Abstract
The action of the main ciguatoxin involved in ciguatera fish poisoning in the Pacific region (P-CTX-1b) was studied in myotubes originated from rat skeletal muscle cells kept in primary culture.
The effect of P-CTX-1b on sodium currents at short times of exposure (up to 1 min) showed a moderate increase in peak Na+ current. During prolonged exposures, P-CTX-1b decreased the peak Na+ current. This action was always accompanied by an increase of leakage currents, tail currents and outward Na+ currents, resulting in an intracellular Na+ accumulation. This effect is blocked by prior exposure to tetrodotoxin (TTX) and becomes evident only after washout of TTX.
Low to moderate concentrations of P-CTX-1b (2–5 nM) partially blocked potassium currents in a manner that was dependent on the membrane potential.
P-CTX-1b (2–12 nM) caused a small membrane depolarization (3–5 mV) and an increase in the frequency of spontaneous action potential discharges that reached in general low frequencies (0.1–0.5 Hz).
P-CTX-1b (10 nM) caused a transient increase of intracellular inositol 1,4,5-trisphosphate (IP3) mass levels, which was blocked by TTX.
In the presence of P-CTX-1b (10 nM) and in the absence of external Ca2+, the intracellular Ca2+ levels show a transient increase in the cytoplasm as well as in the nuclei. The time course of this effect may reflect the action of IP3 over internal stores activated by P-CTX-1b-induced membrane depolarization.
Keywords: Skeletal muscle, sodium currents, potassium currents, Pacific ciguatoxin-1b, IP3, intracellular Ca2+, intranuclear Ca2+
Introduction
The ciguatoxins family is a group of lipid-soluble, heat stable, cyclic polyether molecules isolated from carnivorous fish inhabiting tropical and sub-tropical areas of the Indo–Pacific Oceans (Yasumoto & Murata, 1993; Satake et al., 1998) and the Caribbean Sea (Lewis et al., 1998). These toxins are involved in a complex human food poisoning known as ciguatera, that is endemic to the region, and is elicited after the ingestion of a variety of reef fish that have accumulated them (Bagnis et al., 1979; Pottier et al., 2001). The main ciguatoxin extracted from these fish from the Pacific region is known as P-CTX-1b or P-CTX-1 (Murata et al., 1990; Lewis et al., 1991; 2000).
Ciguatoxins have a chemical structure reminiscent of brevetoxins (Baden, 1989) and share a common binding site with them on the neuronal voltage-sensitive sodium channel protein (Lombet et al., 1987; Lewis et al., 1991; Dechraoui et al., 1999). In neuronal membranes P-CTX-1b has been reported to shift the voltage-dependence of sodium channel activation to more negative values, and to enhance membrane excitability (Benoit et al., 1986; 1996; Hogg et al., 1998; Strachan et al., 1999). At vertebrate neuromuscular junctions P-CTX-1b was reported to induce repetitive endplate potentials and indirectly elicited muscle action potentials up to frequencies of 60 to 100 Hz upon a single nerve stimulus (Molgó et al., 1990). In skeletal muscle fibres the effect of the toxin seemed to differ from that reported in nerve cells although it was not previously studied in detail (reviewed by Molgó et al., 1992). Membrane potential changes in muscle cells trigger two types of voltage sensor-mediated calcium release signals (Jaimovich et al., 2000; Powell et al., 2001). In these cells calcium – besides its classical role in muscle contraction – is involved in other signalling processes with different time-domains unrelated to it, which appear to be regulated by the IP3 signalling pathway.
The aim of the present study was to determine the action of highly purified P-CTX-1b on primary cultures of rat skeletal muscle cells in order to determine its membrane effects and its eventual use as a tool to activate second messenger signalling pathways.
Methods
Primary culture of rat myotubes
Myotubes were obtained from the culture of the satellite cells dissected from hind-limb muscles of neonatal Fisher 344 rats, as previously described (Hidalgo et al., 1995). In brief, cell dissociation was performed with collagenase type VI treatment (1 mg ml−1) and continuous stirring for 15 min at 37°C. Cells recovered by centrifugation were resuspended, counted and plated over glass coverslips at a density of about 3×105 cells per 35 mm culture dish. Cell cultures were kept in a 95% air, 5% CO2 atmosphere at 37°C in a medium that was a 1 : 1 mixture of Dulbecco's modified Eagle medium (DMEM) and F-12 supplemented with 10% bovine serum and 2.5% foetal calf serum. To prevent fibroblast overgrowth, primary cultures were treated during 24 h with 10 μM cytosine arabinoside at the second or third day of culture. Myotubes were generally used for data acquisition after 6–10 days in culture. In all cases, these myotubes, did not show an organized sarcomere structure, although contraction in response to membrane depolarization was evident most of the time.
Electrophysiological recordings
The coverslip containing the myotubes was placed as the bottom of a chamber that was mounted on the stage of an inverted microscope (Nikon Diaphot-2, Japan). Patch-clamp recordings were performed with fire-polished glass pipettes (Drummond Microcaps, Broomall, PA, U.S.A.) of 2–5 MΩ resistance. Whole-cell current-clamp recordings were done using the nystatin perforated patch-clamp technique (Horn & Marty, 1988).
For current recordings, the standard physiological solution used to replace the culture medium had the following composition (in mM): NaCl 140, KCl 5, CaCl2 1, MgCl2 1, HEPES-Na 10, glucose 1 mg ml−1, pH 7.4; a nominally Ca2+-free version of this solution was obtained by replacement of CaCl2 by MgCl2 without the addition of Ca2+ chelators. Intrapipette solutions contained either (in mM): KCl 145; HEPES 10 in acid form and MgCl2 1 (adjusted to pH 7.4 with KOH) or tetraethylammonium-OH (TEA) 155, HEPES 10 in acid form, MgCl2 1 (adjusted to pH 7.4 with methanesulphonic acid) to block and reduce potassium and chloride currents.
The whole-cell configuration was achieved by either perforating the area under the patch through the addition of nystatin (final concentration 1 mg ml−1) to the patch electrode solution or by gentle mechanical suction of the membrane patch. In the latter case, ATP-Mg (2–4 mM) was added to the solution. Patch pipettes were mounted on the head-stage of an Axopatch 1-D amplifier (Axon Instruments, Union City, CA, U.S.A.). A Labmaster–DMA data acquisition and pulse generator board and a 486-PC-based microcomputer with pClamp 5.5 and Clampfit 6.0 software (Axon Instruments) were used to stimulate, acquire and analyse the data.
In voltage-clamp experiments the net current was obtained by cancellation of the linear components by the addition of 8 hyperpolarizing pulses of 1/8th the amplitude to the depolarizing test pulse (−P/8 protocol). Preliminary voltage clamp data showed that the standard amount of Na+ in the external saline (ca. 145 mM) elicited sodium currents that were too large to be processed accurately by the recording system. This problem was solved by two complementary approaches. First, the myotubes used for recording were selected among the smallest available (30–150 pF) in comparison to the standard size achievable by these cells during a week or more in culture conditions, and second, by the replacement of 75% of the external solution by a tetraethylammonium-methanesulphonate solution (TEA–MES). Under these altered ionic conditions (external Na+ concentration≈36 mM) and small size cells, the space-clamp difficulties were minimized and reproducible voltage-clamp recordings were routinely obtained.
P-CTX-1b was added to the extracellular bathing solution from X 100–500 stocks made in distilled water. All experiments were carried out at room temperature (22–24°C).
Intracellular calcium signal measurements
Myotubes attached to glass coverslips mounted in a 1 ml capacity chamber bathed with standard physiological solution were loaded for 30–45 min with the acetoxymethyl ester of the calcium-sensitive fluorescent dye fluo-3 (dish final concentration: 5.4 μM) (Molecular Probes, Eugene, OR, U.S.A.) containing 0.1% pluronic acid. This esterified dye is permeable through the cell membrane and is converted to the free form intracellularly by endogenous esterases (Minta et al., 1989). Before their use for imaging, myotubes were washed with a dye-free external solution without Ca2+ for about 15 min.
Fluorescent myotube images were obtained with an inverted confocal laser scanning microscope (Carl Zeiss Axiovert, 135 M-LSM microsystems, Germany) controlled through the manufacturer-supplied software and workstation. The 488 nm wavelength line of an Argon-ion laser was used for excitation of the fluo-3 dye. Images were collected using either a ×20 dry objective lens (0.5 numerical aperture) or with a ×40 oil immersion lens (1.3 numerical aperture). The aperture setting of the confocal pinhole was maintained constant in a given experiment. Images were digitized into a maximal array of 512×512 pixels. The optical sections of a given myotube before and after P-CTX-1b treatment were collected every 0.3–1.0 s and analysed frame by frame with ImageJ, a public domain image analysis software package (NIH, Bethesda, U.S.A.). The photomultiplier gain was kept constant in a given experiment and toxin effects were quantified on the same myotube before and during the action of the toxin. Images from each experiment were processed identically.
Determination of inositol 1,4,5-trisphosphate (IP3) levels
IP3 levels were measured using the method described by Bredt et al. (1989) with slight modifications, as described by Liberona et al. (1998). Briefly, a crude rat cerebellum membrane preparation was obtained after homogenization in 50 mM Tris-HCl pH 7.7, 1 mM EDTA, 2 mM β-mercaptoethanol and centrifugation at 20,000×g during 15 min. This procedure was repeated three times, re-suspending the final pellet in the same solution plus 0.3 M sucrose and freezing it at −80°C until use. The membrane preparation was calibrated for IP3 binding with 1.6 nM 3H-IP3 and 1–120 nM cold IP3, carrying out the sample analysis in a similar way, but adding an aliquot of the neutralized supernatant instead of cold IP3. The remaining membrane-bound 3H-IP3 radioactivity was measured by liquid scintillation.
Toxins and drugs used
Pacific ciguatoxin-1b (M.W. 1111.7) was a kind gift from Dr A.M. Legrand, and was extracted from Gymnothorax javanicus moray-eel liver, and purified at the Institute Louis Malardé, Papeete, Tahiti, French Polynesia by using procedures previously described (Legrand et al., 1989; Murata et al., 1990). Due to the limited amount of P-CTX-1b available, no extensive dose-response studies could be performed. Tetrodotoxin, nystatin, collagenase and cytosine arabinoside were purchased from Sigma Chemicals (St. Louis, MO, U.S.A.). All salts used were of analytical grade.
Data analysis
Graphs were produced with Origin 6 software (Microcal Software Inc., Northampton, MA, U.S.A.). When the data represents the average of several samples, the values are expressed as the mean±s.e.mean.
Results
Effect of P-CTX-1b on sodium currents
Rat myotubes in primary culture were selected for size to have small currents (see Methods) and voltage-clamped. In all six cells studied, within the first 10 min of P-CTX-1b (1 nM) treatment, there was a slight increase in peak sodium current and the tail current became more evident. Longer toxin exposures, enhanced the tail current, but the peak current decreased, and the inactivation phase of this smaller current was longer than in control conditions, indicating that sodium channels remain open during the test pulse. This pattern of action also occurred at higher toxin concentrations, but at shorter times of toxin exposure. Representative families of Na+ currents recorded in the absence and presence of 10 nM P-CTX-1b are shown in Figure 1. As shown in Figure 1b and b′, after 1 min of P-CTX-1b addition to the medium, there was practically no change in the net peak amplitude of fast transient (inward and outward) sodium currents. No changes were recorded either in the kinetic parameters for Na+ current activation and inactivation when fitted to the classical Hodgkin & Huxley (1952) model (m3h paradigm) (data not shown). At longer times of exposure (>10 min), there was a sustained current, more apparent at strong membrane depolarizations, as well as a pronounced tail current (Figure 1c). In the majority of these experiments, an important rise in the holding leakage current was observed after P-CTX-1b action, which usually attained values in the range 20–25 pA/pF, and its development was blocked by the presence of TTX (10 μM). This offset current is absent in the traces shown because the data acquisition protocol corrected the trace to zero current at the prepulse baseline. Concomitant to this increase in leakage current, the peak sodium current appeared significantly reduced. From all the data collected at 1, 5, 10 and 20 nM, the percentage of inhibition of the maximal Na+ peak current had a range 60–80% after at least 15 min of P-CTX-1b exposure. A conceivable explanation for this effect is that Na+ channels are kept permanently opened once they bind P-CTX-1b. Therefore, it is likely that the kinetics of the P-CTX-1b-modified Na+ channels would evolve from an increased open probability (immediately after toxin addition) to a permanently open state. As shown on the current-voltage (I-V) relation (Figure 1d), a 10 mV negative shift in the Na+ reversal potential was observed during the action of P-CTX-1b with respect to control data. This result implies that the concentration gradient for sodium across the membrane is reduced, and may reflect the action of permanently opened Na+ channels.
Figure 1.
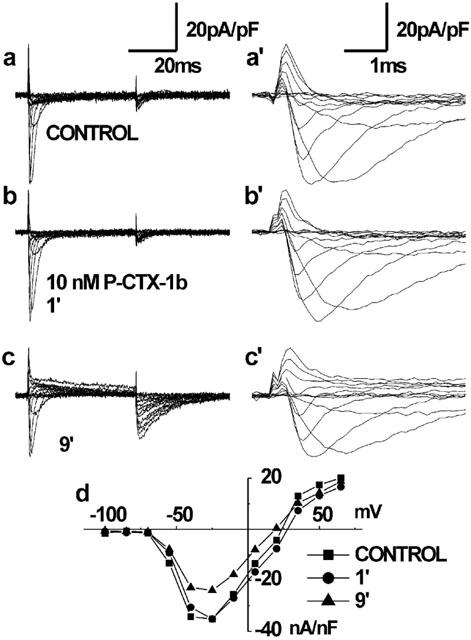
Effect of P-CTX-1b on sodium currents normalized by capacitance, obtained from a whole-cell recording in a small myotube (67 pF) with a reduced Na+ gradient. The pulse protocol corresponds to a sequence of voltage–clamp steps ranging from −100 to +65 mV at 15 mV increments from a holding potential of −90 mV. The external NaCl concentration was reduced to 36 mM and the remainder 109 mM was replaced by TEA-methanesulphonate. Control currents (a), and at a 1 min (b) and 9 min (c) after the addition of 10 nM P-CTX-1b to the bath. Panels a′, b′ and c′ show at an expanded time-base the same current traces shown in a, b and c. Note, in c and c′, the decrease (33%) in peak Na+ current after 9 min exposure to P-CTX-1b, as well as the sustained outward Na+ current and the large tail currents. Panel d summarizes the normalized peak Na+ current-voltage relationship obtained under control conditions, and after 1 and 9 min of P-CTX-1b exposure.
In order to determine whether TTX prevented the effect of P-CTX-1b on the Na+ current under normal concentration gradient conditions, experiments were performed in myotubes bathed in a nominally Ca2+-free standard external solution (with 145 mM Na+) containing 10 μM TTX. Under these quasi-physiological conditions, TTX addition to the bath, blocked Na+ currents by 97–99% (Figure 2a). When a local perfusion system delivered directly on top of the studied cell a TTX-free standard external solution, this blockade was almost immediately relieved, as depicted in Figure 2b. In the presence of TTX (no local perfusion), the addition of 4 nM P-CTX-1b to the bath had no significant effect on leakage currents (Figure 2c). However, as shown in Figure 2g, in the presence of P-CTX-1b, the peak K+ current was transiently decreased by about 10% in a voltage-dependent manner, and fully recovered once the cell was again perfused locally with the toxin-free saline, washing out TTX and eventually P-CTX-1b. While this toxin washout is taking place, a clear increase in peak Na+ currents was seen (Figure 2d,e), making evident a sustained effect of P-CTX-1b. This maintained action of P-CTX-1b on Na+ channels was accompanied by a 13 mV negative shift in the Na+ reversal potential (Figure 2f), indicative of an intracellular accumulation of Na+, transitory in nature, due to the whole cell recording configuration.
Figure 2.
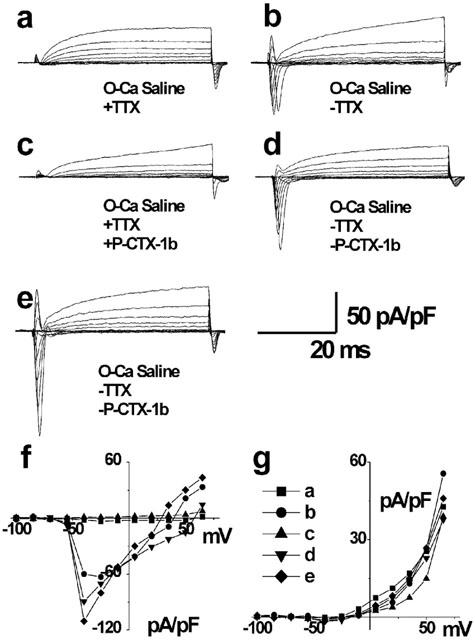
Effect of P-CTX-1b and TTX on a family of current traces obtained in a myotube with the whole-cell configuration of the patch–clamp technique. The external saline contained 145 mM Na+ and was nominally Ca2+-free, and the pipette medium contained 140 mM K+ and 2 mM ATP-Mg. Traces were normalized by the cell capacitance (85.5 pF). (a) Control traces in the presence of 10 μM TTX in the bath. (b) Washout of TTX action by the local perfusion of the cell with a TTX-free external saline. (c) Currents recorded 30 s after the addition of 4 nM P-CTX-1b in the presence of TTX. (d) Onset of the local washout of both TTX and P-CTX-1b. (e) Recordings obtained 2 min after the onset of the toxins washout. (f and g) I-V curves for the conditions presented in panels a–e, for the peak sodium current (f), and the potassium currents (g).
In addition, the present results suggest that TTX did not prevent the accessibility of P-CTX-1b to its binding site in the muscle Na+ channel protein, and that TTX was more easily reversible than P-CTX-1b upon removal.
Effect of P-CTX-1b on potassium currents
To explore the effect of P-CTX-1b upon K+ currents, rat myotubes were bathed in standard saline containing 10 μM TTX to block voltage-gated Na+ channels. The voltage clamp stimulation protocol used was a family of depolarizing voltage-steps (200 ms duration) that ranged from −100 to +65 mV separated by 15 mV increments, starting from a holding potential of −90 mV. A representative experiment is shown in Figure 3a,b. In this case, the addition of 5 nM P-CTX-1b to the bath caused an overall reduction close to 50% of the K+ currents. Control experiments (not shown) indicate that such reduction cannot be attributed to a K+ current rundown, because repeated test-pulse protocols under control conditions elicited the same amount of current, even after periods longer than 30 min. The blockade of the net K+ currents at this concentration (5 nM, Figure 3b), showed an increased inhibition with stronger depolarizations, this effect was also found at lower concentrations (2 nM, data not shown). This behaviour may indicate a voltage-dependency for the inhibitory effect of the toxin (see also Figure 3e). The fact that the tail current obtained after the addition of the toxin was not reduced (Figure 3c) in the same proportion also supports this notion. The comparison of superimposed normalized K+ current traces obtained at +35 mV, for control and in the presence of 5 nM P-CTX-1b, revealed no significant differences in their activation kinetics (Figure 3d). We have already mentioned that most frequently P-CTX-1b, in the absence of TTX, had an additional effect of increasing the membrane current leakage. The inhibitory effect of P-CTX-1b over K+ currents was observed whether or not an increased membrane current leakage was detected.
Figure 3.
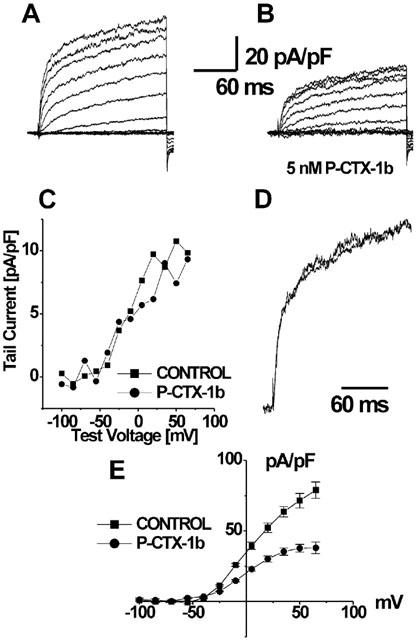
Effect of P-CTX-1b on potassium currents under whole-cell patch–clamp conditions. Panels a and b show the respective current traces before (a), and after addition of 5 nM P-CTX-1b to the external medium (b). The current has been normalized by the capacitance of the cell (46.7 pF) and 65% of the series resistance (12 MΩ) was compensated. Panel c depicts the averaged current amplitude of the last five points at the end of the recording, which corresponds to the recovery phase of the tail current at −90 mV. Panel d shows the superimposed traces at +35 mV, normalized with respect to their respective maximum values. The same result was obtained for all the traces; it is apparent that in these conditions, no major changes in the activation kinetics were present. Panel e shows the I–V relationship of the average current obtained from the last 5.8 ms of the test-pulse, before and after P-CTX-1b addition, each value is the mean±s.e.mean of data from four determinations. Notice that the blockade of K+ current is more pronounced at stronger membrane depolarization.
Effect of P-CTX-1b on myotubes under current-clamp conditions
With the reported effects of P-CTX-1b on sodium and potassium conductances, it can be expected an overall increased excitability of myotubes. To test this assumption, current–clamp experiments were carried out with the antibiotic-performated patch mode, to prevent the internal dialysis inherent to the whole-cell patch–clamp configuration. Membrane current injection through the patch electrode to myotubes elicited action potentials which had overshoots of 20–30 mV at a holding membrane potential of −90 mV, as shown in a typical recording obtained from one of four cells under the same conditions (Figure 4a). P-CTX-1b at 2 and 12 nM, when added to the external standard physiological bathing solution, decreased the threshold for action potential generation, but had little or no effect on the amplitude or time course of directly elicited action potentials (Figure 4b,c). In addition, spontaneous action potentials were recorded during the action of P-CTX-1b (12 nM) either following direct myotube stimulation (Figure 4d), or in the absence of stimulation (Figure 4e) in all four cells. This repetitive firing of the myotubes attained in general low frequencies (0.1–0.5 Hz). Furthermore, spontaneous transient membrane depolarization of 3–10 mV amplitude were always recorded in the presence of P-CTX-1b at different time intervals between the spontaneous action potentials (Figure 4e). About 50% of such spontaneous occurrences did not reach the threshold membrane potential for action potential generation. P-CTX-1b (2–12 nM) caused a moderate membrane depolarization of the myotubes (3–5 mV) during short times of exposure (<15 min). Very low frequency spontaneous transient membrane depolarization or spontaneous action potentials were detected under control conditions in just one of the four cells used in this series of experiments.
Figure 4.
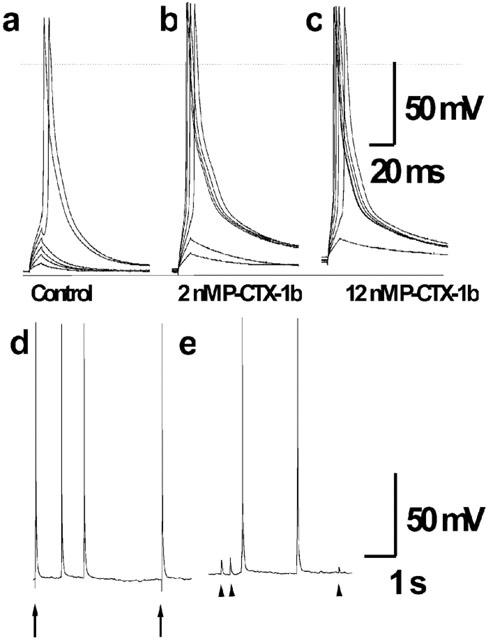
Effect of P-CTX-1b on action potentials recorded under current-clamp conditions (holding potential: −90 mV) in a cultured rat myotube using the nystatin perforated patch technique. Depolarizing current pulses of 10 ms duration and increasing intensity (in steps of 200 pA) were applied before (a), and after addition of 2 nM (b) or 10 nM P-CTX-1b (c) to the external physiological solution. Note, that using the same pulse-protocol (0.25 Hz) the threshold potential for action potential generation was attained in the fifth pulse during controls (a), and during the third and second pulse in the presence of 2 and 12 nM P-CTX-1b respectively (b and c). The dashed line indicates the zero membrane potential level, the continuous solid line indicates as reference the −95 mV membrane potential. Evoked (arrows) and repetitive spontaneous action potentials (d), and spontaneous transient membrane depolarization (arrow heads) and action potentials (e), recorded from the same myotube at a holding membrane potential of −90 mV after toxin addition.
Effect of P-CTX-1b on intracellular Ca2+ signals
Previous reports indicate that rat myotubes loaded with fluo-3 upon depolarization with high K+ external solutions, display two kinds of calcium transients: a fast one, which is associated with the contractile response, and a slower one which may last for several seconds and whose underlying function is not yet fully understood. Under confocal fluorescence microscopy and standard saline, or nominally calcium-free saline conditions, there is a detectable low rate of spontaneous activity (one out of four cells, data not shown). The addition of 10 nM P-CTX-1b to the saline induced not only an increase in the number of myotubes exhibiting fast Ca2+ transients (as was the case under current–clamp conditions), but also an increase in the frequency of such transients. Apart from the frequency, no differences in the fast Ca2+ transients were evident between control and P-CTX-1b-treated cells. In addition to these spike-associated changes during the action of P-CTX-1b (2–10 nM), some myotubes exhibited slower Ca2+ transients lasting several seconds in which the fluorescence was mainly localized in a limited and small oval area of the cytosol which corresponded to the myotube nuclei. Because these signals were recorded in the absence of external Ca2+, they represent the release of Ca2+ from internal stores. Figure 5a shows a sequence of selected confocal fluorescence images, from a series of images, captured at 1 s intervals after P-CTX-1b (10 nM) was added to the external bath. Figure 5b collects the fluorescence signal in arbitrary units of all the sequence taken from two selected regions of interest located in one of the nucleus and in the cytoplasm. After the initial rise in fluorescence associated with contraction, there is a second rise of calcium, which oscillates, most prominently inside one of the nuclei. As previously noted, in the presence of 10 μM TTX, no P-CTX-1b evoked calcium signals could be detected.
Figure 5.
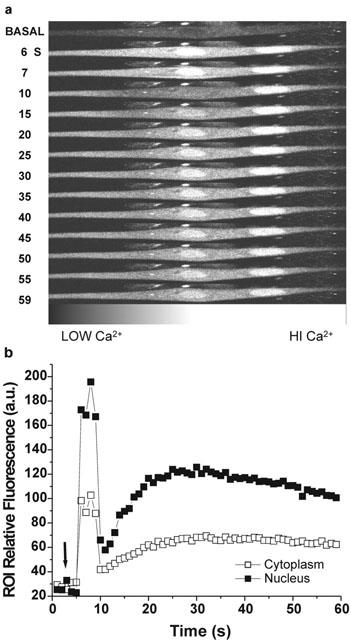
Fast and slow Ca2+ signals evoked by P-CTX-1b in a rat myotube loaded with the calcium indicator fluo-3. Selected series of fluorescence images (in grayscale) captured by confocal laser scanning microscopy before (basal), and at the times indicated after the addition of 10 nM P-CTX-1b to the medium (a). Time-course of the relative fluorescence changes for two regions of interest (25 by 25 pixels) one located in the nucleus and the other in the cytoplasm (b). The arrow indicates the moment at which P-CTX-1b was added to the bathing medium. Note that after the addition of P-CTX-1b there is a persistent increase in fluorescence at the nuclear region to values higher than those found at the cytosol. Note also that the fluorescence in the left nucleus oscillated after the first few seconds of toxin action.
Effect of P-CTX-1b on intracellular IP3 levels
It has been previously reported that calcium signals associated with cell nuclei are related to changes in the intracellular concentration of IP3 (Liberona et al., 1997; 1998; Jaimovich et al., 2000). Therefore, it was of interest to search for eventual changes in IP3 levels in the presence of P-CTX-1b.
The addition of P-CTX-1b (10 nM) to the incubation medium, resulted in a significant and transient increase in the total level of IP3 in the myotubes. As shown in Figure 6, after the addition of P-CTX-1b, the IP3 concentration increased nearly 3 fold, with a peak at 15 s and then returned, within 60 s, to values close to basal levels. This effect of P-CTX-1b on IP3 mass levels could be completely prevented by the pre-incubation myotubes with 1 μM TTX (Figure 6, inset), suggesting that changes in membrane excitability induced by P-CTX-1b may be mediating this action.
Figure 6.
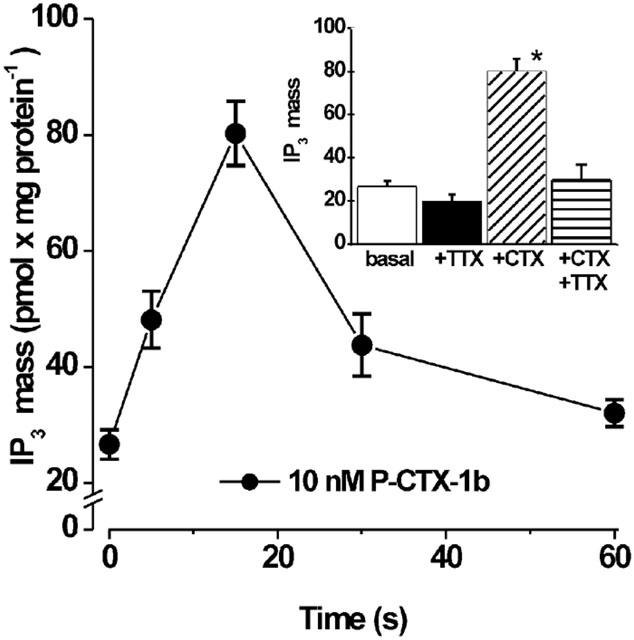
Effect of P-CTX-1b on IP3 mass levels in rat myotubes. Confluent plates of rat myotubes were washed three times with PBS, and incubated for the times indicated with 10 nM P-CTX-1b. The mass of IP3 in the extract once neutralized was measured by a radioligand receptor assay, as described in Methods. (Inset) Plates of rat myotubes were rinsed with PBS and incubated with 10 nM P-CTX-1b for 15 s in the absence or in the presence of 1 μM TTX. Each column represents the mean value±s.e.mean obtained from three to six different experiments done in triplicate. (*) denotes P<0.0001 Student's t-test.
Discussion
To the best of our knowledge this is the first description of the effects of P-CTX-1b, the main ciguatoxin involved in fish poisoning in the Pacific region, in primary cultures of rat skeletal muscle cells devoid of motor innervation. The effect of P-CTX-1b on Na+ channels in myotubes is totally compatible with the mechanism proposed for myelinated axons and neuronal cells, i.e. persistent activation of voltage-dependent sodium channels at the resting membrane potential by modification of the voltage-dependence of a fraction of the channels (Benoit et al., 1986; 1996; Hogg et al., 1998; Strachan et al., 1999). In those studies, Na+ currents under P-CTX-1b can be decomposed into an early component with unmodified kinetics and voltage dependence, and a late current component with different kinetics and a shift to the left in the voltage-dependence for activation.
In addition, other types of effects of P-CTX-1b were evident in rat myotubes. One of the effects is characterized by a small membrane depolarization, and a low to moderate increase in the frequency of spontaneous action potentials that never reached the high frequency levels (60–100 Hz) of those reported on myelinated axons (Benoit et al., 1986), motor nerve terminals (Molgó et al., 1990) or preganglionic terminals of sympathetic ganglia (Hamblin et al., 1995). This difference may be due to the fact that muscle cells have an overall higher chloride conductance (Kwiecinski et al., 1984) than nerve cells, which will act as a dampening factor to the depolarizing tendency of the P-CTX-1b persistent effect over Na+ channels. The effect of P-CTX-1b on Na+ currents could be explained by assuming that, as described for neural cells, only a fraction of the Na+ channels interact with the toxin, but unlike neural cells, they would become permanently open after a period of time in which an actual increase in peak Na+ current is apparent. Several features are consistent with this interpretation. In the electrophysiological recordings, the leakage current as well as both the steady-state current and the tail current should increase as a consequence of permanently opened channels, and the peak N+ current should decrease reflecting a reduced number of available unmodified Na+ channels. It remains to be established if there are different populations of Na+ channels with respect to their ability to bind P-CTX-1b. The notion that toxins may affect differentially distinct Na+ channel populations is not new; in muscle fibres several toxins affect selectively only a fraction of active Na+ channels; these phenomena have been described for both scorpion toxins (Jaimovich et al., 1982) and for TTX derivatives (Jaimovich et al., 1983). Pharmacological differences between Na+ channels from nerve and muscle cells have been described (Moczydlowski et al., 1986; Le Gall et al., 1999) and the effects described by us for P-CTX-1b also support such differences. If our interpretation is correct, the main difference between Na+ channels from nerve and muscle would reside in the kinetics of the Na+ current through channels modified by the toxin. Upon P-CTX-1b binding, neuronal Na+ channels with altered but functional gating mechanisms, will increase their open probability and produce a slow current, visible under voltage-clamp. On the other hand, muscle cell Na+ channels, upon binding P-CTX-1b will increase their open probability during several seconds, and then switch to a permanently open mode that will be detected as both a leakage current, and as an increase in both steady-state and tail currents. Increased Na+ leak currents, during P-CTX-1b action, were reflected as a moderate accumulation of Na+ near the membrane, that was detected by the negative shift in the Na+ reversal potential. Increased leakage Na+ current will probably trigger an increase of the Na+/Ca2+ exchange process in the sense of Ca2+ entry (or reduced Ca2+ extrusion), precluding both a major depolarization and a significant increase of the intracellular Na+ concentration.
P-CTX-1b also affected other voltage-dependent ion channels. Indeed, the K+ currents showed a manifest reduction in their amplitude without significant changes in their activation kinetics. The inhibitory effect over the K+ currents is consistent with the increased excitability found in myotubes under current–clamp experiments. This effect would place the membrane potential closer to the action potential threshold due to the absence of the hyperpolarizing nature of K+ conductances either at rest, or those voltage-dependent.
Another type of effect of P-CTX-1b was seen as both an increase of intracellular IP3 and a slow and transient rise of intracellular Ca2+ within the cell nuclei, closely coupled in time of occurrence. These two effects are likely to be part of the same phenomena, since slow, nucleoplasmic Ca2+ signals, associated with IP3 transients and nuclear IP3 receptors have already been described in cultured muscle cells (Liberona et al., 1998; Jaimovich et al., 2000; Powell et al., 2001). It is unlikely then, that one effect of P-CTX-1b is to increase IP3 concentration, and Ca2+ would be released from IP3-sensitive stores. The IP3 receptors are located, among other internal membranes, in the nuclear envelope of cultured muscle cells (Jaimovich et al., 2000; Powell et al., 2001). Calcium signalling to the nucleus has been proposed to be mediated by IP3 receptors in various cell systems (Stehno-Bittel et al., 1995; Lui et al., 1998) and to have a role in the control of gene expression (Karin & Hunter, 1995; Gallin & Greenberg, 1995; Cahill et al., 1996). There is already a study showing an effect of ciguatoxin on the expression of mRNA for the c-fos gene product in nerve cells (Peng et al., 1995). Whether a similar action occurs in muscle cells remains to be determined.
Another line of evidence has shown that the IP3 increase may be triggered by high K+-induced depolarization in cultured myotubes (Liberona et al., 1998; Jaimovich et al., 2000; Powell et al., 2001; Estrada et al., 2001). We have shown that P-CTX-1b also caused membrane depolarization. However, even if the change in membrane potential detected in our current–clamp experiments was much less pronounced as that reported for neuronal tissues, it may be significant enough to play a role in the increase of IP3 levels. The fact that TTX was capable of blocking the IP3-increase induced by P-CTX-1b strongly suggests that membrane depolarization as such is involved in its generation. Membrane depolarization (Molgó et al., 1990) or increased Ca2+ entry through the reversed activation of the Na+/Ca2+ exchange system, as described in cholinergic synaptosomes (Morot Gaudry et al., 1996) could contribute to trigger the IP3 increase.
In conclusion, P-CTX-1b besides interacting with the Na+ channels, as it does in other excitable tissues, it also interacts with potassium channels, the combined effect of which activates a calcium-release signal–transduction pathway in muscle cells, mediated by membrane depolarization and IP3. These effects suggest that P-CTX-1b may be a useful tool in further studies of the events involved in the excitation–transcription coupling in skeletal muscles.
Acknowledgments
We thank Dr Anne-Marie Legrand, Dr Mireille Chinain and the members of the Institut Territorial de Recherches Médicales Louis Malardé, Papeete, Tahiti, French Polynesia for kindly providing the Pacific ciguatoxin-1b used in the present study. We are indebted to Dr E Benoit for helpful discussions and careful reading of the manuscript. This work was made possible by an exchange program (ECOS Sud-CONICYT, C99B03) and was supported in part by grant no. 8980010 from FONDECYT (to E. Jaimovich) and by a grant from the Direction des Sistèmes de forces et de la Prospective (to J. Molgó).
Abbreviations
- IP3
inositol 1,4,5-trisphosphate
- P-CTX
Pacific ciguatoxin
- TEA
tetraethylammonium
- TTX
tetrodotoxin
References
- BADEN D.G. Brevetoxins: unique polyether dinoflagellate toxins. FASEB J. 1989;3:1807–1817. doi: 10.1096/fasebj.3.7.2565840. [DOI] [PubMed] [Google Scholar]
- BAGNIS R., KUBERSKI T., LAUGIER S. Clinical observations on 3009 cases of ciguatera (fish poisoning) in the South Pacific. Am. J. Trop. Med. Hyg. 1979;28:1067–1073. doi: 10.4269/ajtmh.1979.28.1067. [DOI] [PubMed] [Google Scholar]
- BENOIT E., JUZANS P., LEGRAND A.M., MOLGÓ J. Nodal swelling produced by ciguatoxin-induced selective activation of sodium channels in myelinated nerve fibers. Neuroscience. 1996;71:1121–1131. doi: 10.1016/0306-4522(95)00506-4. [DOI] [PubMed] [Google Scholar]
- BENOIT E., LEGRAND A.M., DUBOIS J.M. Effects of ciguatoxin on current and voltage clamped frog myelinated nerve fibre. Toxicon. 1986;24:357–364. doi: 10.1016/0041-0101(86)90195-9. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., MOUREY R.J., SNYDER S.H. A simple sensitive and specific radioreceptor assay for inositol 1,4,5-triphosphate in biologial tissues. Biochem. Biophys. Res. Commun. 1989;159:976–982. doi: 10.1016/0006-291x(89)92204-3. [DOI] [PubMed] [Google Scholar]
- CAHILL M., JANKNECHT R., NORDHEIM A. Signalling pathways: Jack of all cascades. Curr. Biol. 1996;6:16–19. doi: 10.1016/s0960-9822(02)00410-4. [DOI] [PubMed] [Google Scholar]
- DECHRAOUI M.Y., NAAR J., PAUILLAC S., LEGRAND A.M. Ciguatoxins and brevetoxins, neurotoxic polyether compounds active on sodium channels. Toxicon. 1999;37:125–143. doi: 10.1016/s0041-0101(98)00169-x. [DOI] [PubMed] [Google Scholar]
- ESTRADA M., CÁRDENAS C., LIBERONA J.L., CARRASCO M.A., MIGNERY G.A., ALLEN P.D., JAIMOVICH E. Calcium transients in 1B5 myotubes lacking ryanodine receptors are related to inositol trisphosphate receptors. J. Biol. Chem. 2001;276:22868–22874. doi: 10.1074/jbc.M100118200. [DOI] [PubMed] [Google Scholar]
- GALLIN W., GREENBERG M. Calcium regulation of gene expression in neurons: the mode of entry matters. Curr. Opin. Neurobiol. 1995;5:367–374. doi: 10.1016/0959-4388(95)80050-6. [DOI] [PubMed] [Google Scholar]
- HAMBLIN P.A., MCLACHLAN E.M., LEWIS R.J. Sub-nanomolar concentrations of ciguatoxin-1 excite preganglionic terminals in guinea pig sympathetic ganglia. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:236–246. doi: 10.1007/BF00176780. [DOI] [PubMed] [Google Scholar]
- HIDALGO J., NIEMEYER M.I., JAIMOVICH E. Voltage control of calcium transients elicited by caffeine and tetracaine in cultured rat muscle cells. Cell Calcium. 1995;18:140–154. doi: 10.1016/0143-4160(95)90005-5. [DOI] [PubMed] [Google Scholar]
- HODGKIN A.L., HUXLEY A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGG R.C., LEWIS R.J., ADAMS D.J. Ciguatoxin (CTX-1) modulates single tetrodotoxin-sensitive sodium channels in rat parasympathetic neurones. Neurosci. Lett. 1998;252:103–106. doi: 10.1016/s0304-3940(98)00575-8. [DOI] [PubMed] [Google Scholar]
- HORN R., MARTY A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAIMOVICH E., CHICHEPORTICHE R., LOMBET A., LAZDUNSKI M., ILDEFONSE M., ROUGIER O. Differences in the properties of Na+ channels in muscle surface and T-tubular membranes revealed by tetrodotoxin derivatives. Pflügers Arch. 1983;397:1–5. doi: 10.1007/BF00585159. [DOI] [PubMed] [Google Scholar]
- JAIMOVICH E., ILDEFONSE M., BARHANIN J., ROUGIER O., LAZDUNSKI M. Centruroides toxin, a selective blocker of surface Na+ channels in skeletal muscle: voltage-clamp analysis and biochemical characterisation of the receptor. Proc. Natl. Acad. Sci. U.S.A. 1982;79:3896–3900. doi: 10.1073/pnas.79.12.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAIMOVICH E., REYES R., LIBERONA J.L., POWELL J.A. IP3 receptors, IP3 transients and nucleus-associated calcium signals in cultured skeletal muscle. Amer. J. Physiol. 2000;278:C998–C1010. doi: 10.1152/ajpcell.2000.278.5.C998. [DOI] [PubMed] [Google Scholar]
- KARIN M., HUNTER T. Transcriptional control by protein phosphorylation signal transmission from the cell surface to the nucleus. Curr. Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- KWIECINSKI H., LEHMANN-HORN F., RUDEL R. The resting membrane parameters of human intercostal muscle at low, normal and high extracellular potassium. Muscle & Nerve. 1984;7:60–65. doi: 10.1002/mus.880070110. [DOI] [PubMed] [Google Scholar]
- LE GALL F., FAVREAU P., BENOIT E., MATTEI C., BOUET F., MENOU J.L., MENEZ A., LETOURNEUX Y., MOLGÓ J. A new conotoxin isolated from Conus Consors venom acting selectively on axons and motor nerve terminals through a Na+-dependent mechanism. Eur. J. Neurosci. 1999;11:3134–3142. doi: 10.1046/j.1460-9568.1999.00732.x. [DOI] [PubMed] [Google Scholar]
- LEGRAND A.M., LITAUDON M., GENTHON J.N., BAGNIS R., YASUMOTO T. Isolation and some properties of ciguatoxin. J. Appl. Phycol. 1989;1:183–188. [Google Scholar]
- LEWIS R.J., MOLGÓ J., ADAMS D.J.Ciguatera toxins: pharmacology of toxins involved in ciguatera and related fish poisonings Seafood and Freshwater Toxins 2000New York: Marcel Dekker; 419–447.Botana, L. (ed) pp [Google Scholar]
- LEWIS R.J., SELLIN M., POLI M.A., NORTON R.S., MACLEOD J.K., SHEIL M.M. Purification and characterisation of ciguatoxins from moray eel (Lycodontis javanicus, muraenidae) Toxicon. 1991;29:1115–1127. doi: 10.1016/0041-0101(91)90209-a. [DOI] [PubMed] [Google Scholar]
- LEWIS R.J., VERNOUX J.P., BRERETON M. Structure of Caribbean ciguatoxin isolated from Caranx latus. J. Am. Chem. Soc. 1998;120:5914–5920. [Google Scholar]
- LIBERONA J.L., CAVIEDES P., TASCON S., HIDALGO J., GIGLIO J.R., SAMPAIO S.V., CAVIEDES R., JAIMOVICH E. Expression of ion channels during differentiation of a human skeletal muscle cell line. J. Muscle Res. Cell. Motil. 1997;18:587–598. doi: 10.1023/a:1018671520294. [DOI] [PubMed] [Google Scholar]
- LIBERONA J.L., POWELL J.A., SHENOI S., PETHERBRIDGE L., CAVIEDES R., JAIMOVICH E. Differences in both inositol 1,4,5-trisphosphate mass and inositol 1,4,5-trisphosphate receptros between normal and dystrophic skeletal muscle cell lines. Muscle & Nerve. 1998;21:902–909. doi: 10.1002/(sici)1097-4598(199807)21:7<902::aid-mus8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- LOMBET A., BIDARD J.N., LAZDUNSKI M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987;219:355–359. doi: 10.1016/0014-5793(87)80252-1. [DOI] [PubMed] [Google Scholar]
- LUI P.P.Y., KONG S.K., FUNG K.P., LEE C.Y. The rise of nuclear and cytosolic Ca2+ can be uncoupled in HELA cells. Pflügers Arch. Eur. J. Physiol. 1998;436:371–376. doi: 10.1007/s004240050645. [DOI] [PubMed] [Google Scholar]
- MINTA A., KAO JPY, A., TSIEN R.Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- MOCZYDLOWSKI E., OLIVERA B.M., GRAY W.R., STRICHARTZ G.R. Discrimination of muscle and neuronal Na-channel subtypes by binding competition between [3H]saxitoxin and mu-conotoxins. Proc. Natl. Acad. Sci. U.S.A. 1986;83:5321–5325. doi: 10.1073/pnas.83.14.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLGÓ J., BENOIT E., COMELLA J.X., LEGRAND A.M.Ciguatoxin: a tool for research on sodium-dependent mechanisms Methods in Neuroscience, Neurotoxins 19928New York: Academic Press; 149–164.Conn, P.M. (ed) pp [Google Scholar]
- MOLGÓ J., COMELLA J.X., LEGRAND A.M. Ciguatoxin enhances quantal transmitter release from frog motor nerve terminals. Br. J. Pharmacol. 1990;99:695–700. doi: 10.1111/j.1476-5381.1990.tb12991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOROT-GAUDRY TALARMAIN Y., MOLGÓ J., MEUNIER F.A., MOULIAN N., LEGRAND A.M. Reversed mode Na+-Ca2+ exchange activated by ciguatoxin (CTX-1b) enhances acetylcholine from Torpedo cholinergic synaptosomes. Ann. N.Y. Acad. Sci. 1996;779:404–406. doi: 10.1111/j.1749-6632.1996.tb44814.x. [DOI] [PubMed] [Google Scholar]
- MURATA M., LEGRAND A.M., ISHIBASHI Y., FUKUI M., YASUMUTO T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990;112:4380–4386. [Google Scholar]
- PENG Y.G., TAYLOR T.B., FINCH R.E., MOELLER P.D., RAMSDELL J.S. Neuroexcitatory actions of ciguatoxin on brain regions associated with thermoregulation. NeuroReport. 1995;6:305–309. doi: 10.1097/00001756-199501000-00020. [DOI] [PubMed] [Google Scholar]
- POTTIER I., VERNOUX J.P., LEWIS R.J. Ciguatera fish poisoning in the Caribbean islands and Western Atlantic. Rev. Environ. Contam. Toxicol. 2001;168:99–141. doi: 10.1007/978-1-4613-0143-1_3. [DOI] [PubMed] [Google Scholar]
- POWELL J.A., CARRASCO M.A., ADAMS D.S., DROUET B., RIOS J., MULLER M., ESTRADA M., JAIMOVICH E. IP3 receptor function and localization in myotubes: an unexplored Ca2+ signaling pathway in skeletal muscle. J. Cell Sci. 2001;114:3673–3683. doi: 10.1242/jcs.114.20.3673. [DOI] [PubMed] [Google Scholar]
- SATAKE M., FUKUI M., LEGRAND A.M., CRUCHET P., YASUMOTO T. Isolation and structures of new ciguatoxin analogs, 2,3-dihydroxyCTX3C and 51-hydroxyCTX3C, accumulated in tropical reef fish. Tetrahedron Lett. 1998;39:1197–1198. [Google Scholar]
- STEHNO-BITTEL L., LUCKHOFF A., & CLAPHAM D.E. Calcium release from the nucleus by InsP3. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- STRACHAN L.C., LEWIS R.J., NICHOLSON G.M. Differential actions of Pacific ciguatoxin-1 on sodium channel subtypes in mammalian sensory neurons. J. Pharmacol. Exper. Ther. 1999;288:379–388. [PubMed] [Google Scholar]
- YASUMOTO T., MURATA M. Marine toxins. Chem. Rev. 1993;93:1897–1909. [Google Scholar]


