Abstract
The present study was undertaken to elucidate whether PKCα plays a role in the mechanism of the stretch-induced contraction potentiated by 20-hydroxyeicosatetraenoic acid (20-HETE). The effects of 20-HETE on the canine basilar artery were compared with those of iberiotoxin, a blocker of large conductance Ca2+-activated K+ channels (KCa channels), as this blocker was shown earlier to sensitize these arteries to mechanical stretch.
Slow stretch at rates of 0.1 to 3 mm s−1 did not produce any contraction in normal physiological solution.
In the presence of 20-HETE, the slow stretch could produce contraction, which was inhibited by nicardipine, a 1,4-dihydropyridine Ca2+ channel blocker, and gadolinium, a blocker of stretch-activated cation channels.
20-HETE inhibited whole-cell K+ current and depolarized the membrane by approximately 10 mV. These effects of 20-HETE were similar to those of iberiotoxin.
Calphostin C, an inhibitor of protein kinase C (PKC), inhibited the action of 20-HETE, but not that of iberiotoxin.
In response to 20-HETE PKCα isoform was translocated from the cytosol to the membrane fraction, which translocation was inhibited by calphostin C.
These results suggest that 20-HETE induced sensitization of the canine basilar artery to stretch was caused by PKCα-mediated inhibition of KCa channel activity.
Keywords: 20-hydroxyeicosatetraenoic acid, mechanical stretch, myogenic contraction, Ca2+-activated K+ current, membrane potential, protein kinase C isoform, vascular smooth muscle, canine cerebral artery
Introduction
Mechanical stretch of the vascular wall acts as a facilitating stimulus on the medial smooth muscle to intensify its activity, eliciting myogenic contraction of blood vessels. The myogenic response contributes to the autoregulation of blood flow, and it is also considered to be one of the primary mechanisms responsible for the basal tone of blood vessels (Bayliss, 1902; Nakayama, 1982; Osol, 1995; Davis & Hill, 1999; Hill et al., 2001). However, there is much to be clarified as to the mechanism of mechanotransduction, i.e., how the vascular wall is receptive to a mechanical stress and how it produces various responses, including contraction. We previously reported that large conductance Ca2+-activated K+ channel (KCa channel) blockers, including iberiotoxin, charybdotoxin, and tetraethylammonium, sensitized the canine basilar artery to mechanical stretch (Obara et al., 2001). Thereby, these KCa channel blockers shifted the relationship between stretch rate and contraction parallel toward the left, i.e., the blockers sensitized the artery to the rate of stretch.
Arachidonic acid metabolites generated by the cytochrome P450 mono-oxygenase pathway were reported to play a major role in the modulation of vascular tone in the cerebral and renal circulation (Roman & Harder, 1993; Harder et al., 1995). For instance, epoxyeicosatrienoic acids (EETs), which are cytochrome P450 metabolites of arachidonic acid, released from the endothelium in response to acetylcholine, were shown to hyperpolarize smooth muscle, open KCa channels, and relax arteries (Rosolowsky & Campbell, 1993; Campbell et al., 1996; Gebremedhin et al., 1998a). Thus EETs have been considered as a candidate of endothelium-derived hyperpolarizing factor (EDHF) (Campbell et al., 1996), though the chemical identity of EDHF is controversial (Fukao et al., 1997; Van de Voode & Vanheel, 1997; McGuire et al., 2001; Triggle & Ding, 2002). Another major metabolite of arachidonic acid catalysed by cytochrome P450 hydroxylase in the cerebral and renal vasculature is 20-hydroxyeicosatetraenoic acid (20-HETE) (Ma et al., 1993; Harder et al., 1994; Imig et al., 1996), which is a potent vasoconstrictor in isolated cat cerebral and rat renal microvessels. 20-HETE has also been considered to be an endogenous mediator of myogenic tone of cerebral vessels (Harder et al., 1994; Imig et al., 1996). As to the underlying cellular-ionic mechanism of 20-HETE, the contractile response was considered to be attributable to the depolarization-induced influx of Ca2+ secondary to the inhibition of KCa channels (Harder et al., 1994; Ma et al., 1993; Zou et al., 1996). Consequently, 20-HETE was reported to activate L-type calcium channels in a concentration-dependent manner, which activation was antagonized by nicardipine (Harder et al., 1997; Gebremedhin et al., 1998b). As to an alternative action of 20-HETE, Lange et al. (1997) reported that 20-HETE activated protein kinase C (PKC), by which the KCa channel activity in cat cerebral vascular smooth muscle was inhibited. However, the signal transduction pathway by which 20-HETE exerts these effects still remains unclear.
There are at least 12 isoforms of PKC subspecies (Nishizuka, 1992). Of these, we identified four isoforms in the canine basilar artery, i.e., PKCα, δ, ζ and η (Nishizawa et al., 2000). Furthermore, we found that PKCα was particularly involved in the maintenance of delayed vasospasm after subarachnoid haemorrhage in the canine model (Nishizawa et al., 2000).
The present study was thus undertaken to elucidate whether PKCα plays a role in the mechanism underlying the stretch-induced contraction potentiated by 20-HETE. Therefore, the effects of iberiotoxin on the canine basilar artery were compared with those of 20-HETE. Our results indicate that 20-HETE made the basilar artery more sensitive to mechanical stretch, in which the inhibition of KCa channel activity by PKCα was involved as an important mechanism.
Methods
General
The present study was reviewed by the Ethics Committee on Animal Experiments at the University of Shizuoka, and was carried out in accordance with the Institutional Guideline for Animal Experiments of the University of Shizuoka, the guiding principle of the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society, and the Law (No. 105) and Notification (No. 6) of Care and Protection of Animals established by Japanese Government.
Isolation of basilar artery and single smooth muscle cells
Healthy mongrel dogs of either sex weighing 7–15 kg were used. The dogs were anaesthetized with pentobarbital sodium (30 mg kg−1, i.v.) and exsanguinated by bleeding from the carotid arteries. A cylindrical segment of the basilar artery, 2 cm long, was isolated and cut into ring segments about 0.5 mm wide. The preparation was horizontally mounted in an organ bath containing 5 ml of Tyrode solution containing (mM): NaCl 158.3, KCl 4, CaCl2 2, MgCl2 1.05, NaHCO3 10, NaH2PO4 0.42, and glucose 5.6 bubbled with 97% O2 and 3% CO2 (pH 7.35 at 35°C).
Single smooth muscle cells were isolated from the canine basilar artery by the method reported previously (Kimura et al., 2000). In brief, the artery was cut into small segments (1–2 mm), and these segments were incubated for 60 min at 37°C in calcium-free Hank's solution. Then, they were digested for 15–25 min at 37°C in calcium-free Hank's solution containing 1.5 mg ml−1 collagenase (type IA, Sigma), 0.1 mg ml−1 protease, 0.2 mg ml−1 Na2ATP, 2 mg ml−1 trypsin inhibitor, and 2 mg ml−1 BSA. After digestion, the supernatant was discarded; and the softened muscle segments were transferred again into calcium-free Hank's solution and incubated for 15 min at 37°C. Single cells were dispersed by gentle agitation with a wide-bore glass pipette. Isolated canine basilar myocytes were kept in Kraft-Brühe (K-B) solution (Isenberg & Klöckner, 1982) at 4°C until used.
Mechanical stimulation
For the mechanical stimulation, ring segments of artery were mounted between the force transducer and an arm whose position was controlled by a mechanical stimulator (DPS-256, Dia Medical System Co., Tokyo, Japan), and the length of the ring segments was controlled. The wall length of a ring segment was adjusted to its initial length (Li), at which no measurable increase in the passive tension was observed (Nakayama, 1982). In usual experiments, an amount of stretch equal to 1.5 Li, i.e., 150% of the initial length (=100%), given to the artery rings evoked maximum and reproducible responses. In the present study, thus, the artery ring was stretched from Li to 1.5 Li at various rates between 0.1 mm s−1 to 30 mm s−1. The maximum tensions in the absence and the presence of 100 nM 20-HETE were produced by stretch at rate of 30 mm s−1 and 1 mm s−1, respectively (see Figure 2A). In most present experiments, therefore, stretches at a rate of 30 mm s−1 and 1 mm s−1 were designated as the quick stretch and the slow one, respectively. Papaverine, a putative cAMP phosphodiesterase inhibitor, was used to totally eliminate the vascular tone (Nakayama, 1982). The active tension produced by stretch was defined as the difference in amplitude between the tension in the absence of papaverine (100 μM) and that in the presence of papaverine, as previously described (Obara et al., 2001). In order to secure the responsiveness of the artery, we produced 80 mM KCl-induced contraction at the beginning of each experiment. The resting tension was adjusted to 2 mN (about 1.5 Li) before application of 80 mM KCl. Isotonic 80 mM KCl–Tyrode solution was prepared by replacement of NaCl with an equimolar amount of KCl. To eliminate the effects of endothelial factors, we rubbed the intimal layer of the artery with a moist cotton pledget. The effectiveness of the endothelial removal was established functionally by the absence of acetylcholine (30 nM)-induced relaxation of the artery precontracted with U-46619 (10 nM). The definition of sensitization of the artery in response to agonist was applied to that in response to the stretch rate.
Figure 2.

(A) Effect of 20-HETE on the relationship between rate of stretch and tension. The artery was stretched at various rates in the absence and presence of 100 nM 20-HETE. The ER50 and Hill coefficient in the presence of 100 nM 20-HETE were 0.43 mm sec−1 and 0.59, respectively. The ER50 and Hill coefficient in the absence of 20-HETE were 12.49 mm s−1 and 2.01, respectively. Each point represents the mean±s.e.mean. The number in parentheses represents the number of experiments. (B) Effects of nicardipine, gadolinium, and calphostin C on the slow stretch-induced contraction in the presence of 20-HETE or iberiotoxin. The artery was pretreated with 100 nM nicardipine, 1 μM gadolinium or 1 μM calphostin C in the presence of 100 nM 20-HETE or 100 nM iberiotoxin for 10 min and then was stretched at the rate of 1 mm s−1. The maximum response to 80 mM KCl was taken as 100% on the ordinate. Each point represents the mean±s.e.mean of five experiments. *P<0.05 and **P<0.01 compared with the corresponding vehicle-treated group.
Whole-cell K+ current recording and measurement of resting membrane potential
Whole-cell K+ current and resting membrane potential of basilar arterial muscle cells were measured with an AXOPATCH-10 (Axon Instruments, Foster City, CA, U.S.A.) amplifier by using the patch-clamp technique described by Hamill et al. (1981).
Outward whole-cell K+ currents were recorded under voltage clamp condition using patch pipettes containing (mM): KCl 140, CaCl2 1.8, MgCl2 1, EGTA 5, MgATP 2, GTP 0.1 and HEPES 10, with the final pH adjusted to 7.2 with KOH. The external solution bathing the cell was composed of (mM) NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1, glucose 10 and HEPES 5 with the pH adjusted to 7.4 with NaOH. Outward whole-cell K+ currents were elicited every 1 s by depolarizing pulses of 250-ms duration from a holding potential of −70 mV to +60 mV in 10 mV increments.
Resting membrane potential was measured under the current clamp condition by the method reported previously (Obara et al., 2001). Patch pipettes were filled with (mM) KCl 140, EGTA 0.5, MgATP 4 and HEPES 5, with the pH adjusted to 7.4 with NaOH. The external solution contained (mM) NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1, glucose 10, and HEPES 5 at pH 7.4. The patch pipettes had a tip resistance of 2–7 MΩ. Electrode capacitance and series resistance were partially compensated electrically. Drugs were applied by adding them to the perfusing bath solution. Experiments were carried out at room temperature (20–23°C).
Measurement of translocation of protein kinase C isoforms
Translocation of protein kinase C (PKC) was measured by Western blot analysis as described previously (Obara et al., 1999). Briefly, canine basilar artery rings were homogenized with a Polytron in ice-cold homogenization buffer composed of (mM) Tris/HCl 50, ethylenediamine tetraacetic acid (EDTA) 5, EGTA 10, phenylmethylsulphonyl fluoride 1, dithiothreitol 5, benzamide 10, leupeptin 25 mg ml−1, and sucrose 250, and then centrifuged at 100,000×g for 30 min at 4°C. The ‘cytosolic' and ‘crude membrane' fractions were derived from the supernatant and the pellet, respectively. PKC isoforms were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis on 10% acrylamide gels, transferred to nitrocellulose membranes, and blotted with anti-PKC isoforms antibodies (Sigma, St. Louis, MO, U.S.A.). The amount of PKC isoform was quantified from densitometric scans of immunostained nitrocellulose blots obtained by use of a scanner (Dual-Wavelength Scanner, CS-9000, Shimazu, Tokyo, Japan).
Drugs
The drugs used in the present studies were the following: acetylcholine chloride, ethylenglycol-bis (β-aminoethylether)-N, N′-tetraacetic acid (EGTA), gadolinium chloridehexahydrate, 20-hydroxyeicosatetraenoic acid (20-HETE), N - (2 - hydroxyethyl)piperazine - N′ - (4- butanesulphonic acid) (HEPES), iberiotoxin, nicardipine hydrochloride, and 9,11-dideoxy-11α, 9α-epoxymethano prostaglandin F2α (U46619) from Sigma (St. Louis, MO, U.S.A.), and calphostin C from Wako (Osaka, Japan). Calphostin C, 20-HETE, and U46619 were stocked as 1 mM solutions in 100% dimethyl sulphoxide (DMSO). The final concentrations of DMSO did not exceed 0.1% in the organ bath. In the preliminary study, this concentration (0.1%) of DMSO had no significant effect on the stretch-induced contraction and whole-cell K+ current. Other drugs were dissolved in distilled water. All other drugs used in the present study were reagent grade.
Data analysis
Data were expressed as the mean±s.e.mean. Statistical analysis was made by the paired or unpaired Student's t-test, or by Turkey's test after analysis of variance (ANOVA). A P value of less than 0.05 was considered statistically significant. For fitting the curves representing the relationship between dose or rate of stretch and response, Hill plots were used according to the following formula: Rexp=Rmax/{1+(EC50 or ER50/X)n}, where X is the concentration of the drug or the rate of stretch, EC50 is a 50% effective concentration of the drug, ER50 is a 50% effective concentration of the drug, ER50 is a 50% effective rate of stretch, n is the Hill coefficient, Rexp is the expected response, and Rmax is the maximum response.
Results
Effect of 20-HETE on mechanical response to stretch
Figure 1 shows the effect of 20-HETE on the mechanical response of a canine basilar artery in ring form to a slow stretch. When the artery was treated with 100 nM 20-HETE for 10 min, a slow stretch (from Li to 1.5 Li, at a rate of 1 mm s−1 and a stimulus period of 15 min) produced an initial rise and subsequent fall of passive tension, which was followed by a delayed contraction (Figure 1A, trace a). The contraction reached maximum (11.8±3.9 mN, n=5) in about 2 min, and gradually declined. In the presence of 100 μM papaverine, however, the contraction was totally eliminated; and only the passive increase in tension appeared (Figure 1A, trace b). A slow stretch by itself did not produce any appreciable contraction, but it produced only passive tension (Figure 1A, trace c). Furthermore, 20-HETE (100 nM) had no apparent effect on the basal tension. Thus, in the following experiments, the active component of stretch-induced contraction (arrow in Figure 1A) was designated as the difference in tension amplitudes in the presence or absence of papaverine.
Figure 1.

Effects of 20-HETE on the mechanical response of canine basilar artery to slow stretch. (A) Typical tracings of mechanical responses to slow stretch. The artery was treated with 100 nM 20-HETE (trace a) or 100 μM papaverine (trace b) for 10 min. Isometric tension of the artery was measured in the absence (trace c) of 100 nM 20-HETE. Trace ‘a' was superimposed on the passive increase in tension (b) or on that without 20-HETE (c). The active tension was produced by slow stretch at a rate of 1 mm s−1, amount of stretch from Li to 1.5 Li, and a stimulus period of 15 min. (B) Effect of various concentrations of 20-HETE on slow stretch-induced contraction. Artery was treated with various concentrations of 20-HETE for 10 min, and then stretched at a rate of 1 mm s−1 to 1.5 Li. The maximum response to 80 mM KCl was taken as 100% on the ordinate. The EC50 and Hill coefficient were 28.6 nM and 1.48, respectively. Each point represents the mean±s.e.mean. The number in parentheses represents the number of experiments. *P<0.05 and **P<0.01 compared with the corresponding vehicle-treated group.
As shown in Figure 1B, 20-HETE at a concentration less than 10 nM had no apparent effect on the mechanical response of the artery to slow stretch at a rate of 1 mm s−1. 20-HETE (over 10 nM), however, augmented the stretch-induced contraction in a concentration-dependent manner. The value of EC50 of 20-HETE for a 50% increase in tension in response to stretch was estimated as 28.6 nM. The concentration-response curve for 20-HETE appeared steep, indicating a narrow range for the mode of action. 20-HETE at concentrations over 300 nM triggered a spontaneous contraction of the artery. Thus, we chose the 100 nM concentration of 20-HETE as a contractile seizure in the following experiments.
Next, we assessed how 20-HETE affected the mechanical activity of the basilar artery in response to various rates of stretch (Figure 2A). The stretch at various rates from 0.1–30 mm s−1 and an amount of stretch up to 1.5 Li produced contraction in a rate-dependent manner in the presence of 100 nM 20-HETE. The stretch-induced contraction reached maximum at rates over 1 mm s−1. The stretch at a rate over 3 mm s−1 by itself produced contraction in a rate-dependent manner even in the absence of 20-HETE, which reached maximum at a rate of 30 mm s−1. The amplitude of maximum tension produced by a slow stretch rate of 1 mm s−1 in the presence of 100 nM 20-HETE was 6.9±5.9 mN (n=5), whereas that by a quick stretch rate of 30 mm s−1 was 7.8±4.9 mN (P>0.05, n=5), respectively. Fifty per cent effective rates (ER50) for stretch-induced contraction in the presence and absence of 20-HETE were estimated as 0.44 mm s−1 and 12.49 mm s−1, respectively. Thus, the 20-HETE-treated artery was about 30 times more sensitive to the rate of stretch than the untreated artery.
Then, we tested the effects of nicardipine, gadolinium, and calphostin C on the stretch-induced contraction augmented by 20-HETE. The slow stretch-induced contraction augmented by 20-HETE was inhibited by nicardipine (100 nM), a 1,4-dihydropyridine Ca2+ channel blocker, gadolinium (1 μM), a blocker of stretch-activated cation channels (SA channels), and calphostin C (1 μM), a protein kinase C (PKC) inhibitor (Figure 2B). The stretch-induced contraction of the iberiotoxin-treated artery was also inhibited by nicardipine and gadolinium, but not by calphostin C (Figure 2B).
Effects of 20-HETE on whole-cell outward current and resting membrane potential
The effect of 20-HETE on whole-cell K+ current in freshly-dispersed canine basilar artery smooth muscle cells was studied by use of a whole-cell voltage clamp technique. 20-HETE (30–100 nM) added to the bathing solution significantly inhibited the peak whole-cell K+ current by 37.5±7.0% (P<0.01 versus vehicle-treated myocyte, n=4) at 30 nM and by 61.5±4.8% (P<0.01, n=5) at 100 nM (Figure 3A). However, 20-HETE did not appear to shift the current-voltage relationship, suggesting that 20-HETE decreased the whole-cell current amplitude by decreasing either the probability of channel opening or the number of active channels. The removal of Ca2+ from the bathing solution (with 2 nM EGTA) inhibited the peak whole-cell K+ current by 62.7±5.4% (P<0.01, n=5), and 20-HETE (100 nM) had no apparent effect on the Ca2+-insensitive component of the whole-cell K+ current (data not shown). Iberiotoxin (100 nM) also inhibited the peak whole-cell K+ current, by 60.3±3.8% (P<0.01, n=5; Figure 3B). The vehicle for 20-HETE and iberiotoxin had no apparent effect on the current (data not shown). Iberiotoxin (100 nM) combined with 20-HETE (1–100 nM) did not produce any additional change in the amplitude of the whole-cell K+ current. 20-HETE and iberiotoxin (each 100 nM) and 20-HETE plus iberiotoxin (each 100 nM) similarly depolarized the membrane about 10 mV (Table 1). These results indicate that 20-HETE acted mainly as an inhibitor of large conductance KCa channels.
Figure 3.

Effects of 20-HETE and iberiotoxin on whole-cell K+ current. (A) Effect of 20-HETE. Upper panel, representative tracings of outward whole-cell K+ current elicited by depolarizing pulses to +60 mV from a holding potential of −70 mV in the absence or presence of 30 nM or 100 nM 20-HETE. Lower panel, current-voltage relationships of whole-cell K+ current for the same cell shown in the upper panel in the absence or the presence of 30 nM or 100 nM 20-HETE. Whole-cell K+ current was activated by incremental 10 mV depolarizing steps from a holding potential of −70 mV to +60 mV. (B) Effect of iberiotoxin. Upper panel, representative tracings of outward whole-cell K+ current elicited by depolarizing pulses to +60 mV from a holding potential of −70 mV in the absence or presence of 100 nM iberiotoxin or 100 nM 20-HETE plus 100 nM iberiotoxin. Lower panel, current-voltage relationships of whole-cell K+ current for the same cell shown in the upper panel in the absence or the presence of 100 nM iberiotoxin or 100 nM 20-HETE plus 100 nM iberiotoxin. Each point represents the mean±s.e.mean of five experiments.
Table 1.
Effects of 20-HETE, iberiotoxin and calphostin C on the resting membrane potentials of single smooth muscle cells isolated from canine basilar artery

Since there is a study showing that 20-HETE inhibited the activity of KCa channels through a mechanism involving PKC (Lange et al., 1997), we assessed the effect of calphostin C, a conventional and novel PKC inhibitor, on the inhibitory action of 20-HETE on the whole-cell K+ current. 20-HETE (100 nM) or iberiotoxin (100 nM) inhibited the whole-cell K+ current by about 60% (each n=5; Figure 4A,B). Calphostin C (1 μM) alone did not change the amplitude of the whole-cell K+ current or the resting membrane potential as compared with the control (Figure 4A,B, and Table 1). In the presence of calphostin C, 20-HETE failed to inhibit whole-cell K+ current and depolarize the resting membrane potential (Figure 4A and Table 1), whereas iberiotoxin (100 nM) significantly inhibited the whole-cell K+ current by 65.1±5.4% (P<0.01, n=5) and depolarized the membrane about 10 mV (Figure 4B and Table 1).
Figure 4.
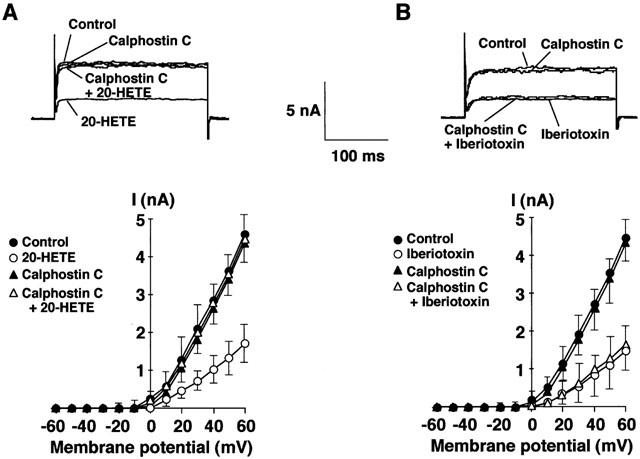
Effects of calphostin C on whole-cell K+ current inhibited by 20-HETE and iberiotoxin. (A) Effect on 20-HETE-induced inhibition of whole-cell K+ current. (B) Effect on iberiotoxin-induced inhibition of whole-cell K+ current. Upper panel, representative tracings of outward whole-cell K+ current elicited by depolarizing pulses to +60 mV from a holding potential of −70 mV in the absence (in A and B) or presence of 100 nM 20-HETE (in A), 1 μM calphostin C (in A and B), 1 μM calphostin C plus 100 nM 20-HETE (in A) or 1 μM calphostin C plus 100 nM iberiotoxin (in B). Lower panel, current-voltage relationships of whole-cell K+ current for the same cell shown in the upper panel in the absence and presence of 100 nM 20-HETE (in A), 100 nM iberiotoxin (in B), 1 μM calphostin C (in A and B), 1 μM calphostin C plus 100 nM 20-HETE (in A) or 1 μM calphostin C plus 100 nM iberiotoxin (in B). Whole-cell K+ current was activated by incremental 10 mV depolarizing steps from a holding potential of −70 mV to +60 mV. Each point represents the mean±s.e.mean of five experiments.
Effect of 20-HETE on translocation of PKCα isoform
In the canine basilar artery, we previously identified at least four PKC isoforms, i.e., PKC α, δ, ζ and η (Nishizawa et al., 2000). In the resting state, PKC α, δ, and ζ were abundant in the cytosol fraction (PKCα, 59.0±4.5%; PKCδ, 69.1±4.4%; PKCζ, 65.4±5.4%; each n=5), whereas PKCη was distributed almost equally in both cytosol (50.9±4.9%, n=5) and membrane (49.1±4.9%, n=5) fractions (Figure 5B). Of the four isoforms, 20-HETE (100 nM) caused only PKCα to be translocated from the cytosol to the membrane fraction (Figure 5A,B). Whereas, 20-HETE had no apparent effect on the distribution of the other three PKC isoforms in the cytosol and membrane fractions. Calphostin C (1 μM) effectively inhibited the translocation of PKCα produced by 20-HETE (Figure 5A,C). Iberiotoxin (100 nM), on the other hand, had no appreciable effect on the distribution of any PKC isoforms in the cytosol and membrane fractions (data not shown).
Figure 5.
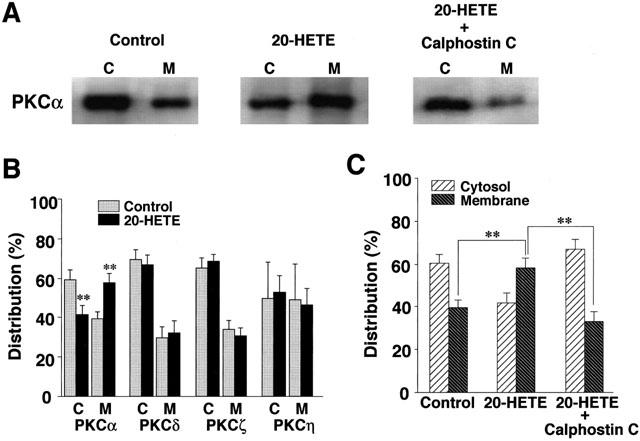
Effect of 20-HETE on the subcellular distribution of PKC isoforms in canine basilar artery. (A) Representative subcellular distribution of PKCα isoform in the cytosol (C) and the membrane (M) fractions detected by Western blot analysis with isoform-specific antibodies to PKC. (B) The subcellular distribution of PKC isoforms. The artery was treated with 100 nM 20-HETE or vehicle for 10 min. The results are expressed as a percentage of the total amount of each PKC isoform. (C) Effect of calphostin C on 20-HETE-induced translocation of PKCα. The artery was treated with 100 nM 20-HETE, 100 nM 20-HETE plus 1 μM calphostin C, or vehicle for 10 min. The results are expressed as a percentage of the total amount of PKCα isoform. Data are the mean±s.e.mean of five individual experiments. **P<0.01 compared with the corresponding control value.
Discussion
The present results showed that 20-HETE sensitized the basilar artery to mechanical stretch by inhibiting KCa channel activity. Furthermore, PKCα seemed to play an important role in the inhibition of the channel activity.
The graphic representation of the relationship between the concentration (dose) of a drug and its effect is the first step to elucidate the pharmacological action of the drug. In a similar manner, in order to elucidate the mechanism for mechanotransduction, it is important to know how the parameters of mechanical stress are related to its effect. Our previous study indicated that the amount and rate of stretch, as well as the interval between stretches, were the main parameters to produce myogenic contraction of cerebral artery in response to mechanical stretch (Nakayama, 1982). Of these, we reported that the rate of stretch was pivotal for mobilization of activator Ca2+ in the stretch-induced contraction, based on our assessment of the stretch-rate dependency of canine basilar artery (Obara et al., 2001). In the present study, 20-HETE caused a parallel shift to the left of the relationship between the rate of stretch and contraction of the canine basilar artery, whereas it had no apparent effect on the maximum contraction (Figure 2A). These results indicate that 20-HETE sensitized the basilar artery to mechanical stretch.
We previously reported that the blockade of KCa channel activity by iberiotoxin, charybdotoxin, and TEA sensitized the canine basilar artery to mechanical stretch (Obara et al., 2001). In the present study by use of the whole-cell patch clamp technique, we confirmed the previous studies reporting that 20-HETE attenuated the KCa channel activity (Harder et al., 1994; Ma et al., 1993; Zou et al., 1996); i.e., 20-HETE, in the same way as iberiotoxin, inhibited the whole-cell KCa current (Figure 3). Both 20-HETE and iberiotoxin depolarized the membrane approximately 10 mV (Table 1). 20-HETE in combination with iberiotoxin produced neither additional depolarization nor enhanced inhibition of KCa current (Table 1 and Figure 3B). Moreover, removal of Ca2+ from bathing solution inhibited the whole-cell K+ current by about 60%, which was almost the same amount produced by 20-HETE or iberiotoxin. Also, 20-HETE and iberiotoxin had no apparent effect on the Ca2+-intensive component of the whole-cell K+ current. These results suggest that 20-HETE inhibited the KCa channel activity.
Large conductance KCa channels are expressed at high density in the cell membrane of canine basilar artery (Asano et al., 1993) and the activation of these channels increases K+ efflux, thereby producing a hyperpolarization of the membrane of artery (Nelson & Quayle, 1995). Vascular smooth muscle cells including canine basilar artery have a deep membrane potential of about −60 mV (Kuriyama et al., 1995). In the present study, the resting membrane potential was approximately −55 mV (Table 1), which was almost the same as that reported previously in canine basilar artery by use of microelectrode (Fujiwara et al., 1982). Iberiotoxin and 20-HETE similarly produced contraction in response to the slow stretch (Figures 1 and 2), and they almost equally depolarized about 10 mV in canine basilar artery myocytes (Table 1). Davis et al. (1992) reported that stretch of isolated porcine coronary smooth muscle cells elicited sustained depolarization with magnitude of 10–30 mV, which was well correlated with the degree of cells stretch. The significant increase in the open probability of voltage-dependent calcium channel (VDC channel) is generally considered to occur at −35 mV (Nelson et al., 1990). Considering that the slow stretch-induced contraction was stretch rate-dependent and was nicardipine-sensitive (Figure 2B), it is reasonable to expect that the slow stretch elicited depolarization in a rate-dependent manner. A small amount of depolarization induced by slow stretch seems to be sufficient to activate VDCs in the presence of large conductance KCa channel blockers, which leads to contraction.
It has been reported that stretch elicited a sustained depolarization in isolated smooth muscle cells from porcine coronary artery (Davis et al., 1992). Stretch-activated channels (SA channels) identified in a variety of cell types, including smooth muscles, are considered to be sensitive to Gd3+ (Kirber et al., 1988; Davis et al., 1992; Langton, 1993). In the presence of 20-HETE or iberiotoxin, we showed that the slow stretch-induced contraction was abolished by either nicardipine or Gd3+ in the canine basilar artery (Figure 2B). Consequently, it is possible that the slow stretch activates the Gd3+-sensitive SA channel, which causes a long-lasting depolarization of membrane leading to the opening of L-type VDCs and a contraction. Recently, Welsh et al. (2002) reported that the pressure-induced depolarization of rat cerebral artery involved a transient receptor potential channel (TRPC6) activation, which was mediated by phospholipase C (PLC) activation and diacylglycerol (DAG) activity. TRPC6 activation (Inoue et al., 2001) and the pressure-induced depolarization (Welsh et al., 2000) were inhibited by Gd3+. In the present study, slow stretch-induced contraction in the presence of 20-HETE or iberiotoxin was inhibited by Gd3+ (Figure 2B) but not by U-73122, an inhibitor of PLC (data not shown). Moreover, 1 mM Gd3+, whose concentration was used in the present study, did not affect the 80 mM KCl-induced contraction (Obara et al., 2001). Therefore, the present results suggest that slow stretch-induced contraction involves the SA channel activity but not TRPC6 activity.
It has been reported that several cis-unsaturated fatty acids, including arachidonic acid and their metabolites, activate PKC (Hansson et al., 1986; Murakami et al., 1986; Sekiguchi et al., 1987). The activated PKC inhibited KCa channels (Minami et al., 1993; Ribalet & Eddlestone, 1995; Zhang et al., 1995; Shipston & Armstrong, 1996), and promoted vasoconstriction (Walsh et al., 1994; Lange et al., 1997). In the present study, calphostin C per se did not change the amplitude of the whole-cell K+ current as compared with the control (Figure 4A). However, in the presence of calphostin C, 20-HETE failed to inhibit the current, indicating that the blockade of PKC counteracted the inhibitory action of 20-HETE on K+ current. As a consequence, calphostin C inhibited the slow stretch-induced contraction in the presence of 20-HETE (Figure 2B). Taken together, it seems possible that PKC plays a regulatory role in the activity of the KCa channel.
At least 12 isoforms of PKC have been identified, and we have recently found four of them (PKCα, δ, ζ, and η) in the canine basilar artery (Nishizawa et al., 2000). Of these isoforms, only PKCα was translocated from the cytosol to the membrane fraction by 20-HETE, indicating an activation of the kinase; and this translocation was inhibited by calphostin C (Figure 5). On the other hand, iberiotoxin showed a different mode of action as the toxin had no apparent effect on the translocation of PKCα (data not shown). Therefore, our results strongly suggest that 20-HETE inhibited KCa channel activity through activation of PKCα, whereas iberiotoxin directly blocks KCa channel. Although the exact site of phosphorylation is not clear, analysis of rat, human, and mouse KCa channel alpha subunit sequences using Prosite data base search engines demonstrates the existence of 16 potential phosphorylation sites for PKC (Harder et al., 1995). It is still unknown whether the KCa channel subunits are directly phosphorylated by PKCα. Further studies are required to clarify the underlying mechanism at the molecular level.
In summary, we have provided the first evidence demonstrating that 20-HETE sensitized the canine basilar artery to mechanical stretch via PKCα-mediated inhibition of KCa channels.
Acknowledgments
The present study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by grants from the Shizuoka Research and Development Foundation.
Abbreviations
- DAG
diacylglycerol
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- ECL
enhanced chemiluminescence
- EDHF
endothelium-derived hyperpolarizing factor
- EET
epoxyeicosatrienoic acid
- ER50
50% effective rates
- 20-HETE
20-hydroxyeicosatetraenoic acid
- KCa channel
large conductance Ca2+-activated K+ channel
- PKC
protein kinase C, SA channel, stretch-activated cation channel
- SDS
sodium dodecyl sulphate
- TBS
Tris-buffered saline
- TRPC
transient receptor potential channel
- U-46619
9,11-dideoxy-11α, 9α-epoxymethano prostaglandin F2α
References
- ASANO M., MASUZAWA-ITO K., MATSUDA T., SUZUKI Y., OYAMA H., SHIBUYA M., SUGITA K. Functional role of charybdotoxin-sensitive K+ channels in the resting state of cerebral, coronary and mesenteric arteries of the dog. J. Pharmacol. Exp. Ther. 1993;267:1277–1285. [PubMed] [Google Scholar]
- BAYLISS W.M. On the local reaction of the arterial wall to change of internal pressure. J. Physiol. (Lond.) 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL W.B., GEBREMEDHIN D., PRATT P.F., HARDER D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- DAVIS M.J., DONOVITZ J.A., HOOD J.D. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am. J. Physiol. 1992;262:C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- DAVIS M.J., HILL M.A. Signaling mechanism underlying the vascular myogenic response. Physiol. Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- FUKAO M., HATTORI Y., KANNO M., SAKUMA I., KITABATAKE A. Evidence against a role of cytochrome P450-derived arachidonic acid metabolites in endothelium-dependent hyperpolarization by acetylcholine in rat isolated mesenteric artery. Br. J. Pharmacol. 1997;120:439–446. doi: 10.1038/sj.bjp.0700932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJIWARA S., ITO Y., ITOH T., KURIYAMA H., SUZUKI H. Diltiazem-induced vasodilatation of smooth muscle cells of the canine basilar artery. Br. J. Pharmacol. 1982;75:455–467. doi: 10.1111/j.1476-5381.1982.tb09162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEBREMEDHIN D., HARDER D.R., PRATT P.F., CAMPBELL W.B. Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: role of a cytochrome P450 metabolite. J. Vasc. Res. 1998a;35:274–284. doi: 10.1159/000025594. [DOI] [PubMed] [Google Scholar]
- GEBREMEDHIN D., LANGE A.R., NARAYANAN J., JACOBS E.R., HARDER D.R. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J. Physiol. (Lond.) 1998b;507:771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER M.V., SAKMAN B., SIGWORTH F.J. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HANSSON A., SERHAN C.N., HAEGGSTROM J., INGEKMA-SUNDBERG M., SAMUELSSON B. Activation of protein kinase C by lipoxin A and other eicosanoids. Intracellular action of oxygenation products of arachidonic acid. Biochem. Biophys. Res. Commun. 1986;134:1215–1222. doi: 10.1016/0006-291x(86)90380-3. [DOI] [PubMed] [Google Scholar]
- HARDER D.R., CAMPBELL W.B., ROMAN R.J. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J. Vasc. Res. 1995;32:79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- HARDER D.R., GEBREMEDHIN D., NARAYANAN J., JEFCOAT C., FALCK J.R., CAMPBELL W.B., ROMAN R. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am. J. Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- HARDER D.R., LANGE A.R., GEBREMEDHIN D., BIRKS E.K., ROMAN R.J. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J. Vasc. Res. 1997;34:237–243. doi: 10.1159/000159228. [DOI] [PubMed] [Google Scholar]
- HILL M.A., ZOU H., POTOCNIK S.J., MEININGER G.A., DAVIS M.J. Arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J. Appl. Physiol. 2001;91:973–983. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- IMIG J.D., ZOU A.P., STEC D.E., HARDER D.R., FALCK J.R., ROMAN R.J. Formation and actions of 20-hydroxyeicosatetraenoic acid in the renal microcirculation. Am. J. Physiol. 1996;270:R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- INOUE R., OKADA T., ONOUE H., HARA Y., SHIMIZU S., NAITOH S., ITO Y., MORI Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ. Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- ISENBERG G., KLÖCKNER U. Calcium tolerant ventricular myocytes prepared by pre-incubation in a ‘K-B medium'. Pflügers Arch. 1982;424:431–438. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- KIMURA M., OBARA K., SASASE T., ISHIKAWA T., TANABE Y., NAKAYAMA K. Specific inhibition of stretch-induced increase in L-type calcium channel currents by herbimycin A in canine basilar arterial myocytes. Br. J. Pharmacol. 2000;130:923–931. doi: 10.1038/sj.bjp.0703360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBER M.T., WALSH J.V., JR., SINGER J.J. Stretch-activated ion channels in smooth muscle; a mechanism for the initiation of stretch-induced contraction. Pflügers Arch. 1988;412:339–345. doi: 10.1007/BF01907549. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H., KITAMURA K., NABATA H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol. Rev. 1995;47:387–573. [PubMed] [Google Scholar]
- LANGE A., GEBREMEDHIN D., NARAYANAN J., HARDER D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J. Biol. Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- LANGTON P.D. Calcium channel currents recorded from isolated myocytes of rat basilar artery are stretch sensitive. J. Physiol. (Lond.) 1993;471:1–11. doi: 10.1113/jphysiol.1993.sp019887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA Y.H., GEBREMEDHIN D., SCHWARTZMAN M.L., FALCK J.R., CLARK J.E., MASTERS B.S., HARDER D.R., ROMAN R.J. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ. Res. 1993;72:126–136. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- MCGUIRE J.J., DING H., TRIGGLE C.R. Endothelium-derived relaxing factors: A focus on endothelium-derived hyperpolarizing factor(s) Can. J. Physiol. Pharmacol. 2001;79:443–470. [PubMed] [Google Scholar]
- MINAMI K., FUKUZAWA K., NAKAYA Y. Protein kinase C inhibits the Ca2+-activated K+ channel of cultured porcine coronary artery smooth muscle cells. Biochem. Biophys. Res. Commun. 1993;190:263–269. doi: 10.1006/bbrc.1993.1040. [DOI] [PubMed] [Google Scholar]
- MURAKAMI K., CHAN S.Y., ROUTTENBERG A. Protein kinase C activation by cis-fatty acid in the absence of Ca2+ and phospholipids. J. Biol. Chem. 1986;261:15424–15429. [PubMed] [Google Scholar]
- NAKAYAMA K. Calcium-dependent contractile activation of cerebral artery produced by quick stretch. Am. J. Physiol. 1982;242:H760–H768. doi: 10.1152/ajpheart.1982.242.5.H760. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., PATLAK J.B., WORLEY J.F., STANDEN N.B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- NISHIZAWA S., OBARA K., NAKAYAMA K., KOIDE M., YOKOYAMA T., YOKOTA N., OHTA S. Protein kinase Cδ and α are involved in the development of vasospasm after subarachnoid hemorrhage. Eur. J. Pharmacol. 2000;398:113–119. doi: 10.1016/s0014-2999(00)00311-3. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- OBARA K., HATA S., SATO K., KOIDE M., ISHII K., NAKAYAMA K. Contractile potentiation by endothelin-1 involves protein kinase C-δ activity in the porcine coronary artery. Jpn. J. Physiol. 1999;49:175–183. doi: 10.2170/jjphysiol.49.175. [DOI] [PubMed] [Google Scholar]
- OBARA K., SAITO M., YAMANAKA A., UCHINO M., NAKAYAMA K. Involvement of different activator Ca2+ in the rate-dependent stretch-induced contractions of canine basilar artery. Jpn. J. Physiol. 2001;51:327–335. doi: 10.2170/jjphysiol.51.327. [DOI] [PubMed] [Google Scholar]
- OSOL G. Mechanotransduction by vascular smooth muscle. J. Vasc. Res. 1995;32:275–292. doi: 10.1159/000159102. [DOI] [PubMed] [Google Scholar]
- RIBALET B., EDDLESTONE G.T. Characterization of the G protein coupling of SRIF and beta-adrenergic receptors to the maxi KCa channel in insulin-secreting cells. J. Membr. Biol. 1995;148:111–125. doi: 10.1007/BF00207268. [DOI] [PubMed] [Google Scholar]
- ROMAN R.J., HARDER D.R. Cellular and ionic signal transduction mechanisms for the mechanical activation of renal arterial vascular smooth muscle. J. Am. Soc. Nephrol. 1993;4:986–996. doi: 10.1681/ASN.V44986. [DOI] [PubMed] [Google Scholar]
- ROSOLOWSKY M., CAMPBELL W.B. Role of PGI2 and EETs in the relaxation of bovine coronary arteries to arachidonic acid. Am. J. Physiol. 1993;280:H2470–H2477. doi: 10.1152/ajpheart.1993.264.2.H327. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI K., TSUKUDA M., OGITA K., KIKKAWA U., NISHIZUKA Y. Three distinct forms of rat brain protein kinase C: differential response to unsaturated fatty acids. Biochem. Biophys. Res. Commun. 1987;145:797–802. doi: 10.1016/0006-291x(87)91035-7. [DOI] [PubMed] [Google Scholar]
- SHIPSTON M.J., ARMSTRONG D.L. Activation of protein kinase C inhibits calcium-activated potassium channels in rat pituitary tumour cells. J. Physiol. (Lond.) 1996;493:665–672. doi: 10.1113/jphysiol.1996.sp021413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRIGGLE C.R., DING H. Endothelium-derived hyperpolarizing factor: Is there a novel chemical mediator. Clin. Exp. Pharmacol. Physiol. 2002;29:153–160. doi: 10.1046/j.1440-1681.2002.03632.x. [DOI] [PubMed] [Google Scholar]
- VAN DE VOORDE J., VANHEIL B. Influence of cytochrome P-450 inhibitors on endothelium-dependent nitro-L-arginine-resistant relaxation and cromakalim-induced relaxation in rat mesenteric arteries. J. Cardiovasc. Pharmacol. 1997;29:827–832. doi: 10.1097/00005344-199706000-00018. [DOI] [PubMed] [Google Scholar]
- WALSH M.P., ANDREA J.E., ALLEN B.G., CLEMENT-CHOMIENNEP O., COLLINS E.M., MORGAN K.G. Smooth muscle protein kinase C. Can. J. Physiol. Pharmacol. 1994;72:1392–1399. doi: 10.1139/y94-201. [DOI] [PubMed] [Google Scholar]
- WELSH D.G., MORIELLI A.D., NELSON M.T., BRAYDEN J.E. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- WELSH D.G., NELSON M.T., ECKMAN D.M., BRAYDEN J.E. Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure. J. Physiol. (Lond.) 2000;527:139–148. doi: 10.1111/j.1469-7793.2000.t01-1-00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H., WEIR B., DANIEL E.E. Activation of protein kinase C inhibits potassium currents in cultured endothelial cells. Pharmacology. 1995;50:247–256. doi: 10.1159/000139289. [DOI] [PubMed] [Google Scholar]
- ZOU A.P., FLEMING J.T., FALCK J.R., JACOBS E.R., GEBREMEDHIN D., HARDER D.R., ROMAN R.J. 20-HETE is an endogenous inhibitor of the large conductance Ca2+-activated K+ channel in renal arterioles. Am. J. Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]


