Abstract
In the canine a single brief (5 min) coronary artery occlusion protects the myocardium against the severe ventricular arrhythmias and reduces the ischaemic changes that result from a subsequent, more prolonged (25 min) occlusion. The main purpose of the present study was to examine whether mitochondrial KATP channels are involved in this protection.
In chloralose-urethane anaesthetized dogs, preconditioning (PC) was induced by a single 5 min period occlusion of the left anterior descending (LAD) coronary artery, 20 min prior to a 25 min occlusion of the same artery. In some of these PC dogs 5-hydroxydecanoate (5-HD; 150 μg kg−1 min−1 by intracoronary infusion) was given over a period of 30 min either before, or after PC. In other dogs the mitochondrial KATP channel opener diazoxide (1 mg kg−1; i.c.) was given, either alone or in the presence of 5-HD. Control dogs (infused with saline) were simply subjected to a 25 min occlusion and reperfusion.
Compared to controls, both PC and diazoxide significantly reduced the number of ventricular premature beats (VPBs; 295±67 to 89±28 and 19±11, respectively; P<0.05), the number of episodes of ventricular tachycardia (VT; 8.3±4.2 to 1.6±0.9 and 0.2±0.1; P<0.05) and the incidences of VT (100 to 43 and 33%; P<0.05) and ventricular fibrilation (VF; 60 to 0 and 17%; P<0.05) during the 25 min occlusion of the LAD. Further, 43% of the PC dogs and 58% of the diazoxide treated dogs survived the combined ischaemia-reperfusion insult (cp. 0% in the controls; P<0.05). The protection afforded by PC and diazoxide was abolished by 5-HD, especially when it was given prior to the PC occlusion. In the presence of 5-HD, three out of 10 dogs fibrillated during the PC occlusion and another three dogs died following reperfusion. Furthermore, there were no survivors in this group from the prolonged ischaemia/reperfusion insult. 5-HD given after PC only attenuated the antiarrhythmic protection.
Opening of mitoKATP channels prior to ischaemia by preconditioning and diazoxide protects the myocardium against ischaemia and reperfusion-induced arrhythmias. This protection is abolished if the opening of these channels is prevented by the prior administration of 5-HD but only attenuated if 5-HD is given after preconditioning. The results indicate that opening of mitoKATP channels prior to ischaemia is mandatory for protection against ischaemia and reperfusion-induced arrhythmias.
Keywords: Mitochondrial KATP channels, ventricular arrhythmia, ischaemia, sudden death, 5-hydroxydecanoate, diazoxide
Introduction
Gross and Auchampach proposed in 1992 that KATP channels are involved in the cardioprotective effects of ischaemic preconditioning (Gross & Auchampach, 1992). They showed, in a canine model of ischaemia-reperfusion, that the protection against infarct size that resulted from preconditioning was completely abolished by the KATP channel blocker glibenclamide, when it was given either before or after preconditioning (Gross & Auchampach, 1992). This finding was confirmed by further studies showing that opening of KATP channels prior to ischaemia mimics (Gross et al., 1994; Grover, 1994; Schulz et al., 1994), whereas blockade of this channel attenuates (Auchampach et al., 1992; Toombs et al., 1993; Schulz et al., 1994), the cardioprotective effects of preconditioning. It was suggested therefore that the mechanisms of this cardioprotection, resulting either from the administration of KATP channel openers or from short periods of ischaemia (preconditioning), involves the opening of sarcolemmal KATP channels and the subsequent shortening of the action potential. This would reduce calcium entry, depress contractility and reduce myocardial oxygen demand during a subsequent and more prolonged period of ischaemia (Nichols et al., 1991). More recent evidence casts doubt on this hypothesis. For example, the degree of action potential shortening does not correlate with the degree of protection (Grover et al., 1995). Thus, Grover and colleagues showed that dofetilide, which abolishes cromakalim-induced action potential shortening, does not modify the cardioprotective effects either of cromakalim or of preconditioning (Grover et al., 1995; 1996). Similarly, Yao & Gross (1994) showed that bimakalim, given in a dose that induced protection against infarct size in dogs, does not shorten monophasic action potential duration. Furthermore, we have found that in anaesthetized dogs the protective effect of preconditioning against arrhythmias (Végh et al., 1992a) was not modified by glibenclamide (which acts somewhat preferentially on sarcolemmal KATP channels; O'rourke, 2000), given either before or after preconditioning (Végh et al., 1993). We were also unable to modify ischaemia-induced arrhythmias by the administration of cromakalim (Végh et al., 1997). Our conclusion, therefore, was that it is unlikely that sarcolemmal KATP channels contribute significantly to the antiarrhythmic effects of preconditioning (reviewed by Parratt & Kane, 1994).
More recent evidence suggests that mitochondrial, rather than sarcolemmal, KATP channels are involved in the cardioprotective effects associated with preconditioning (Liu et al., 1998; Downey & Cohen, 2000; 2001; Gross & Fryer, 2000; O'rourke, 2000; Fryer et al., 2001). These mitochondrial channels are also susceptible to various KATP channel agonists and antagonists (Inoue et al., 1991). Indeed the KATP channel opener diazoxide, which is about 1000-times more potent in opening mitochondrial than sarcolemmal KATP channels, has been found to be cardioprotective in a concentration that opens only mitochondrial(mito) KATP channels (Garlid et al., 1997). Furthermore, the selective mitoKATP channel inhibitor 5-hydroxydecanoate (5-HD) abolishes the protection that results either from the administration of diazoxide or from ischaemic preconditioning (McCullough et al., 1991; Downey & Cohen, 2000). Such studies, however, have only investigated the infarct size reducing effect of preconditioning as an end-point of protection, and except for a study (Asemu et al., 1999) in rat isolated hearts (a model in which ventricular fibrillation, the most serious ischaemia-induced arrhythmia, could not be demonstrated), there have been no attempts to investigate the role of mitoKATP channels in the protective effect of preconditioning against the arrhythmias that result from myocardial ischaemia. The aim of the present study was, therefore, to investigate whether mitoKATP channels are involved in the antiarrhythmic effect of preconditioning in a well established canine model of ischaemia and reperfusion. We did this in two ways; first we examined whether the antiarrhythmic effects of preconditioning could be blocked by the administration of the selective mitoKATP channel inhibitor 5-hydroxydecanoate; second, we examined whether diazoxide can also protect the heart against arrhythmias resulting from ischaemia and reperfusion.
Methods
In vivo studies in anaesthetized dogs
These were similar to those already described in detail elsewhere (Végh et al., 1992a). In brief, adult mongrel dogs of both sexes, weighing between 18 and 26 kg (mean: 23±2 kg) were anaesthetized with a mixture of chloralose and urethane (60 and 200 mg kg−1 i.v., respectively) and ventilated with room air using a Harvard Respirator at a rate and volume sufficient to maintain arterial blood gases and pH within normal limits (Végh et al., 1992a). If necessary, additional chloralose and urethane (10 and 25 mg kg−1 h−1, respectively) were infused to maintain anaesthesia. Temperature was recorded from the mid-oesophagus and maintained at 37±0.5°C by means of a heating pad.
Polyethylene catheters were inserted into the right femoral artery (for monitoring blood pressure) and into the right femoral vein (for drug and anaesthetic administration). Another catheter was introduced into the cavity of the left ventricle, through the left femoral artery, for the measurement of left ventricular systolic (LVSP) and end-diastolic (LVEDP) pressures, as well as changes in positive and negative dP/dtmax.
The animals were thoractomized at the fifth intercostal space and the anterior descending branch of the left coronary artery (LAD) prepared for occlusion just proximal to the first main diagonal branch. The circumflex branch of the left coronary artery (LCX) was also dissected free to allow the measurement of coronary blood flow by means of a 2.0 mm electromagnetic flow probe attached to a Statham SP2202 flowmeter. Epicardial ST-segment changes and the degree of inhomogeneity of electrical activation were measured from the left ventricular wall distal to the occlusion site using a ‘composite' electrode described previously (Végh et al., 1992a). This gives a summarized recording of R-waves from 30 epicardial measuring points. In the adequately perfused and oxygenated myocardium all sites are activated almost simultaneously, resulting in a single large spike. However, following occlusion, widening and fractionation of the summarized R-wave occurs, indicating that adjacent fibres are not simultaneously activated because of inhomogeneity of conduction. We expressed this as the greatest delay in activation (ms) within the ischaemic area.
All these parameters, together with a limb lead electrocardiogram, systemic arterial and left ventricular systolic and end-diastolic pressures (Statham P23XL transducers) and LV dP/dt were recorded on an eight channel Medicor R81 recorder.
In these experiments a side branch of the LAD was catheterized immediately proximal to the proposed occlusion site (Végh et al., 1996). This was used for the local intracoronary administration of drugs or saline.
Ventricular arrhythmias during coronary artery occlusion and following reperfusion were assessed as previously described (Végh et al., 1992a). In brief, the total number of ventricular premature beats (VPBs), the incidence and number of episodes of ventricular tachycardia (VT; defined as a run of four or more ventricular premature beats at a rate faster than the resting heart rate), and the incidence of ventricular fibrillation (VF) were evaluated. Following reperfusion, only the incidence of VF was assessed. Those dogs were pronounced survivors if they were still alive, and predominantly in sinus rhythm, 10 min after reperfusion. These animals were euthanized by an excess of anaesthetic.
The risk area following coronary artery occlusion was assessed in each dog at the end of the experiment by injecting patent blue V dye into the re-occluded coronary artery. It was expressed as a percentage of the left ventricular wall together with the septum (Kis et al., 1999).
Experimental protocol
The experimental protocol is illustrated in Figure 1. Six groups of dogs were used. The animals were randomly assigned to control or treated groups by providing an equal distribution of males and females in each group. In all groups a 30 min recovery period was allowed to stabilize after surgery. Ten dogs served as controls. In these dogs saline was infused into a side branch of the left anterior descending coronary artery (LAD) proximal to the occlusion site, at a rate of 0.5 ml min−1 over a period of 30 min. After this time the dogs were subjected to a 25 min occlusion of the LAD; if the dogs had survived up to this time the ischaemic myocardium was then rapidly reperfused. Three groups of dogs were subjected to preconditioning by occluding the LAD for 5 min 20 min prior to a prolonged (25 min) period of ischaemia. In some of these preconditioned dogs (n=14) saline was infused 30 min prior to prolonged ischaemia. In a further group of preconditioned dogs 5-hydroxydecanoate (5-HD) was given in a dose of 150 μg kg−1 min−1 by intracoronary infusion over a period of 30 min, commencing either 5 min prior to (n=10) or immediately after (n=10) the 5 min preconditioning occlusion. Another thirteen dogs were given 1 mg kg−1 diazoxide, administered slowly (over a 2 min period) into the coronary artery, 20 min prior to prolonged occlusion; a further six dogs were subjected to this same protocol but in the presence of an infusion of 5-HD.
Figure 1.
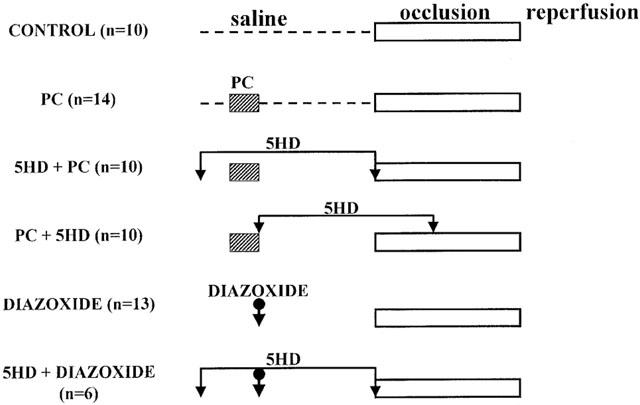
Experimental protocol for the studies examining the role of mitochondrial KATP channels in the antiarrhythmic effects of ischaemic preconditioning in anaesthetized dogs. Preconditioning was induced by a 5 min occlusion of the left anterior descending (LAD) coronary artery 20 min prior to a 25 min occlusion of the same artery (PC group, n=14). Two groups of preconditioned dogs were given 5-hydroxydecanoate (5-HD) in a dose of 150 μg kg−1 min−1 by intracoronary infusion over a 30 min period, either before (5-HD+PC); n=10) or immediately after (PC+5-HD; n=10) preconditioning. In some dogs diazoxide was injected, by the intracoronary route, in a dose of 1 mg kg−1 20 min prior to the prolonged occlusion either alone (n=3) or in the presence of 5-HD (n=6). Control dogs (n=10) were given intracoronary saline (0.5 ml min−1) over a period of 30 min and then subjected to prolonged (25 min) ischaemia.
The dose of 5-hydroxydecanoate was taken from the original paper of Gross & Auchampach (1992) who gave this same dose (150 μg kg−1 min−1) by the same intracoronary route, and also in dogs, to selectively block mitochondrial (mito)KATP channels. The dose of diazoxide (1 mg kg−1) was calculated from that used in rat isolated hearts to selectively open mitoKATP channels by Garlid et al. (1997).
Ethics
The origin and upkeep of these dogs were in accord with Hungarian law (XXVIII, chapter IV, paragraph 31) regarding large experimental animals, which comply with those of the European Commission as described in the regulations dated December 16, 1991.
Statistical evaluation
All the data are expressed as means±s.e.mean and differences between means compared by a Student's t-test corrected for multiple comparisons using a two-way ANOVA. Ventricular premature beats were compared using the Mann–Whitney Rank Sum test, and the incidences of arrhythmias (such as VT, VF) and survival from the combined ischaemia–reperfusion insult were compared using the Fisher Exact test. Differences between groups were considered significant when P<0.05.
Results
Haemodynamic effects of 5-hydroxydecanoate and of diazoxide
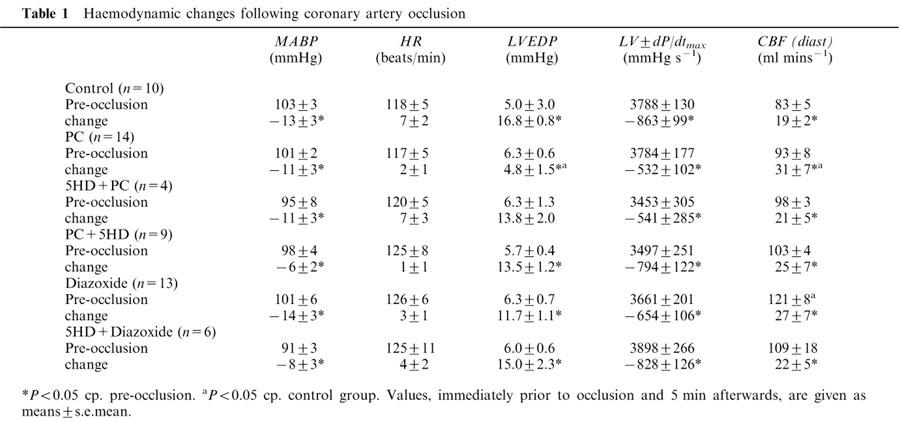
There were no significant haemodynamic changes following the intracoronary administration of either 5-HD and diazoxide, except that diazoxide markedly increased coronary blood flow in the circumflex branch of the left coronary artery (from 118±8 to 174±26 ml min−1; P<0.05) compared to the control dogs infused with saline (from 99±8 to 96±7) and reduced coronary vascular resistance during diastole (from 0.73±0.03 to 0.47±0.06 mmHg ml−1 min−1; P<0.0.5 cf. controls from 0.97±0.10 to 0.95±0.1mmHg ml−1 min−1).
Haemodynamic changes following the 25 min occlusion of the LAD
The changes in the most important haemodynamic parameters are illustrated in Table 1. There were no significant changes between groups in any of these parameters when measured immediately prior to coronary artery occlusion. There was only one exception; in dogs given diazoxide the diastolic coronary blood flow was higher prior to occlusion than in the other groups. Coronary artery occlusion resulted in similar haemodynamic changes in all groups; arterial blood pressure and LVdP/dtmax were significantly reduced, whereas LVEDP was markedly increased, except in dogs subjected to preconditioning. In these dogs the elevation of LVEDP was significantly less than in the controls (Table 1). Following occlusion of the LAD there was a marked increase in LCX coronary blood flow in all the groups, but especially in the dogs that had been subjected to preconditioning. This ‘compensatory' increase in coronary blood flow was not modified by 5-HD (Table 1).
Table 1.
Haemodynamic changes following coronary artery occlusion
The severity of ventricular arrhythmias during a preconditioning coronary artery occlusion; effects of the prior administration of 5-HD
In normal dogs there are relatively few ventricular premature beats during a 5 min coronary artery occlusion. For example, in the present study, of the 24 dogs subjected to such a single (preconditioning) occlusion period there was a mean of only 18±14 ventricular premature beats (VBPs). Further, two dogs exhibited just a single episode of VT (Figure 2), no dog fibrillated during the preconditioning occlusion, and only one of these 24 dogs fibrillated during reperfusion. However, when the 5 min preconditioning procedure was performed in the presence of 5-HD, the number of VPBs and of episodes of VT were both significantly increased (to 76±33 and 4.1±1.2 episodes, respectively). Indeed, it proved difficult to precondition dogs in the presence of 5-HD, since seven out of the 10 dogs exhibited VT and three out of 10 of the animals fibrillated during the preconditioning occlusion. Further, another three dogs out of the remaining seven dogs fibrillated during reperfusion. Thus, only four of the 10 dogs in this group survived to be subjected to prolonged ischaemia.
Figure 2.
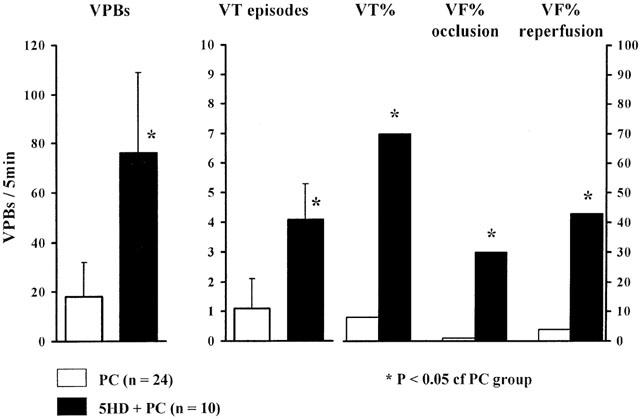
The severity of ventricular arrhythmias (number of VPBs, VT episodes, incidences of VT, VF and survival) during the 5 min preconditioning procedure in dogs given either saline (PC) or 5-HD (5-HD +PC). It was difficult to precondition dogs in the presence of 5-HD. In these dogs the number of VPBs and VT episodes, and the incidence of VT, were markedly increased during the 5 min preconditioning occlusion and there was a high incidence of VF both during occlusion and reperfusion. Values are means±s.e.mean. *P<0.05 compared to preconditioned dogs without 5-HD.
The severity of ventricular arrhythmias during the 25 min coronary artery occlusion
This is illustrated in Figures 3 and 4. In control dogs, there was marked ventricular ectopic activity (VPBs 295±67) and a high incidence (100%) and many episodes (8.3±4.2) of VT during coronary artery occlusion (Figure 3). In contrast, these arrhythmias were significantly less in dogs subjected to preconditioning. Thus, there were only 89±28 VPBs, 1.6±0.9 episodes of VT (P<0.05) and a 43% incidence of VT during the prolonged occlusion in these preconditioned dogs. The incidences of VF during ischaemia and reperfusion were also modified by preconditioning (Figure 4). During coronary artery occlusion VF occurred in 60% of the control dogs and no dog survived the combined ischaemia-reperfusion insult (Figure 4). In contrast, no preconditioned dog died during occlusion and there was a 43% survival from the occlusion/reperfusion insult.
Figure 3.
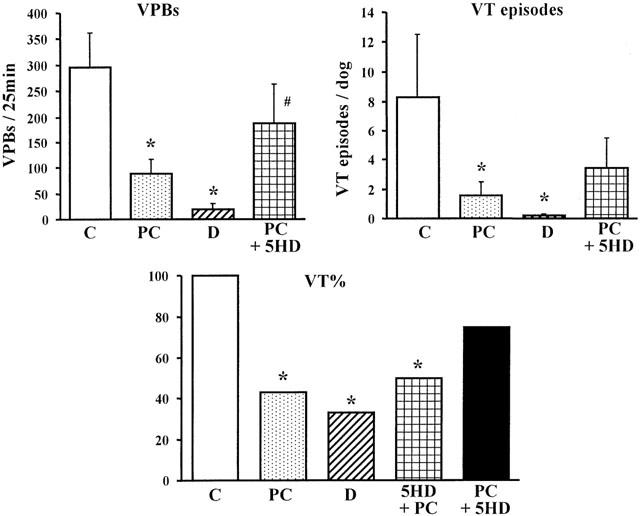
Ventricular premature beats (VPBs) and the incidence (%) and number of episodes of ventricular tachycardia (VT) in control dogs (C), in preconditioned dogs (PC), in dogs given diazoxide (D) and in preconditioned dogs given 5-hydroxydecanoate after preconditioning (PC+5-HD). In dogs given 5-HD prior to preconditioning (5-HD+PC) only the incidence of VT is shown. The antiarrhythmic effect of PC is prevented by 5-HD. Values are means±s.e.mean. *P<0.05 compared to controls; #P<0.05 compared to preconditioned dogs.
Figure 4.
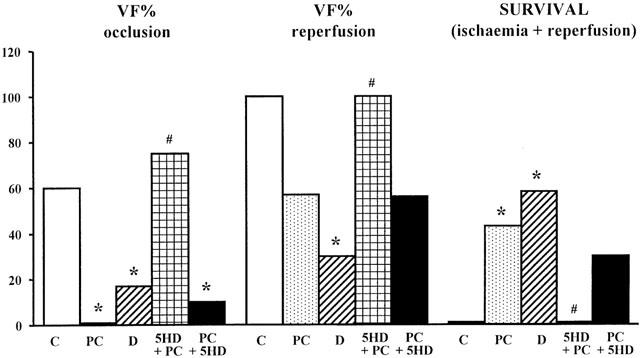
Ventricular fibrillation (VF; %) during a 25 min coronary artery occlusion (VF occlusion), following reperfusion (VF reperfusion) and survival following the combined ischaemia-reperfusion insult in control dogs (C), in preconditioned dogs (PC), in dogs given diazoxide (D), and in preconditioned dogs given 5-HD either prior to, or immediately after, the preconditioning occlusion. The reduction in VF and the increase in survival following ischaemic preconditioning is prevented by the administration of 5-HD prior to preconditioning. *P<0.05 compared to controls; #P<0.05 compared to preconditioned dogs.
A protection similar to that following preconditioning was achieved against ischaemia-induced arrhythmias with diazoxide. Thus, there were only few VPBs (19±11) and episodes of VT (0.2±0.1) and a much lower incidence of VT (33%) during the prolonged period of ischaemia (Figure 3). Furthermore, no dog given diazoxide fibrillated during occlusion, and 58% of these dogs survived the combined ischaemia-reperfusion insult (Figure 4; all P<0.05 compared to controls).
Administration of 5-HD prior to the prolonged occlusion attenuated, but did not abolish, the antiarrhythmic effects of preconditioning (Figure 4). For example, only one of these dogs fibrillated during the prolonged occlusion (P<0.05 c.p. controls) and 30% survived reperfusion. In those four dogs given 5-HD prior to the preconditioning occlusion (see above) and which survived long enough to be subjected to a prolonged occlusion, VF occurred in three of these dogs (Figure 4), in each case preceded by a brief period of VT (Figure 3), and the remaining dog fibrillated during reperfusion. 5-HD also completely abolished the protective effect of diazoxide; there were many VPBs (754±139) and all the dogs exhibited VT with many episodes (37.5±2.5). The greater number of VPB's in these dogs compared to controls (ns) was due entirely to an exceedingly high number (2053 ectopic beats) and many more episodes of VT (109 episodes) in one particular dog. Only 50% of these dogs survived the ischaemic period (P<0.05 c.p. those dogs given diazoxide alone) and no dog survived reperfusion (compared to 58% in those dogs given diazoxide but not 5-HD; P<0.05).
The severity of myocardial ischaemia during a 25 min occlusion of the LAD
This was assessed in two ways; by changes in the ST-segment recorded from epicardial electrodes and in the degree of inhomogeneity of electrical activation, both measured from the ischaemic area. Compared to controls, both preconditioning and diazozide markedly reduced both epicardial ST-segment elevation (Figure 5) and the degree of inhomogeneity (Figure 6). These protective effects against ischaemia were attenuated or abolished by 5-HD, depending on the timing of its administration.
Figure 5.
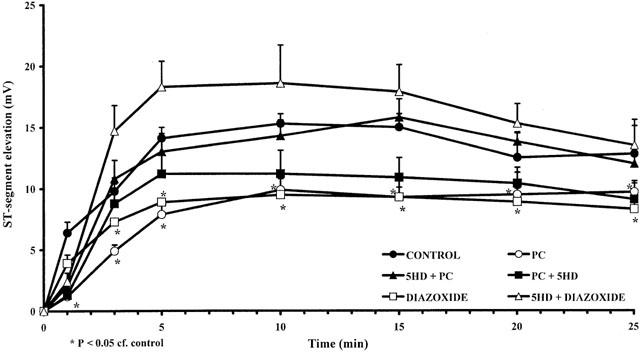
Changes in epicardial ST-segment elevation (mV) during a 25 min coronary artery occlusion in control (C) dogs, in preconditioned dogs (PC), in preconditioned dogs given 5-HD before (5-HD+PC) or after (PC+5-HD) preconditioning, and in dogs treated with diazoxide alone (D) or in the presence of 5-HD (5-HD+D). The preconditioning and diazoxide-induced reductions in this index of ischaemia severity are prevented by 5-HD. Values are means±s.e.mean. *P<0.05 compared to control.
Figure 6.
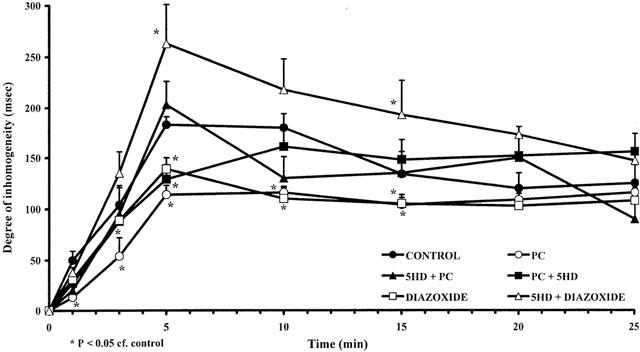
Changes in the degree of inhomogeneity of electrical activation (ms) within the ischaemic area during a 25 min coronary artery occlusion in control dogs (C), in preconditioned dogs (PC), in preconditioned dogs given 5-HD before (5-HD+PC) or after (PC+5-HD) preconditioning, and in dogs treated with diazoxide alone (D) or in the presence of 5-HD (5-HD+D). The preconditioning and diazoxide-induced reductions in this index of ischaemia severity are prevented by 5-HD. Values are means±s.e.mean. *P<0.05 compared to control.
There were no significant differences in the area at risk between controls (38±2%), preconditioned dogs (41±3%) preconditioned dogs given 5-HD prior to preconditioning (35±2%) or prior to prolonged occlusion (36±1%), or in dogs given diazoxide in the absence (38±1%) or in the presence of 5-HD (36±2%).
Discussion
In the present study we confirm our previous findings that preconditioning induced by brief (5 min) periods of coronary artery occlusion, protects the myocardium against the severe ventricular arrhythmias that occur during a subsequent, more prolonged period of ischaemia (Végh et al., 1990; 1992a). We now demonstrate that even a single preconditioning occlusion of 5 min is profoundly antiarrhythmic. This, as with other manifestations of the protection afforded by ischaemic preconditioning (such as infarct size reduction and the enhanced recovery of contractile function following a period of ischaemia and reperfusion) probably involves the opening of mitochondrial KATP channels. This observation is important because it shows, we think for the first time, that all three major protective effects of preconditioning involve a similar common mechanism. In the past this has been the subject of debate (reviewed by Parratt & Kane, 1994).
The evidence for the involvement of these channels in arrhythmia suppression following preconditioning is that (1) blocking these channels with 5-hydroxydecanoate abolishes the protection against arrhythmias resulting from preconditioning and (2) that opening these channels by pharmacological means with diazoxide results in a similar protection against arrhythmias during ischaemia and reperfusion as that resulting from ischaemic preconditioning. The marked protection afforded by diazoxide is also 5-HD sensitive; it is abolished if diazoxide is given in the presence of 5-HD.
We found, and this is in apparent contrast to others, that it was difficult to precondition dogs in the presence of 5-HD. Few of the dogs survived the preconditioning occlusion since, during attempts to precondition with even a single 5 min occlusion in the presence of 5-HD, arrhythmias were severe; this was true both during this brief period of ischaemia (occlusion) and during reperfusion (Figure 2). It seems that mitoKATP channels are opened by even such a brief (5 min) period of ischaemia and that this is necessary for the effectiveness of preconditioning to reduce the severity of those life-threatening arrhythmias which result from a subsequent, more prolonged, coronary artery occlusion commencing 20 min later. MitoKATP channel opening is thus (following mediator release?) a prerequisite (‘trigger') for the protection. What is not so clear from the present results is whether, for protection, they need to remain open during the subsequent more prolonged ischaemic period.
Although the antiarrhythmic effect of preconditioning was still evidence when 5-HD was given after the preconditioning occlusion but before (and during) the prolonged (25 min) period of ischaemia we also do not know whether, once opened, the channels can be closed by 5-HD administration. Presumably other events downstream to mitoKATP channel opening have already taken place and it is these events that lead to protection against arrhythmias. This important question, that is whether these channels are involved, not only in the trigger mechanism for protection but are also as the end-target (mediator) of this protection, has been recently discussed by Patel & Gross (2001). They have suggested that the opening of these channels generates reactive oxygen species (ROS) and that these are part of the cascade leading to cardioprotection. They have discussed the evidence suggesting that the opening of mitoKATP channels is indeed upstream to ROS generation.
The difficulty we found in preconditioning in the presence of 5-HD is similar to our previous studies when, prior to the preconditioning occlusion(s), drugs had already been given to block the important initial (i.e. trigger) chemical mediators of this protection, namely bradykinin (Végh et al., 1994), prostacyclin (Végh et al., 1990) and nitric oxide (Végh et al., 1992b). In this regard, a role for nitric oxide is supported by the finding that, albeit in a different species and system, a NO donor (S-nitroso-N-acetylpenicillamine; SNAP) directly activates mitochondrial KATP systems and indeed, also potentiates the ability of diazoxide to open these same channels by a cGMP-independent mechanism (Sasaki et al., 2000). This has led to speculation that NO might function as an endogenous mitochondrial KATP channel opener (Horimoto et al., 2000). Our suggestion would be that it is the bradykinin-mediated release of NO leading to the opening of these channels that is a key triggering mechanism leading to arrhythmia protection (Parratt et al., 1997). It is of interest that Wang et al. (2001) have recently demonstrated, albeit in a model of late (delayed) cardioprotection, that the protective effects of diazoxide are not observed in iNOS knockout mice.
The present studies, using arrhythmia severity rather than infarct size as the endpoint, suggest to us that not only do the mitochondrial KATP channels have to be opened (by preconditioning or diazoxide) to provide protection, a conclusion that is generally accepted, but also, since 5-HD given after preconditioning and continued well into the prolonged ischaemic period fails to significantly modify preconditioning-induced protection against arrhythmias, that mitoKATP channels are a trigger, but not the end-effector of this protection. This accords well with the conclusion of Downey's group (Downey & Cohen, 2000; 2001; Pain et al., 2000) that the opening of these channels, like activation of protein kinase C, tyrosine kinase and the generation of nitric oxide, is involved in the cascade(s) leading to protection but that these channels are not the end-effector, which remains elusive. The evidence leading to the proposal, that mitochondrial KATP channels are both the trigger and the end-effector of protection by ischaemic preconditioning has been recently summarized by Gross & Fryer (2000). However, neither we nor Downey's group (Pain et al., 2000), have been successful in demonstrating convincingly that these channels are indeed also the end-effector. The reasonable conclusion (Gross & Fryer, 2000) that ‘obviously, additional studies are necessary to determine the critical juncture at which mitochondrial KATP channel activation elicits its important cardioprotective function' still stands.
Acknowledgments
This work was supported by the Hungarian Scientific Research Foundation (OTKA; Project numbers: T032542 and T025301), the Health Scientific Committee of the Hungarian Ministry of Health (ETT; Project number 09/2000) and by the Research and Education Division of the Hungarian Ministry of Education (FKFP; Project number: 1290/1997). Professor Parratt is presently the holder of a Leverhulme Trust Emeritus Fellowship. We appreciate the technical assistance of Erika Bakó and Maria Györfi during these experiments.
Abbreviations
- DABP
diastolic arterial blood pressure
- 5 - HD
5-hydroxydecanoate
- HR
heart rate
- KATP - channel
ATP-dependent K+ channel
- LAD
left anterior descendent coronary artery
- LCX
left circumflex
- LVEDP
left ventricular end-diastolic pressure
- LVSP
left ventricular systolic pressure
- MABP
mean arterial blood pressure
- PC
preconditioning
- SABP
systolic arterial blood pressure
- VF
ventricular fibrillation
- VPBs
ventricular premature beats
- VT
ventricular tachycardia
References
- ASEMU G., PAPOUSEK F., OSTADAL B., KOLAR F. Adaptation to high altitude hypoxia protects the rat heart against ischaemia-induced arrhythmias. Involvement of mitochondrial KATP channels. J. Mol. Cell Cardiol. 1999;31:1821–1831. doi: 10.1006/jmcc.1999.1013. [DOI] [PubMed] [Google Scholar]
- AUCHAMPACH J.A., GROVER G.J., GROSS G.J. Blockade of ischaemic preconditioning in dogs by the novel ATP dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc. Res. 1992;26:1054–1062. doi: 10.1093/cvr/26.11.1054. [DOI] [PubMed] [Google Scholar]
- DOWNEY J.M., COHEN M.V. Do mitochondrial KATP channels serve as triggers rather than end-effectors of ischaemic preconditioning's protection. Basic Res. Cardiol. 2000;95:272–274. doi: 10.1007/s003950070045. [DOI] [PubMed] [Google Scholar]
- DOWNEY J.M., COHEN M.V. Mitochondrial KATP channel opening during index ischaemia and following myocardial reperfusion in ischemic rat hearts. J. Mol. Cell. Cardiol. 2001;33:651–653. doi: 10.1006/jmcc.2000.1352. [DOI] [PubMed] [Google Scholar]
- FRYER R.M., HSU A.K., GROSS G.J. Mitocondrial KATP channel opening is important during index ischemia and following myocardial reperfusion in ischemic preconditioned rat hearts. J. Mol. Cell. Cardiol. 2001;33:831–834. doi: 10.1006/jmcc.2001.1350. [DOI] [PubMed] [Google Scholar]
- GARLID K.D., PAUCEK P., YAROV-YAROVOY V., MURRAY H.N., DARBENZIO R.B., D'ALONZO A.J., LODGE N.J., SMITH M.A., GROVER G.J. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ. Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- GROSS G.J., AUCHAMPACH J.A. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ. Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- GROSS G.J., FRYER R.M. Mitochondrial KATP channels. Triggers or distal effectors of ischemic or pharmacological preconditioning. Circ. Res. 2000;87:431–433. doi: 10.1161/01.res.87.6.431. [DOI] [PubMed] [Google Scholar]
- GROSS G.J., YAO Y., AUCHAMPACH J.A.Role of ATP-sensitive potassium channels in ischaemic preconditioning Ischaemic Preconditioning: The concept of endogenous Cardioprotection. Developments in Cardiovascular Medicine 1994Boston Mass: Kluwer Academic Publishers; 125–135.ed. Przyklenk, K. & Kloner, R.A. pp [Google Scholar]
- GROVER G.J. Protective effects of ATP-sensitive potassium channel openers in models of myocardial ischaemia. Cardiovasc. Res. 1994;28:778–782. doi: 10.1093/cvr/28.6.778. [DOI] [PubMed] [Google Scholar]
- GROVER G.J., D'ALONZO A.J., DZWONCZYK S., PARHAM C.S., DARBENZIO R.B. Preconditioning is not abolished by the delayed rectifier K+ blocker dofetilide. Am. J. Physiol. 1996;40:H1207–H1214. doi: 10.1152/ajpheart.1996.271.3.H1207. [DOI] [PubMed] [Google Scholar]
- GROVER G.J., D'ALONZO A.J., PARHAM C.S., DARBENZIO R.B. Cardioprotection with the KATP channel opener cromakalim is not correlated with ischaemic myocardial action potential shortening. J. Cardiovasc. Pharmacol. 1995;26:145–152. doi: 10.1097/00005344-199507000-00023. [DOI] [PubMed] [Google Scholar]
- HORIMOTO H., GAUDETTE G.R., SALTMAN A.E., KRUKESKAMP I.B. The role of nitric oxide, K+ATP channels, and cGMP in the preconditioning response of the rabbit. J. Surg. Res. 2000;92:56–63. doi: 10.1006/jsre.2000.5845. [DOI] [PubMed] [Google Scholar]
- INOUE I., NAGASE H., KISHI K., HIGUTI T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- KIS A., VÉGH Á., PAPP J.G.Y., PARRATT J.R. Pacing-induced delayed protection against arrhythmias is attenuated by aminoguanidine, an inhibitor of nitric oxide synthase. Br. J. Pharmacol. 1999;127:1545–1550. doi: 10.1038/sj.bjp.0702695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y., SATO T., O'ROURKE B., MARBÁN E. Mitochondrial ATP-dependent potassium channels. Novel effectors of cardioprotection. Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- MCCULLOUGH J.R., NORMANDIN D.E., CONDER M.L., SLEPH P.G., DZWONZYK S., GROVER G.J. Specific block of the antiischemic actions of cromakalim by sodium 5-hydroxy-decanoate. Circ. Res. 1991;69:949–958. doi: 10.1161/01.res.69.4.949. [DOI] [PubMed] [Google Scholar]
- NICHOLS C.G., RIPOLL C., LEDERER W.J. ATP sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ. Res. 1991;68:280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- O'ROURKE B. Mitochondrial KATP channels in preconditioning. Circ. Res. 2000;87:845–855. doi: 10.1161/01.res.87.10.845. [DOI] [PubMed] [Google Scholar]
- PAIN T., YANG X.-M., CRITZ S.D., YUE Y., NAKANO A., LIU G.S., HEUSCH G., COHEN M.V., DOWNEY J.M. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R., KANE K.A. KATP channels in ischaemic preconditioning. Cardiovasc. Res. 1994;26:783–787. doi: 10.1093/cvr/28.6.783. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R., VÉGH Á., ZEITLIN I.J., AHMAD M., OLDROYD K., KASZALA K., PAPP J.G.Y.Bradykinin and endothelial-cardiac myocyte interactions in ischaemic preconditioning Am. J. Cardiol. 199780124A–131A.3A [DOI] [PubMed] [Google Scholar]
- PATEL H.H., GROSS G.J. Diazoxide induced cardioprotection: what comes first, KATP channels or reactive oxygen species. Cardiovasc. Res. 2001;51:633–636. doi: 10.1016/s0008-6363(01)00396-0. [DOI] [PubMed] [Google Scholar]
- SASAKI N., SATO T., OHLER A., O'ROURKE B., MARBÁN E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–445. doi: 10.1161/01.cir.101.4.439. [DOI] [PubMed] [Google Scholar]
- SCHULZ R., ROSE J., HEUSCH G. Involvement of activation of ATP-dependent potassium channels in ischaemic preconditioning in swine. Am. J. Physiol. 1994;267:H1341–H1352. doi: 10.1152/ajpheart.1994.267.4.H1341. [DOI] [PubMed] [Google Scholar]
- TOOMBS C.F., MOORE T.L., SHEBUSKI R.J. Limitation of infarct size in the rabbit by ischaemic preconditioning is reversible with glibenclamide. Cardiovasc. Res. 1993;27:617–622. doi: 10.1093/cvr/27.4.617. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., GYÖRGY K., PAPP J.G.Y., SAKAI K., PARRATT J.R. Nicorandil suppresses ventricular arrhythmias in a canine model of myocardial ischaemia. Eur. J. Pharmacol. 1996;305:163–168. doi: 10.1016/0014-2999(96)00166-5. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., KOMORI S., SZEKERES L., PARRATT J.R. Antiarrhythmic effects of preconditioning in anaesthetised dogs and rats. Cardiovasc. Res. 1992a;26:487–495. doi: 10.1093/cvr/26.5.487. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., PAPP J.G.Y., GYÖRGY K., KASZALA K., PARRATT J.R. Does opening of ATP-sensitive K+ channels modify ischaemia-induced ventricular arrhythmias in anaesthetised dogs. Eur. J. Pharmacol. 1997;333:33–38. doi: 10.1016/s0014-2999(97)01088-1. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., PAPP J.G.Y., PARRATT J.R. Attenuation of the antiarrhythmic effects of ischaemic preconditioning by blockade of bradykinin B2 receptors. Br. J. Pharmacol. 1994;113:1167–1172. doi: 10.1111/j.1476-5381.1994.tb17120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VÉGH Á., PAPP J.G.Y., SZEKERES L., PARRATT J.R. Are ATP sensitive potassium channels involved in the pronounced antiarrhythmic effects of preconditioning. Cardiovasc. Res. 1993;27:638–643. doi: 10.1093/cvr/27.4.638. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., SZEKERES L., PARRATT J.R. Protective effects of preconditioning of the ischaemic myocardium involve cyclo-oxygenase products. Cardiovasc. Res. 1990;24:1020–1023. doi: 10.1093/cvr/24.12.1020. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., SZEKERES L., PARRATT J.R. Preconditioning of the ischaemic myocardium; involvement of the L-arginine–nitric oxide pathway. Br. J. Pharmacol. 1992b;107:648–652. doi: 10.1111/j.1476-5381.1992.tb14501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG L., CHEREDNICHENKO G., HERNANDEZ L., HALOW J., CAMACHO S.A., FIGUERERO V., SCHAEFER S. Preconditioning limits mitochondrial Ca2+ during ischemia in rat hearts: role of KATP channels. Am. J. Physiol. 2001;280:H2321–H2328. doi: 10.1152/ajpheart.2001.280.5.H2321. [DOI] [PubMed] [Google Scholar]
- YAO Z., GROSS G.J. Effects of the KATP channel opener bimakalim on coronary blood flow, monophasic action potential duration, and infarct size in dogs. Circulation. 1994;89:1769–1775. doi: 10.1161/01.cir.89.4.1769. [DOI] [PubMed] [Google Scholar]


