Abstract
Xestospongin-C isolated from a marine sponge, Xestospongia sp., has recently been shown to be a membrane-permeable IP3 receptor inhibitor. In this study we examined the effects of this compound on smooth muscle from guinea-pig ileum.
In guinea-pig ileum permeabilized with α-toxin, xestospongin-C (3 μM) inhibited contractions induced by Ca2+ mobilized from sarcoplasmic reticulum (SR) with IP3 or carbachol with GTP, but not with caffeine.
In intact smooth muscle tissue, xestospongin-C (3–10 μM) inhibited carbachol- and high-K+-induced increases in [Ca2+]i and contractions at sustained phase.
It also inhibited voltage-dependent inward Ba2+ currents in a concentration-dependent manner with an IC50 of 0.63 μM. Xestospongin-C (3–10 μM) had no effect on carbachol-induced inward Ca2+ currents via non-selective cation channels; but it did reduce voltage-dependent K+ currents in a concentration-dependent manner with an IC50 of 0.13 μM.
These results suggest that xestospongin-C inhibits the IP3 receptor but not the ryanodine receptor in smooth muscle SR membrane. In intact smooth muscle cells, however, xestospongin-C appears to inhibit voltage-dependent Ca2+ and K+ currents at a concentration range similar to that at which it inhibits the IP3 receptor. Xestospongin-C is a selective blocker of the IP3 receptor in permeabilised cells but not in cells with intact plasma membrane.
Keywords: Xestospongin-C; inositol 1,4,5-triphosphate receptor; smooth muscle; voltage-dependent Ca2+ channel
Introduction
Inositol 1,4,5-triphosphate (IP3), boosted by activation of a phosphoinositide signalling cascade, enhances Ca2+ efflux from the sarcoplasmic reticulum (SR) in smooth muscle. Understanding the role of IP3 is important because SR Ca2+ release is involved in receptor-mediated signal transduction, and it would be useful to have drugs that interfere with IP3-mediated Ca2+ release. Although heparin and decavanadate are currently used for this purpose, these agents are non-selective and cell membrane impermeable (for a review, see Wilcox et al., 1998). Xestospongin-C isolated from the sponge Xestospongia sp. has recently been shown to be a membrane-permeable inhibitor of IP3-mediated Ca2+ release (Gafni et al., 1997; Wilcox et al., 1998). This compound appears to be a potent and highly sensitive inhibitor of the IP3 receptor with an IC50 of 0.35 μM, which is 30 times lower than that for the ryanodine receptor (Gafni et al., 1997). Consequently, this compound has been recognized as potentially valuable in the investigation of IP3-dependent responses in intact cell preparations, and it has been used as a selective pharmacological tool (Kiselyov et al., 1998; Hu et al., 1999; Netzeband et al., 1999; Holden et al., 2000; Miyamoto et al., 2000; Ueda & Inoue, 2000; Oka et al., 2002). However, the pharmacological features of this compound have not been extensively studied. In the present study we examined the selectivity of xestospongin-C for ion channels responsible for smooth muscle contraction.
Methods
Fura-2 loading and simultaneous measurements of muscle force and [Ca2+]i
Male guinea-pigs weighing 300–400 g were sacrificed with a sharp blow to the neck followed by exsanguination. The ileal longitudinal muscle was isolated, cut into strips of 8–10 mm length and placed in a physiological salt solution (PSS) (in mM): NaCl 136.9, KCl 5.4, CaCl2 1.5, MgCl2 1.0, NaHCO3 23.8, glucose 5.5. Ethylenediaminetetraacetic acid (EDTA) (0.01 mM) was also added to chelate trace amounts of heavy metal ion contamination. A high-K+ solution was prepared by replacing NaCl with equimolar KCl. These solutions were saturated with 95% O2–5% CO2 at 37°C to maintain the pH at 7.2. The force of contraction was recorded isometrically. The muscle preparations were attached to a holder under a resting force of 5 mN and equilibrated for 60–90 min. Meanwhile, 40 mM K+ was applied repeatedly until the sustained force became reproducible. [Ca2+]i was measured according to the method described by Ozaki et al. (1997) using the fluorescent Ca2+ indicator fura-2. The muscle strips were exposed to the acetoxymethyl ester of fura-2 (fura-2/AM 10 μM) in the presence of 0.02% cremophor EL for 5–6 h at room temperature and then transferred to the muscle bath integrated in the fluorimeter (CAF-110, JASCO, Japan). The muscle strips were illuminated alternately (48 Hz) with 340 and 380 nm lights. We collected the light emitted from the muscle strips with the aid of a photomultiplier through a 500 nm filter.
Preparation of cells for patch clamp analysis
The muscle tissue was cut into small pieces and placed in Ca2+-free PSS, which was then replaced with PSS containing 30 μM Ca2+ (low-Ca2+ PSS), and 30 min incubations at 37°C were performed in fresh low-Ca2+ PSS containing collagenase (0.3 mg ml−1), papain (0.3 mg ml−1) and bovine serum albumin (2 mg ml−1). After digestion by these enzymes the tissue fragments were suspended in a fresh 120 μM Ca2+ containing PSS and gently agitated. The resulting suspension was centrifuged at 600×g for 2 min, and the cells were re-suspended in 0.5 mM Ca2+ containing PSS. The cell suspensions were placed on glass cover-slips and stored in a moist atmosphere at 4°C for 2–8 h. Experiments were performed at room temperature (22–24°C).
Whole-cell voltage clamp
We recorded the whole-cell membrane current and potential at room temperature using standard patch-clamp techniques. The patch pipette had a resistance of 3–6 MΩ when filled with a pipette solution. Membrane currents were measured with an Axopatch 1C voltage-clamp amplifier (Axon Instruments, U.S.A.). Command pulses were applied using an IBM-compatible computer and pCLAMP (version 5.5) software. The data was filtered at 5 kHz and displayed on an oscilloscope, a computer monitor and a pen recorder.
The PSS used for the patch-clamp experiments was composed of (mM): NaCl 126, KCl 6, CaCl2 2, MgCl2 1.2, glucose 14 and N-[2-hydroxyethyl]piperazine-N′-2-ethan-sulphonic acid (HEPES) 10.5 (pH 7.2 with NaOH). The patch pipette solution for the outward K+ currents was composed of (mM): KCl 134, MgCl2 1.2, ATP 1, GTP 0.1, EGTA 0.05, glucose 14, HEPES 10.5 (pH 7.2 with KOH). The inward currents were isolated by suppressing K+ currents using a patch pipette filled with (mM): CsCl 140, glucose 14, EGTA 0.05, HEPES 10.5 and Na2ATP 4 (pH 7.2 with CsOH). In experiments with Ca2+ currents, CaCl2 in the bath solution was replaced with BaCl2.
Skinned muscle preparations
Small bundles of muscle tissue (120–150 μm in diameter and 1–2 mm in length) were obtained from guinea-pig ileal longitudinal muscle. The bundles were skinned with 80 μg ml−1 α-toxin for 20–30 min in a relaxing solution (see below), and then both ends of each bundle were tied with monofilament silk thread. One end of each bundle was connected to an isometric transducer, and the other end was fixed with a stainless steel pin to the siliconized floor of a chamber under a resting tension of 0.5 mN and equilibrated for 60–90 min. Meanwhile, 1 μM Ca2+ was repeatedly applied until the peak force became reproducible. Experiments were carried out at room temperature (ca. 22°C).
The relaxing solution contained (in mM): magnesium (methansulphonate)2 7.1, potassium methansulphonate 108.0, ATP 5.9, PIPES 20, phosphocreatine 10, 20 units ml−1 creatine phosphokinase, 1 μM FCCP, 1 mg ml−1 E-64 and 10 mM EGTA. The ionic strength was adjusted to 0.2 M, and the pH was adjusted to 7.1 at 20°C. Free Ca2+ concentration was changed by adding an appropriate amount of Ca2+-(methansulphonate)2. Contraction due to Ca2+ release from the SR was introduced by adding caffeine (3–20 mM), IP3 (30 μM) or carbachol (10 μM) with GTP (100 μM) in a low-EGTA (0.05 mM) solution. Ca2+ loading into the SR was performed by adding pCa 6 solution for 15 min, and the muscle was treated with a Ca2+-free low-EGTA solution for 15 min. Contractions due to Ca2+ release from the SR were reproducible at least four times under these experimental conditions. The ionic composition of these solutions was calculated with the aid of a slight modification of a computer program developed by Fabiato (1981). The area of the force oscillations during a one-minute period was used for the quantitative assessment of the effects of caffeine, carbachol, IP3 and xestospongin-C.
Chemicals
The chemicals used were carbachol, verapamil (Sigma, St. Louis, MO, U.S.A.), caffeine, EGTA (Wako Pure Chemicals, Tokyo, Japan) and IP3 (Dojindo Laboratories, Kumamoto, Japan). Xestospongin C was isolated and purified from an Okinawan marine sponge, Xestospongia sp., (Kobayashi et al., 1998) and dissolved in 100% ethanol as stock solution. Staphylococcus aureus α toxin was donated by Dr I. Kato (Chiba University, Chiba, Japan).
Statistical analysis
Data was presented in the form of the mean value±standard error. We used Student's t-test for the comparison of methods (P<0.05 being considered significant).
Results
Ca2+ release due to caffeine, carbachol and IP3 in the permeabilized muscle
In muscle permeabilized with α-toxin, 20 mM of caffeine induced considerable transient contraction in the absence of Ca2+ and in the presence of 0.05 mM EGTA, indicating that the SR function is intact. The addition of IP3 (30 μM) induced reproducible transient contractions in the permeabilized muscle. Xestospongin-C (3 μM) added 5 min before the addition of IP3 inhibited the IP3-induced contraction, as shown in Figure 1A. Carbachol (10 μM) added in the presence of GTP (100 μM) also induced transient contractions in the absence of Ca2+. Xestospongin-C (3 μM) similarly inhibited the carbachol-induced transient contractions (Figure 1B). In contrast, contractions induced with caffeine (3 mM) were not affected by xestospongin-C at 10 μM. These results are summarized in Figure 1D.
Figure 1.
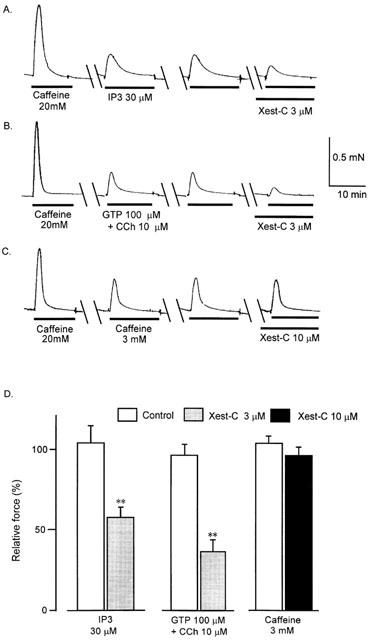
Effects of xestospongin-C (Xest-C, 3 and 10 μM) on transient contractions due to Ca2+ release from the sarcoplasmic reticulum in α-toxin-skinned muscle fibres. Prior to the experiment the muscle had been conditioned by adding 20 mM caffeine. We then induced contractions with IP3 (30 μM), GTP (100 μM)+carbachol (10 μM) and caffeine (3 mM) twice to demonstrate the reproducibility of these responses. (A) Typical tracing of the effects of 3 μM xestospongin-C on transient contraction induced by 30 μM IP3 in Ca2+-free solution. (B) Typical tracing of the effects of 3 μM xestospongin-C on a transient contraction induced by 10 μM carbachol in the presence of 100 μM GTP. (C) Typical tracing of the effects of 3 μM xestospongin-C on a transient contraction induced by 3 mM caffeine. (D) The area of force oscillations during a 15 min period was used for quantitative assessment of the effects of caffeine, carbachol and IP3, and the summarized data of (A), (B) and (C) is shown (n=6 each). **P < 0.01 vs control. 100% represents the first control response before the addition of xestospongin-C.
Effects of xestospongin-C on carbachol-stimulated muscle contraction and [Ca2+]i in intact tissue
Figure 2 shows changes in muscle contraction and fura-2-Ca2+ fluorescence induced by the addition of carbachol in the ileal longitudinal muscle. These muscles were well loaded with fura-2 and exhibited stable signals for at least 30 min. Carbachol (1 μM) induced sustained increases in [Ca2+]i and contraction. During stimulation with carbachol, [Ca2+]i and tension sometimes changed to small rhythmic oscillations. Xestospongin-C at 3 μM added during the plateau contractions had no effect on [Ca2+]i or contraction; however, a higher concentration of xestospongin-C (10 μM) partially inhibited the stimulated [Ca2+]i and tension. Further addition of verapamil (10 μM) decreased [Ca2+]i and tension almost to the baseline.
Figure 2.
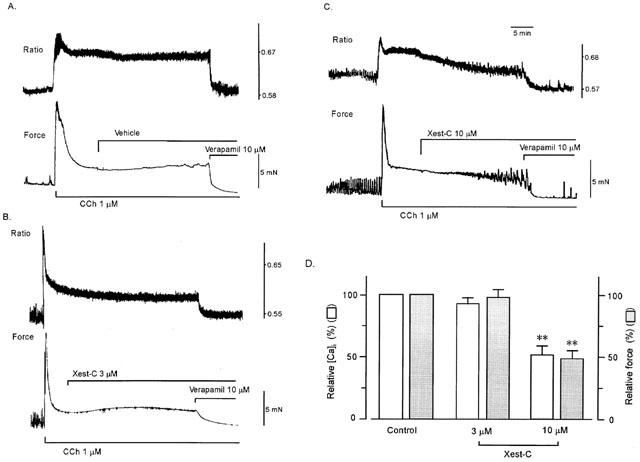
Effects of xestospongin-C on carbachol-stimulated [Ca2+]i (upper tracing) and muscle tension (lower tracing) in ileal smooth muscle. When [Ca2+]i and muscle tension induced with carbachol reached a steady state level, the vehicle (ethanol 0.2%) (A), 3 (B) or 10 (C) μM xestospongin-C and 10 μM verapamil were added sequentially. (D) Summarized data on (B) and (C) (n=5 and 6, respectively). In (D), 100% represents carbachol-induced increases in [Ca2+]i and force at steady state before the addition of xestospongin-C. Values were obtained 25 min after the addition of xestospongin-C. **P < 0.01 vs control.
Effects of xestospongin-C on high-K+-stimulated muscle force and [Ca2+]i in intact tissue
Figure 3 shows changes in muscle tension and fura-2-Ca2+ fluorescence in the ileal longitudinal muscle induced by the addition of a high-K+ solution. The addition of 65.4 mM K+ produced initial transient increases followed by sustained increases in [Ca2+]i and muscle tension. Xestospongin-C (3 and 10 μM) added during the plateau contractions inhibited [Ca2+]i and tension in a concentration-dependent manner. Further addition of verapamil (10 μM) decreased [Ca2+]i and tension almost to the baseline.
Figure 3.
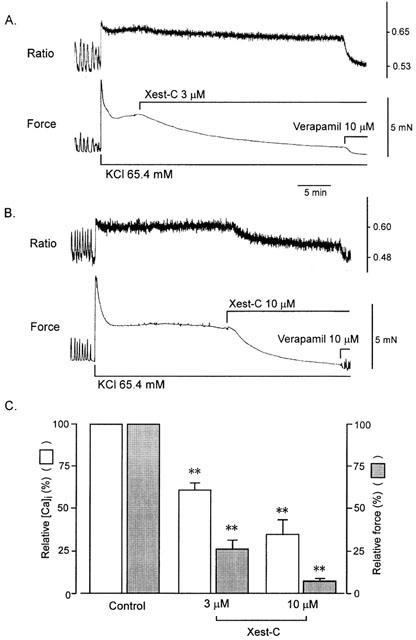
Effects of xestospongin-C on high-K+-stimulated [Ca2+]i (upper tracing) and muscle tension (lower tracing) in ileal smooth muscle. When [Ca2+]i and muscle tension induced with high K+ reached a steady state level, 3 (A) or 10 (B) μM xestospongin-C and 10 μM verapamil were added sequentially. (C) Summarized data on (A) and (B) (n=6). In (C), 100% represents high K+-induced increases in [Ca2+]i and force at steady state before the addition of xestospongin-C. Values were obtained 20–30 min after the addition of 3 μM xestospongin-C and 15 min after the addition of 10 μM xestospongin-C. **P < 0.01 vs control.
Effects of xestospongin-C on voltage-dependent Ca2+ currents
We examined the effect of xestospongin-C on Ca2+ currents through voltage-dependent Ca2+ channels in single ileal smooth muscle cells in a solution containing 2 mM Ba2+. The use of Ba2+ instead of Ca2+ as the charge carrier caused IBa to be elicited through depolarization from a holding potential of −80 mV to a test potential of 0 mV for 80 ms at 0.1 Hz. Application of 3 μM xestospongin-C reduced peak IBa amplitude by 79.3±8.2% (n=5). IBa was significantly reduced within 3 min of the start of the application (Figure 4). After washout of xestospongin-C, IBa was partially recovered. Figure 4 illustrates the relationship between concentration and response with regard to the inhibitory effect of xestospongin-C on IBa (IC50=0.63 μM).
Figure 4.
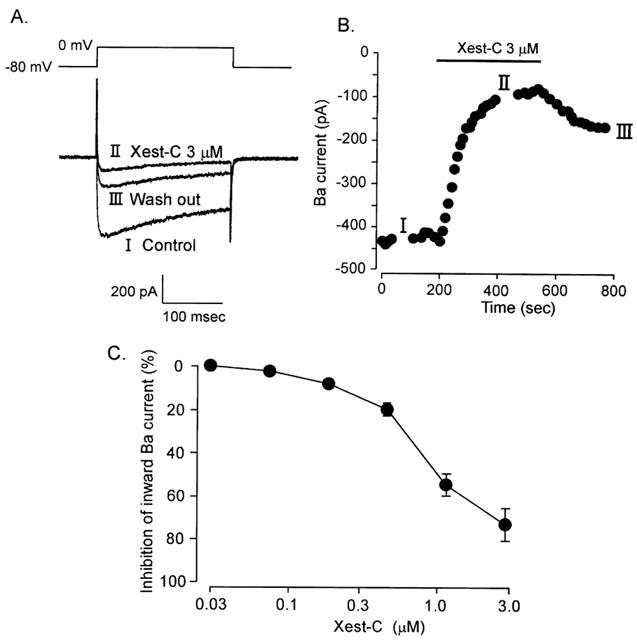
Effects of xestospongin-C on IBa through Ca2+ channels in single guinea-pig ileal smooth muscle cells. IBa was elicited by 80 ms depolarisation from −60 to 0 mV at 0.1 Hz. (A) Typical tracings of IBa in the presence or absence of 3 μM xestospongin-C. (B) Changes in peak amplitude of IBa with time. Xestospongin-C (3 μM) was applied during the period indicated by a horizontal bar. Time 0 min indicates the start of IBa recording under conditions in which the K+ current was blocked by internal diffusion of Cs from the recording pipette for about 3 min after the patch membrane was ruptured. (C) Effects of various concentrations of xestospongin-C on IBa (n=4–6).
Effects of xestospongin-C on carbachol-induced non-selective cation currents
In ileal smooth muscle cells clamped at a holding potential of −40 mV, the application of 50 μM carbachol induced a slow inward current with an increase in baseline fluctuation (Figure 5). In most cells the response continued for 10 min or longer. As is shown in Figure 5A, the carbachol-induced inward current was only slightly inhibited (by 1.8±1.1%, n=5) by 3 μM xestospongin-C (n=4). The data are summarized in Figure 5B.
Figure 5.
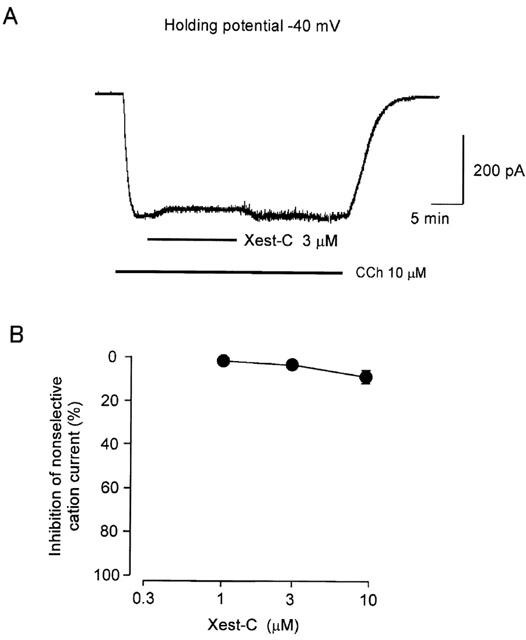
Typical tracing of the effects of xestospongin-C on carbachol-induced inward currents in ileal smooth muscle cell. Once a whole cell had been prepared, the current was clamped at −40 mV. When the carbachol (50 μM)-induced inward current reached steady state, xestospongin-C (3 μM) was added to the bathing medium (A). Summarized data on the effects of xestospongin-C at various concentrations are presented in (B) (n=4–6).
Effects of xestospongin-C on outward K+ currents
We measured the amplitude of the whole-cell K+ current before and after exposure to xestospongin-C. Xestospongin-C (0.01–3 μM) reduced the net outward current in a concentration-dependent manner with an IC50 of 0.13 μM (Figure 6). Maximum effect was observed at 3 μM.
Figure 6.
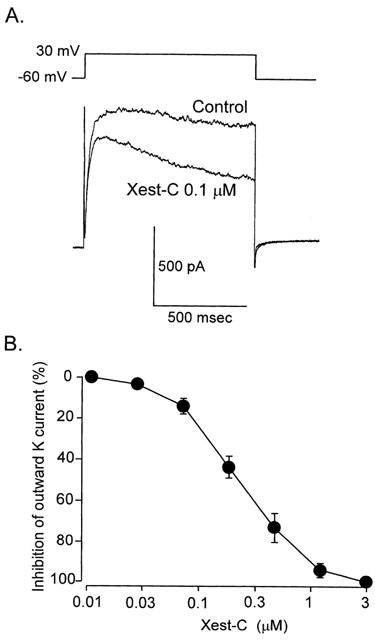
Effects of xestospongin-C on outward K+ currents recorded from ileal smooth muscle cells. The cells were held at −60 mV, and test depolarisation with a duration of 900 ms was applied from −40 to +50 mV in increments of 10 mV. A pipette containing 0.05 mM EGTA was used to record currents. (A) Effect of 1 μM xestospongin-C on whole-cell currents. Summarized data on the effects of xestospongin-C (0.03–3 μM) at various concentrations on whole-cell currents (n=6) are presented in (B), in which cells had been pretreated with xestospongin-C for 10 min.
Discussion
As illustrated in Figure 1, the results of the present study show that xestospongin-C at 3 μM strongly inhibited IP3- and muscarinic agonist-mediated Ca2+ release from the SR as estimated by the contractile response in membrane-permeabilized smooth muscle. However, xestospongin-C at this concentration and at a concentration of 10 μM did not affect caffeine-induced Ca2+ release (Ca2+-induced Ca2+ release). These results agree with those of a previous report which showed that xestospongins selectively inhibit Ca2+ release from the endoplasmic reticulum in cerebellar microsomes (Gafni et al., 1997). Unexpectedly, however, we found that xestospongin-C (3 μM) inhibited high-K+-induced contraction at a sustained phase in intact tissue, which is considered to be mediated via the Ca2+ influx through voltage-dependent L-type Ca2+ channels (Karaki et al., 1997). We further analysed the mechanism of inhibition, using the patch clamp method, and found that xestospongin-C inhibited voltage-dependent Ca2+ channel activity at a submicromolar concentration range. These results suggest that xestospongin-C is not selective against IP3-mediated Ca2+ releasing channels in intact cells. Miyamoto et al. (2000) have suggested that although 3 μM xestospongin-C is selective against the IP3 receptor in cardiac muscle, higher concentrations (>10 μM) of xestospongin-C interfere with other components of cardiac contractile responses.
In the guinea-pig ileum, inhibitors of voltage-dependent Ca2+ channels such as verapamil and nifedipine completely suppress carbachol-induced contraction (data not shown), suggesting that carbachol-induced contraction is mediated by Ca2+ influx through voltage-dependent Ca2+ channels. It is also known that stimulation of muscarinic receptors with carbachol in intestinal smooth muscle cells induces a non-selective cation current. This current acts to depolarize the membrane, resulting in activation of L-type Ca2+ channels. It is conceivable that xestospongin-C inhibits carbachol-induced contraction by suppressing this channel; however, xestospongin-C had no effect on the carbachol-induced cation current in the ileal myocytes, so we concluded that it inhibits carbachol-induced contraction by directly suppressing L-type Ca2+ channels following depolarization of the membrane.
Because 3 μM xestospongin-C inhibits high-K+-induced contraction by suppressing voltage-dependent Ca2+ channels (Figure 3), we expected it to equally inhibit carbachol-induced contraction. However, 3 μM xestospongin-C failed to exhibit any effect on carbachol-induced contraction (Figure 2C). Thus we speculated that xestospongin-C may inhibit channels, which counteracts the depolarizing effect of carbachol. As we expected, we saw that xestospongin-C inhibited the voltage-dependent outward K+ current (IC50=0.13 μM) at a lower concentration range than that at which voltage-dependent Ca2+ channels (IC50=0.63 μM) were inhibited.
Xestospongins do not appear to act as competitive antagonists at the IP3 receptors, but they may act as functional antagonists possibly by sterically blocking the channel pores (Gafni et al., 1997). Xestospongins have a chemical structure comprised of an elongated lipophilic core with two charged nitrogen groups. This may provide an ideal lipophilic/hydrophilic moiety to fit into the IP3 receptor channel pore. Although the mechanism responsible for the inhibition of ion fluxes through the Ca2+ and K+ channels is not known, it is possible that the L-type Ca2+ channel, IP3 receptor and voltage-dependent K+ channel, although not the ryanodine receptor and non-selective cation channel, share a xestospongin recognition site within their ion-conducting pores.
de smet et al. (1999) reported that xestospongin-C is not only an inhibitor of IP3-induced Ca2+ release but also an equally potent blocker of the endoplasmic-reticulum Ca2+ pump in permeabilized A7r5 smooth muscle cells, and thus they suggest that xestospongin-C cannot be considered as a selective blocker of IP3 receptors. In the present study, however, we were unable to observe any inhibitory effect of xestospongin-C on caffeine-induced Ca2+ release, which suggests that the Ca2+ pump is intact in the presence of xestospongin-C in our preparations.
In conclusion, we confirmed that xestospongin-C inhibits the IP3 receptor without affecting the ryanodine receptor in the smooth muscle SR. However, we also found that xestospongin-C inhibits both voltage-dependent Ca2+ channels and voltage-dependent K+ channels in the plasma membrane without affecting agonist-induced non-selective cation channels. Xestospongin-C acts as a selective blocker of the IP3 receptor in permeabilised cells but not in cells with intact plasma membrane.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture and Science, and the Programme for the Promotion of Basic Research Activities for Innovative Biosciences (BRAIN).
Abbreviations
- CICR
Ca2+-induced Ca2+-release
- IP3
inositol 1,4,5-triphosphate
- PSS
physiological salt solution
- SR
sarcoplasmic reticulum
References
- DE SMET P., PARYS J.B., CALLEWAERT G., WEIDEMA A.F., HILL E., DE SMEDT H., ERNEUX C., SORRENTINO V., MISSIAEN L. Xestospongin-C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca2+ pumps. Cell Calcium. 1999;26:9–13. doi: 10.1054/ceca.1999.0047. [DOI] [PubMed] [Google Scholar]
- FABIATO A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J. Gen. Physiol. 1981;78:457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAFNI J., MUNSCH J.A., LAM T.H., CATLIN M.C., COSTA L.G., MOLINSKI T.F., PESSAH L.N. Xestospongins: Potent membrane permeable blockers of the inositol 1,4,5-triphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- HU Q., DESHPANDE S., IRANI K., ZIEGELSTEIN R.C. [Ca2+]i oscillation frequency regulates agonist-stimulated NF-κB transcriptional activity. J. Biol. Chem. 1999;274:33995–33998. doi: 10.1074/jbc.274.48.33995. [DOI] [PubMed] [Google Scholar]
- HOLDEN C.P., HAUGHEY N.J., DOLHUN B., SHEPEL P.N., NATH A., GEIGER J.D. Diadenosine pentaphosphate increases levels of intracellular calcium in astrocytes by a mechanism involving release from caffeine/ryanodine- and IP3-sensitive stores. J. Neuroscience Res. 2000;59:276–282. [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MITSUI-SAITO M., AMANO K., HARADA K., MIYAMOTO S., NAKAZAWA H., WON K.-J., SATO K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- KISELYOV K., XU X., MOZHAYEVA G., KUO T., PESSAH I., MIGNERY G., ZHU X., BIRNBAUMER L., MUALLEM S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI M., MIYAMOTO Y., AOKI S., MURAKAMI N., IN K., ISHIDA T. Isomerization of dimeric 2,9-disubstituted 1-oxa-quinolizidine alkaloids and structural revision of araguspongines B and E, isolated from a marine sponge of Xestospongia sp. Heterocycles. 1998;47:195–203. [Google Scholar]
- MIYAMOTO S., IZUMI M., HORI M., KOBAYASHI M., OZAKI H., KARAKI H. Xestospongin-C, a selective and membrane-permeable inhibitor of IP3 receptor, attenuates the positive inotropic effect of α-adrenergic stimulation in guinea-pig papillary muscle. Br. J. Pharmacol. 2000;130:650–654. doi: 10.1038/sj.bjp.0703358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETZEBAND J.G., CONROY S.M., PARSONS K.L., GRUOL D.L. Cannabinoids enhance NMDA-elicited Ca2+ signals in cerebellar granule neurons in culture. J. Neurosci. 1999;19:8765–8777. doi: 10.1523/JNEUROSCI.19-20-08765.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKA T., SATO K., HORI M., OZAKI H., KARAKI H. FcεRI cross-linking-induced actin assembly mediates calcium signalling in RBL-2H3 mast cells. Br. J. Pharmacol. 2002;135:1959–1966. doi: 10.1038/sj.bjp.0704788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAKI H., SATO K., KARAKI H. Simultaneous recordings of calcium signals and mechanical activity using fluorescent dye fura-2 in isolated strips of vascular smooth muscle. Jpn. J. Pharmacol. 1987;45:429–433. doi: 10.1254/jjp.45.429. [DOI] [PubMed] [Google Scholar]
- UEDA H., INOUE M. In vivo signal transduction of nociceptive response by kyotorphin (tyrosine-arginine) through Gαi- and inositol trisphosphate-mediated Ca2+ influx. Mol. Pharmacol. 2000;57:108–115. [PubMed] [Google Scholar]
- WILCOX R.A., PRIMROSE W.U., NAHORSKI S.R., CHALLISS R.A.J. New developments in the molecular pharmacology of the myo-inositol 1,4,5-triphosphate receptor. Trends Pharmacol. Sci. 1998;19:467–475. doi: 10.1016/s0165-6147(98)01260-7. [DOI] [PubMed] [Google Scholar]


