal peristalsis and whether the peristaltic motor responses to U-46,619 and LTD4 are blocked by selective antagonists for TP and cysLT1 receptors, respectively.
Methods
Recording of peristalsis
The small intestine (jejunum and ileum) of adult guinea-pigs (TRIK strain, either sex, 350–450 g body weight) was isolated, flushed of luminal contents and placed, for up to 4 h, in Tyrode solution kept at room temperature and oxygenated with a mixture of 95% O2 and 5% CO2 (Shahbazian et al., 2001). The composition of the Tyrode solution was (mM): NaCl 136.9, KCl 2.7, CaCl2 1.8, MgCl2 1.0, NaHCO3 11.9, NaH2PO4 0.4 and glucose 5.6. The small intestine (jejunum and ileum) was divided into eight segments, each being approximately 10 cm long. Since the baseline peristaltic parameters recorded here (see below) did not significantly differ between segments taken from the proximal jejunum and distal ileum (n=9), the segments were assigned randomly to the pharmacological treatments under study. Four intestinal segments were set up on parallel and secured horizontally in organ baths containing 30 ml of Tyrode solution at 37°C. In order to elicit propulsive peristalsis, pre-warmed Tyrode solution was continuously infused into the lumen of the segments at a rate of 0.5 ml min−1 (Shahbazian et al., 2001). The intraluminal pressure at the aboral end of the segments was measured with a pressure transducer whose signal was, via an analogue/digital converter, fed into a personal computer and recorded and analysed with the software Peristal 1.0 (Heinemann et al., 1999). The fluid passing through the gut lumen was directed into a vertical outlet tubing which ended 4 cm above the fluid level in the organ bath. When fluid was infused, the intraluminal pressure rose slowly until it reached a threshold at which peristalsis was triggered (Figure 1). The aborally moving wave of peristaltic contraction resulted in a spike-like increase in the intraluminal pressure (the peristaltic wave), which caused emptying of the segment if the maximal pressure of the peristaltic wave exceeded the level of 400 Pa as set by the position of the outlet tubing.
Figure 1.
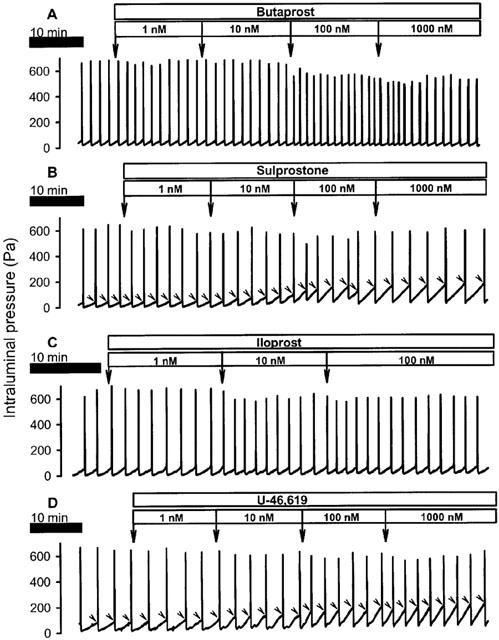
Recordings of the effects of butaprost (A), sulprostone (B), iloprost (C) and U-46,619 (D) on peristalsis. The drugs were administered to the organ bath at the specified concentrations. As can be seen, sulprostone and U-46,619 increased the peristaltic pressure threshold (indicated by arrow heads) whereas butaprost lowered it. Iloprost tended to lower the amplitude of the peristaltic waves.
Experimental protocols
The preparations were equilibrated in the organ bath for a period of 30 min, during which they were kept in a quiescent state. Thereafter the bath fluid was renewed and peristaltic motility initiated by intraluminal perfusion of the segments. After baseline peristalsis had been recorded for a 30 min period, the drugs to be tested were added to the bath, i.e., to the serosal surface of the intestinal segments, at volumes not exceeding 1% of the bath volume. The corresponding vehicle solutions were devoid of any effect. Three sets of experiments were performed. Firstly, the motor effects of BW-245C, butaprost, PGA2, PGE1, PGE2, sulprostone (1–1000 nM), PGD2 (3–300 nM) and iloprost (1–100 nM) were studied, these substances being added in a cumulative manner at 15 min intervals (Heinemann et al., 1999; Shahbazian et al., 2001). These intervals were long enough for agonist effects to reach a maximum, but too short for agonist effects to decline before the segments were exposed to the next agonist concentration (Figure 1). Furthermore, the test period of 15 min enabled us to observe agonist effects on at least 4 peristaltic waves, even when the frequency of peristaltic waves was as low as 0.3 Hz (Figure 1).
Secondly, the motor responses to PGD2 (3–300 nM), butaprost, PGE1, sulprostone, U-46,619 (1–1000 nM) and LTD4 (10–100 nM) were studied in the presence of vehicle, the EP1 receptor antagonist SC-51,089 (1 μM), the DP/EP1/EP2 receptor antagonist AH-6809 (30 μM), the TP receptor antagonist SQ-29,548 (10 μM) or the cysLT1 receptor antagonist tomelukast (10 μM). The receptor antagonists or their vehicle were added to the organ bath 15 min before the recording of the cumulative concentration–response curves for the agonists was begun. Thirdly, the susceptibility of the peristaltic motor effects of PGE1 and U-46,619 (1–1000 nM) to the α2 adrenoceptor antagonist yohimbine (0.3 μM, pre-exposure time 15 min) were investigated. Each protocol was carried out with at least five segments from five different guinea-pigs.
Evaluation of peristalsis
The recordings of peristalsis were analysed with the software Peristal 1.0 with regard to four different parameters: the peristaltic pressure threshold (PPT), the amplitude (maximal pressure) of the peristaltic waves, the maximal acceleration of the peristaltic waves, and the residual baseline pressure. PPT (Pa) is the intraluminal pressure at which a peristaltic wave is triggered. The residual baseline pressure (Pa) equals the minimal intraluminal pressure that is achieved after completion of each peristaltic wave and thus reflects the emptying capacity of the peristaltic waves (Shahbazian et al., 2001). Further indices of peristaltic performance are the amplitude of the peristaltic waves (Pa) and the maximal acceleration of the peristaltic waves (Pa s−2), which is determined not only by the speed with which the muscle contracts but also by the speed with which the contraction moves aborally to empty the segments. The peristalsis parameters of three consecutive peristaltic waves that took place immediately before administration of any substance were averaged to determine the characteristics of peristalsis at baseline. An analogous procedure was applied to calculate the responses to the receptor antagonists and agonists under study. Thus, the influence of receptor antagonists on peristalsis was deduced from the mean parameters of three peristaltic waves that were recorded 15 min post-administration, immediately before the recording of the cumulative agonist concentration–response curves was begun. The motor effects of receptor agonists were evaluated by measuring the peak changes that occurred during the 15 min observation periods and averaging the parameters of three peristaltic waves at the peak effect.
Drugs and solutions
The sources of the drugs used here were as follows. PGD2, PGE1, PGE2, LTD4, [1S-[1α,2α(Z),3α,4α]]-7-[3-[[2-[(phenylamino)carbonyl]hydrazino]methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptanoic acid (SQ-29,548) and yohimbine hydrochloride were obtained from Sigma (Vienna, Austria). Butaprost, (±)-3-(3-cyclohexyl-3-hydroxypropyl)-2,5-dioxo-,4-imidazolidineheptanoic acid (BW-245C), 9,11-dideoxy-9α,11α-methanoepoxy-PGF2α (U-46,619), 6-isopropoxy-9-oxoxanthene-2-carboxylic acid (AH-6,809), PGA2 and sulprostone were bought from Cayman (Ann Arbor, MI, U.S.A.). 8-Chlorodibenz[b,f][1,4]oxazepine-10(11H)-carboxylic acid, 2-[1-oxo-3(4-pyridinyl)propyl]hydrazide monohydrochloride (SC-51,089) and tomelukast (LY-171,883) were purchased from Biomol (Hamburg, Germany). Iloprost was a gift of Schering AG (Berlin, Germany). The drugs were dissolved with appropriate media, the concentrations given hereafter in parentheses referring to the stock solutions. BW-245C (3 mM), butaprost (1 mM), PGD2 (3 mM), SQ-29,548 (10 mM), sulprostone (1 mM) and tomelukast (10 mM) were dissolved in ethanol, SC-51,089 (10 mM) in distilled water, yohimbine hydrochloride (1 mM) in Tyrode solution, AH-6,809 and U-46,619 (10 mM) in dimethyl sulphoxide, and PGE1 (1 mM) and PGE2 (1 mM) in methanol. PGA2 (1 mM) was supplied in a methanol/ammonium acetate buffer of pH 5.6, while iloprost was provided in aqueous solution (100 μg ml−1). The stock solutions were diluted with Tyrode solution as required. Care was taken that none of the organic solvents reached concentrations higher than 0.3% in the bathing solution.
Statistics
The data are presented as means±s.e.mean of n experiments, n referring to the number of guinea-pigs used in the test. The results were evaluated with Student's paired t-test or one-way analysis of variance (ANOVA) for repeated measures followed by Dunnett's test, as appropriate. Probability values of P<0.05 were regarded as significant.
Results
Baseline peristalsis
Quantitative estimates of the peristalsis parameters at baseline were: PPT, 71±2 Pa; residual baseline pressure, 13±1 Pa; maximal pressure of peristaltic waves, 622±7 Pa; and maximal acceleration of peristaltic waves 317±7 Pa s−2 (n=147).
Peristaltic motor effects of selective PG receptor agonists
The EP1/EP3 receptor agonist sulprostone, the EP2 receptor agonist butaprost and the EP1/IP receptor agonist iloprost caused distinct and concentration-dependent changes in the peristalsis parameters under study (Figures 1A,B,C and 2). Thus, PPT was decreased by butaprost (1–1000 nM), left unchanged by iloprost (1–100 nM), and increased by sulprostone (1–1000 nM). The maximal pressure (amplitude) and the maximal acceleration of the peristaltic waves was concentration-dependently attenuated by sulprostone, butaprost and iloprost (Figures 1A,B,C and 2). A further parameter of peristaltic performance, the residual baseline pressure, remained unaltered by butaprost but was enhanced by sulprostone and iloprost (Figure 2B). It should not go unnoticed that all peristaltic motor changes elicited by iloprost were quantitatively minor (Figures 1C and 2).
Figure 2.
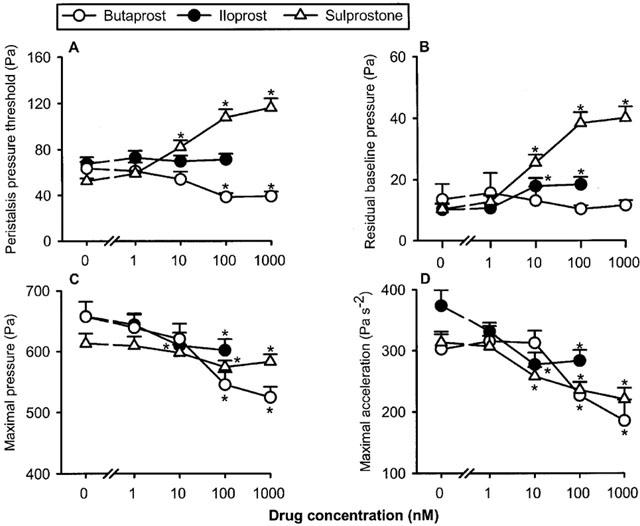
Concentration-dependent effects of butaprost, iloprost and sulprostone to alter the peristaltic pressure threshold (A), residual baseline pressure (B), maximal pressure (C) and maximal acceleration (D) of the peristaltic waves. The concentration–response curves were recorded in a cumulative manner at 15 min intervals. The values represent means+s.e.mean, n=12 for butaprost, n=13 for iloprost, n=16 for sulprostone. *P<0.05 versus parameters recorded immediately before drug administration when the drug concentration was ‘0' (ANOVA for repeated measures followed by Dunnett's test).
The selective DP receptor agonist BW-245C (1, 10, 100 and 1000 nM) failed to alter PPT, the residual baseline pressure and the maximal acceleration of the peristaltic waves (n=6, data not shown). The maximal pressure of the peristaltic waves was also left unchanged by BW-245C at the concentrations of 1–100 nM, while 1000 nM BW-245C enhanced this parameter to 109±3.4% of that measured under baseline conditions (n=6, P<0.05, ANOVA for repeated measures followed by Dunnett's test).
Peristaltic motor effects of natural PG receptor agonists
Exposure of the intestinal segments to PGA2, PGD2, PGE1 and PGE2 caused various patterns of peristaltic motor changes (Figure 3). PPT remained practically unchanged by PGA2, PGE1 and PGE2 (1–1000 nM) and was slightly, but significantly, increased by PGD2 (3–300 nM). The maximal pressure of the peristaltic waves was attenuated by PGE1 and PGE2, but not significantly altered by PGA2 and PGD2, whereas the maximal acceleration of the peristaltic waves was decreased by PGE1, PGE2 and PGD2 but not PGA2 (Figure 3). PGE1 and PGE2 also increased the residual baseline pressure, while PGA2 and PGD2 were without significant influence on this parameter (Figure 3B).
Figure 3.
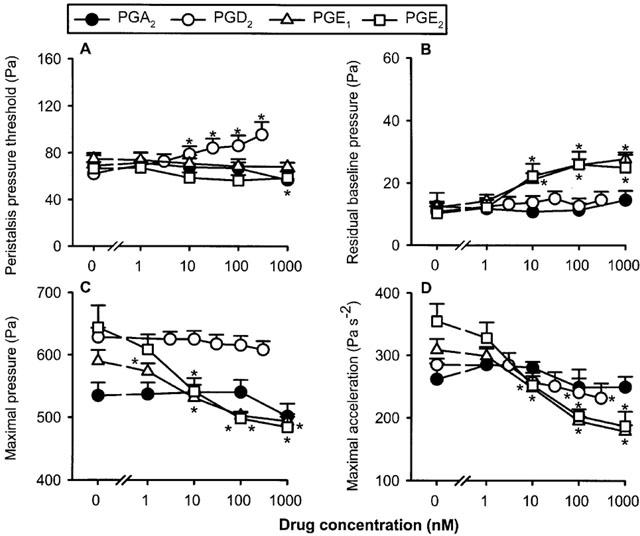
Concentration-dependent effects of prostaglandin A2 (PGA2), PGD2, PGE1 and PGE2 to alter the peristaltic pressure threshold (A), residual baseline pressure (B), maximal pressure (C) and maximal acceleration (D) of the peristaltic waves. The concentration–response curves were recorded in a cumulative manner at 15 min intervals. The values represent means+s.e.mean, n=5 for PGA2, n=12 for PGD2, n=27 for PGE1, n=10 for PGE2. *P<0.05 versus parameters recorded immediately before drug administration when the drug concentration was ‘0' (ANOVA for repeated measures followed by Dunnett's test).
Peristaltic motor effects of PG receptor agonists in the presence of PG receptor antagonists
Exposure of intestinal segments to the EP1 receptor antagonist SC-51,089 (1 μM) failed to alter baseline peristalsis to any significant degree, as deduced from a lack of effect on PPT (Table 1) and the other parameters of peristalsis under study (not shown). In contrast, the DP/EP1/EP2 receptor antagonist AH-6809 (30 μM) increased PPT (Table 1) and decreased the maximal pressure and acceleration of the peristaltic waves to a significant extent (P<0.01, n=22, data not shown). Neither SC-51,089 nor AH-6809 was able to modify the peristaltic motor effects of sulprostone, i.e., to enhance PPT and the residual baseline pressure and to attenuate the maximal pressure and the maximal acceleration of the peristaltic waves (Figure 4). SC-51,089 likewise failed to alter the peristaltic motor changes elicited by 1–1000 nM butaprost (n=7, data not shown) and 1–1000 nM PGE1 (n=7, data not shown). The peristaltic motor responses to 3–300 nM PGD2 were not modified by AH-6809 (n=10, data not shown).
Table 1.
Effect of SC-51,089, AH-6,809, SQ-29,548, tomelukast and yohimbine on the peristaltic pressure threshold (PPT)
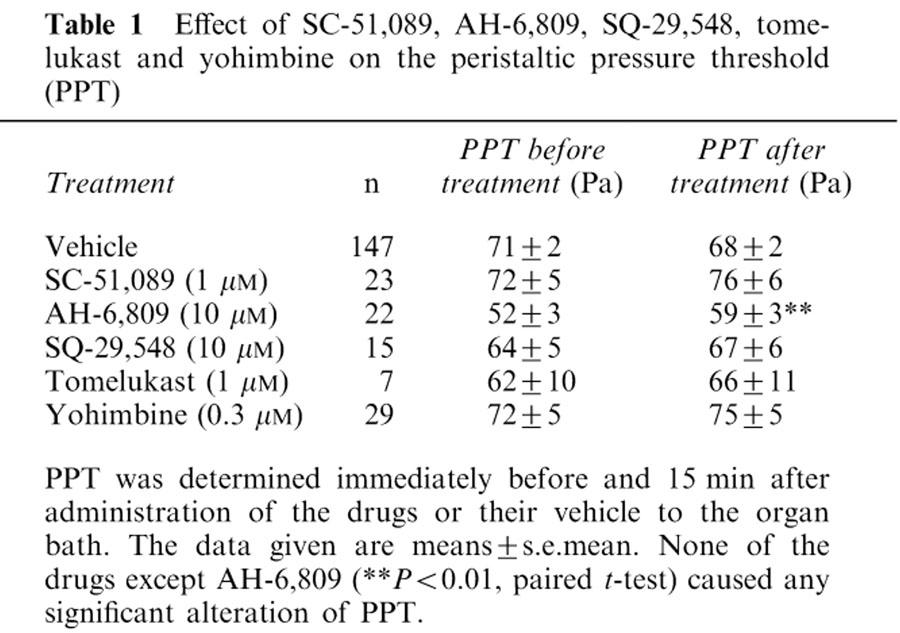
Figure 4.
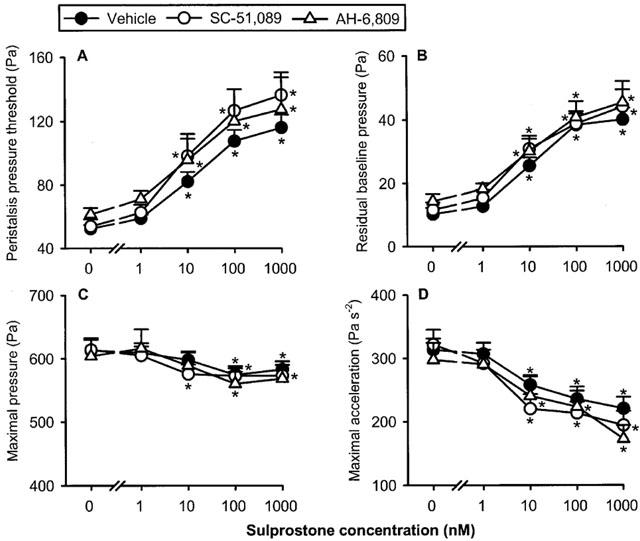
Concentration-dependent effect of sulprostone to alter the peristaltic pressure threshold (A), residual baseline pressure (B), maximal pressure (C) and maximal acceleration (D) of the peristaltic waves as assessed in the presence of vehicle (n=16), SC-51,089 (n=9) and AH-6809 (n=7). The concentration–response curves were recorded in a cumulative manner at 15 min intervals. The values represent means+s.e.mean. *P<0.05 versus parameters recorded immediately before addition of sulprostone as indicated by ‘0' (ANOVA for repeated measures followed by Dunnett's test).
Peristaltic motor effects of PGE1 in the presence of yohimbine
The α2-adrenoceptor antagonist yohimbine (0.3 μM) was devoid of any influence on baseline peristalsis (Table 1) and failed to alter the peristaltic motor changes elicited by 1–1000 nM PGE1 (n=14, data not shown).
Peristaltic motor effects of U-46,619 in the absence and presence of SQ-29,548 and yohimbine
The TP receptor agonist U-46,619 caused distinct changes in the peristalsis parameters under study (Figures 1D and 5). Thus, PPT and the residual baseline pressure were enhanced by U-46,619 (1–1000 nM) whereas the maximal acceleration of the peristaltic waves was reduced. In contrast, the maximal pressure of the peristaltic waves was not significantly altered by U-46,619 (Figure 5) although in some experiments this parameter of peristalsis was attenuated (Figure 1D). The action of U-46,619 to alter peristaltic motility was suppressed by the TP receptor antagonist SQ-29,548 (10 μM). Thus, the U-46,619-induced increase in PPT and residual baseline pressure and decrease in the maximal acceleration of the peristaltic waves were no longer seen when SQ-29,548 was present in the bath (Figure 5). However, baseline peristalsis was not influenced by SQ-29,548 (Table 1). The α2-adrenoceptor antagonist yohimbine (0.3 μM) failed to modify the peristaltic motor effects of U-46,619 (n=7, data not shown).
Figure 5.
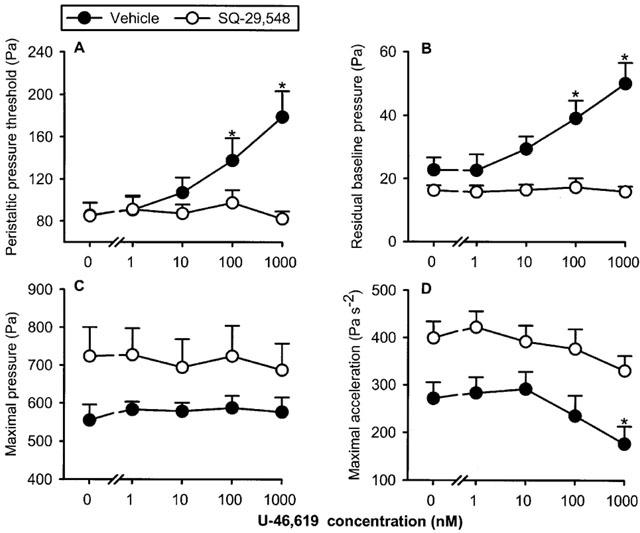
Concentration-dependent effect of U-46,619 to alter the peristaltic pressure threshold (A), residual baseline pressure (B), maximal pressure (C) and maximal acceleration (D) of the peristaltic waves as assessed in the presence of vehicle (n=6) and SQ-29,548 (n=6). The concentration–response curves were recorded in a cumulative manner at 15 min intervals. The values represent means+s.e.mean. *P<0.05 versus parameters recorded immediately before addition of U-46,619 as indicated by ‘0' (ANOVA for repeated measures followed by Dunnett's test).
Peristaltic motor effects of LTD4 in the absence and presence of tomelukast
LTD4 (10–100 nM) enhanced PPT and the residual baseline pressure in a concentration-dependent manner, although the magnitude of these peristaltic motor changes was minor (Figure 6). The maximal pressure and the maximal acceleration of the peristaltic waves were not modified by LTD4. Exposure of the intestinal segments to the cysLT1 receptor antagonist tomelukast (10 μM) failed to alter baseline peristalsis to any significant degree as deduced from a lack of effect on PPT (Table 1) and the other parameters of peristalsis under study (data not shown). Tomelukast, however, prevented the peristaltic motor alterations caused by LTD4 (Figure 6).
Figure 6.
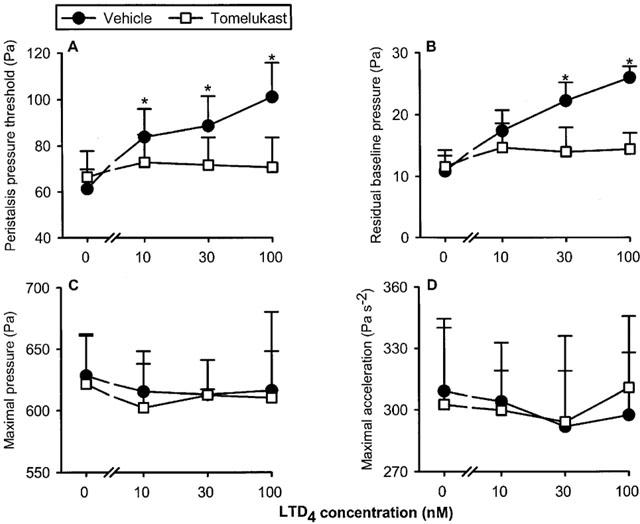
Concentration-dependent effect of leukotriene D4 (LTD4) to alter the peristaltic pressure threshold (A), residual baseline pressure (B), maximal pressure (C) and maximal acceleration (D) of the peristaltic waves as assessed in the presence of vehicle (n=7) and tomelukast (n=7). The concentration–response curves were recorded in a cumulative manner at 15 min intervals. The values represent means+s.e.mean. *P<0.05 versus parameters recorded immediately before addition of LTD4 as indicated by ‘0' (ANOVA for repeated measures followed by Dunnett's test).
Discussion
The effects of prostanoid receptor agonists and LTD4 on propulsive peristalsis in segments from the guinea-pig small intestine were examined with a method that had previously been employed to analyse drug actions on this clinically relevant motor pattern of the gut (Heinemann et al., 1999; Shahbazian et al., 2001). Elicited by mechanical stimulation of the mucosa or distension of the intestinal wall, peristalsis involves ascending excitatory, descending inhibitory and descending excitatory reflexes within the enteric nervous system (Costa et al., 2000). These reflexes result in an aborally moving pattern of temporally and spatially coordinated contractions and relaxations of the circular muscle. The focus of our in vitro set-up was to refine the analysis of drug-induced peristaltic motor alterations by recording and calculating four parameters of peristalsis. Changes in PPT reflect modifications in the sensitivity of the peristaltic nerve-muscle circuitry to distension, whereas alterations in the maximal pressure (amplitude), maximal acceleration and/or residual baseline pressure of the peristaltic waves indicate modifications of peristaltic motor performance. In this way, it was possible to differentiate between four categories of drug-induced peristaltic motor alterations: a decrease in distension sensitivity and peristaltic performance (sulprostone and U-46,619); a decrease in distension sensitivity without a major change in peristaltic performance (PGD2 and LTD4); a decrease in peristaltic performance without a major change in distension sensitivity (PGE1, PGE2 and iloprost); and a decrease in peristaltic performance associated with an increase in distension sensitivity (butaprost).
In view of this heterogeneity of prostanoid and LTD4 actions on intestinal peristalsis it was the major aim of this study to define the particular patterns of peristaltic motor effects elicited by selective DP, EP1, EP2, EP3, IP, TP and LT receptor activation. This goal was addressed by the use of receptor-selective agonists and antagonists. We did, however, not resort to the comparative study of agonist potencies, because the relative potency of the test compounds in our in vitro preparation is determined both by their receptor affinity and pharmacokinetic behaviour. This is because compounds administered into the organ bath need to penetrate the serosa and longitudinal muscle before they reach the myenteric plexus and circular muscle. In addition, it need to be taken into account that the peristalsis-modifying effect of the test compounds reflects their net action on multiple receptors at multiple sites of the nerve-muscle system subserving peristalsis.
In a first step, the DP receptor agonist BW-245C (Town et al., 1983), the EP1/EP3 receptor agonist sulprostone (Racké et al., 1995; Narumiya et al., 1999; Walch et al., 2001; Kim et al., 2002), the EP2 receptor agonist butaprost (Lawrence et al., 1992; Narumiya et al., 1999), the EP1/IP receptor agonist iloprost (Dong et al., 1986; Lawrence et al., 1992) and the TP receptor agonist U-46,619 (Eglen & Whiting, 1988; Narumiya et al., 1999) were characterized in their concentration-dependent actions on intestinal peristalsis. This agonist-directed identification of receptors was complemented by the use of effective, yet selective concentrations of the EP1 receptor antagonist SC-51,089 (1 μM; Hallinan et al., 1993), the DP/EP1/EP2 receptor antagonist AH-6,809 (30 μM; Eglen & Whiting, 1988; Walch et al., 2001), the TP receptor antagonist SQ-29,548 (10 μM; Ogletree et al., 1985) and the cysLT1 receptor antagonist tomelukast (10 μM; Fleisch et al., 1985).
The first conclusion derived from the current study is that EP2 receptor activation by the selective agonist butaprost (Lawrence et al., 1992; Narumiya et al., 1999) attenuates peristaltic performance in parallel with an increase in distension sensitivity, i.e., a decrease in PPT. Since EP2 receptors have been suggested to occur on sensory and other neurons of the enteric nerve plexuses (Lawrence et al., 1992; Dekkers et al., 1997) it is tempting to postulate that butaprost enhances distension sensitivity by an action on enteric neurons. In contrast, the butaprost-induced attenuation of peristaltic performance is likely to be mediated by EP2 receptors on intestinal muscle, given that muscular EP2 receptors have been reported to bring about relaxation (Botella et al., 1993). Another important conclusion deduced from this study is that EP3 receptor activation results in a decrease in distension sensitivity and peristaltic performance. Our inference is based on the observation that the EP1 receptor antagonists SC-51,809 and AH-6809 failed to inhibit the peristaltic motor response to the EP1/EP3 receptor agonist sulprostone. Consequently, the sulprostone-evoked decrease in distension sensitivity and peristaltic performance is most probably mediated by EP3 receptors. Such a pattern of peristaltic motor modification was impossible to predict from the reported effects of sulprostone on intestinal nerve and muscle which bear EP3 receptors (Lawrence et al., 1992; Botella et al., 1993; Morimoto et al., 1997; Northey et al., 2000; Okada et al., 2000). This is because peristaltic motility involves excitatory and inhibitory motor reflexes (Costa et al., 2000) and, as a consequence, the peristaltic motor alterations caused by the test compounds cannot be categorized as actions mediated by contraction- or relaxation-promoting prostanoid receptors (Coleman et al., 1994; Narumiya et al., 1999). Because any drug that blocks nerve activity eliminates peristalsis (Johnson et al., 1996; Holzer et al., 1998), it was not possible to sort out in our preparation whether the test compounds influence propulsive motility by a neural or muscular site of action.
The inability of the EP1 receptor antagonists SC-51,089 and AH-6809 to inhibit the peristaltic motor effects of sulprostone, PGE1, and, expectedly, butaprost, is important to consider with respect to the role of EP1 receptors in intestinal peristalsis. From our data it would appear that EP1 receptors, which are abundantly present in the guinea-pig ileum (Eglen & Whiting, 1988; Lawrence et al., 1992), have no bearing on propulsive motility. If a role of EP1 receptors in peristaltic motility of the guinea-pig small intestine is negated, the peristaltic motor response to the EP1/IP receptor agonist iloprost (Dong et al., 1986; Lawrence et al., 1992) must be regarded as being due solely to IP receptor activation. Thus, we conclude that IP receptor activation leads to a decrease in peristaltic performance without any change in distension sensitivity. This pattern of peristaltic motor modification is in keeping with the ability of IP receptor agonists to relax intestinal muscle by a direct action on myocytes (Lawrence et al., 1992).
The effects of PGE1 and PGE2 to decrease peristaltic performance without changing distension sensitivity are in line with the reported action of PGE1 to inhibit peristalsis-like contractions in the guinea-pig ileum (Radmanovic, 1972) and of the PGE2 analogue enprostil to impair antroduodenal motility in man (Hausken et al., 1991). A comparison with the motor responses to butaprost and sulprostone suggests that both EP2 and EP3 receptors contribute to the net effects of PGE1 and PGE2 on propulsive motility. Since PGA2 was without major influence on peristalsis, we assume that PGE2 modifies peristaltic motility per se rather than through an action of its dehydration metabolite PGA2. Major differences in the induction, recording, analysis and interpretation of peristaltic motility explain why our finding of an inhibitory action of PGE1 and PGE2 on propulsive peristalsis is at variance with reports that PGs of the E and F series induce or stimulate peristalsis-like motility in the guinea-pig isolated ileum and colon (Ishizawa & Miyazaki, 1975; Eley et al., 1977; Fontaine et al., 1997; Sanger & Watt, 1978; Grbovic & Radmanovic, 1987). The effect of PGD2 to lower distension sensitivity without impairing peristaltic performance differed markedly from the motor actions of PGE1 and PGE2. It can be ruled out, however, that the motor response to PGD2 is brought about by DP receptors because only low levels of DP receptors occur in this small intestine (Eglen & Whiting, 1988; Narumiya et al., 1999), the DP receptor agonist BW-245C (Town et al., 1983) was without any appreciable influence on peristalsis and the DP/EP1/EP2 receptor antagonist AH-6809 (Eglen & Whiting, 1988; Walch et al., 2001) failed to prevent the peristaltic motor effect of PGD2. Concentrations of this weak DP antagonist higher than 30 μM could not be employed because of solubility limitations.
Characteristic modifications of peristaltic motility were not only induced by PG receptor agonists but also by the TP receptor agonist U-46,619 (Eglen & Whiting, 1988; Narumiya et al., 1999) and LTD4. The decrease in distension sensitivity and peristaltic performance caused by U-46,619 was prevented by SQ-29,548, which indicates that its action was indeed mediated by TP receptors. cysLT1 receptors appear to be responsible for the ability of LTD4 to decrease distension sensitivity without attenuating peristaltic performance, since the motor response to LTD4 was prevented by the cysLT1 receptor antagonist tomelukast (Fleisch et al., 1985). Our results are in overall agreement with other reports that functional TP and cysLT1 receptors are present in the gut (Sanger & Bennett, 1980; Gardiner et al., 1990; Metters, 1995; Sametz et al., 2000).
The α2-adrenoceptor antagonist yohimbine (0.3 μM; Marcoli et al., 1985) was included in this study because it had previously been proposed that PGE1 inhibits peristalsis in the guinea-pig ileum via activation of sympathetic neurons (Radmanovic, 1972), and the inhibitory motor action of sympathetic nerve stimulation is primarily brought about by α2-adrenoceptors on myenteric neurons (Marcoli et al., 1985). In addition, prostanoids can modulate the release of noradrenaline from sympathetic nerve fibres in the gut (Racké et al., 1995). In this study we failed, however, to reveal any contribution of α2-adrenoceptors to the peristaltic motor effects of PGE1 as well as of U-46,619.
The inability of SC-51,089, SQ-29,548 and tomelukast to alter peristaltic motor activity at effective concentrations indicates that endogenous ligands at EP1, TP and cysLT1 receptors do not participate in the physiological control of peristalsis. Whether the effect of AH-6,809 to lower distension sensitivity and peristaltic performance points to a physiological role of DP and/or EP2 receptors in peristaltic motor regulation or reflects a non-specific action awaits to be elucidated. It seems conceivable that exacerbated production of prostanoids and LTs under pathological conditions modulates peristaltic motility as deduced from the high activity of PGD2, PGE1, PGE2, butaprost, sulprostone, iloprost, U-46,619 and LTD4 to modify peristaltic performance and/or intestinal sensitivity to distension. This conjecture is consistent with a permissive action of prostaglandins in the fine tuning of peristaltic motility as deduced from the peristaltic motor effects of cyclo-oxygenase inhibitors (Shahbazian et al., 2001). However, the magnitude of the peristaltic motor modifications brought about by prostanoid and LT receptor agonists is fairly small. If our data can be extrapolated to the human gut, it would appear that the ability of PGs to induce diarrhoea, which is their major effect in the intestine, is due to their prosecretory (Bunce & Spraggs, 1990; Beubler & Schuligoi, 2000) but not peristaltic motor action.
Acknowledgments
This study was supported by the Austrian Research Funds (FWF grant P14295-MED) and the Federal Ministry of Education, Science and Culture of the Republic of Austria (grant GZ 70.065/2-Pr/4/2000). The authors thank Schering AG (Berlin, Germany) for kindly providing a sample of iloprost.
Abbreviations
- LT
leukotriene
- PG
prostaglandin
- PPT
peristaltic pressure threshold
References
- AN S., YANG J., XIA M., GOETZL E.J. Cloning and expression of the EP2 subtype of human receptors for prostaglandin E2. Biochem. Biophys. Res. Commun. 1993;197:263–270. doi: 10.1006/bbrc.1993.2470. [DOI] [PubMed] [Google Scholar]
- BASTIEN L., SAWYER N., GRYGORCZYK R., METTERS K.M., ADAM M. Cloning, functional expression, and characterization of the human prostaglandin E2 receptor EP2 subtype. J. Biol. Chem. 1994;269:11873–11877. [PubMed] [Google Scholar]
- BENNETT A., ELEY K.G., STOCKLEY H.L. Inhibition of peristalsis in guinea-pig isolated ileum and colon by drugs that block prostaglandin synthesis. Br. J. Pharmacol. 1976;57:335–340. [PMC free article] [PubMed] [Google Scholar]
- BEUBLER E., SCHULIGOI R. Mechanisms of cholera toxin-induced diarrhea. Ann. New York Acad. Sci. 2000;915:339–346. doi: 10.1111/j.1749-6632.2000.tb05262.x. [DOI] [PubMed] [Google Scholar]
- BOTELLA A., DELVAUX M., FIORAMONTI J., FREXINOS J., BUENO L. Stimulatory (EP1 and EP3) and inhibitory (EP2) prostaglandin E2 receptors in isolated ileal smooth muscle cells. Eur. J. Pharmacol. 1993;237:131–137. doi: 10.1016/0014-2999(93)90102-n. [DOI] [PubMed] [Google Scholar]
- BRUCH H.P., SCHMIDT E., LAVEN R., KEHRER G., WASNER K.H. The role of prostaglandins in peristalsis of the human colon. Acta Hepatogastroenterol. 1978;25:303–307. [PubMed] [Google Scholar]
- BUNCE K.T., SPRAGGS C.F. Prostanoid stimulation of anion secretion in guinea-pig gastric and ileal mucosa is mediated by different receptors. Br. J. Pharmacol. 1990;101:889–895. doi: 10.1111/j.1476-5381.1990.tb14176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- COSTA M., BROOKES S.J.H., HENNIG G.W. Anatomy and physiology of the enteric nervous system. Gut. 2000;47 Suppl. IV:iv15–iv19. doi: 10.1136/gut.47.suppl_4.iv15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKKERS J.A., AKKERMANS L.M., KROESE A.B. Effects of the inflammatory mediator prostaglandin E2 on myenteric neurons in guinea pig ileum. Am. J. Physiol. 1997;272:G1451–G1456. doi: 10.1152/ajpgi.1997.272.6.G1451. [DOI] [PubMed] [Google Scholar]
- DONG Y.J., JONES R.L., WILSON N.H. Prostaglandin E receptor subtypes in smooth muscle: agonist activities of stable prostacyclin analogues. Br. J. Pharmacol. 1986;87:97–107. doi: 10.1111/j.1476-5381.1986.tb10161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., WHITING R.L. The action of prostanoid receptor agonists and antagonists on smooth muscle and platelets. Br. J. Pharmacol. 1988;94:591–601. doi: 10.1111/j.1476-5381.1988.tb11565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELEY K.G., BENNETT A., STOCKLEY H.L. The effects of prostaglandins E1, E2, F1α and F2α on guinea-pig ileal and colonic peristalsis. J. Pharm. Pharmacol. 1977;29:276–280. doi: 10.1111/j.2042-7158.1977.tb11311.x. [DOI] [PubMed] [Google Scholar]
- FLEISCH J.H., RINKEMA L.E., HAISCH K.D., SWANSON-BEAN D., GOODSON T., HO P.P., MARSHALL W.S. LY171883, 1-less than 2-hydroxy-3-propyl-4-less than 4-(1H-tetrazol-5-yl) butoxy greater than phenyl greater than ethanone, an orally active leukotriene D4 antagonist. J. Pharmacol. Exp. Ther. 1985;233:148–157. [PubMed] [Google Scholar]
- FONTAINE J., VAN NUETEN J.M., REUSE J.J. Effects of prostaglandins on the peristaltic reflex of the guinea-pig ileum. Arch. Int. Pharmacodyn. Ther. 1977;226:341–343. [PubMed] [Google Scholar]
- GARDINER P.J., ABRAM T.S., CUTHBERT N.J. Evidence for two leukotriene receptor types in the guinea-pig isolated ileum. Eur. J. Pharmacol. 1990;182:291–299. doi: 10.1016/0014-2999(90)90288-h. [DOI] [PubMed] [Google Scholar]
- GRBOVIC L., RADMANOVIC B.Z. The action of prostaglandin E2 on the rhythmic activity of circular muscle of the isolated guinea-pig ileum. Arch. Int. Pharmacodyn. Ther. 1987;285:166–176. [PubMed] [Google Scholar]
- HALLINAN E.A., HAGEN T.J., HUSA R.K., TSYMBALOV S., RAO S.N., VANHOECK J.P., RAFFERTY M.F., STAPELFIELD A., SAVAGE M.A., REICHMAN M. N-substituted dibenzoxazepines as analgesic PGE2 antagonists. J. Med. Chem. 1993;36:3293–3299. doi: 10.1021/jm00074a010. [DOI] [PubMed] [Google Scholar]
- HAUSKEN T., ODEGAARD S., BERSTAD A. Antroduodenal motility studied by real-time ultrasonography. Effect of enprostil. Gastroenterology. 1991;100:59–63. doi: 10.1016/0016-5085(91)90582-6. [DOI] [PubMed] [Google Scholar]
- HEINEMANN A., SHAHBAZIAN A., BARTHO L., HOLZER P. Different receptors mediating the inhibitory action of exogenous ATP and endogenously released purines on guinea-pig intestinal peristalsis. Br. J. Pharmacol. 1999;128:313–320. doi: 10.1038/sj.bjp.0702808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P., LIPPE I.T., HEINEMANN A., BARTHO L. Tachykinin NK1 and NK2 receptor-mediated control of peristaltic propulsion in the guinea-pig small intestine in vitro. Neuropharmacology. 1998;37:131–138. doi: 10.1016/s0028-3908(97)00195-0. [DOI] [PubMed] [Google Scholar]
- ISHIZAWA M., MIYAZAKI E. Effect of prostaglandin F2α on propulsive activity of the isolated segmental colon of the guinea-pig. Prostaglandins. 1975;10:759–768. doi: 10.1016/0090-6980(75)90004-0. [DOI] [PubMed] [Google Scholar]
- JOHNSON P.J., BORNSTEIN J.C., YUAN S.Y., FURNESS J.B. Analysis of contributions of acetylcholine and tachykinins to neuro-neuronal transmission in motility reflexes in the guinea-pig ileum. Br. J. Pharmacol. 1996;118:973–983. doi: 10.1111/j.1476-5381.1996.tb15495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONSSON E.W.Functional characterisation of receptors for cysteinyl leukotrienes in smooth muscle Acta. Physiol. Scand. 19986411–55.Suppl [PubMed] [Google Scholar]
- KIM T.W., BECKETT E.A., HANNA R., KOH S.D., ORDOG T., WARD S.M., SANDERS K.M. Regulation of pacemaker frequency in the murine gastric antrum. J. Physiol. (London) 2002;538:145–157. doi: 10.1113/jphysiol.2001.012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWRENCE R.A., JONES R.L., WILSON N.H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br. J. Pharmacol. 1992;105:271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCOLI M., LECCHINI S., DE PONTI F., D'ANGELO L., CREMA A., FRIGO G.M. Subsensitivity of enteric cholinergic neurones to α2-adrenoceptor agonists after chronic sympathetic denervation. Naunyn Schmiedeberg's Arch. Pharmacol. 1985;329:271–277. doi: 10.1007/BF00501879. [DOI] [PubMed] [Google Scholar]
- METTERS K.M. Leukotrienic receptors. J. Lipid Mediat. Cell Signal. 1995;12:413–427. doi: 10.1016/0929-7855(95)00027-n. [DOI] [PubMed] [Google Scholar]
- MORIMOTO K., SUGIMOTO Y., KATSUYAMA M., OIDA H., TSUBOI K., KISHI K., KINOSHITA Y., NEGISHI M., CHIBA T., NARUMIYA S., ICHIKAWA A. Cellular localization of mRNAs for prostaglandin E receptor subtypes in mouse gastrointestinal tract. Am. J. Physiol. 1997;272:G681–G687. doi: 10.1152/ajpgi.1997.272.3.G681. [DOI] [PubMed] [Google Scholar]
- NARUMIYA S., SUGIMOTO Y., USHIKUBI F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- NORTHEY A., DENIS D., CIRINO M., METTERS K.M., NANTEL F. Cellular distribution of prostanoid EP receptors mRNA in the rat gastrointestinal tract. Prostaglandins Other Lipid Mediat. 2000;62:145–156. doi: 10.1016/s0090-6980(00)00058-7. [DOI] [PubMed] [Google Scholar]
- OGLETREE M.L., HARRIS D.N., GREENBERG R., HASLANGER M.F., NAKANE M. Pharmacological actions of SQ 29,548, a novel selective thromboxane antagonist. J. Pharmacol. Exp. Ther. 1985;234:435–441. [PubMed] [Google Scholar]
- OKADA Y., HARA A., MA H., XIAO C.-Y., TAKAHATA O., KOHGO Y., NARUMIYA S., USHIKUBI F. Characterization of prostanoid receptors mediating contraction of the gastric fundus and ileum: studies using mice deficient in prostanoid receptors. Br. J. Pharmacol. 2000;131:745–755. doi: 10.1038/sj.bjp.0703627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKÉ K., BERRINO L., MÖLIG A., JÄGER R., GRIEPENKERL I., BRÄUTIGAM M., REIMANN A. Modulation of noradrenaline release in rat isolated stomach by prostanoids, but not by histaminergic mechanisms. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:631–639. doi: 10.1007/BF00171322. [DOI] [PubMed] [Google Scholar]
- RADMANOVIC B.Z. Effect of prostaglandin E1 on the peristaltic activity of the guinea-pig isolated ileum. Arch. Int. Pharmacodyn. Ther. 1972;200:396–404. [PubMed] [Google Scholar]
- SAMETZ W., HENNERBICHLER S., GLASER S., WINTERSTEIGER R., JUAN H. Characterization of prostanoid receptors mediating actions of the isoprostanes, 8-iso-PGE2 and 8-iso-PGF2α, in some isolated smooth muscle preparations. Br. J. Pharmacol. 2000;130:1903–1910. doi: 10.1038/sj.bjp.0703522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER G.J., BENNETT A. Regional differences in the responses to prostanoids of circular muscle from guinea-pig isolated intestine. J. Pharm. Pharmacol. 1980;32:705–708. doi: 10.1111/j.2042-7158.1980.tb13043.x. [DOI] [PubMed] [Google Scholar]
- SANGER G.J., WATT A.J. The effect of PGE1 on peristalsis and on perivascular nerve inhibition of peristaltic activity in guinea-pig isolated ileum. J. Pharm. Pharmacol. 1978;30:762–765. doi: 10.1111/j.2042-7158.1978.tb13388.x. [DOI] [PubMed] [Google Scholar]
- SHAHBAZIAN A., SCHULIGOI R., HEINEMANN A., PESKAR B.A., HOLZER P. Disturbance of peristalsis in the guinea-pig isolated small intestine by indomethacin, but not cyclo-oxygenase isoform-selective inhibitors. Br. J. Pharmacol. 2001;132:1299–1309. doi: 10.1038/sj.bjp.0703940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOWN M., CASALS-STENZEL J., SCHILLINGER E. Pharmacological and cardiovascular properties of a hydantoin derivative, BW 245 C, with high affinity and selectivity for PGD2 receptors. Prostaglandins. 1983;25:13–28. doi: 10.1016/0090-6980(83)90131-4. [DOI] [PubMed] [Google Scholar]
- WALCH L., DE MONTPREVILLE V., BRINK C., NOREL X. Prostanoid EP1- and TP-receptors involved in the contraction of human pulmonary veins. Br. J. Pharmacol. 2001;134:1671–1678. doi: 10.1038/sj.bjp.0704423. [DOI] [PMC free article] [PubMed] [Google Scholar]


