Abstract
Conflicting results have been reported regarding the influence of nitric oxide (NO) and peroxynitrite on dopamine (DA) uptake and release. In the present study, effects of NO donors were studied in rat C6 glioma cells expressing human DA transporter.
[3H]-DA uptake was inhibited by S-nitroso-thiol S-nitroso-N-acetylpenicillamine, spermine/NO, diethylamine/NO (DEA/NO), (Z)-1-[N-(3-ammoniopropyl)-N-(n-propyl)-amino]/NO (PAPA/NO), and 3-morphosynodiomine (SIN-1) in a rank order correlating with their half lives as NO donors, whereas no effect was observed for diethylenetriamine/NO and dipropylenetriamine/NO, which release NO very slowly.
Hydroxycobalamin, a NO scavenger, but not superoxide dismutase and catalase, enzymes that metabolize superoxide and hydrogen peroxide, respectively, abolished the inhibitory effect of DEA/NO and SIN-1, indicating that they inhibit DA uptake through a mechanism related to the production of NO but unrelated to the formation of peroxynitrite. In consonance, peroxynitrite did not alter DA uptake in the present system.
DEA/NO and PAPA/NO reduced [3H]-MPP+ uptake, whereas the release of [3H]-MPP+ was not modified, demonstrating that NO can inhibit uptake of DA transporter substrate without accelerating DA transporter-mediated reverse transport of substrate under the same conditions.
Keywords: Dopamine transporter, nitric oxide, dopamine, N-methyl-4-phenylpyridinium (MPP+), peroxynitrite
Introduction
Nitric oxide (NO) is generally recognized to have a wide range of effects in various physiological and pathophysiological processes. In the central nervous system, NO is generated by various neurons and acts distally as a free radical gas transmitter affecting neighbouring nerve cells including dopamine (DA) neurons (see West et al., 2002). Long-term elevation of NO can be neurotoxic or neuroprotective in rat mesencephalic DA neuron-enriched cultures and human neuroblastoma catecholamine-rich NB69 cells, depending on NO concentration generated as measured by the formation of nitrite, the oxidation end product of NO (Rodriguez-Martin et al., 2000; Canals et al., 2001). Short-term, NO plays a regulatory role in various, including dopaminergic, neurotransmission systems, influencing DA transport and release (Kiss & Vizi, 2001). In rat synaptosomal preparations and cultured neurons, NO and NO donors typically inhibit DA uptake (Lonart & Johnson, 1994; Pogun et al., 1994; Cook et al., 1996). Surprisingly, a recent study of Kaye et al. (2000) shows a lack of effect on DA uptake of the NO donor S-nitroso-thiol S-nitroso-N-acetylpenicillamine (SNAP) in Chinese hamster ovary (CHO) cells expressing human DAT. This raises the possibility that DA uptake by DAT in non-neuronal host cells is not sensitive to NO as opposed to DA uptake in situ, or that human DAT is less susceptible than rat DAT to this particular activity of NO. It is well known that NO can react with superoxide anion to form the toxic compound peroxynitrite (Dawson et al., 1993; Feelisch, 1998), but the role of peroxynitrite in DA transport effects, by itself or as a product of the reaction of NO with superoxide anion, has been a matter of debate, with Lonart & Johnson (1994) reporting only a minor contribution of peroxynitrite to NO-induced inhibition of DA uptake by rat striatal synaptosomes and Park et al. (2002) demonstrating a profound effect of peroxynitrite on DA uptake by EM4 cells expressing human DAT.
Another major point of debate has been the contribution of DA releasing activity (by reversed DA transport) of NO to its DA uptake inhibitory effect (Lonart & Johnson, 1994; Pogun et al., 1994), and to our knowledge no studies are available that demonstrate inhibition of DA uptake without releasing activity by NO monitored in the same system. Clearly, NO can exert DA releasing effects mediated by reversed DA transport in vitro (Lonart & Johnson, 1994; Büyükuysal, 1997a, b), and a number of in vivo observations with microdialysis fit in with enhanced DA release, although other in vivo findings suggest that NO, endogenously, can suppress reversed DA transport (see Kiss & Vizi, 2001), pointing to the involvement of additional, important complexities existing in vivo (see also West et al., 2002).
The present study was undertaken to (1) assess whether DA uptake by human DAT in non-neuronal host cells is inhibited by NO as has been reported for rat DAT in situ or in cultured neurons; if so, (2) determine the role of peroxynitrite in the NO effect; and (3) assess the contribution of DA transport reversal to the DA uptake inhibitory effect. The design of the experiments takes advantage of the advent of the recently developed diazeniumdiolates (NONOates) that reliably release NO upon contact with water milieu and include various compounds with widely different half lives of NO generation (Feelisch, 1998; Fitzhugh & Keefer, 2000).
Methods
Materials
[3H]-DA (54.8 Ci mmol−1), [3H]-2β-carbomethoxy-3β-(4-fluorophenyl)tropane (WIN 35,428) (86 Ci mmol−1), [3H]-Glycine (86 Ci mmol−1) and [3H]-N-methyl-4-phenylpyridinium (MPP+) (78 Ci mmol−1) were purchased from New England Nuclear (Boston, MA, U.S.A.). Diethylamine (DEA)/NO, (Z)-1-[N-(3-ammoniopropyl)-N-(n-propyl)-amino] (PAPA)/NO and peroxynitrite were obtained from Alexis Chemical Co. (San Diego, CA, U.S.A.). Most other reagents were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Tropolone was dissolved in assay buffer immediately before use, whereas NONOate stock solutions were prepared fresh each day in ice-cold 0.01 N NaOH and stored on ice for use.
Rat C6 glioma cells stably expressing human DAT
The cloning of a human DAT cDNA and its transfection into C6 glioma cells were performed in the laboratory of Dr. Aaron Janowsky, Amie Eshleman and Kim Neve at Oregon Health Sciences University, Portland, OR, U.S.A. (Eshleman et al., 1994). Cells stably expressing the human DAT were grown in Dulbecco's modified Eagle's medium supplemented with 5% foetal bovine serum, 5% bovine calf serum, and 1 μg ml−1 puromycin as described previously (Reith et al., 1996). For transport experiments, C6 cells were seeded and grown in 96-well plates. After 2–3 days, when the cells were confluent, the medium was replaced with buffer as described below.
[3H]-dopamine uptake
The general procedures were as described by us previously (Reith et al., 1996). Briefly, the medium was removed from each well and cells were washed twice with ‘Wash Buffer' (mM: NaCl 122, KCl 5, CaCl2 1, MgSO4 1.2, glucose 10, Na2HPO4 15, pH 7.4). Cells were preincubated with ‘Assay Buffer' (identical to Wash Buffer plus 0.1 mM tropolone (see Eshleman et al., 1997)) and appropriate drugs at room temperature (21°C); except where indicated, the preincubation time was 15 min. Uptake was initiated by the addition of 20 nM [3H]-DA and incubation continued for 8 min. The final assay volume was 250 μl well−1. Cocaine (0.1 mM) was used to define nonspecific uptake. Assays were terminated by removing the medium and washing twice with ice-cold Wash Buffer. Trichloroacetic acid (3%, v v−1) was added to each well, and the radioactivity in the acid extract was determined by conventional liquid scintillation spectrometry. All experiments were conducted with triplicate determinations.
[3H]-MPP+ uptake
The procedures were as for [3H]-DA uptake with the following exceptions. The preincubation time with drugs was 2 min, and uptake of [3H]-MPP+ (2 nM) was allowed to proceed for 30 min. Preincubation and uptake occurred in Wash Buffer.
[3H]-MPP+ release
The protocol of the release experiments was described before by Johnson et al. (1998), with modifications. In brief, loading of the cells was carried out at room temperature for 40 min by adding Wash Buffer and [3H]-MPP+ (2 nM). In order to define the portion of tritium taken up specifically by the DAT, parallel samples contained 0.1 mM cocaine to block DAT. The Wash Buffer was removed and cells were washed twice with ‘Release Buffer', which was identical to Wash Buffer except that it lacked calcium (see also Janowsky et al., 1998). Release Buffer and drugs (300 μl final volume at room temperature) were added to the wells initiating release (zero time). [3H]-MPP+ remaining in the cells was measured at six time points ranging from 1 to 90 min. At each time point, release was terminated by aspirating the buffer from the well. Trichloroacetic acid (3%, v v−1) was added, and radioactivity remaining in the cells was determined by liquid scintillation spectrometry.
[3H]-glycine uptake
The procedures were as for [3H]-DA uptake with the following exceptions. The preincubation time with drugs was 5 min, and uptake of [3H]-glycine (0.1 μM) was allowed to proceed for 10 min. Preincubation and uptake occurred in Wash Buffer. Background 0-min uptake was deducted. This protocol is similar to that used by Gomeza et al. (1995) in C6 cells.
[3H]-WIN 35,428 binding
Binding assays were performed as [3H]-DA uptake assays with the following exception. Drugs and [3H]-WIN 35,428 were added together to wells containing DAT cells, and incubation was allowed to proceed for 15 min.
Statistical analysis
Data were analysed by parametric statistical tests as indicated in figure legends. The accepted level of significance was 0.05.
Results
Inhibitory effect of DO donors on DA uptake in relation to their half-life
[3H]-DA uptake was reduced to less than 50% of its normal value in the presence of the NO generators DEA/NO or PAPA/NO at concentrations of 0.08 mM and up, both after 2- and 15-min preincubation (Figure 1 top and middle panel). In contrast to these diazeniumdiolates with short half-lives in the minute range (Fitzhugh & Keefer, 2000), S-nitroso-thiol S-nitroso-N-acetylpenicillamine (SNAP), which has a half-life of 4–6 h (Roy et al., 1994; Zamora et al., 1997; Babich & Zuckerbraun, 2001), left approximately 70% of [3H]-DA uptake undisturbed across the tested concentration range of 0.1–1.6 mM with 15 min of preincubation being as ineffective as 2 min (Figure 1 bottom panel). All subsequent experiments involving [3H]-DA uptake were done with 15-min preincubation.
Figure 1.
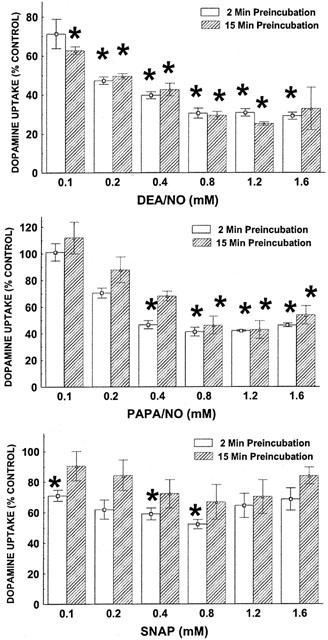
Effect of DEA/NO, PAPA/NO and SNAP on [3H]-DA uptake after preincubation for 2 or 15 min. Data represent specific uptake and are expressed as percentage of amount of [3H]-DA in vehicle-treated control wells (on average 14,800 c.p.m.). Results are mean±s.e.mean (vertical bar) of three independent experiments, each performed in triplicate. *P<0.05, compared with 100% of vehicle-treated cells (one-sample Student's t-test, with Bonferroni correction for number (6) of comparisons with same control).
Various diazeniumdiolates and 3-morphosynodiomine (SIN-1), with half-lives ranging from 2 min to 20 h (Zamora et al., 1997; Fitzhugh & Keefer, 2000; Babich & Zuckerbraun, 2001), were tested for their effect on [3H]-DA uptake (Figure 2). Diethylenetriamine (DETA)/NO (t1/2, 20 h) and dipropylenetriamine (DPTA)/NO (t1/2, 5 h) were ineffective over the entire concentration range. Spermine (SPER)/NO (t1/2, 0.5–2.3 h) displayed some uptake inhibitory activity at the highest concentration tested (0.8 mM), whereas the strongest effects were observed for PAPA/NO (t1/2, 15 min), DEA/NO (t1/2, 2 min), and SIN-1 (t1/2, 10 min).
Figure 2.
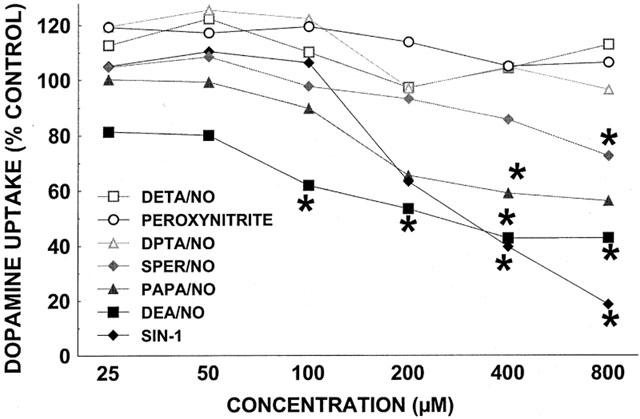
Effect of various NO donors and peroxynitrite on [3H]-DA uptake. Data represent specific uptake and are expressed as percentage of amount of [3H]-DA in vehicle-treated control wells (on average 14,800 c.p.m.). Results are mean of three independent experiments, each performed in triplicate. For all data points, the s.e.means, expressed as % Control, were between 1 and 22% with the bulk of the points falling below the 8% mark. *P<0.05, compared with 100% of vehicle-treated cells (one-sample Student's t-test, with Bonferroni correction for number (6) of comparisons with same control).
Reduced NO donor-induced inhibition of DA uptake as a result of scavenging of NO but not counteracting the formation of peroxynitrite and breaking down hydrogen peroxide
The NO scavenger hydroxycobalamin (1 mM) abolished the inhibitory activity on [3H]-DA uptake of DEA/NO (0.8 mM) or SIN-1 (0.8 mM), without itself affecting DA uptake (Figure 3), consonant with a role for NO in the action of the NO donors.
Figure 3.
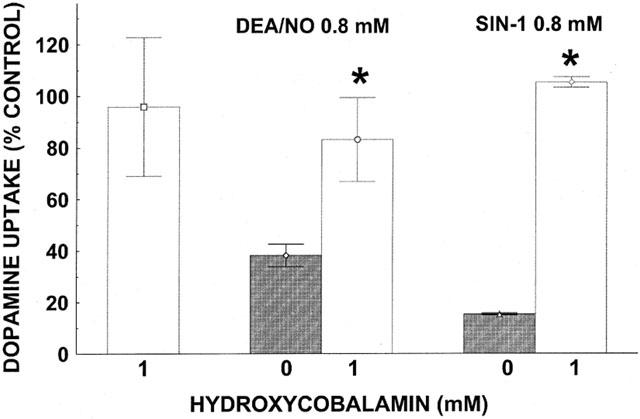
Hydroxycobalamin-induced reversal of inhibition of [3H]-DA uptake by DEA/NO and SIN-1. Data represent specific uptake and are expressed as percentage of amount of [3H]-DA in vehicle-treated control wells (on average 13,800 c.p.m.). Results are mean±s.e.mean (vertical bar) of three independent experiments, each performed in triplicate. *P<0.01, compared with corresponding control (hydroxocobalamine 0 mM) value (Student's t-test).
Because NO can react with superoxide anion to form the toxic compound peroxynitrite, superoxide dismutase (SOD) (50 or 100 U ml−1) was included to diminish superoxide and thereby prevent the formation of peroxynitrite, along with catalase (100 or 200 U ml−1) to convert the produced hydrogen peroxide (Figure 4). Inhibition of [3H]-DA uptake induced by DEA/NO (0.8 mM), PAPA/NO (0.8 mM), or SIN-1 (0.8 mM), was not antagonized by the combination of SOD and catalase which when added by themselves were without effect. Also consonant with the lack of involvement of peroxynitrite under the conditions of these experiments was the ineffectiveness of peroxynitrite co-incubated with DAT expressing cells before and during the [3H]-DA uptake assay (Figure 2).
Figure 4.
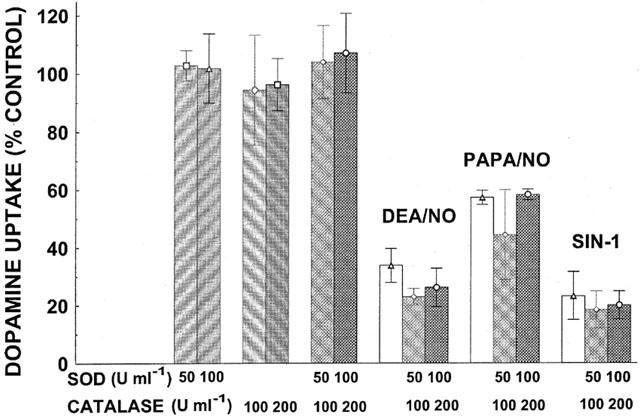
Lack of effect of SOD and catalase on inhibition of [3H]-DA uptake by DO donors. DEA/NO, PAPA/NO, or SIN-1 were present at 0.8 mM. Data represent specific uptake and are expressed as percentage of amount of [3H]-DA in vehicle-treated control wells (on average 14,600 c.p.m.). Results are mean±s.e.mean (vertical bar) of three independent experiments, each performed in triplicate. There were no statistically significant differences between NO donors by themselves or in combination with SOD plus catalase (two-way ANOVA followed by Duncan Multiple Comparison test).
Inhibitory activity of NO donors on uptake but not release of MPP+
Releasing activity of drugs impacts measurement of [3H]-DA uptake. Release of [3H]-DA from C6 glioma cells is difficult to assess because of high spontaneous leakage, and we therefore monitored release of [3H]-MPP+ (Janowsky et al., 1998), a DAT substrate (Javitch et al., 1985). DEA/NO and PAPA/NO were as effective in inhibiting the uptake of [3H]-MPP+ (Figure 5) as [3H]-DA (Figure 2). However, under the same conditions, DEA/NO (0.8 mM), PAPA/NO (0.8 mM), or SIN-1 (0.8 mM) had little or no effect on the release of [3H]-MPP+ (Figure 6). In comparison, the known releaser D-amphetamine (3 μM) (Sitte et al., 1998) produced substantial liberation of [3H]-MPP+.
Figure 5.
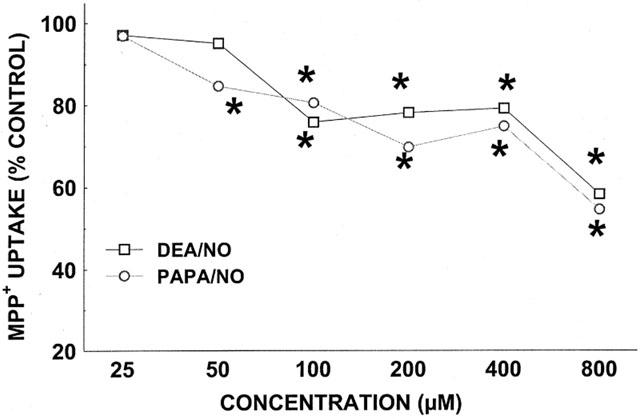
Effect of DEA/NO and PAPA/NO on [3H]-MPP+ uptake. Data represent specific uptake and are expressed as percentage of amount of [3H]-MPP+ in vehicle-treated control wells (on average 3400 c.p.m.). Results are mean of three independent experiments, each performed in triplicate. For all data points, the s.e.means, expressed as % Control, were between 5 and 8%. *P<0.05, compared with 100% of vehicle-treated cells (two-way ANOVA followed by Duncan Multiple Comparison test).
Figure 6.
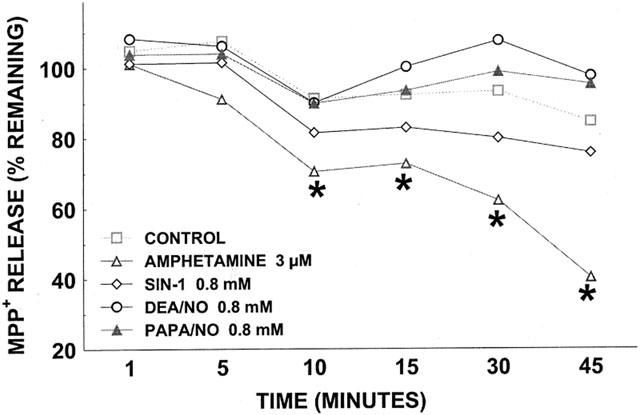
Effect of DEA/NO (0.8 mM), PAPA/NO (0.8 mM), SIN-1 (0.8 mM), and d-amphetamine (3 μM) on [3H]-MPP+ release. Data have been corrected for cell-associated [3H]-MPP+ in the presence of 0.1 mM cocaine for DAT blockade and are expressed as percentage of amount of [3H]-MPP+ remaining at time 0 (on average 4100 c.p.m.). Data for cells treated with vehicle in release phase are shown in dashed line. Results are mean of three independent experiments, each performed in triplicate. For all data points, the s.e.means, expressed as % Control, were between 2 and 9%. *P<0.01, compared with corresponding control value at that time point (two-way ANOVA followed by Duncan Multiple Comparison test).
Lack of effect of NO donors on glycine uptake
The present C6 glioma cell line endogenously expresses the glycine transporter GLYT1 (Gomeza et al., 1995), allowing measurement of glycine uptake under the same conditions as those used for assessment of DAT function. Preliminary experiments showed [3H]-glycine uptake to be linear for approximately 30 min, and drugs were tested with a 10-min uptake time; under these conditions, the majority of [3H]-glycine uptake represents high-affinity Na+-dependent uptake by GLYT1 (Gomeza et al., 1995). DEA/NO (0.025–1.6 mM) and PAPA/NO (0.025–1.6 mM) had no effect at all on [3H]-glycine uptake (data not shown).
Lack of effect of NO donors on WIN 35,428 binding
The binding of [3H]-WIN 35,428 was measured to assess whether NO donors interact with the DA-sensitive cocaine analogue recognition site. Concentrations of 0.025–0.8 mM of SNAP, PAPA/NO, DEA/NO, or peroxynitrite were without effect (data not shown).
Discussion
NO release by NO donors
The relationship between the half-life of an NO donor and the release of NO under certain experimental conditions depends on a number of factors, such as the time of the assay in relation to half-life, the concentration of donor applied, the stoichiometry of NO production from the donor, and the presence of cells known to enhance NO inactivation (Ferrero et al., 1999). For donors with half-lives less than our assay time (15 min), such as DEA/NO (2 min) and SIN-1 (10 min), the absolute NO release will start levelling off before the end of the assay. Because of all of these factors, the correlation between donor activity and half-life holds only in a qualitative sense. Indeed, in a study comparing donors in delivering NO and enhancing cyclic GMP in chromaffin cells, a general correlation was observed between the half-maximal donor concentration for cyclic GMP production and half-life, and also between donor concentration needed for maximal cyclic GMP and NO production on the one hand and half-life on the other hand (Ferrero et al., 1999). In the same study, one finds a general relationship between NO production and half-life when comparing donors applied at the same concentration for the same amount of time. Consistent with these findings are our results for donors with half-lifes ranging from minutes to hours (Figures 1 and 2).
For SNAP, the tendency to reduce DA uptake in our experiments became statistically significantly only at shorter preincubation times and concentrations of 0.8 mM or lower (Figure 1 bottom panel). It was observed previously that SNAP, even though it released small amounts of NO in a seemingly linear fashion with time up to 30 min, stopped elevating cyclic GMP early on in chromaffin cells; in addition, SNAP concentration curves were biphasic for cyclic GMP production with higher levels becoming inhibitory (Ferrero et al., 1999). It is possible that a similar inhibition played a role in our finding that the small DA uptake inhibitory effect of SNAP was lost upon longer preincubation times with SNAP, and upon application of higher concentrations of SNAP. Another confounding factor is the kinetically rather complex generation of NO from SNAP and its susceptibility to trace-metal mediated redox processes (Fitzhugh & Keefer, 2000).
Sensitivity of DAT to NO in non-neuronal host cells
The report of Kaye et al. (2000) questioned the sensitivity of DA uptake to NO when measured in CHO cells expressing human DAT as opposed to DA uptake measured in rat striatal synaptosomes or cultured neurons. The present observations in C6 glioma cells expressing human DAT indicate that the reasons for the lack of effect of the NO donor SNAP in DAT-CHO cells (Kaye et al., 2000) did not originate in the non-neuronal host cell used, or the species of the DAT examined. The present results provide an explanation for the results of Kaye et al. (2000) with SNAP. As an NO donor, SNAP has a relatively long half life of 4–6 h (Roy et al., 1994; Zamora et al., 1997; Babich & Zuckerbraun, 2001), releasing NO slowly, and therefore is rather ineffective in inhibiting DA transport (Figure 1). The fact that SNAP, under the same conditions, suppressed norepinephrine (NE) uptake by CHO cells expressing NE transporter (NET), agrees with previous results of Pogun et al. (1994) who showed a more severe effect of SNAP on NE than DA uptake in rat striatal synaptosomes. This may not be caused by a generally higher sensitivity of NET than DAT because another NO donor, sodium nitroprusside, was without effect on NE uptake under the same conditions showing inhibition of DA uptake (Pogun et al., 1994). It is possible that other chemical effects of the earlier generation of NO donors may have played a role, and the present study therefore made use of the more recently developed diazeniumdiolates which reliably release NO upon contact with water milieu. The inhibition of DA uptake observed in the present experiments is likely due to NO because (i) the effect of various NO donors correlated with their half life, i.e. the rate of NO generation, and (ii) NO scavenging reversed the DA uptake inhibition. The lack of effect of NO donors on the binding of [3H]-WIN 35,428 in our system agrees with the reported ineffectiveness of sodium nitroprusside on the binding of [3H]-WIN 35,428 to rat striatal membranes (Pogun et al., 1994) and suggests that NO, or the NO generating diazeniumdiolates, do not interfere with the portion of the DA recognition domain on DAT that overlaps with the WIN 35,428 binding site.
A concern in using diazeniumdiolates could be that they are amine derivatives (diethylamine, spermine) and have the potential of interacting with a modulatory site on the N-methyl-D-aspartate (NMDA) receptor facilitating NMDA-mediated transmission (Maciver et al., 1991; Williams et al., 1991). However, this would require the presence of endogenous transmitter the action of which could then be facilitated, which is unlikely to be the case in the present cultured C6 glioma cells. In situ in brain, there is evidence that NO can promote glutamate release (Lonart et al., 1992; Guevara-Guzman et al., 1994), and glutamate is known to promote DA reversed transport both in vitro (Lonart & Zigmond, 1991) and in vivo (Falkenburger et al., 2001). Transporter reversal is thought to occur by the depolarization resulting from Na+ influx through glutamate-gated, glutamate receptor-linked channels and through voltage-dependent sodium channels (Falkenburger et al., 2001). This type of interaction between glutamate receptors and DAT differs from that between DA D2 receptors and DAT (Wu et al., 2002) which is voltage-independent (Mayfield & Zahniser, 2001). Be that as it may, a role for NMDA receptors in the present astrocytic C6 cells is unlikely as there still is no reliable evidence for functional NMDA receptors in astrocytes (Verkhratsky & Steinhäuser, 2000). This, along with the other evidence discussed above supports the notion that NO mediates the activity of diazeniumdiolates under the conditions of the present experiments. In agreement with this, preliminary experiments with diethylamine, N-propyl-1,3-propane diamine, and spermine (the endproducts of DEA/NO, PAPA/NO, and spermine/NO degradation in aqueous solution, respectively) did not uncover dopamine uptake inhibitory activity (data not shown). This negative finding for spermine contrasts with the inhibitory activity reported by Ritz et al. (1994) on dopamine transport in rat striatum. It is possible that spermine does not act at the DAT itself but rather at an accessory protein not present in C6 cells expressing human DAT.
Role of peroxynitrite or dopamine quinone
The observations of Lonart & Johnson (1994) with SOD and catalase in rat striatal synaptosomes did not suggest a major contribution of peroxynitrite to the inhibitory effect of NO on DA uptake. Likewise, our results with these enzymes, applied to C6 glioma cells expressing human DAT, do not indicate an important role for peroxynitrite in the NO-induced inhibition of DA uptake. The lack of effect of added peroxynitrite (0.025–0.8 mM) in our system is in contrast with the profound inhibition (at 1 mM) observed by Park et al. (2002) in HEK-293-derived, EM4 cells expressing human DAT. There are some differences between the amino acid sequence of the DAT in the present study as compared with the report of Park et al. (2002): Met instead of Arg at position 344 and the presence of the first 22 amino acids instead of the Flag-HA tag. Position 344 is close by Cys342 which is shown by Park et al. (2002) to be oxidized by peroxynitrite; both Cys342 and another nearby residue, Asp345, are conformationally sensitive (Chen & Reith, 2002). It is not known whether these differences in DAT sequences are important for the peroxinitrite effect. It is also possible that the high instability of peroxynitrite resulted in effectively lower concentrations than aimed for in the current study. This does not negate the above interpretation of the SOD/catalase experiments regarding the lack of involvement of peroxynitrite in the action of NO in our system. The lack of a role for peroxynitrite in the effect of NO also agrees with the lack of effect of NO donors on the uptake of [3H]-glycine by GLYT1 in the present human DAT-C6 cells. Peroxynitrite is toxic and could affect general cell householding processes such as energy production which is needed for the maintenance of Na+ gradients by Na+,K+-ATPase. Both DAT and GLYT1 function depend on the Na+ gradient, and it is important to note that uptake of DA, but not glycine, was inhibited by NO donors.
Although it has been reported that NO can oxidize DA to form DA o-semiquinone and DA quinone in rat mesencephalic primary cultures (Cook et al., 1996), the same study also demonstrates that inhibition of DA uptake by NO is not mediated by such oxidative products because protection against oxidation by ascorbate and other reagents did not remove the DA uptake inhibitory effect of NO (Cook et al., 1996). Furthermore, DA quinone has been reported not to be transported by DAT, and reaction of DA quinone with external Cys residues on DAT does not inhibit DA transport function (Park et al., 2002). It is known that DA quinone can react with internally located Cys342 in human DAT to prevent ligand binding (Whitehead et al., 2001); however, the role of this reaction upon exposure to NO in the DA uptake assay, in terms of inhibiting DA uptake, is probably minor as DA uptake was still inhibited by NO when DA was protected against oxidation during NO treatment (Cook et al., 1996). Moreover, the inhibition observed in the present experiments was not only found with DA, but also with MPP+ as the DAT substrate.
Inhibition of uptake of DAT substrate without enhancement of reversed transport
In general, reversal of DAT operation can be achieved in vitro by changing transmembrane ion gradients or by adding substrates to the external medium such as D-amphetamine (see Chen & Reith, 2002). In vivo, DAT reversal has been implicated in physiopathological situations such as ischaemia and neurodegeneration, and it may play a role in maintaining extracellular DA levels in a paracrine fashion (for recent review see Leviel, 2001). A recent study by Falkenburger et al. (2001) shows that stimulation of glutamate input into the substantia nigra produces dendritic DA efflux by DAT reversal, reducing membrane excitability by dendrodendritic inhibition. It has been argued that, in DA terminal regions, NO inhibits the function of DAT operating either in normal or reversed direction (Kiss & Vizi, 2001). However, the evidence for the ‘inhibitory' NO action on reversed transport came from in vivo observations which are compounded by complicating factors such as release of DA through Ca2+-dependent release mechanisms (Lonart et al., 1993; West & Galloway, 1998) and impact of striatonigral feedback loops (West et al., 2002). In vitro release designs have consistently shown enhancement of Ca2+-independent (Black et al., 1994) and reversed DA (Lonart & Johnson, 1994; Büyükuysal, 1997a, b) transport in rat striatal slices. Compounds that enhance reversed DA transport are known to be potent inhibitors in [3H]-DA uptake assays because such assays cannot distinguish uptake inhibitors from releasers (Bönisch, 1998). Most studies on NO and the DAT measured either uptake or release, and the one study that monitored both, showed an effect on uptake as well as release (Lonart & Johnson, 1994). This left the basic question as to whether the observed uptake effects were, at least in part, due to releasing effects. The present results show that it is possible for NO to inhibit inward DAT function unidirectionally without promoting substrate efflux. An explanation for the lack of effect on reversed transport in the present system comes from the work of Büyükuysal (1997b) who found that the stimulation of reversed DA transport in rat striatal slices by NO was highly dependent on the activation of voltage-dependent sodium channels. Although the non-neuronal astrocytic C6 glioma cells used here, do have voltage-dependent Na+ channels, K+ channels and K+ permeability predominate such that action potentials cannot be generated as opposed to the situation in rat striatal slices (Nicholls et al., 1992; Verkhratsky & Steinhäuser, 2000). The current cell system for expressing human DAT therefore will not give information regarding regulatory mechanisms impacting DAT if these mechanisms involve changes in membrane potential through activation of voltage-dependent Na+ channels. Although DAT reversal can occur upon depolarization, the latter is not required for reversal as is exemplified by DA release induced by releasers such as D-amphetamine.
Thus, our finding that NO can inhibit DA uptake without promoting substrate release shows that DAT reversal is not a prerequisite for uptake effects demonstrated in other studies. Furthermore, inhibition of DA uptake in the present study was observed with NO donors including the recently developed diazeniumdiolates (NONOates) in accordance with their half lives as NO donors and unrelated to the formation of peroxynitrite.
Acknowledgments
This research is supported by grant from National Institute on Drug Abuse (DA 11978) to M.E.A. Reith. The authors thank Janet Berfield for her technical assistance.
Abbreviations
- DA
dopamine
- DAT
DA transporter
- DEA/NO
diethylamine/NO
- DETA/NO
diethylenetriamine/NO
- DPTA/NO
dipropylenetriamine/NO
- MPP+
N-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NO
nitric oxide
- PAPA/NO
(Z)-1-[N-(3-ammoniopropyl)-N-(n-propyl)-amino]/NO
- SIN-1
3-morphosynodiomine
- SNAP
S-nitroso-thiol S-nitroso-N-acetylpenicillamine
- SOD
superoxide dismutase
- SPER/NO
spermine/NO
References
- BABICH H., ZUCKERBRAUN H.L. In vitro cytotoxicity of glyco-S-nitrosothiols, a novel class of nitric oxide donors. Toxicology in Vitro. 2001;15:181–190. doi: 10.1016/s0887-2333(01)00006-6. [DOI] [PubMed] [Google Scholar]
- BLACK M.D., MATTHEWS E.K., HUMPHREY P.P.A. The effects of a photosensitive nitric oxide donor on basal and electrically-stimulated dopamine efflux from the rat striatum in vitro. Neuropharmacology. 1994;33:1357–1365. doi: 10.1016/0028-3908(94)90037-x. [DOI] [PubMed] [Google Scholar]
- BÖNISCH H. Transport and drug binding kinetics in membrane vesicle preparation. Methods Enzymol. 1998;296:259–278. doi: 10.1016/s0076-6879(98)96020-7. [DOI] [PubMed] [Google Scholar]
- BÜYÜKUYSAL R.L. Effect of nitric oxide donors on endogenous dopamine release from striatal slices. I: Requirements to antioxidants in the medium. Fundam. Clin. Pharmacol. 1997a;11:519–527. doi: 10.1111/j.1472-8206.1997.tb00856.x. [DOI] [PubMed] [Google Scholar]
- BÜYÜKUYSAL R.L. Effect of nitric oxide donors on endogenous dopamine release from striatal slices. II: The role of voltage-dependent sodium channels, calcium channel activation, reverse transport mechanism, guanylate cyclase and endogenous glutamate. Fundam. Clin. Pharmacol. 1997b;11:528–536. doi: 10.1111/j.1472-8206.1997.tb00857.x. [DOI] [PubMed] [Google Scholar]
- CANALS S., CASAREJOS M.J., RODRIGUEZ-MARTIN E., DE BERNARDO S., MENA M.A. Neurotrophic and neurotoxic effects of nitric oxide on fetal midbrain cultures. J. Neurochem. 2001;76:56–68. doi: 10.1046/j.1471-4159.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- CHEN N.-H., REITH M.E.A.Structure-function relationships for biogenic amine neurotransmitter transporters Neurotransmitter Transporters, Structure, Function, and Regulation 2002Totowa, NJ: Humana Press; 53–109.2nd edn. ed. Reith, M.E.A. pp [Google Scholar]
- COOK J.A., WINK D.A., BLOUNT V., KRISHNA M.C., HANBAUER I. Role of antioxidants in the nitric oxide-elicited inhibition of dopamine uptake in cultured mesencephalic neurons. Insights into potential mechanisms of nitric oxide-mediated neurotoxicity. Neurochem. Int. 1996;28:609–617. doi: 10.1016/0197-0186(95)00125-5. [DOI] [PubMed] [Google Scholar]
- DAWSON V.L., DAWSON T.M., BARTLEY D.A., UHL G.R., SNYDER S.H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J. Neurosci. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHLEMAN A.J., HENNINGSEN R.A., NEVE K.A., JANOWSKY A. Release of dopamine via the human transporter. Mol. Pharmacol. 1994;45:312–316. [PubMed] [Google Scholar]
- ESHLEMAN A.J., STEWART E., EVENSON A.K., MASON J.N., BLAKELY R.D., JANOWSKY A., NEVE K.A. Metabolism of catecholamines by catechol-O-methyltransferase in cells expressing recombinant catecholamine transporters. J. Neurochem. 1997;69:1459–1466. doi: 10.1046/j.1471-4159.1997.69041459.x. [DOI] [PubMed] [Google Scholar]
- FALKENBURGER B.H., BARSTOW K.L., MINTZ I.M. Dendrodendritic inhibition through reversal of dopamine transport. Science. 2001;293:2465–2467. doi: 10.1126/science.1060645. [DOI] [PubMed] [Google Scholar]
- FEELISCH M. The use of nitric oxide donors in pharmacological studies. Naunyn-Schmiedebergs Arch. Pharmacol. 1998;358:113–122. doi: 10.1007/pl00005231. [DOI] [PubMed] [Google Scholar]
- FERRERO R., RODRIGUEZ-PASCUAL F., MIRAS-PORTUGAL M.T., TORRES M. Comparative effects of several nitric oxide donors on intracellular cyclic GMP levels in bovine chromaffin cells: correlation with nitric oxide production. Br. J. Pharmacol. 1999;127:779–787. doi: 10.1038/sj.bjp.0702607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZHUGH A.L., KEEFER L.K. Diazeniumdiolates: pro- and antioxidant applications of the ‘NONOates'. Free Radic. Biol. Med. 2000;28:1463–1469. doi: 10.1016/s0891-5849(00)00251-3. [DOI] [PubMed] [Google Scholar]
- GOMEZA J., ZAFRA F., OLIVARES L., GIMENEZ C., ARAGON C. Regulation by phorbol esters of the glycine transporter (GLYT1) in glioblastoma cells. Biochim. Biophys. Acta. 1995;1233:41–46. doi: 10.1016/0005-2736(94)00249-o. [DOI] [PubMed] [Google Scholar]
- GUEVARA-GUZMAN R., EMSON P.C., KENDRICK K.M. Modulation of in vivo striatal transmitter release by nitric oxide and cyclic GMP. J. Neurochem. 1994;62:807–810. doi: 10.1046/j.1471-4159.1994.62020807.x. [DOI] [PubMed] [Google Scholar]
- JANOWSKY A., NEVE K., ESHLEMAN A.J.Uptake and release of neurotransmitters Current Protocols in Neuroscience 1998suppl. 2New York: John Wiley & Sons, Inc; 1–22.unit 7.9. eds. Crawley, J.N., Gerfen, C.R., Rogawski, M.A., Sibley, D.R. & Skolnick, P. pp [DOI] [PubMed] [Google Scholar]
- JAVITCH J.A., D'AMATO R.J., STRITTMATTER S.M., SNYDER S.H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. U.S.A. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R.A., ESHLEMAN A.J., MEYERS T., NEVE K.A., JANOWSKY A. [3H]substrate- and cell-specific effects of uptake inhibitors on human dopamine and serotonin transporter-mediated efflux. Synapse. 1998;30:97–106. doi: 10.1002/(SICI)1098-2396(199809)30:1<97::AID-SYN12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- KAYE D.M., GRUSKIN S., SMITH A.I., ESLER M.D. Nitric oxide mediated modulation of norepinephrine transport: identification of a potential target for S-nitrosylation. Br. J. Pharmacol. 2000;130:1060–1064. doi: 10.1038/sj.bjp.0703416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISS J.P., VIZI E.S. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001;24:211–215. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- LEVIEL V. The reverse trasnport of DA, what physiological significance. Neurochem. Int. 2001;38:83–106. doi: 10.1016/s0197-0186(00)00076-0. [DOI] [PubMed] [Google Scholar]
- LONART G., CASSELS K.L., JOHNSON K.M. Nitric oxide induces calcium-dependent [3H]dopamine release from striatal slices. J. Neurosci. Res. 1993;35:192–198. doi: 10.1002/jnr.490350210. [DOI] [PubMed] [Google Scholar]
- LONART G., JOHNSON K.M. Inhibitory effects of nitric oxide on the uptake of [3H]dopamine and [3H]glutamate by striatal synaptosomes. J. Neurochem. 1994;63:2108–2117. doi: 10.1046/j.1471-4159.1994.63062108.x. [DOI] [PubMed] [Google Scholar]
- LONART G., WANG J., JOHNSON K.M. Nitric oxide induces neurotransmitter release from hippocampal slices. Eur. J. Pharmacol. 1992;220:271–272. doi: 10.1016/0014-2999(92)90759-w. [DOI] [PubMed] [Google Scholar]
- LONART G., ZIGMOND M.J. High glutamate concentrations evoke Ca(++)-independent dopamine release from striatal slices: a possible role of reverse dopamine transport. J. Pharmacol. Exp. Ther. 1991;256:1132–1138. [PubMed] [Google Scholar]
- MACIVER C.R., BEDNAR D.L., KARBON E.W. Opposite effects of spermine and arcaine on responses of N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Neurosci. Lett. 1991;132:146–150. doi: 10.1016/0304-3940(91)90288-5. [DOI] [PubMed] [Google Scholar]
- MAYFIELD R.D., ZAHNISER N.R. Dopamine D(2) receptor regulation of the dopamine transporter expressed in xenopus laevis oocytes is voltage-independent. Mol. Pharmacol. 2001;59:113–121. doi: 10.1124/mol.59.1.113. [DOI] [PubMed] [Google Scholar]
- NICHOLLS J.G., MARTIN A.R., WALLACE B.G. From Neuron to Brain. Sunderland, MA: Sinauer Associates, Inc; 1992. pp. 159–160. [Google Scholar]
- PARK S.U., FERRER J.V., JAVITCH J.A., KUHN D.M. Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: potential mechanism of neurotoxicity in dopamine neurons. J. Neurosci. 2002;22:4399–4405. doi: 10.1523/JNEUROSCI.22-11-04399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGUN S., BAUMANN M.H., KUHAR M.J. Nitric oxide inhibits [3H]dopamine uptake. Brain Res. 1994;641:83–91. doi: 10.1016/0006-8993(94)91818-x. [DOI] [PubMed] [Google Scholar]
- REITH M.E.A., XU C., ZHANG L., COFFEY L.L. Translocation of dopamine and binding of WIN 35,428 measured under identical conditions in cells expressing the cloned human dopamine transporter. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:295–304. doi: 10.1007/BF00171060. [DOI] [PubMed] [Google Scholar]
- RITZ M.C., MANTIONE C.R., LONDON E.D. Spermine interacts with cocaine binding sites on dopamine transporters. Psychopharmacology (Berl) 1994;114:47–52. doi: 10.1007/BF02245443. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-MARTIN E., CASAREJOS M.J., BAZAN E., CANALS S., HERRANZ A.S., MENA M.A. Nitric oxide induces differentiation in the NB69 human catecholamine-rich cell line. Neuropharmacology. 2000;39:2090–2100. doi: 10.1016/s0028-3908(00)00049-6. [DOI] [PubMed] [Google Scholar]
- ROY B., DU MOULINET D'HARDEMARE A., FONTECAVE M. New thionitrites: synthesis, stability, and nitric oxide generation. J. Org. Chem. 1994;59:7019–7026. [Google Scholar]
- SITTE H.H., HUCK S., REITHER H., BOEHM S., SINGER E.A., PIFL C. Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J. Neurochem. 1998;71:1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- VERKHRATSKY A., STEINHÄUSER C. Ion channels in glial cells. Brain Res. Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- WEST A.R., GALLOWAY M.P. Nitric oxide and potassium chloride-facilitated striatal dopamine efflux in vivo: role of calcium-dependent release mechanisms. Neurochem. Int. 1998;33:493–501. doi: 10.1016/s0197-0186(98)00054-0. [DOI] [PubMed] [Google Scholar]
- WEST A.R., GALLOWAY M.P., GRACE A.A. Regulation of striatal dopamine neurotransmission by nitric oxide: Effector pathways and signaling mechanisms. Synapse. 2002;44:227–245. doi: 10.1002/syn.10076. [DOI] [PubMed] [Google Scholar]
- WHITEHEAD R.E., FERRER J.V., JAVITCH J.A., JUSTICE J.B. Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J. Neurochem. 2001;76:1242–1251. doi: 10.1046/j.1471-4159.2001.00125.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS K., HANNA J.L., MOLINOFF P.B. Developmental changes in the sensitivity of the N-methyl-D-aspartate receptor to polyamines. Mol. Pharmacol. 1991;40:774–782. [PubMed] [Google Scholar]
- WU Q., REITH M.E.A., WALKER Q.D., CARROL F.I., GARRIS P.A. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: An in vivo voltammetric study. J. Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMORA R., MATTHYS K.E., HERMAN A.G. The protective role of thiols against nitric oxide-mediated cytotoxicity in murine macrophage J774 cells. Eur. J. Pharmacol. 1997;321:87–96. doi: 10.1016/s0014-2999(96)00918-1. [DOI] [PubMed] [Google Scholar]


