Abstract
Peripheral lesion to the trigeminal nerve may induce severe pain states. Several lines of evidence have suggested that the antimigraine effect of the triptans with 5-HT1B/1D receptor agonist properties may result from inhibition of nociceptive transmission in the spinal nucleus of the trigeminal nerve by these drugs. On this basis, we have assessed the potential antinociceptive effects of sumatriptan and zolmitriptan, compared to dihydroergotamine (DHE), in a rat model of trigeminal neuropathic pain.
Chronic constriction injury was produced by two loose ligatures of the infraorbital nerve on the right side. Responsiveness to von Frey filament stimulation of the vibrissal pad was used to evaluate allodynia.
Two weeks after ligatures, rats with a chronic constriction of the right infraorbital nerve displayed bilateral mechanical hyper-responsiveness to von Frey filament stimulation of the vibrissal pad with a mean threshold of 0.38±0.04 g on the injured side and of 0.43±0.04 g on the contralateral (left) side (versus ⩾12.5 g on both sides in the same rats prior to nerve constriction injury).
Sumatriptan at a clinically relevant dose (100 μg kg−1, s.c.) led to a significant reduction of the mechanical allodynia-like behaviour on both the injured and the contralateral sides (peak-effects 6.3±1.1 g and 4.4±0.7 g, respectively). A more pronounced effect was obtained with zolmitriptan (100 μg kg−1, s.c.) (peak-effects: 7.4±0.9 g and 3.2±1.3 g) whereas DHE (50–100 μg kg−1, i.v.) was less active (peak-effect ∼1.5 g).
Subcutaneous pretreatment with the 5-HT1B/1D receptor antagonist, GR 127935 (3 mg kg−1), prevented the anti-allodynia-like effects of triptans and DHE. Pretreatment with the 5-HT1A receptor antagonist, WAY 100635 (2 mg kg−1, s.c.), did not alter the effect of triptans but significantly enhanced that of DHE (peak effect 4.3±0.5 g).
In a rat model of peripheral neuropathic pain, which consisted of a unilateral loose constriction of the sciatic nerve, neither sumatriptan (50–300 μg kg−1) nor zolmitriptan (50–300 μg kg−1) modified the thresholds for paw withdrawal and vocalization in response to noxious mechanical stimulation.
These results support the rationale for exploring the clinical efficacy of brain penetrant 5-HT1B/1D receptor agonists as analgesics to reduce certain types of trigeminal neuropathic pain in humans.
Keywords: Antinociception, 5-HT1B/1D receptor agonists, sumatriptan, zolmitriptan, dihydroergotamine, trigeminal neuropathic pain
Introduction
Trigeminal neuropathic pain, i.e. pain resulting from functional changes in peripheral and central pathways subsequent to injury to the trigeminal nervous system, offers a most difficult challenge to therapy. To date, only antidepressants and anticonvulsants may be beneficial in the treatment of trigeminal neuralgia, albeit up to one-half of the patients become refractory or intolerant to these medications (Swerdlow, 1984).
In a recently developed rat model of trigeminal pain, neuropathy is produced by loosely ligaturing the infraorbital nerve. Most rats with this constrictive ligature of the third branch of the trigeminal nerve consistently display signs of abnormal spontaneous pain-related behaviour (Kryzhanovski et al., 1992; 1993) as well as mechanical (Vos et al., 1994; Idänpään-Heikkilä & Guilbaud, 1999; Benoliel et al., 2001) and thermal hypersensitivity (Kryzhanovski et al., 1992; Imamura et al., 1997). Earlier studies have investigated the effects of various pharmacological treatments in such a model in rats. The behavioural abnormalities of this animal model of trigeminal neuropathic pain seem difficult to treat, in close resemblance with the clinical disorders in human subjects suffering from trigeminal neuralgia. Thus, single or repeated injections of morphine or the tricyclic antidepressants amitriptyline and clomipramine were devoid of any effects on the mechanical hyper-responsiveness in rats bearing ligatures on the infraorbital nerve (Idänpään-Heikkilä & Guilbaud, 1999). The anticonvulsant carbamazepine resulted in motor incoordination, a major drawback at the dose-levels required to modify the mechanical hyper-responsiveness (Idänpään-Heikkilä & Guilbaud, 1999), while the novel antiepileptic compound gabapentin was found to exert some antinociceptive effects (Christensen et al., 2001). Repeated injections of the GABAB receptor agonist, baclofen, partially alleviated the pain-related behaviour, suggesting that functional deficits in the GABAergic system might contribute to trigeminal neuropathic pain in this model (Idänpään-Heikkilä & Guilbaud, 1999). In addition, the combination of the glycine/NMDA receptor antagonist (+)-HA-966 ((+)-3-amino-1-hydroxy-2-pyrrolidone) and morphine was also found to attenuate pain-related behaviour in rats bearing ligatures on the infraorbital nerve (Christensen et al., 1999), probably through the resulting decreased glutamate neurotransmission associated with the promotion of opioidergic mechanisms.
Migraine headache is thought to result from an abnormal distension of meningeal blood vessels and the consequent activation of the trigeminal nervous system (Edvinsson, 2001; Goadsby, 2001). The observation that hyperalgesia and allodynia of the face and the scalp often accompany migraine headache suggests that sensitization may occur in spinal nuclei of the trigeminal nerve in response to sustained noxious inputs from distended intracranial blood vessels (Cumberbatch et al., 1999; Burstein et al., 2000a, b; Goadsby, 2001). Migraine is efficiently treated by drugs acting at 5-hydroxytryptamine (5-HT)1B/1D receptors, especially the so-called triptans which are rather selective agonists at these receptors (Pauwels & John, 1999). Triptans and other 5-HT1B/1D receptor agonists inhibit the activity of trigeminal nucleus neurons evoked by direct electrical stimulation of the superior sagittal sinus or neurogenic inflammation of the meninges (Nozaki et al., 1992; Goadsby & Hoskin, 1996; Goadsby & Knight, 1997a; Martin, 1997; Cumberbatch et al., 1998a; Storer et al., 2001), an effect which very probably accounts for the efficacy of these drugs to reduce pain associated with migraine. In addition, triptans are effective in the treatment of cluster headache (Plosker & McTavish, 1994; Pini & Morelli, 1999; Besson & Dananchet, 2000; Edvinsson, 2001). This led us to analyse the effects of triptans in the rat model of trigeminal neuropathic pain described above (Kryzhanovski et al., 1992; 1993; Vos et al., 1994). Appropriate mechanical nociceptive tests were used to assess the potential antinociceptive effects of two of these drugs, sumatriptan and zolmitriptan, compared to the classical antimigraine ergot derivative, dihydroergotamine (DHE). In addition, the possible implication of 5-HT1A and 5-HT1B/1D receptors in these effects was assessed by investigating whether they could be prevented by selective antagonists. Finally, because pain caused by trigeminal mechanisms may differ from peripheral pain implicating the spinal cord with regard to their respective sensitivity to analgesic drugs, we also investigated the possible effect of triptans in the mononeuropathic pain model of Bennett & Xie (1988), in which one sciatic nerve is ligatured.
Methods
The ethical guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP, 1983) were adhered to in these studies.
Animals
Male Sprague-Dawley rats (Iffa-Credo, Lyon, France), n=174, weighing 175–200 g on arrival, were used. Animals were housed at six in an appropriate cage on a 12 h light/12 h dark cycle. The ambient temperature was kept at 22±1°C, and the rats had free access to standard laboratory food and tap water. The animals were allowed to habituate to the housing facilities for at least 1 week before starting the experiments.
Induction of mononeuropathy
Rats were anaesthetized with an interaperitoneal (i.p.) injection of sodium pentobarbital (Nembutal, 50 mg kg−1). The unilateral chronic constriction injury to the right intraorbital nerve was performed under direct visual control using a Zeiss microscope (×10–25) essentially as described by Vos et al. (1994). The head of the rat was fixed in a stereotaxic frame and a mid-line scalp incision was made, exposing skull and nasal bone. The infraorbital part of the right infraorbital nerve was then exposed using a surgical procedure adapted from Gregg (1973) and Jacquin & Zeigler (1983). The edge of the orbit, formed by the maxillary, frontal, lacrimal, and zygomatic bones, was dissected free. To give access to the infraorbital nerve, the orbital contents were gently deflected. The infraorbital nerve was dissected free at its most rostral extent in the orbital cavity, just caudal to the infraorbital foreamen. Two chromic catgut (5–0) ligatures were tied loosely (with about 2 mm spacing) around the nerve. Under our own conditions, a ‘slip knot' was tied at the two ends of the chromic catgut filament which allowed the perfect control of the degree of constriction of the infraorbital nerve. To obtain the desired degree of constriction, the criterion formulated by Bennett & Xie (1988) was applied: the ligatures reduced the diameter of the nerve by a just noticeable amount and retarded, but did not interrupt, the epineural circulation. Blood circulation through epineural vessels was checked under direct visual control using the Zeiss operation microscope. In order to confirm that mechanical hyper-responsiveness in operated animals was specific for the chronic constriction injury to the infraorbital nerve, sham-operated animals were also tested in the series of experiments reported herein. In these rats, the right infraorbital nerve was exposed using the same procedure, but the nerve was not ligatured.
Unilateral peripheral mononeuropathy was produced on the right hindpaw according to the method described by Bennett & Xie (1988). The common sciatic nerve was exposed by blunt dissection at the level of the midthigh and four chromic catgut (5–0) ligatures were tied loosely (with about 1 mm spacing) around the nerve. As for the infraorbital nerve, care was taken to avoid any interruption of the epineural circulation. In the sham-operated rats, the right common sciatic nerve was exposed using the same procedure, but the nerve was not ligatured.
Nociceptive test procedures
Infraorbital nerve-ligatured rats were placed individually in plastic cages (35×20×15 cm), where they adapted to the testing environments for 2 h. During this period, the experimenter entered slowly his hand into the cage to touch the walls of the cage with a plastic rod, similar to the ones on which the von Frey filaments are mounted. After the 2 h-habituation period, rats were in a sniffing/no locomotion state (with the four paws placed on the ground neither moving nor freezing) and the stimulation session was started. Mechanical sensitivity was determined with a graded series of 10 von Frey filaments (Semmes-Weinstein monofilaments, Stoelting, Wood Dale, IL, U.S.A.). The filaments produced a bending force of 0.217, 0.445, 0.745, 0.976, 2.35, 4.19, 4.64, 6.00, 7.37 and 12.5 g. The 12.5 g filament, the bending force of which already turned the head of the rat, was chosen as the cut-off. Stimuli were applied within the infraorbital nerve territory, near the centre of the vibrissal pad, on the hairy skin surrounding the mystacial vibrissae. These areas were stimulated on both sides of the face, ipsilateral and contralateral to the constricted nerve. In all cases, the stimulation was applied first on the contralateral side with the filament producing the lowest force. Each stimulation consisted of three consecutive applications (1 s apart) of the stimulus filament. For each session, the complete series of von Frey filaments was applied following an increasing force order, until a well defined behavioural response was triggered. As previously described by Vos et al. (1994), this response consisted of a brisk withdrawal of the head or/and an attack/escape reaction. Brisk withdrawal of the head was often followed by an uninterrupted series of at least three face wash strokes directed to the stimulated facial area. In the case of attack/escape reaction, the rat avoided further contact with the filament either passively by moving its body away from the stimulating object to a crouching position against cage wall, or actively by attacking the stimulating object, making biting and grabbing movements. The minimal force applied through von Frey filaments to trigger at least one of these behaviours was considered as the mechanical response threshold. Thresholds to stimulation of the face by von Frey filaments were determined 2 days before and 14 days after surgery, at a time when hyper-responsiveness to mechanical stimulation had fully developed (Vos et al., 1994; Idänpään-Heikkilä & Guilbaud, 1999).
In sciatic nerve-ligatured rats, nociceptive thresholds, expressed as grams (g), were measured with a Ugo Basile analgesimeter (Bioseb, Chaville, France) by applying an increasing pressure to the nerve-injured and the contralateral hindpaw of unrestrained rats until a paw withdrawal (paw withdrawal threshold) and thereafter a squeak (vocalisation threshold) were obtained (Le Bars et al., 2001). We systemically compared the effects of compounds on the paw withdrawal to pressure, a spinally coordinated reflex, and the vocalization threshold to paw pressure, a supra-spinal integrated response (Kayser & Christensen, 2000) in order to explore the relative contribution of spinal versus supra-spinal mechanisms to their antinociceptive effects. In each rat, thresholds to paw pressure were determined one day before and 14 days after sciatic nerve ligatures in order to assess the development of mechanical allodynia after surgery.
Pharmacological treatments
Sciatic nerve constricted rats (n=42) received subcutaneous (s.c.) injections of either (n=6 for each treatment) saline, sumatriptan (50, 100 and 300 μg kg−1) or zolmitriptan (50, 100 and 300 μg kg−1). The doses of triptans were chosen to match those employed in human migraine (6 mg s.c., Sumatriptan Investigators' Brochure, GlaxoWelcome). Testing sessions, beginning at 09:30 h, were conducted in a quiet room by the same person, blind to the injected solution. Rats were randomly assigned in groups of 4–6 for a given series of tests. Each animal received only one dose of a given drug or the vehicle, and was used in only one experiment. Mechanical response thresholds were measured at 10 min intervals for the first hour after administration of sumatriptan, zolmitriptan or saline, then every hour over a 4 h period.
Infraorbital nerve-constricted rats (n=120) were divided into two groups. In order to determine the effects of various drug treatments, the first group (n=72) was subdivided into three subgroups (n=24 each). In the first subgroup, the effects of intravenous (i.v.) injections of DHE (25, 50 and 100 μg kg−1) were analysed. The doses of DHE and route of administration were based on previous experiments showing a pronounced reduction in the firing of central trigeminal neurons within 30 min of DHE administration (Hoskin et al., 1996a). Mechanical response thresholds were measured at 15 min intervals for the first hour after DHE administration, then at 30 min intervals for the second hour and finally at the third and fourth hour after the treatment. A control group (saline, 0.1 ml kg−1) was tested simultaneously.
In the other two subgroups, the effects of s.c. injections of either sumatriptan or zolmitriptan (50, 100 and 300 μg kg−1) were analysed. Mechanical response thresholds were measured at 1 h intervals over a 9 h period. Control rats injected with saline (0.1 ml kg−1) were tested in parallel.
The second group of rats (n=48) was subdivided into two subgroups (n=24 each) for assessing the possible involvement of 5-HT1B/1D and 5-HT1A receptors in the antinociceptive effects of the drugs tested. Rats of the first subgroup were pretreated with the 5-HT1B/1D receptor antagonist GR 127935 (3 mg kg−1, s.c., Skingle et al., 1996) 20 min before i.v. injection of DHE (50 μg kg−1, n=6) or s.c. injection of sumatriptan (100 μg kg−1, n=6), zolmitriptan (100 μg kg−1, n=6) or saline (n=6). Rats of the second subgroup were pretreated with the 5-HT1A receptor antagonist WAY 100635 (2 mg kg−1, s.c., Fletcher et al., 1996) also 20 min before administration of saline (n=6), DHE (n=6), sumatriptan (n=6) or zolmitriptan (n=6) at the same doses as indicated above. Mechanical response thresholds were measured at the same time points as those selected in experiments were no antagonist was injected (see above).
Data presentation and statistical analyses
Data are expressed as the means±s.e.m. The non-parametric Kruskall & Wallis (1952) one-way analysis of variance was used and comparisons between the groups were performed using the Mann–Whitney U-test. The areas under the time-course curves (AUCs) were calculated using the trapezoidal rule. The t-test was used to compare overall effects (AUCs) between two groups. The significance level was P<0.05.
Drugs
Sumatriptan succinate and GR 127935 (2′-methyl-4′-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid [4-methoxy-3-(4-methyl-piperazine-1-yl)-phenyl]amide hydrochloride) were from GlaxoWelcome (Taplow, UK); zolmitriptan was from Astra-Zeneca (Södertälje, Sweden); DHE mesylate was from Sanofi-Synthelabo (Bagneux, France), and WAY 100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl-N-(2-pyridinyl)-cyclohexanecarboxamide dihydrochloride) was from Wyeth Res. (Princeton, NJ, U.S.A.). All compounds were dissolved in 0.9% NaCl. Solutions were prepared immediately before use.
Results
Mechanical response thresholds in nerve-injured rats
The mechanical response threshold on the nerve-injured side of rats with an infraorbital nerve ligature was 0.38±0.04 g on postoperative day 14, a value considerably less than the 12.5 g threshold observed in sham-operated rats (n=6, not shown) and the 12.5 g pre-ligature threshold (Figure 1A). The mechanical response threshold to stimulation of the territory of the contralateral infraorbital nerve was also markedly decreased (0.43±0.04 g) vs sham-operated rats (12.5 g), and in fact, both sides appeared equally hypersensitive to mechanical stimulation (Figure 1A).
Figure 1.
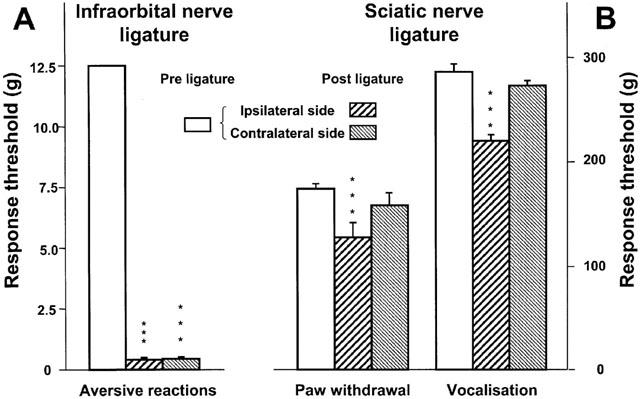
Mechanical response thresholds before (‘Pre ligature') and 2 weeks after (‘Post ligature') ligature of (A) the right infraorbital nerve or (B) the right sciatic nerve. Response thresholds were determined in the territories ipsilateral and contralateral to the ligature. Data (means±s.e.m., A: n=138, B: n=18) are expressed as grams (g). ***P<0.001 versus pre ligature value.
As illustrated in Figure 1B, the paw withdrawal threshold measured on the ipsilateral side of sciatic nerve ligatures (127±7 g) was significantly lower than the pre-ligature value (175±5 g). In addition, the vocalization threshold to ipsilateral paw pressure on the 14th postoperative day (221±9 g) was also significantly decreased compared to that measured before the surgery (286±9 g) (Figure 1B). In contrast, the paw withdrawal and the vocalization thresholds measured on the contralateral side of sciatic nerve ligatures did not significantly differ between the pre- and the post-operative tests (Figure 1B). In the sham-operated rats, no variations could be detected in the responsiveness to mechanical stimulation of the ipsilateral paw on postoperative day 14 (paw withdrawal threshold 173±7 g and vocalization threshold 280±8 g, compared to 174±6 g and 284±7 g, respectively, before sham operation; n=6).
Effects of DHE, sumatriptan and zolmitriptan on mechanical response thresholds in infraorbital nerve-ligatured rats
Figure 2 shows that i.v. injection of saline or DHE at 25 μg kg−1 had no effect on the response threshold to mechanical stimulation applied ipsilaterally or contralaterally to the ligatured infraorbital nerve. By contrast, higher doses of DHE, 50 and 100 μg kg−1, raised the mechanical response thresholds to values up to ∼1.5 g. Although the maximal increase was similar at both doses, the change in the response threshold reached statistical significance earlier after 50 μg kg−1 than after 100 μg kg−1 of the drug (Figure 2). At both ipsilateral and contralateral sides, the effects of DHE lasted for about 2 h after the injection.
Figure 2.
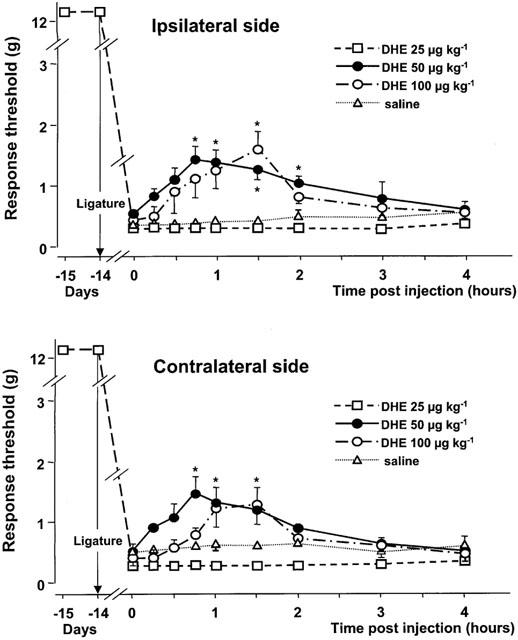
Time-course curves of the effects of various doses of dihydroergotamine (DHE) or saline on the mechanical response thresholds in the infraorbital nerve territory ipsilateral and contralateral to the ligature. Data (means±s.e.m., n=6) are expressed as grams (g). *P<0.05 versus saline.
Of the three doses tested, only the dose of 100 μg kg−1 s.c. of sumatriptan resulted in a significant elevation of the response threshold at both the nerve-injured and the contralateral side (Figure 3). This effect reached statistical significance already at 1 h after the injection and lasted for about 5 h. The maximal increase in the response thresholds at both the nerve-injured (6.3±1.1 g) and the contralateral (4.4±0.7 g) side was observed 3 h after the injection. The overall effect of the dose of 100 μg kg−1 on both the nerve-injured and the contralateral sides did not differ (P=0.027). In contrast with the lack of behavioural changes in rats treated with 50 or 100 μg kg−1 of sumatriptan, those treated with the 300 μg kg−1 dose appeared hyperreactive to external stimuli (noises) with signs of increased locomotor activity.
Figure 3.
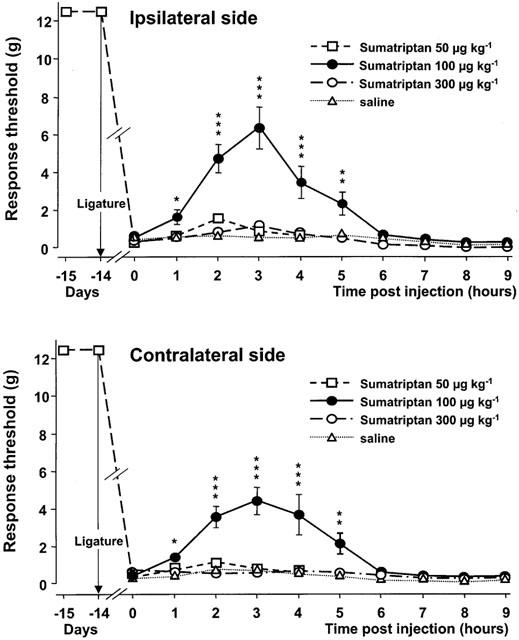
Time-course curves of the effects of various doses of sumatriptan or saline on the mechanical response thresholds in the infraorbital nerve territory ipsilateral and contralateral to the ligature. Data (means±s.e.m., n=6) are expressed as grams (g). *P<0.05, **P<0.01, ***P<0.001 versus saline.
Figure 4 illustrates the effect of zolmitriptan. Subcutaneous injections of this drug at 50 and 100 μg kg−1 increased the mechanical response threshold at both the nerve-injured and the contralateral side. Peak effects for both the nerve-injured (2.3±0.5 g) and the contralateral (1.2±0.2 g) side were reached 3 h after injection of the lowest dose (50 μg kg−1), and a significant increase in response threshold was still observed 1 h later. Also at the dose of 100 μg kg−1, the maximal increase in the response thresholds at the nerve-injured (7.4±0.9 g) and the contralateral (3.2±1.3 g) side caused by zolmitriptan was reached 3 h after the injection. However, at this dose, the effect lasted for up to 8 h at the nerve-injured side. Comparison of the overall effect of zolmitriptan at 50 and 100 μg kg−1 at the nerve-injured versus the contralateral side indicated a significantly larger increase in response threshold on the injured side (P=0.03). At the highest dose tested, 300 μg kg−1, zolmitriptan induced only minor changes in mechanical response thresholds (Figure 4). However, at this dose, but not at the other two, rats were hyperreactive to external stimuli.
Figure 4.
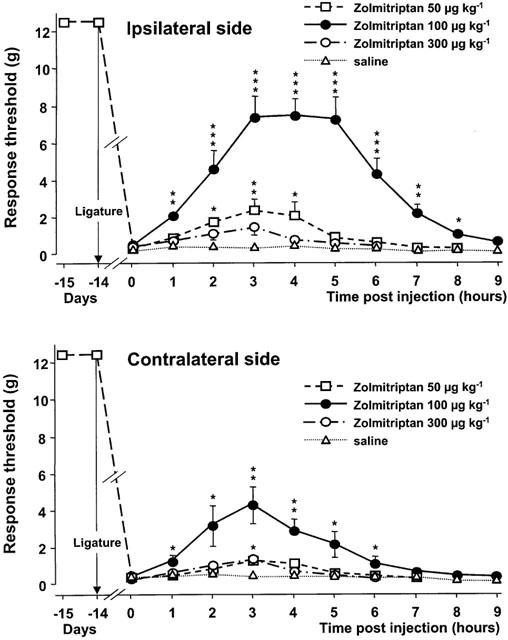
Time-course curves of the effects of various doses of zolmitriptan or saline on the mechanical response thresholds in the infraorbital nerve territory ipsilateral and contralateral to the ligature. Data (means±s.e.m., n=6) are expressed as grams (g). *P<0.05, **P<0.01, ***P<0.001 versus saline.
Effects of sumatriptan and zolmitriptan on mechanical response thresholds in sciatic nerve-ligatured rats
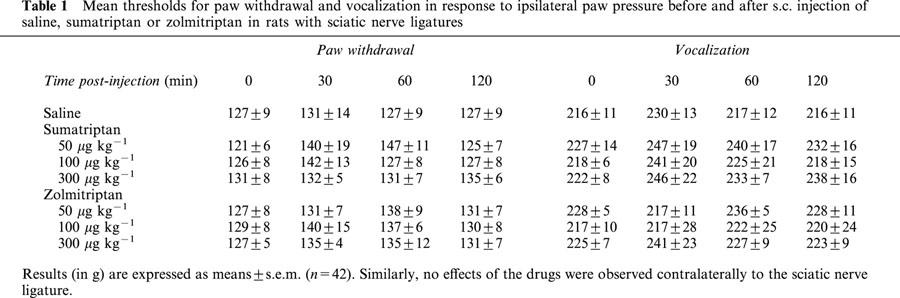
Table 1 summarizes the results obtained from experiments aimed at determining paw withdrawal and vocalisation thresholds prior to and 30, 60 and 120 min following s.c. injection of sumatriptan (50, 100 and 300 μg kg−1) or zolmitriptan (50, 100 and 300 μg kg−1) compared to saline in rats with sciatic nerve ligatures. In contrast to that observed in rats with infraorbital nerve ligatures, none of these drugs at the three doses tested reduced the mechanical allodynia in rats bearing unilateral ligatures of the sciatic nerve.
Table 1.
Mean thresholds for paw withdrawal and vocalization in response to ipsilateral paw pressure before and after s.c. injection of saline, sumatriptan or zolmitriptan in rats with sciatic nerve ligatures
Prevention by GR 127935 of DHE- and triptan-induced increase in mechanical response thresholds in infraorbital nerve-ligatured rats
As depicted in Figure 5, the apparent rank order of potency of drugs tested against mechanical hyper-responsiveness in the territory of the ipsilateral infraorbital nerve was DHE<sumatriptan<zolmitriptan. For the contralateral side, this order was DHE<sumatriptan=zolmitriptan.
Figure 5.
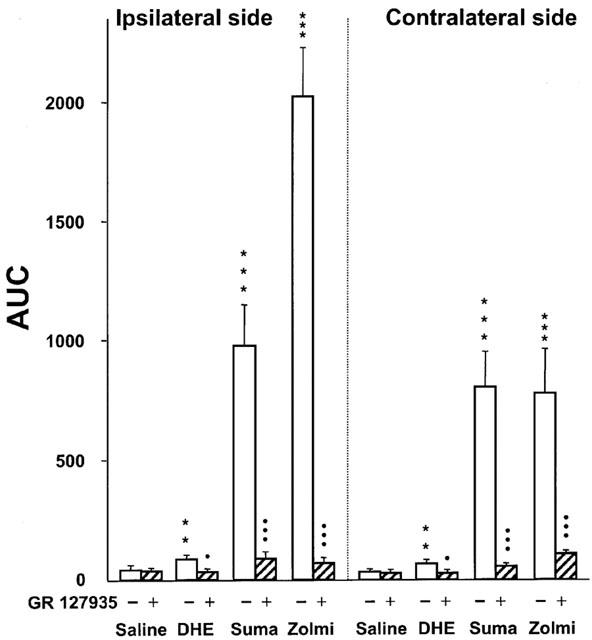
Areas under the curves (AUC, g×min, means±s.e.m.) of the respective time-course curves in Figures 2,3,4 (open bars) and effect of pretreatment with GR 127935 (3 mg kg−1, s.c. 20 min before, hatched bars) on mechanical response thresholds in the infraorbital nerve territory ipsilateral and contralateral to the ligature after administration of dihydroergotamine (DHE, 50 μg kg−1, i.v.), sumatriptan (Suma, 100 μg kg−1, s.c.) or zolmitriptan (Zolmi, 100 μg kg−1, s.c.). **P<0.01, ***P<0.001 versus saline, •P<0.05, •••P<0.001 versus treatment with DHE, sumatriptan or zolmitriptan alone, n=6 in each group.
When administered 20 min prior to saline, GR 127935 (3 mg kg−1, s.c.) did not affect the mechanical hyper-responsiveness in infraorbital nerve-constricted rats. However, this treatment completely prevented the increase in mechanical response thresholds caused by 50 μg kg−1 DHE (not shown) and markedly reduced those caused by sumatriptan (100 μg kg−1) and zolmitriptan (100 μg kg−1) in the operated rats (Figure 5). As illustrated in Figure 5, the effects of GR 127935 were similar on the nerve-injured and the contralateral side.
Effects of WAY 100635 on DHE- and triptan-induced increase in mechanical response thresholds in infraorbital nerve-ligatured rats
As shown in Figure 6, WAY 100635 (2 mg kg−1, s.c.) had no effect on the responses at either side, when administered 20 min prior to saline. However, pretreatment with WAY 100635 markedly increased the enhancing effect of DHE (50 μg kg−1) on mechanical response threshold both on the ipsilateral and the contralateral side of the ligatured nerve (Figure 6). Indeed, calculation of the corresponding AUCs indicated that the amplitude of the DHE-induced effect was increased by at least 2 fold after pretreatment with WAY 100635. By contrast, this pretreatment did not significantly affect the increase in mechanical response thresholds caused by sumatriptan or zolmitriptan at 100 or 300 μg kg−1 (P>0.05) (not shown).
Figure 6.
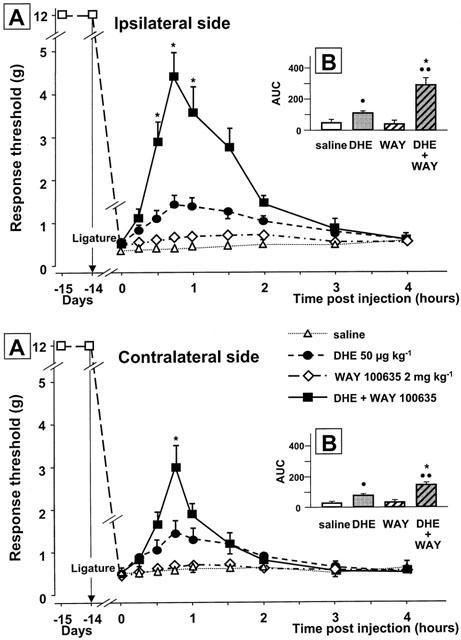
Effect of pretreatment with WAY 100635 (2 mg kg−1, s.c., 20 min before) on dihydroergotamine (DHE, 50 μg kg−1, i.v.)-induced changes in the mechanical response thresholds in the infraorbital nerve territory ipsilateral or contralateral to the ligature. Results are expressed as (A) the time-course curves of the mean±s.e.m., in grams, of the response thresholds and (B) the mean±s.e.m. of areas under the time-course curves (AUC) calculated by the trapezoidal rule (see Methods). •P<0.05, •••P<0.01 versus saline; *P<0.05 versus DHE (50 μg kg−1, i.v.) alone, n=6 in each group.
Discussion
Several lines of evidence have suggested that the antimigraine effect of the triptans with 5-HT1B/1D receptor agonist properties may result from inhibition of central nociceptive transmission in the spinal nucleus of the trigeminal nerve by these drugs (Edvinsson, 2001; Goadsby, 2001). In this work, we thus tested the action of sumatriptan and zolmitriptan, at doses analogous to those employed in human migraine, in rats with a chronic constriction injury to the infraorbital nerve or the sciatic nerve. Studies comparing the different triptans on the one hand and triptans with other medications on the other hand should ideally be the basis for assessing their actual interest in pain therapy. We therefore compared the efficacy of sumatriptan and zolmitriptan to DHE. The major findings were that: (1) clinically effective antimigraine agents attenuated the hyper-responsiveness to mechanical stimulation of the face in the rat model of trigeminal neuropathic pain, but neither reduced allodynia nor produced antinociception in sciatic nerve-ligatured rats. The apparent rank order of potency derived from the present experiments was zolmitriptan>sumatriptan >DHE; (2) GR 127935, a selective 5-HT1B/1D receptor antagonist (Skingle et al., 1996), suppressed antinociception evoked by DHE and the triptans in infraorbital nerve-ligatured rats; and (3) WAY 100635, a selective 5-HT1A receptor antagonist (Fletcher et al., 1996), enhanced DHE- but did not affect triptan-induced response.
Characteristics of neuropathic pain models
In agreement with Bennett & Xie (1988), unilateral ligature of the sciatic nerve produced a significant decrease in threshold pressures to trigger withdrawal of the ipsilateral hindpaw and vocalization, as expected from the occurrence of mechanical allodynia on the injured side. In contrast, mechanical stimulation of the contralateral side produced the same responses as in intact rats indicating unchanged sensitivity to such a stimulation (Bennett & Xie, 1988).
Interestingly, markedly different observations were made in rats bearing a unilateral ligature of the infraorbital nerve because mechanical response thresholds were found to be decreased on both the ipsilateral and the contralateral, non-operated, side. Previous behavioural studies on this model also showed pronounced mechano-allodynia (Vos et al., 1994; Idänpään-Heikkilä & Guilbaud, 1999; Christensen et al., 1999; 2001) or heat-hyperalgesia (Kryzhanovski et al., 1992; Imamura et al., 1997) on both sides of the face. In addition, electrophysiological changes expected from the occurrence of hyper-responsiveness also on the side without nerve ligation were observed in the corresponding ventropostero-thalamic nucleus and in the primary somatosensory cortex in rats with the same chronic constriction injury to the infraorbital nerve as that made in our studies (Benoist et al., 1999; Vos et al., 2000). In particular, on the intact side, about 50% of the vibrissa neurons exhibited an enlarged peripheral receptive field including more than two vibrissae (Benoist et al., 1999; Vos et al., 2000). In line with these observations, projections from the trigeminal ganglion have been shown to reach the medullary and cervical dorsal horns on both sides (Pfaller & Arvidsson, 1988; Jacquin et al., 1990), and evidence has been reported that unilateral stimulation of the trigeminal ganglion activates neurones in both the ipsilateral and the contralateral caudal trigeminal nucleus (Ingvardsen et al., 1997; Samsam et al., 2001). Accordingly, anatomical and functional organizations of the trigeminal complex fully explain why unilateral injury of the infraorbital nerve produced mechanical allodynia on both sides of the face.
Effect of triptans
In the present study, we clearly demonstrated that both sumatriptan and zolmitriptan were effective in reducing mechanical hypersensitivity after chronic constriction injury to the infraorbital nerve. However, at the optimal dose of 100 μg kg−1, sumatriptan maximally increased the response threshold from 0.4 g to 6.3 g, a value still markedly less than that found in intact animals (>12.5 g). Zolmitriptan was more potent in this regard because the response threshold reached values close to 8 g in rats treated with 100 μg kg−1 of this drug. One possible explanation of the difference between the two compounds is that zolmitriptan crosses the blood brain barrier and reaches brain sites much more easily than sumatriptan (Humphrey et al., 1991; Kaube et al., 1993; Goadsby & Knight, 1997b; Martin, 1997; Johnson et al., 2001). Indeed, a central rather than a peripheral action can be suspected for both drugs since they prevented mechanical allodynia on both the injured and the intact side. Direct investigations (by electrophysiologial recordings or c-fos immunolabelling) of the effects of both sumatriptan and zolmitriptan on neuronal activity within the right (lesioned side) and the left (intact side) spinal nuclei of the trigeminal nerve are needed to provide a clear-cut demonstration of their central action under the present experimental conditions. In any case, the reduction by triptans of the mechanical hyper-responsiveness appeared to be mediated through 5-HT1B/1D receptor stimulation since it could be prevented by the 5-HT1B/1D receptor antagonist GR 127935 (Skingle et al., 1996). In line with these observations, clear-cut evidence has been reported that 5-HT1B/1D receptor agonists prevent the excitation of spinal trigeminal nucleus neurons evoked by direct electrical stimulation of the superior sagittal sinus or neurogenic inflammation of the meninges (Nozaki et al., 1992; Goadsby & Knight, 1997a; Cumberbatch et al., 1998a; Storer et al., 2001), which supports the concept that activation of central 5-HT1B/1D receptors is antinociceptive. Whether only the 5-HT1B or the 5-HT1D receptors, or both receptor types, mediate the antiallodynia effect of triptans cannot be answered yet, but studies with selective antagonists such as SB-224289 (Selkirk et al., 1998) and BRL-15572 (Price et al., 1997) for the 5-HT1B and 5-HT1D receptors, respectively, should provide relevant data to solve this question.
Sumatriptan, as well as zolmitriptan, at the same doses as those used in the infraorbital nerve-ligatured rats, were unaffected in rats with sciatic nerve ligatures. Similarly, sumatriptan (300 μg kg−1 intraperitoneally) was reported to exert no effect on thermal hyperalgesia induced by sciatic nerve ligature in the rat (Bingham et al., 2001). In addition, Cumberbatch et al. (1998b) demonstrated that, at the dose 300 μg kg−1 i.v., the 5-HT1B/1D receptor agonist naratriptan inhibited responses of single trigeminal neurons to noxious electrical and mechanical stimulation of the dura and face, but did not affect spinal dorsal horn neuronal responses to noxious mechanical stimulation of the hind-paw. In addition, Skingle et al. (1990) reported that sumatriptan, up to 10 mg kg−1 s.c., was totally inactive in various validated nociceptive tests based on peripheral noxious stimulations in rodents. However, other data showed that sumatriptan inhibits neurogenic inflammation induced by capsaicin applied to the rat sciatic epineurium (Zochodne & Ho, 1994) at doses comparable to those used in trigeminal extravasation studies. Furthermore, high doses of sumatriptan (>5 mg kg−1 s.c.), much larger than those used in the present study, were needed to produce peripheral antinociceptive effects through cholinergic mechanisms in both rats and mice (Ghelardini et al., 1996b; Jain & Kulkarni, 1998). At lower doses, comparable to those used in migraine models, sumatriptan only prevented hyperalgesia induced by morphine withdrawal (Ghelardini et al., 1996a) and attenuated the hypersensitivity to noxious thermal stimuli induced by intraplantar carrageenin in mice (Bingham et al., 2001). Under our conditions, it may be that peripheral lesion of the trigeminal nerve produces persistent pain that is more sensitive to triptans than the pain-related behaviour caused by chronic constriction injury to the sciatic nerve, in which a single injection of triptan appeared to be ineffective. Indeed, anatomical and embryological differences exist between trigeminal and spinal nerves (Le Douarin & Kalcheim, 1999), which may account for the difference in sensitivity to triptans. While the sciatic nerve is a mixed sensory-motor nerve, the infraorbital nerve is purely sensory and processes, in contrast to spinal nerves, distinct modalities of sensory information independently and from a well-defined and restricted region of the face (Paxinos, 1995).
Within the medulla oblongata as well as in the lumbar enlargement of the spinal cord, 5-HT1B/1D receptors are located on the terminals of both primary afferent fibres and serotonergic fibres, and on intrinsic neurons (interneurons and neurons projecting to brain nuclei) (Bruinvels et al., 1992; 1993; Laporte et al., 1995; Hamon & Bourgoin, 1999). However, the respective quantitative distribution of these receptors in these three distinct neuronal compartments is not precisely known, and differences between the medulla oblongata and the spinal cord might possibly account for the differential effects of triptans on mechanical hyper-responsiveness caused by injury of the infraorbital nerve compared to the sciatic nerve. Clearly, further data on the precise cellular and subcellular distribution of 5-HT1B/1D receptors at trigeminal versus spinal level are needed to possibly solve this question.
Effect of dihydroergotamine
DHE (50 and 100, but not 25 μg kg−1 i.v.) was also found to raise the response threshold in rats with unilateral ligature of the infraorbital nerve, but to a much lower extent than triptans. The effect of DHE was observed on both the nerve-injured and the contralateral side and was mediated through 5-HT1B/1D receptors since it could be prevented by GR 127935. However, DHE is known to bind with high affinity not only to these receptors but also to 5-HT1A, 5-HT2A, 5-HT2C, D1, D2 dopaminergic, α1- and α2-adrenergic receptors (McCarthy & Peroutka, 1989) and with a relatively lower affinity to 5-HT1F receptors (Johnson et al., 1997). Interestingly, the anti-allodynia-like effect of DHE in infraorbital nerve-ligated rats appeared to be markedly higher after the blockade of 5-HT1A receptors by WAY 100635. Thus, DHE alone increased the response threshold from 0.2 g to 1.5 g, whereas this threshold rose up to 4.3 g when DHE was administered after WAY 100635 pretreatment. These data suggest that 5-HT1A receptor stimulation by DHE was in fact pronociceptive, and counteracted its 5-HT1B/1D receptor-mediated antinociceptive action. In line with this interpretation, Alhaider & Wilcox (1993) already reported that 5-HT1A receptor agonists increase sensitivity to noxious stimulation, and Millan et al. (1996) noted that 5-HT1A receptor blockade by WAY 100135 has clear-cut antinociceptive effects in the formalin and writhing tests in rodents. In addition, Ardid et al. (2001) recently showed potentiation of the antinociceptive effect of a serotonergic antidepressant by WAY 100635 in a neuropathic pain model in rats. However, the role of 5-HT1A receptors in nociceptive control mechanism is still a matter of debate because other authors reported clear-cut antinociceptive effects of 5-HT1A receptor stimulation in various relevant paradigms (Gjerstad et al., 1996; Lin et al., 1996). In the present case, experiments with selective 5-HT1A receptor agonists have to be performed in order to further assess the potential worsening action of 5-HT1A receptor stimulation on trigeminal neuropathic pain induced by infraorbital nerve ligature.
A final point to be discussed concerns the lack of pain relief by the highest dose, 300 μg kg−1, of triptans, which may limit our conclusion concerning the antinociceptive effect of these drugs. Indeed, the mechanisms responsible for the disappearance of this effect at this dose cannot be completely inferred from the present investigations. However, it is worth noting that this dose of triptans rendered the rats hyperreactive to external stimuli, and the resulting behavioural alterations might have masked a potential antinociceptive effect. Indeed, central 5-HT1B receptor stimulation is well known to both reduce habituation to auditory stimuli (Dulawa et al., 1997) and increase locomotor activity (Rempel et al., 1993), i.e. produces behavioural changes which might well interfere with assessment of sensitivity to mechanical stimuli under our experimental conditions. On the other hand, it cannot be excluded that at the highest dose used, 300 μg kg−1, both sumatriptan and zolmitriptan interacted not only with 5-HT1B/1D receptors, but also with other receptor types, and that the resulting non selective effects have masked the expected 5-HT1B/1D receptor-mediated antiallodynia effect.
Conclusion
The data reported herein show that antimigraine 5-HT1B/1D receptor agonists exerted anti-allodynic effects in the rat model of trigeminal neuropathic pain which suggests that they might be useful in the treatment of some trigeminal neuropathic pain disorders. Of the three drugs tested, zolmitriptan appeared as the most promising compound for this purpose. Further exploration of the mechanisms involved in the antinociceptive effects of zolmitriptan, sumatriptan and DHE should improve our understanding of nociceptive processing in trigeminal neuropathic pain.
Acknowledgments
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, and the Bristol-Myers Squibb Foundation (Unrestricted Biomedical Research Grant Program). We are grateful to pharmaceutical companies (Astra-Zeneca, GlaxoWelcome, Sanofi-Synthelabo, Wyeth) for generous gifts of drugs used in these studies. B Aubel was recipient of fellowships from the Société Française de Pharmacologie and the Fondation Midy during performance of this work.
Abbreviations
- AUC
areas under the curve
- DHE
dihydroergotamine
- GABA
γ-aminobutyric acid
- 5-HT
5-hydroxytryptamine, serotonin
- NMDA
N-methyl-D-aspartate
References
- ALHAIDER A.A., WILCOX G.L. Differential roles of 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptor subtypes in modulating spinal nociceptive transmission in mice. J. Pharmacol. Exp. Ther. 1993;265:378–385. [PubMed] [Google Scholar]
- ARDID D., ALLOUI A., BROUSSE G., JOURDAN D., PICARD P., DUBRAY C., ESCHALIER A. Potentiation of the antinociceptive effect of clomipramine by a 5-HT1A antagonist in neuropathic pain in rats. Br. J. Pharmacol. 2001;132:1118–1126. doi: 10.1038/sj.bjp.0703897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT G.J., XIE Y.K.A. Peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- BENOIST J.M., GAUTRON M., GUILBAUD G. Experimental model of trigeminal pain in the rat by constriction of one infraorbital nerve: changes in neuronal activities in the somatosensory cortices corresponding to the infraorbital nerve. Exp. Brain Res. 1999;126:383–398. doi: 10.1007/s002210050745. [DOI] [PubMed] [Google Scholar]
- BENOLIEL R., ELIAV E., IADAROLA M.J. Neuropeptide Y in trigeminal ganglion following chronic constriction injury of the rat infraorbital nerve: is there correlation to somatosensory parameters. Pain. 2001;91:111–121. doi: 10.1016/s0304-3959(00)00417-6. [DOI] [PubMed] [Google Scholar]
- BESSON G., DANANCHET Y. Neurologic diagnosis and therapy of headaches. Rev. Stomatol. Chir. Maxillofac. 2000;101:119–128. [PubMed] [Google Scholar]
- BINGHAM S., DAVEY P.T., SAMMONS M., RAVAL P., OVEREND P., PARSONS A.A. Inhibition of inflammation-induced thermal hypersensitivity by sumatriptan through activation of 5-HT1B/1D receptors. Exp. Neurol. 2001;167:65–73. doi: 10.1006/exnr.2000.7521. [DOI] [PubMed] [Google Scholar]
- BRUINVELS A.T., LANDWEHRMEYER B., MOSKOWITZ M.A., HOYER D. Evidence for the presence of 5-HT1B receptor messenger RNA in neurons of the rat trigeminal ganglia. Eur. J. Pharmacol. (Mol. Pharmacol. Sect.) 1992;227:357–359. doi: 10.1016/0922-4106(92)90017-p. [DOI] [PubMed] [Google Scholar]
- BRUINVELS A.T., PALACIOS J.M., HOYER D. Autoradiographic characterization and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- BURSTEIN R., CUTRER M.F., YARNITSKY D. The development of cutaneous allodynia during a migraine attack. Clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000a;123:1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- BURSTEIN R., YARNITSKY D., GOOR-ARYEH I., RANSIL B.J., BAJWA Z.H. An association between migraine and cutaneous allodynia. Ann. Neurol. 2000b;47:614–624. [PubMed] [Google Scholar]
- CHRISTENSEN D., GAUTRON M., GUILBAUD G., KAYSER V. Combined systemic administration of the glycine/NMDA receptor antagonist, (+)-HA966, and morphine attenuates pain-related behaviour in a rat model of trigeminal neuropathic pain. Pain. 1999;83:433–440. doi: 10.1016/S0304-3959(99)00126-8. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN D., GAUTRON M., GUILBAUD G., KAYSER V. Effect of gabapentin and lamotrigine on mechanical allodynia-like behaviour in a rat model of trigeminal neuropathic pain. Pain. 2001;93:147–153. doi: 10.1016/S0304-3959(01)00305-0. [DOI] [PubMed] [Google Scholar]
- COMMITTEE FOR RESEARCH AND ETHICAL ISSUE OF THE IASP Ethical standards for investigations of experimental pain in animals. Pain. 1983;16:109–110. [Google Scholar]
- CUMBERBATCH M.J., HILL R.G., HARGREAVES R.J. The effects of 5-HT1A, 5-HT1B and 5-HT1D receptor agonists on trigeminal nociceptive neurotransmission in anaesthetized rats. Eur. J. Pharmacol. 1998a;362:43–46. doi: 10.1016/s0014-2999(98)00764-x. [DOI] [PubMed] [Google Scholar]
- CUMBERBATCH M.J., HILL R.G., HARGREAVES R.J. Differential effects of the 5-HT1B/1D receptor agonist naratriptan on trigeminal versus spinal nociceptive responses. Cephalalgia. 1998b;18:659–663. doi: 10.1046/j.1468-2982.1998.1810659.x. [DOI] [PubMed] [Google Scholar]
- CUMBERBATCH M.J., WILLIAMSON D.J., MASON G.S., HILL R.G., HARGREAVES R.J. Dural vasodilatation causes a sensitization of rat caudal trigeminal neurones in vivo that is blocked by a 5-HT1B/1D agonist. Br. J. Pharmacol. 1999;126:1478–1486. doi: 10.1038/sj.bjp.0702444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULAWA S.C., HEN R., SCEARCE-LEVIE K., GEYER M.A. Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type and serotonin1B knockout mice. Psychopharmacology. 1997;132:125–134. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L. Aspects on the pathophysiology of migraine and cluster headache. Pharmacol. Toxicol. 2001;89:65–73. doi: 10.1034/j.1600-0773.2001.d01-137.x. [DOI] [PubMed] [Google Scholar]
- FLETCHER A., FORSTER E.A., BILL D.J., BROWN G., CLIFFE I.A., HARTLEY J.E., JONES D.E., MCLENACHAN A., STANHOPE K.J., CRITCHLEY D.J.P., CHILDS K.J., MIDDLEFELL V.C., LANFUMEY L., CORRADETTI R., LAPORTE A.M., GOZLAN H., HAMON M., DOURISH C.T. Electrophysiological, biochemical, neurohormonal and behavioral studies with WAY 100635, a potent selective, and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- GHELARDINI C., GALEOTTI N., DONALDSON S., NICOLODI M., SICUTERI F., BARTOLINI A. Prevention by sumatriptan of hyperalgesia induced by morphine withdrawal. Fundam. Clin. Pharmacol. 1996a;10:192. [Google Scholar]
- GHELARDINI C., GALEOTTI N., NICOLODI M., DONALDSON S., SICUTERI F., BARTOLINI A. The central cholinergic system has a role in the antinociception induced in rodents and guinea pigs by the antimigraine drug sumatriptan. J. Pharmacol. Exp. Ther. 1996b;279:884–890. [PubMed] [Google Scholar]
- GJERSTAD J., TJOLSEN A., HOLE K. The effect of 5-HT1A receptor stimulation on nociceptive dorsal horn neurones in rats. Eur. J. Pharmacol. 1996;318:315–321. doi: 10.1016/s0014-2999(96)00819-9. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J.The pathophysiology of headache Wolff's Headache and Other Head Pain 2001Oxford: Oxford University Press; 57–72.7th edn. eds. Silberstein, S.D., Lipton, R.B., Dalessio, D.J. [Google Scholar]
- GOADSBY P.J., HOSKIN K.L. Inhibition of trigeminal neurons by intravenous administration of the serotonin (5-HT)1B/D receptor agonist zolmitriptan (311C90): Are brain stem sites a therapeutic target in migraine. Pain. 1996;67:355–359. doi: 10.1016/0304-3959(96)03118-1. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., KNIGHT Y. Inhibition of trigeminal neurones after intravenous administration of naratriptan through an action at 5-hydroxytryptamine (5-HT)1B/1D receptors. Br. J. Pharmacol. 1997a;122:918–922. doi: 10.1038/sj.bjp.0701456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOADSBY P.J., KNIGHT Y. Direct evidence for central sites of action of zolmitriptan (311C90): an autoradiographic study in cat. Cephalalgia. 1997b;17:153–158. doi: 10.1046/j.1468-2982.1997.1703153.x. [DOI] [PubMed] [Google Scholar]
- GREGG J.M. A surgical approach to the ophthalmic-maxillary nerve trunks in the rat. J. Dent. Res. 1973;52:392. doi: 10.1177/00220345730520024001. [DOI] [PubMed] [Google Scholar]
- HAMON M., BOURGOIN S.Serotonin and its receptors in pain control Novel aspects of pain management – opioids and beyond 1999New York: Wiley-Liss; 203–228.eds. Sawynok, J., Cowan, A. [Google Scholar]
- HOSKIN K.L., KAUBE H., GOADSBY P.J. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiological study. Brain. 1996a;119:249–256. doi: 10.1093/brain/119.1.249. [DOI] [PubMed] [Google Scholar]
- HUMPHREY P.P.A., FENIUK W., MARRIOTT A.S., TANNER R.J.N., JACKSON M.R., TUCKER M.L. Preclinical studies on the anti-migraine drug, sumatriptan. Eur. Neurol. 1991;31:282–290. doi: 10.1159/000116755. [DOI] [PubMed] [Google Scholar]
- IDÄNPÄÄN-HEIKKILÄ J.J., GUILBAUD G. Pharmacological studies on a rat model of trigeminal neuropathic pain: baclofen, but not carbamazepine, morphine or tricyclic antidepressants, attenuates the allodynia-like behaviour. Pain. 1999;79:281–290. doi: 10.1016/s0304-3959(98)00172-9. [DOI] [PubMed] [Google Scholar]
- IMAMURA Y., KAWAMOTO H., NAKANISHI O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp. Brain Res. 1997;116:97–103. doi: 10.1007/pl00005748. [DOI] [PubMed] [Google Scholar]
- INGVARDSEN B.K., LAURSEN H., OLSEN U.B., HANSEN A.J. Possible mechanism of c-fos expression in trigeminal nucleus caudalis following cortical spreading depression. Pain. 1997;72:407–415. doi: 10.1016/s0304-3959(97)00069-9. [DOI] [PubMed] [Google Scholar]
- JACQUIN F.M., NICOLAS L., RHOADES W.R. Trigeminal projections to contralateral dorsal horn: central extent, peripheral origins, and plasticity. Somatosens. Mot. Res. 1990;7:153–183. doi: 10.3109/08990229009144705. [DOI] [PubMed] [Google Scholar]
- JACQUIN F.M., ZIEGLER H.P. Trigeminal orosensation and ingestive behavior in the rat. Behav. Neurosci. 1983;97:62–97. doi: 10.1037//0735-7044.97.1.62. [DOI] [PubMed] [Google Scholar]
- JAIN N.K., KULKARNI S.K. Antinociceptive effect of sumatriptan in mice. Indian J. Exp. Biol. 1998;36:973–979. [PubMed] [Google Scholar]
- JOHNSON D.E., ROLLEMA H., SCHMIDT A.W., MCHARG A.D. Serotonergic effects and extracellular brain levels of eletriptan, zolmitriptan and sumatriptan in rat brain. Eur. J. Pharmacol. 2001;425:203–210. doi: 10.1016/s0014-2999(01)01151-7. [DOI] [PubMed] [Google Scholar]
- JOHNSON K.W., SCHAUS J.M., DURKIN M.M., AUDIA J.E., KALDOR S.W., FLAUGH M.E., ADHAM N., ZGOMBICK J.M., COHEN M.L., BRANCHEK T.A., PHEBUS L.A. 5-HT1F receptor agonist inhibits neurogenic dural inflammation in guinea pigs. Neuroreport. 1997;8:2237–2240. doi: 10.1097/00001756-199707070-00029. [DOI] [PubMed] [Google Scholar]
- KAUBE H., HOSKIN K.L., GOADSBY P.J. Inhibition by sumatriptan of central trigeminal neurones only after blood-brain barrier disruption. Br. J. Pharmacol. 1993;109:788–792. doi: 10.1111/j.1476-5381.1993.tb13643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAYSER V., CHRISTENSEN D. Antinociceptive effect of systemic gabapentin in mononeuropathic rats depends on stimulus characteristics and level of test integration. Pain. 2000;93:147–153. doi: 10.1016/S0304-3959(00)00307-9. [DOI] [PubMed] [Google Scholar]
- KRUSKALL W.H., WALLIS W.A. Use of ranks on one criterion variance analysis. J. Am. Stat. Assoc. 1952;47:583–621. [Google Scholar]
- KRYZHANOVSKI G.N., DOLGIKH V.G., GORIZONTOVA M.P., MIRONOVA I.V. Formation of a pathological system in rats with neuropathic trigeminal neuralgia. Bull. Exp. Biol. Med. 1993;115:623–625. [PubMed] [Google Scholar]
- KRYZHANOVSKI G.N., RESHNYAK V.K., DOLGIKH V.G., GORIZONTOVA M.P., SPERANSKAYA T.V. Trigeminal neuralgia of neuropathic origin. Bull. Exp. Biol. Med. 1992;112:1059–1062. [PubMed] [Google Scholar]
- LAPORTE A.M., FATTACCINI C.M., LOMBARD M.C., CHAUVEAU J., HAMON M. Effects of dorsal rhizotomy and selective lesion of serotonergic and noradrenergic systems on 5-HT1A, 5-HT1B and 5-HT3 receptors in the rat spinal cord. J. Neural Transm. (Gen. Sect.) 1995;100:207–223. doi: 10.1007/BF01276459. [DOI] [PubMed] [Google Scholar]
- LE BARS D., GOZARIU M., CADDEN S.W. Animal models of nociception. Pharmacol. Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- LE DOUARIN N.M., KALCHEIM C. The neural crest. Cambridge: Cambridge University Press; 1999. pp. 1–469. [Google Scholar]
- LIN Q., PENG Y.B., WILLIS W.D. Antinociception and inhibition from the periaqueductal gray are mediated in part by spinal 5-hydroxytryptamine1A receptors. J. Pharmacol. Exp. Ther. 1996;276:958–967. [PubMed] [Google Scholar]
- MARTIN G.R. Pre-clinical pharmacology of zolmitriptan (Zomig™; formerly 311C90), a centrally and peripherally acting 5-HT1B/1D agonist for migraine. Cephalalgia. 1997;18 Suppl:4–14. doi: 10.1177/0333102497017S1802. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B.G., PEROUTKA S.J. Comparative neuropharmacology of dihydroergotamine and sumatriptan (GR-43175) Headache. 1989;29:420–422. doi: 10.1111/j.1526-4610.1989.hed2907420.x. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J., SEGUIN L., HONORÉ P., GIRARDON S., BERVOETS K. Pro- and antinociceptive actions of serotonin (5-HT)1A agonists and antagonists in rodents: relationship to algesiometric paradigm. Behav. Brain Res. 1996;73:69–77. doi: 10.1016/0166-4328(96)00073-3. [DOI] [PubMed] [Google Scholar]
- NOZAKI K., MOSKOWITZ M.A., BOCCALINI P. CP-93,129, sumatriptan, dihydroergotamine block c-fos expression within rat trigeminal nucleus caudalis caused by chemical stimulation of the meninges. Br. J. Pharmacol. 1992;106:409–415. doi: 10.1111/j.1476-5381.1992.tb14348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUWELS P.J., JOHN G.W. Present and future of 5-HT receptor agonists as antimigraine drugs. Clin. Neuropharmacol. 1999;22:123–136. [PubMed] [Google Scholar]
- PAXINOS G.The Rat Nervous System 1995San Diego: Academic Press; >2nd ed [Google Scholar]
- PFALLER K., ARVIDSSON J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J. Comp. Neurol. 1988;268:91–108. doi: 10.1002/cne.902680110. [DOI] [PubMed] [Google Scholar]
- PINI L.A., MORELLI G. Triptans: turning treatment in cluster headache attack. Ital. J. Neurol. Sci. 1999;20:S66–S68. doi: 10.1007/pl00015005. [DOI] [PubMed] [Google Scholar]
- PLOSKER G.L., MCTAVISH D. Sumatriptan: a reappraisal of its pharmacology and therapeutic efficacy in the acute treatment of migraine and cluster headache. Drugs. 1994;47:622–652. doi: 10.2165/00003495-199447040-00006. [DOI] [PubMed] [Google Scholar]
- PRICE G.W., BURTON M.J., COLLIN L.J., DUCKWORTH M., GASTER L., GOTHERT M., JONES B.J., ROBERTS C., WATSON J.M., MIDDLEMISS D.N. SB-216641 and BRL-15572-compounds to pharmacologically discriminate 5-HT1B and 5-HT1D receptors. Naunyn-Schmiedebergs Arch Pharmacol. 1997;356:312–320. doi: 10.1007/pl00005056. [DOI] [PubMed] [Google Scholar]
- REMPEL N.L., CALLAWAY C.W., GEYER M.A. Serotonin1B receptor activation mimics behavioral effects of presynaptic serotonin release. Neuropsychopharmacology. 1993;8:201–211. doi: 10.1038/npp.1993.22. [DOI] [PubMed] [Google Scholar]
- SAMSAM M., COVEÑAS R., CSILLIK B., AHANGARI R., YAJEYA J., RIQUELME R., NARVÁEZ J.A., TRAMU G. Depletion of substance P, neurokinin A and caltitonin gene-related peptide from the contralateral and ipsilateral caudal trigeminal nucleus following unilateral electrical stimulation of the trigeminal ganglion; a possible neurophysiological and neuroanatomical link to generalized head pain. J. Chem. Neuroanat. 2001;21:161–169. doi: 10.1016/s0891-0618(01)00088-6. [DOI] [PubMed] [Google Scholar]
- SELKIRK J.V., SCOTT C., HO M., BURTON M.J., WATSON J., GASTER L.M., COLLIN L., JONES B.J., MIDDLEMISS D.N., PRICE G.W. SB-224289–a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. Br. J. Pharmacol. 1998;125:202–208. doi: 10.1038/sj.bjp.0702059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKINGLE M., BEATTIE D.T., SCOPES D.I.T., STARKEY S.J., CONNOR H.E., FENIUK W., TYERS M.B. GR127935: a potent and selective 5-HT1D receptor antagonist. Behav. Brain Res. 1996;73:157–161. doi: 10.1016/0166-4328(96)00089-7. [DOI] [PubMed] [Google Scholar]
- SKINGLE M., BIRCH P.J., LEIGHTON G.E., HUMPHREY P.P.A. Lack of antinociceptive activity of sumatriptan in rodents. Cephalalgia. 1990;10:207–212. doi: 10.1046/j.1468-2982.1990.1005207.x. [DOI] [PubMed] [Google Scholar]
- STORER R.J., AKERMAN S., CONNOR H.E., GOADSBY P.J. 4991W93, a potent blocker of neurogenic plasma protein extravasation, inhibits trigeminal neurons at 5-hydroxytryptamine (5-HT1B/1D) agonist doses. Neuropharmacology. 2001;40:911–917. doi: 10.1016/s0028-3908(01)00014-4. [DOI] [PubMed] [Google Scholar]
- SWERDLOW M. Anticonvulsivant drugs and chronic pain. Clin. Neuropharmacol. 1984;7:51–82. doi: 10.1097/00002826-198403000-00003. [DOI] [PubMed] [Google Scholar]
- VOS B.P., BENOIST J.M., GAUTRON M., GUILBAUD G. Changes in neuronal activities in the two ventral posterior medial thalamic nuclei in an experimental model of trigeminal pain in the rat by constriction of one infraorbital nerve. Somatosens. Mot. Res. 2000;17:109–122. doi: 10.1080/08990220050020535. [DOI] [PubMed] [Google Scholar]
- VOS B.P., STRASSMAN A.M., MACIEWITZ R.J. Behavioural evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J. Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOCHODNE D.W., HO L.T. Sumatriptan blocks neurogenic inflammation in the peripheral nerve trunk. Neurology. 1994;44:161–163. doi: 10.1212/wnl.44.1.161. [DOI] [PubMed] [Google Scholar]


