Abstract
Matrix metalloproteinase-2 (MMP-2) released during activation of human platelets by aggregating agents and cancer cells is known to stimulate platelet aggregation.
The expression, activity and role of tissue inhibitors of metalloproteinases (TIMPs), natural inhibitors of MMPs, in isolated human platelets were investigated.
Western blot, reverse zymography, immunogold electron microscopy, aggregometry (collagen-, thrombin and HT-1080 human fibrosarcoma cells-induced aggregation), flow cytometry and the release of 14C-serotonin from labelled platelets recruited to the aggregate were used to characterize the presence and function of platelet TIMPs.
TIMP-4 (23 kDa) has been identified as the major MMP inhibitor (12–16 ng per 108 platelets) in human platelets. Platelets expressed lower (<1 ng per 108 platelets) amounts of TIMP-1. No other TIMPs were detected using Western blot analysis.
TIMP-4 co-localized with MMP-2 in resting platelets and was released upon platelet aggregation induced by collagen and thrombin.
Collagen resulted also in the release of higher molecular weight (60 kDa) complexes of TIMP-4.
The release of TIMP-4 was reduced by prostacyclin and S-nitroso-glutathione (GSNO), an NO donor.
Human recombinant TIMP-4 (rTIMP-4), but not human rTIMP-1, inhibited partially both platelet aggregation and recruitment.
The recombinant TIMP-4 potentiated the recruitment inhibitor effects of GSNO.
TIMP-4 was not released during platelet aggregation induced by HT-1080 cells.
Human rTIMP-4 exerted a biphasic effect on HT-1080 cells-induced aggregation.
Thus, TIMP-4 is the major intraplatelet MMP inhibitor and it is involved in regulation of platelet aggregation and recruitment.
Keywords: Matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, platelets, aggregation, platelet recruitment
Introduction
Matrix metalloproteinases (MMPs), a family of matrix-degrading enzymes (Sternlicht & Werb, 2001), regulate the turnover of the extracellular matrix. Recent evidence suggests that in addition to long-lasting remodelling, MMPs may influence acute cellular reactions including regulation of platelet function (Sawicki et al., 1997; 1998; Fernandez-Patron et al., 1999a; Cheung et al., 2000a; Kazes et al., 2000; Galt et al., 2002; Jurasz et al., 2002), vessel wall reactivity (Fernandez-Patron et al., 1999b,c; 2000a, b), cardiac ischaemia-reperfusion injury (Cheung et al., 2000b) and regulation of chemoattractant properties of monocytes (McQuibban et al., 2000). Indeed, we and others have identified MMP-2 in human platelets, and have shown that platelet aggregation stimulated by aggregating agents such as collagen and thrombin leads to the release of platelet MMP-2 (Sawicki et al., 1997; 1998; Fernandez-Patron et al., 1999a; Kazes et al., 2000). This release mediates a non-ADP, non-thromboxane pathway of platelet aggregation. In contrast to MMP-2, MMP-9 serves as an inhibitor of platelet aggregation (Fernandez-Patron et al., 1999a).
In addition to platelet aggregation induced by classical agonists such as collagen and thrombin, we have recently found that MMP-2, when released from platelets, contributes to tumour cell-induced platelet aggregation (TCIPA) (Jurasz et al., 2001a). The process of TCIPA plays an important role in hematogenous spread of cancer facilitating cancer cell embolization in the microvasculature and formation of metastasis (Trousseau, 1865; Gasic et al., 1968; Mehta, 1984; Radomski et al., 1991; Honn et al., 1992; Jurasz et al., 2001b).
Four endogenous tissue inhibitors of metalloproteinases (TIMP-1, TIMP-2, TIMP-3 and TIMP-4) control the activity of MMPs and affect matrix breakdown under physiological and pathological conditions (Brew et al., 2000). It has been also shown that pharmacological administration of human recombinant TIMP-2 (rTIMP-2) inhibits platelet aggregation (Sawicki et al., 1997; Kazes et al., 2000).
For these reasons, we have studied protein expression, regulation and function of TIMPs in human platelets.
Methods
Cells
Human washed platelets (2.5×108 platelets ml−1) were isolated from blood of healthy volunteers (Radomski & Moncada, 1983). Human fibrosarcoma cells HT-1080 (American Type Culture Collection, Rockville, U.S.A.) were grown in culture as previously described (Jurasz et al., 2001a, b). The cells were harvested and suspended in Tyrode's solution (107 cells ml−1).
Platelet aggregation and recruitment
Platelet aggregation was measured in a whole blood ionized calcium lumi aggregometer (Chronolog, Havertown, U.S.A.) following stimulation with collagen (0.5–10 μg ml−1), thrombin (0.01–0.1 u ml−1) or HT-1080 cells (104 cells ml−1) (Sawicki et al., 1997; 1998; Jurasz et al., 2001a, b). To study the effects of TIMPs, S-nitroso-glutathione (GSNO) and prostacyclin (PGI2), platelets were pre-incubated with these agents for 1 min prior to addition of maximally effective concentrations of agonists. Aggregation was then monitored for 9 min and analysed using Aggo-Link data reduction system (Chronolog).
Platelet recruitment was measured by following 14C-serotonin release from the second recruitable population of platelets that were added to collagen-stimulated platelet samples (Freedman et al., 1999). Briefly, platelets in platelet-rich plasma were incubated with 14C-serotonin (1 μM, Amersham, Baie d'Urfe, Canada) for 20 min at room temperature (Holmsen & Dangelmeier, 1989). Under these conditions >95% of 14C-serotonin was incorporated into platelets. The samples of unlabelled platelets (2.5×108 ml−1) were placed in the aggregometer, in the presence of 2 μM imipramin, and stimulated with collagen (10 μg ml−1). After 1 min aggregation 14C-labelled platelets (2.5×107 platelets ml l−1) were added and the reaction followed for 3 min. The release of 14C-serotonin was arrested by ice-cold formaldehyde in 50 mM EDTA. The samples were then centrifuged (10,000×g for 3 min at room temperature) and the 14C-bound radioactivity was measured in the supernatant. To study the effects of TIMPs and NO, human rTIMP-1 (1–100 ng ml−1), rTIMP-4 (1–100 ng ml−1), control IgG (100 ng ml−1) and GSNO (0.1 μM) were preincubated for 1 min prior to the addition of collagen. The results were expressed as percentage release of 14C-serotonin from platelets.
In some experiments, platelet samples prior and following aggregation were centrifuged at 3200×g for 10 min at room temperature and the resultant pellet and platelet releasate used for Western blotting, reverse zymography, flow cytometry and immunogold electron microscopy.
Western blotting
Immunoblot analysis was performed as previously described (Sawicki et al., 1997; 1998; Mayers et al., 2001). Briefly, to study TIMP-1, TIMP-2, TIMP-3 and TIMP-4 expression in unstimulated platelets, the samples were homogenized in homogenization buffer (containing 320 mM sucrose, 10 mM HEPES, 0.1 mM EDTA, 1 mM DL-dithiothreitol, 10 μg ml−1 leupeptin, 10 μg ml−1 soybean trypsin inhibitor and 2 μg ml−1 aprotinin) sonicated, centrifuged at 10,000×g for 20 min at 4°C and the resultant supernatants (65 μg protein per lane) were subjected to 12% SDS–PAGE. To study TIMP-4 during TCIPA, 10 μg of protein was loaded per lane.
To study TIMP-4 release during platelet aggregation both aggregated platelets and releasates were used. The pellets of aggregated platelets were sonicated, centrifuged (10,000×g for 20 min at 4°C) and the resultant supernatant was diluted 10 times. Ten microliters of samples were loaded per lane. Following electrophoresis and transfer of samples onto PVDF membranes (Bio-Rad, Hercules, U.S.A.), the blots were blocked overnight in blocking buffer and then incubated with the primary antibodies (0.1–0.2 μg ml−1). The following antibodies were used: rabbit polyclonal anti-TIMP-1 and mouse monoclonal anti-TIMP-2 were obtained from Oncogene, Cambridge, U.S.A., while rabbit polyclonal anti-TIMP-3 and anti-TIMP-4 antibodies were from Chemicon, Temecula, U.S.A. The immunoreactive bands were visualized with ECL kit. The intensity of bands was measured by densitometry. Human recombinant TIMP standards (except human rTIMP-4 standard that was prepared as described by Bigg et al., 1997) were obtained from respective antibody manufacturers.
Reverse zymography
This was performed as described by Oliver et al. (1997). Briefly, 12% separating SDS gels were copolymerized with 2 mg ml−1 gelatin containing 1% SDS and pro MMP-2 (160 ng ml−1) (Chemicon). The samples of platelet homogenate (21.5 μg protein) releasate (10 μl), and human recombinant TIMP-1, TIMP-2, TIMP-3, and TIMP-4 standards (0.5–5.0 ng) were loaded and subjected to electrophoresis. TIMPs were identified by inhibition of gelatinolysis when compared with standards.
Flow cytometry
Flow cytometry analysis of TIMP-4 was performed essentially as described by Jurasz et al. (2001b). Briefly, platelets were incubated at room temperature in the presence or absence of 3 μg ml−1 collagen for 5 min. To minimize the presence of aggregates in samples, platelets were diluted 10 fold using PBS. Following, platelet samples were incubated with sheep anti-human TIMP-4 antibodies (Oncogene) or sheep IgG as control for 15 min. Next, samples were incubated with FITC-labelled donkey anti-sheep antibodies for 15 min. Flow cytometry was performed using a Becton Dickinson flow cytometer (FACSCalibur, New Jersey, U.S.A.) equipped with a 488 nm wavelength argon laser and with Cell Quest software (Becton Dickinson). Platelets were identified by forward and side scatter signals. Ten thousand platelet specific events were initially analysed by the cytometer, and activated and non-activated platelets were gated so as not to analyse platelet aggregates and microparticles. The gates were then analysed for mean fluorescence. Subsequently, platelet-anti-TMP-4 or platelet-IgG samples were subtracted from their corresponding samples containing FITC-labelled antibodies to account for autofluorescence. Furthermore, platelet-sheep IgG-FITC labelled samples were subtracted from platelet-anti-TIMP-4-FITC labelled samples to account for non-specific binding.
Immunogold electron microscopy
Platelets were fixed and sections prepared as previously described (Sawicki et al., 1998). Briefly, platelets were fixed in a mixture of 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 for 30 min at 21°C. Samples were then embedded in LR gold resin (Polysciences Inc, Warrington, U.S.A.) and thin sections were cut and mounted on uncoated nickel grids. For cytochemical staining the following antibodies were used: monoclonal anti-MMP-2, (Oncogene), polyclonal anti-TIMP-4 (Chemicon) and polyclonal anti MMP-9 antibodies (Fernandez-Patron et al., 1999a). Following double labelling (anti-MMP-2 or anti-MMP-9, and anti-TIMP-4 antibodies, each diluted 1 : 20 v v−1), the samples were treated with mouse (MMP-2 immunodetection) and rabbit (TIMP-4 and MMP-9 immunodetection) anti-IgG-gold (5 and 15 nm, particle size). In control experiments the primary antibodies were omitted. Platelets were examined with a Philips 300 electron microscope.
Statistical analysis
Statistics were performed using Graph Pad Software Prism 3.0 (San Diego, U.S.A.). All means are reported with standard deviation. One-way analysis of variance (ANOVA) and repeated measures ANOVA were performed where appropriate, and a P-value of less than 0.05 was considered as significant.
Reagents
Collagen and thrombin were obtained from Chronolog. If not otherwise indicated all remaining reagents were purchased from Sigma (Oakville, Canada)
Results
TIMPs in resting platelets
In resting platelets, Western blot analysis using specific anti-TIMP-4 antibodies revealed the presence of major TIMP-4 immunoreactive protein migrating at 23 kDa (Figure 1a). A weak TIMP-4-related immunoreactivity band migrating at 29 kDa was also detected. The amounts of platelet TIMP-4, as calculated using TIMP-4 standard (Figure 1b), were in the range of 12–16 ng per 108 platelets (n=6). In addition to TIMP-4, a 28 kDa immunoreactivity band corresponding to TIMP-1 (Figure 1c) was identified; however, TIMP-1 immunoreactivity in platelets was lower than that of TIMP-4 (<1 ng per 108 platelets). Under experimental conditions used, no TIMP-2 or TIMP-3-related immunoreactivity was found in platelets (data not shown, n=6).
Figure 1.
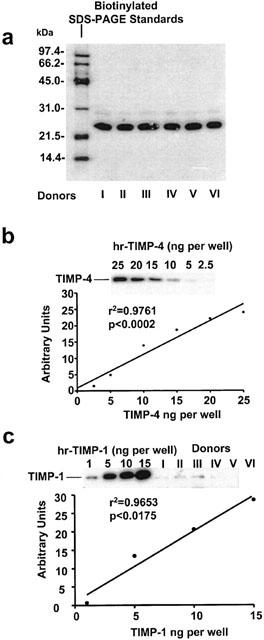
Western blot analysis of TIMPs in resting human platelets. (A and B) show the identification and quantification of TIMP-4, while (C) those of TIMP-1. Molecular weight of TIMP-4 immunoreactivity (23 kDa) was determined using biotinylated SDS–PAGE standards. The amounts of TIMP-4 and TIMP-1 in platelets were measured from regression analysis of TIMP standards.
Release of TIMP-4 during platelet aggregation
Reverse zymography confirmed the presence of TIMP-4 in platelets and showed a single activity band corresponding to TIMP-4 in the releasate of platelets aggregated with collagen (Figure 2a).
Figure 2.
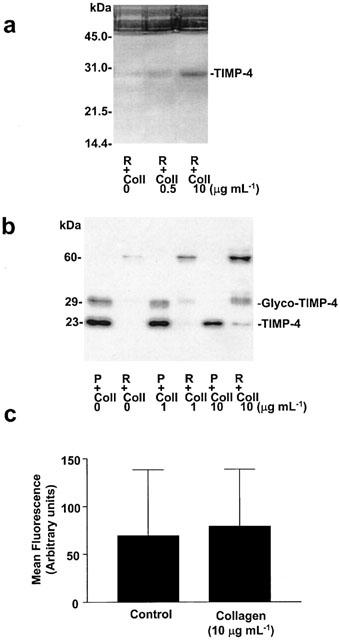
The release of TIMP-4 from platelets aggregated with collagen. Reverse zymography of platelet releasates (A) showing single band of inhibition of MMP-2-induced gelatinolysis. Western blot showing TIMP-4-related immunoreactivity in platelets and platelet releasates (B). Platelet aggregation led to reduction of intraplatelet TIMP-4 and the release of TIMP-4 immunoreactivity migrating at 60 kDa. (C) Flow cytometry analysis of TIMP-4 on platelet surface membranes. Neither resting nor collagen-aggregated platelets showed TIMP-4-related immunoreactivity on the surface. P: platelet pellets, R: platelet releasates and Coll: collagen. Representative reverse zymography and Western gels from four similar experiments. Data shown on C are mean±s.d., n=3.
In addition, collagen-induced platelet aggregation led to a reduction of intraplatelet content of TIMP-4 with the corresponding liberation of this inhibitor into platelet releasate, as shown by Western blot (Figure 2b). The effects of collagen were concentration-dependent. We also found in the releasate a TIMP-4-related immunoreactivity band, migrating at approximately 60 kDa. The presence of this band was increased by platelet aggregation.
In contrast, TIMP-4 could not be detected on the platelet surface using flow cytometry (Figure 2c).
The release of TIMP-4 during platelet aggregation induced by collagen (1 and 10 μg ml−1) was inhibited by PGI2 (0.6–30 nM) and GSNO (0.1–3 μM) (Figure 3a,b).
Figure 3.
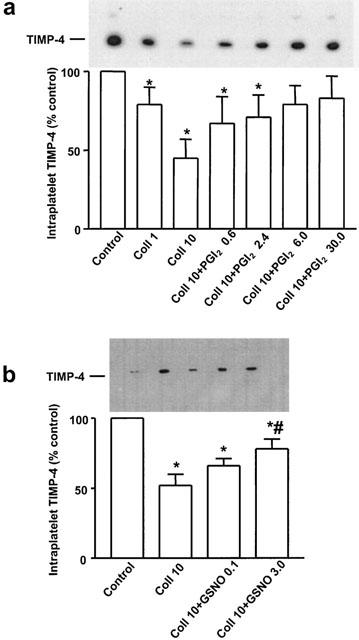
Regulation of collagen-stimulated TIMP-4 release from platelets by PGI2 and NO. Western blot (densitometry and representative insert) showing inhibition of TIMP-4 release by PGI2 (A) and GSNO (B). Data are mean±s.d., n=6. *P<0.05 treatments versus control. #P<0.05 Coll 10 versus Coll 10+GSNO 3. Control: unstimulated platelets; Coll: μg ml−1, PGI2 (nM) and GSNO (μM).
Immunolocalization of TIMP-4, MMP-2 and MMP-9 in platelets
Immunogold electron microscopy of TIMP-4 and MMP-2 demonstrated that these two proteins closely co-localized in resting platelets (Figure 4a). In contrast to MMP-2 that readily localizes to the platelet surface membranes during collagen-induced aggregation (Sawicki et al., 1998), the surface localization of TIMP-4 upon aggregation was not detectable. Co-localization of TIMP-4 with MMP-9 was less apparent (Figure 4b). TIMP-1, TIMP-2 and TIMP-3 were not detected in platelets using this method (data not shown). Control experiments did not show any non-specific immunogold reactivity in platelets (Figure 4a, upper inset).
Figure 4.
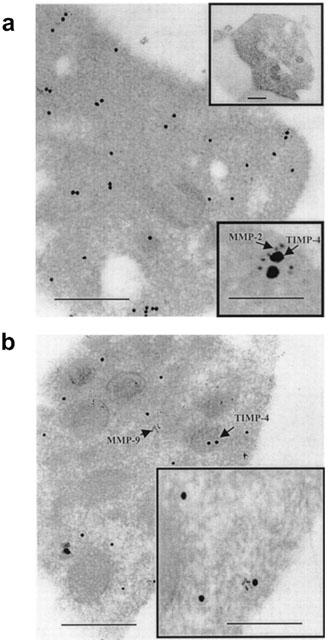
Immunogold electron microscopy examination of TIMP-4, MMP-2 and MMP-9 in non-aggregated platelets. Large gold particles (15 nm) label TIMP-4, while small particles (5 nm) label MMP-2 (A) or MMP-9 (B). TIMP-4 and MMP-2 co-localize as shown by arrows (A, lower inset). No IgG-related immunoreactivity was detected in control experiment (A, upper inset). Co-localization of TIMP-4 and MMP-9 is less apparent. Bar: 0.5 μm, inset bar: 0.1 μm.
Effects of TIMP-4 and TIMP-1 on platelet aggregation and recruitment
Human rTIMP-4 inhibited platelet aggregation induced by collagen (5 μg ml−1) and thrombin (0.1 u ml−1) (Figure 5a,b) with maximal effect at 10 ng ml−1 (Figure 5a). In contrast, collagen-induced platelet aggregation was not inhibited by rTIMP-1 at concentrations up to 100 ng ml−1 (Figure 5c).
Figure 5.
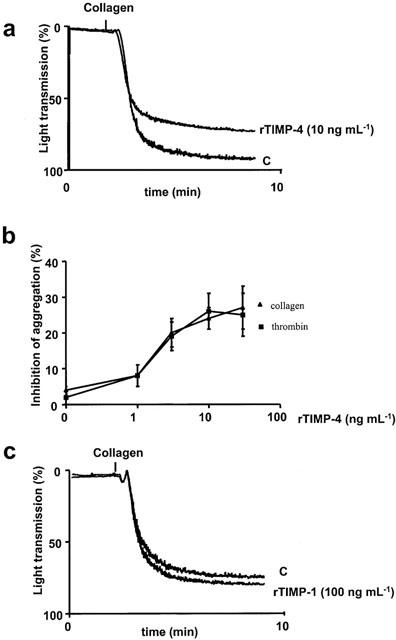
Differential effects of TIMP-4 and TIMP-1 on platelet aggregation. (A and B) show inhibition of aggregation induced by collagen and thrombin. (C) shows that TIMP-1 exerted no significant effects on collagen-induced aggregation. (A and C) superimposed representative traces of four similar experiments. Data in (B) are mean±s.d., n=4.
Prostacyclin (10 nM) was a strong inhibitor of platelet recruitment to the aggregate (Figure 6a). Human rTIMP-4 (1–10 ng ml−1), but not control IgG, significantly reduced collagen-stimulated platelet recruitment; however, its effects were weaker when compared to PGI2. Moreover, the effects of higher concentrations of rTIMP-4 (>30 ng ml−1) were less pronounced than lower (1–10 ng ml−1). Human rTIMP-1 did not exert any significant effects on recruitment (Figure 6a).
Figure 6.
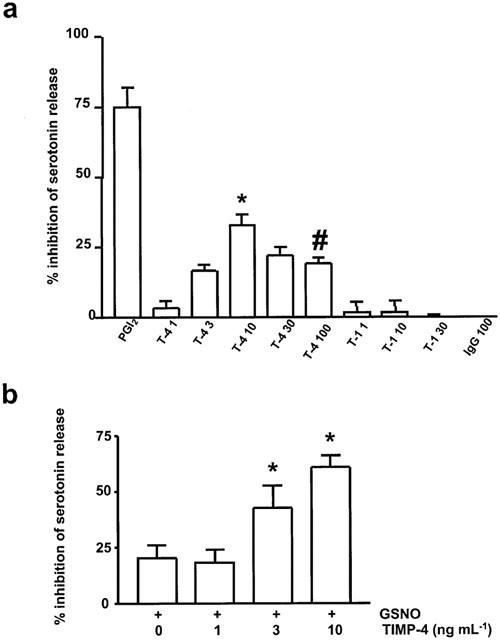
Inhibition of the secondary platelet recruitment by rTIMP-4 and its interactions with NO. (A) shows inhibition of platelet recruitment by rTIMP-4, but not rTIMP-1. (B) shows potentiation of recruitment-inhibitor effects of GSNO by TIMP-4. Human rTIMP-4 (T-4, ng ml−1), rTIMP-1 (T-1, ng ml−1), PGI2: 10 nM, GSNO: 0.1 μM, IgG: 100 ng ml−1. (A) *P<0.01 T-4 10 versus PGI2; #P<0.05 T-4 100 versus T-4 10. (B) *P<0.05 rTIMP-4+GSNO versus GSNO.
The recruitment-inhibitor effects of rTIMP-4 were potentiated by GSNO (Figure 6b).
Effects of TIMP-4 on tumour cell-induced platelet aggregation
Human fibrosarcoma HT-1080 cells expressed TIMP-4 and released small amounts of this protein during incubation (Figure 7a). TIMP-4 was not released during platelet aggregation induced by HT-1080 cells (Figure 7a). Human rTIMP-4 inhibited HT-1080-induced platelet aggregation (Figure 7b). This effect was biphasic and detected at lower (5 and 50 ng ml−1), but not higher (500 ng ml−1) concentrations of rTIMP-4 (Figure 7b).
Figure 7.
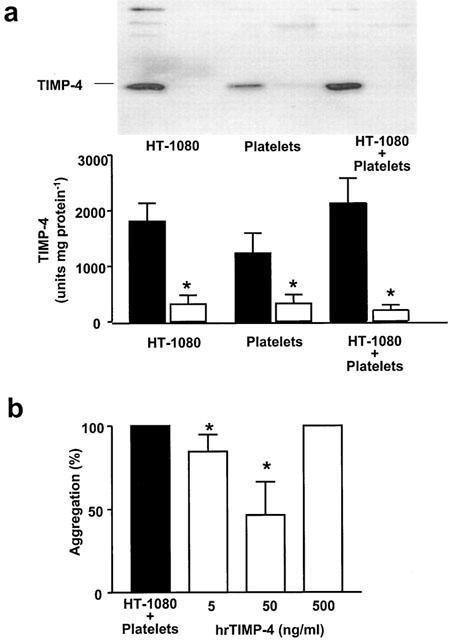
TIMP-4 in tumour cell-induced platelet aggregation. (A) shows Western blot analysis of homogenates of TIMP-4 content in HT-1080 cells and platelets (closed bars) and their respective releasates (open bars) during tumour cell-induced platelet aggregation. Inset shows a representative blot. The results indicate that TIMP-4 was not released during platelet aggregation by HT-1080 cells. Data are mean±s.d., n=4. A biphasic effect of rTIMP-4 on aggregation induced by HT-1080 cells is shown in B. Data are mean±s.d., n=4. *P<0.05 treatments versus control.
Discussion
Cooper et al. (1985) first reported that purified human platelets contain a collagenase inhibitor that appeared to be identical to that produced by human skin fibroblasts. They speculated that this inhibitor might be a significant modulator of collagenase activity following acute tissue injury. However, the molecular identity of this inhibitor was not elucidated. More recently, Murate et al. (1997) found a correlation between platelet count and the levels of TIMP-1 and TIMP-2 in human serum from patients with disorders associated with alterations of platelet levels. In addition, immunohistochemistry of megakaryocytes showed TIMP-1 and TIMP-2 staining in these cells. Moreover, another study found the expression of TIMP-2 mRNA transcripts in isolated human platelets, however, the authors could not detect any TIMP-2 immunoreactivity using Western blot analysis (Kazes et al., 2000).
We have used Western immunoblots, immunogold electron microscopy, and reverse zymography to study the expression of TIMP proteins and their activities in isolated human platelets. Using Western blot we could not detect a significant immunoreactivity of TIMP-2 or TIMP-3 in platelets. In contrast, we found small, but detectable, expression of TIMP-1 (<1 ng per 108 platelets) and a significant expression of TIMP-4 (>10 ng per 108 platelets). These results were subsequently confirmed by immunogold electron microscopy and using reverse zymography that showed a single band of TIMP activity co-migrating with TIMP-4 standard. These data suggest that TIMP-4 is the major TIMP in human platelets.
The TIMP-4 gene was first identified using the expressed sequence tag sequencing approach (Greene et al., 1996). This gene was localized to human chromosome 3p25 (Olson et al., 1998). The analysis of distribution of TIMP-4 cDNA in mice showed the presence of transcripts in various murine tissues including brain, heart, ovary and skeletal muscle (Leco et al., 1997). Human recombinant TIMP-4 inhibits a number of MMPs including MMP-1, MMP-2, MMP-3, MMP-7, MMP-9 and MMP-14 (Liu et al., 1997; Bigg et al., 1997; 2001) and there is evidence for specific, high affinity binding of TIMP-4 to the COOH-terminal hemopexin-like domain of human MMP-2 (Bigg et al., 1997; 2001).
Using immunogold electron microscopy, we have previously shown that MMP-2 is randomly distributed in platelets without apparent association with granules (Sawicki et al., 1998). This study showed that in non-stimulated platelets TIMP-4 co-localizes with MMP-2 in platelet cytoplasm suggesting that TIMP-4 is in complex with MMP-2. Collagen-induced platelet aggregation led to a reduction of intraplatelet levels of TIMP-4 due to its liberation to the platelet releasate. The release was clearly associated with aggregation since this was inhibited by prostacyclin and GSNO, potent inhibitors of this process (Moncada et al., 1976; Radomski et al., 1992). Interestingly, we could not detect the presence of TIMP-4 on the platelet surface during aggregation, as shown by flow cytometry. This is in contrast to MMP-2 release, which leads to the association of this protein with the platelet surface (Sawicki et al., 1997). We suggest that the dissociation of TIMP-4 from its complex with MMP-2 may facilitate the interactions of MMP-2 with platelet integrin GPIb and GPIIb/IIIa (Radomski et al., 2001; Martinez et al., 2001) and stimulate aggregation.
In addition to inhibition of MMPs activity, TIMP-4 appears to be involved in regulation of activation of latent proMMPs to MMPs by inhibiting MT1-MMP-stimulated activation of MMP-2 (Bigg et al., 2001; Hernandez-Barrantes et al., 2001). This is in contrast to TIMP-2 that is known to form a trimolecular complex with cell surface MT1-MMP and proMMP-2, thus activating the latent enzyme (Sato & Saiki, 1996). Recently, Kazes et al. (2000) identified MT1-MMP in human platelet membranes. In addition, we have previously shown that the activated, but not latent, MMP-2 aggregates platelets (Sawicki et al., 1997). However, since we did not identify TIMP-2 in platelets and TIMP-4 appears not to be associated with platelet surface during platelet aggregation in vitro, it is likely that activation of proMMP-2 is not controlled by TIMP-2 under these conditions. Interestingly, Morrison et al. (2001) has recently shown that MMP-2 could be activated by MT2-MMP via a TIMP-2-independent pathway. This, of course, does not preclude a possibility that TIMP-2 and TIMP-4 play an important role in regulation of MMP-2 activation in vivo since both TIMPs are readily detected in the vasculature (Galis et al., 1997; Dollery et al., 1999; Kranzhofer et al., 1999). Finally, we found that the release of TIMP-4 during collagen-induced platelet aggregation was associated with increased formation of TIMP-4-related immunoreactivity bands migrating at approximately 60 kDa. The nature of these bands is currently under investigation.
In addition to agonist-induced release of TIMP-4 from platelets, we have also investigated the effects of tumour cells HT-1080 on the release of this inhibitor during TCIPA. Interestingly, TCIPA was not associated with the release of TIMP-4. This is likely to leave the proaggregatory effects of MMP-2 released from cancer cells largely unopposed, thus promoting formation of tumour cell-platelet thrombi and metastasis (Jurasz et al., 2001a, b).
The results of previous experiments showed that human recombinant TIMP-2 is an effective pharmacological agent that reduced platelet aggregation stimulated by aggregating agents (Sawicki et al., 1997; Kazes et al., 2000). In contrast, TIMP-1 appears to be devoid of ability to inhibit platelet activation. TIMP-4 exerted significant inhibitory effects of collagen- and thrombin-induced platelet aggregation, although its effectiveness was lower than that of TIMP-2. Moroever, TIMP-4 also decreased the secondary platelet recruitment to the aggregate. Interestingly, the effects of TIMP-4 on TCIPA and the secondary platelet recruitment were biphasic: lower concentrations inhibited, while higher were less effective in inhibiting platelet activation. A number of studies have evidenced that TIMPs, including TIMP-4, have also biological actions that are independent of their MMP-inhibitory effects (Sang, 1998; Tummalapalli et al., 2001) including growth modulating and apoptosis-regulating properties. Therefore, the net pharmacological and probably biological effects of TIMPs on platelets may depend on the balance between the MMP-inhibitory and MMP-unrelated effects.
We have previously shown that inhibition of MMP activity with phenanthroline, a synthetic inhibitor of MMP activity, greatly enhances the platelet adhesion-inhibitor effects of NO (Martinez et al., 2001). Similar interactions have been detected between rTIMP-4 and GSNO in relation to their ability to reduce the secondary platelet recruitment to the aggregate. Thus, the simultaneous administration of NO donors and MMP inhibitors may potentially provide a powerful pharmacological strategy to limit vascular disease-induced platelet activation and thrombosis.
In conclusion, our findings are of significance for vascular haemostasis and disease. Under physiological conditions TIMP-4 appears to be the major intraplatelet TIMP controlling the activity of MMP-2-dependent pathway of aggregation. Interestingly, the expression of TIMP-4 in the vascular wall may control the rate of smooth muscle migration and collagen accumulation during experimental rat balloon angioplasty restenosis (Dollery et al., 1999). We postulate that, similar to the heart (Mayers et al., 2001), in platelets, an imbalance between MMP-2 and TIMP-4 activities may contribute to thrombotic complications of vascular disorders, as well as to hematogenous cancer metastasis.
Acknowledgments
This work was supported by a grant (MOP-14074) from the Canadian Institutes of Health Research (CIHR). Paul Jurasz is supported by a Ph.D. studentship from CIHR-Canadian Thoracic Society and Lung Association – Alberta Lung Association. Marek W. Radomski and Christopher M. Overall are supported by CIHR scientist Awards. We thank Ada Chung, Margo Miller, Concetta Carbonaro and Barb Litwinowich for assistance with blood collection.
Abbreviations
- MMP
matrix metalloproteinase
- MT1-MMP
membrane-type 1 matrix metalloproteinase
- NO
nitric oxide
- PGI2
prostacyclin
- human rTIMP
human recombinant tissue inhibitor of metalloproteinase
- TIMP
tissue inhibitor of metalloproteinase
References
- BIGG H.F., MORRISON C.J., BUTLER G.S., BOGOYEVITCH M.A., WANG Z., SOLOWAY P.D., OVERALL C.M. Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res. 2001;61:3610–3618. [PubMed] [Google Scholar]
- BIGG H.F., SHI Y.E., LIU Y.E., STEFFENSEN B., OVERALL C.M. Specific, high affinity binding of tissue inhibitor of metalloproteinases-4 (TIMP-4) to the COOH-terminal hemopexin-like domain of human gelatinase A. TIMP-4 binds progelatinase A and the COOH-terminal domain in a similar manner to TIMP-2. J. Biol. Chem. 1997;272:15496–15500. doi: 10.1074/jbc.272.24.15496. [DOI] [PubMed] [Google Scholar]
- BREW K., DINAKARPANDIAN D., NAGASE H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim. Biophys. Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- CHEUNG P.-Y., SAWICKI G., SALAS E., ETCHES P.C., SCHULZ R., RADOMSKI M.W. The mechanisms of platelet dysfunction during extracorporeal membrane oxygenation in acutely ill neonates. Crit. Care Med. 2000a;28:2584–2590. doi: 10.1097/00003246-200007000-00067. [DOI] [PubMed] [Google Scholar]
- CHEUNG P.-Y., SAWICKI G., WOZNIAK M., WANG W., RADOMSKI M.W., SCHULZ R. Matrix metalloproteinase-2 contributes to the ischemia-reperfusion injury in the heart. Circulation. 2000b;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- COOPER T.W., EISEN A.Z., STRICKLIN G.P., WELGUS H.G. Platelet-derived collagenase inhibitor: characterization and subcellular localization. Proc. Natl. Acad. Sci. U.S.A. 1985;82:2779–2783. doi: 10.1073/pnas.82.9.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLLERY C.M., MCEWAN J.R., WANG M., SANG Q.A., LIU Y.E., SHI Y.E. TIMP-4 is regulated by vascular injury in rats. Ann. New York. Acad. Sci. 1999;878:740–741. doi: 10.1111/j.1749-6632.1999.tb07777.x. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ-PATRON C., MARTINEZ-CUESTA M.A., SALAS E., SAWICKI G., WOZNIAK M., RADOMSKI M.W., DAVIDGE S.T. Differential regulation of platelet aggregation by matrix metalloproteinase-9 and -2. Thromb. Haemostas. 1999a;82:1730–1735. [PubMed] [Google Scholar]
- FERNANDEZ-PATRON C., RADOMSKI M.W., DAVIDGE S.T. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ. Res. 1999b;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ-PATRON C., RADOMSKI M.W., DAVIDGE S.T. Role of matrix metalloproteinase-2 in thrombin-induced vasorelaxation of rat mesenteric arteries. Am. J. Physiol. 2000a;278:H1473–H1479. doi: 10.1152/ajpheart.2000.278.5.H1473. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ-PATRON C., STEWART K.G., ZHANG Y., KOIVUNEN E., RADOMSKI M.W., DAVIDGE S.T. Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin-gene related peptide promotes vasoconstriction. Circ. Res. 2000b;87:670–676. doi: 10.1161/01.res.87.8.670. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ-PATRON C., ZHANG Y., RADOMSKI M.W., HOLLENBERG M.D., DAVIDGE S.T. Rapid release of matrix metalloproteinase (MMP)-2 by thrombin in the rat aorta: modulation by protein tyrosine kinase/phosphatase. Thromb. Haemostas. 1999c;82:1353–1357. [PubMed] [Google Scholar]
- FREEDMAN J.E., SAUTER R., BATTINELLI E.M., AULT K., KNOWLES C., HUANG P.L., LOSCALZO J. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. 1999;84:416–421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- GALIS Z.S., KRANZHOFER R., FENTON J.W., II, LIBBY P. Thrombin promotes activation of matrix metalloproteinase-2 produced by cultured vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1997;17:1837–1844. doi: 10.1161/01.atv.17.3.483. [DOI] [PubMed] [Google Scholar]
- GALT S.W., LINDEMANN S., ALLEN L., MEDD D.J., FALK J.M., MCINTYRE T.M., PRESCOTT S.M., KRAISS L.W., ZIMMERMAN G.A., WEYRICH A.S. Outside-in signals delivered by matrix metalloproteinase-1 regulate platelet function. Circ. Res. 2002;90:1093–1099. doi: 10.1161/01.res.0000019241.12929.eb. [DOI] [PubMed] [Google Scholar]
- GASIC G.J., GASIC T.B., STEWART C.C. Antimetastatic effects associated with platelet reduction. Proc. Natl. Acad. Sci. USA. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENE J., WANG M., LIU Y.E., RAYMOND L.A., ROSEN C., SHI Y.E. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J. Biol. Chem. 1996;271:30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ-BARRANTES S., SHIMURA Y., SOLOWAY P.D., SANG Q.X.A., FRIDMAN R. Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem. Biophys. Res. Commun. 2001;281:126–130. doi: 10.1006/bbrc.2001.4323. [DOI] [PubMed] [Google Scholar]
- HOLMSEN H., DANGELMAIER C.A. Measurement of secretion of serotonin. Meth. Enzymol. 1989;169:205–210. doi: 10.1016/0076-6879(89)69061-1. [DOI] [PubMed] [Google Scholar]
- HONN K.V., TANG D.G., CHEN Y.Q. Platelets and cancer metastasis: more than an epiphenomenon. Sem. Thrombos. Hemostas. 1992;18:392–415. doi: 10.1055/s-2007-1002578. [DOI] [PubMed] [Google Scholar]
- JURASZ P., CHUNG A.W.Y., RADOMSKI A., RADOMSKI M.W. Non-remodelling properties of matrix metalloproteinases: the platelet connection. Circ. Res. 2002;90:1041–1043. doi: 10.1161/01.res.0000021398.28936.1d. [DOI] [PubMed] [Google Scholar]
- JURASZ P., SAWICKI G., DUSZYK M., SAWICKA J., MIRANDA C., MAYERS I., RADOMSKI M.W. Matrix metalloproteinase-2 in tumour-cell induced platelet aggregation: Regulation by NO. Cancer Res. 2001a;61:376–382. [PubMed] [Google Scholar]
- JURASZ P., STEWART M.J., RADOMSKI A., KHADOUR F., DUSZYK M., RADOMSKI M.W. Role of von Willebrand factor in tumour cell-induced platelet aggregation: differential regulation by NO and prostacyclin. Br. J. Pharmacol. 2001b;134:1104–1112. doi: 10.1038/sj.bjp.0704343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZES I., ELALAMY I., SRAER J.-D., HATMI M., NGUYEN G. Platelet release of trimolecular complex components MT1MMP/TIMP2/MMP2: involvement in MMP2 activation and platelet aggregation. Blood. 2000;96:3064–3069. [PubMed] [Google Scholar]
- KRANZHOFER A., BAKER A.H., GEORGE S.J., NEWBY A.C. Expression of tissue inhibitor of metalloproteinase-1, -2, and -3 during neointima formation in organ cultures of human saphenous vein. Arter. Thromb. Vasc. Biol. 1999;19:255–265. doi: 10.1161/01.atv.19.2.255. [DOI] [PubMed] [Google Scholar]
- LECO K.J., APTE S.S., TANIGUCHI G.T., HAWKES S.P., KHOKHA R., SCHULTZ G.A., EDWARDS D.R. Murine tissue inhibitor of metalloproteinases-4 (Timp-4): cDNA isolation and expression in adult mouse tissues. FEBS Lett. 1997;401:213–217. doi: 10.1016/s0014-5793(96)01474-3. [DOI] [PubMed] [Google Scholar]
- LIU Y.E., WANG M., GREENE J., SU J., ULLRICH S., LI H., SHENG S., ALEXANDER P., SANG Q.A., SHI Y.E. Preparation and characterization of recombinant tissue inhibitor of metalloproteinase 4 (TIMP-4) J. Biol. Chem. 1997;272:20479–20483. doi: 10.1074/jbc.272.33.20479. [DOI] [PubMed] [Google Scholar]
- MAYERS I., HURST T., PUTTAGUNTA L., RADOMSKI A., JOHNSON D., SAWICKI G., RADOMSKI M.W. Cardiac surgery increases the activity of matrix metalloproteinases and nitric oxide synthase in humans. J. Thorac. Cardiovasc. Surg. 2001;122:746–752. doi: 10.1067/mtc.2001.116207. [DOI] [PubMed] [Google Scholar]
- MCQUIBBAN G.A., GONG J.H., TAM E.M., MCCULLOCH C.A., CLARK-LEWIS I., OVERALL C.M. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- MARTINEZ M.A., SALAS E., RADOMSKI A., RADOMSKI M.W. Matrix metalloproteinases in platelet adhesion to fibrinogen: interactions with nitric oxide. Medical Science Monitor. 2001;7:646–651. [PubMed] [Google Scholar]
- MEHTA P. Potential role of platelets in the pathogenesis of tumor metastasis. Blood. 1984;63:55–63. [PubMed] [Google Scholar]
- MONCADA S., GRYGLEWSKI R.J., BUNTING S., VANE J.R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- MORRISON C.J., BUTLER G.S., BIGG H.F., ROBERTS C.R., SOLOWAY P.D, OVERALL C.M. Cellular activation of MMP-2 (gelatinase A) by MT2-MMP occurs via a TIMP-2-independent pathway. J. Biol. Chem. 2002;276:47402–47410. doi: 10.1074/jbc.M108643200. [DOI] [PubMed] [Google Scholar]
- MURATE T., YAMASHITA K., ISOGAI C., SUZUKI H., ICHIHARA M., HATANO S., NAKAHARA Y., KINOSHITA T., NAGASAKA T., YOSHIDA S., KOMATSU N., MIURA Y., HOTTA T., FUJIMOTO N., SAITO H., HAYAKAWA T. The production of tissue inhibitors of metalloproteinases (TIMPs) in megakaryopoiesis: possible role of platelet- and megakaryocyte-derived TIMPs in bone marrow fibrosis. Br. J. Haematol. 1997;99:181–189. doi: 10.1046/j.1365-2141.1997.3293146.x. [DOI] [PubMed] [Google Scholar]
- OLIVER G.W., LEFERSON J.D., STETLER-STEVENSON W.G., KLEINER D.E. Quantitative reverse zymography: analysis of picogram amounts of metalloproteinase inhibitors using gelatinase A and B reverse zymograms. Analyt. Biochemistry. 1997;244:161–166. doi: 10.1006/abio.1996.9895. [DOI] [PubMed] [Google Scholar]
- OLSON T.M., HIROHATA S., YE J., LECO K., SELDIN M.F., APTE S.S. Cloning of the human tissue inhibitor of metalloproteinase-4 gene (TIMP4) and localization of the TIMP4 and Timp4 genes to human chromosome 3p25 and mouse chromosome 6, respectively. Genomics. 1998;51:141–151. doi: 10.1006/geno.1998.5362. [DOI] [PubMed] [Google Scholar]
- RADOMSKI A., STEWART M.J., JURASZ P., RADOMSKI M.W. Pharmacological characteristics of solid-phase von Willebrand factor in human platelets. Br. J. Pharmacol. 2001;134:1013–1020. doi: 10.1038/sj.bjp.0704345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADOMSKI M.W., JENKINS D.C., HOLMES L., MONCADA S. Human colorectal adenocarcinoma cells: Differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991;51:6073–6078. [PubMed] [Google Scholar]
- RADOMSKI M.W., MONCADA S. An improved method for washing of human platelets with prostacyclin. Thromb. Res. 1983;30:383–389. doi: 10.1016/0049-3848(83)90230-x. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., REES D.D., DUTRA A., MONCADA S. S-Nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br. J. Pharmacol. 1992;107:745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANG Q.X.A. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998;8:171–177. doi: 10.1038/cr.1998.17. [DOI] [PubMed] [Google Scholar]
- SATO H., SEIKI M. Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J. Biochem. 1996;119:209–215. doi: 10.1093/oxfordjournals.jbchem.a021223. [DOI] [PubMed] [Google Scholar]
- SAWICKI G., SALAS E., MURAT J., MISZTA-LANE H., RADOMSKI M.W. Release of gelatinase A from human platelets mediates aggregation. Nature. 1997;386:616–619. doi: 10.1038/386616a0. [DOI] [PubMed] [Google Scholar]
- SAWICKI G., SANDERS E.S., SALAS E., WOZNIAK M., RODRIGO J., RADOMSKI M.W. Localization and translocation of MMP-2 during aggregation of human platelets. Thromb. Haemostas. 1998;80:836–839. [PubMed] [Google Scholar]
- STERNLICHT M.D., WERB Z. How matrix metalloproteinases regulate cell behaviour. Annu. Rev. Cell. Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROUSSEAU A. Clinique Medicale de L'Hotel-Dieu Paris. London, New Sydenham Society. 1865. pp. 94–96.
- TUMMALAPALLI HEATH B.J., TYAGI S.C. Tissue inhibitor of metalloproteinase-4 instigates apoptosis in transformed cardiac fibroblasts. J. Cell. Biochem. 2001;80:512–521. doi: 10.1002/1097-4644(20010315)80:4<512::aid-jcb1005>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]


