Abstract
The aim of this study was to characterize the 5-HT receptors involved in the 5-HT-induced contraction of longitudinal muscle (LM) strips of porcine proximal stomach. This was done in a classical organ bath set-up for isotonic measurement.
The concentration-contraction curve to 5-HT was not modified by 5-HT3 and 5-HT4 receptor antagonism. Methysergide, ketanserin and mesulergine antagonized the curve to 5-HT. Concomitantly, increasing concentrations of ketanserin and mesulergine progressively revealed a biphasic nature of the 5-HT curve. Ketanserin antagonized the low-affinity receptor while it did not modify the high-affinity receptor.
Tetrodotoxin did not influence the concentration-contraction curve to 5-HT neither in the absence nor presence of ketanserin, indicating that nerves are not involved.
Ketanserin competitively antagonized the monophasic concentration-response curve to α-Methyl-5-HT, yielding a Schild slope that was not significantly different from unity. After constraining the Schild slope to unity, a pKB estimate of 8.23±0.90 was obtained. This affinity estimate of ketanserin closely approximates previously reported affinities at 5-HT2A receptors.
In the presence of ketanserin (0.1 μM; exposing the high-affinity receptor), a wide range of 5-HT receptor antagonists covering all 5-HT receptors known, was tested. Only methysergide and ritanserin inhibited the response to 5-HT, thus expressing affinity for the high-affinity receptor. This did not reveal the identity of the receptor involved.
It can be concluded that 5-HT induces pig proximal stomach (LM) contraction via 5-HT2A receptors located on smooth muscle. A ketanserin-insensitive phase of contractions could not be characterized between the actually known classes of 5-HT receptors with the pharmacological tools that were used.
Keywords: Pig, proximal stomach, fundus, 5-HT2A receptors, ketanserin
Introduction
Many studies have indicated the involvement of various subtypes of 5-HT receptors in proximal stomach motility regulation. Kojima et al. (1992), Meulemans et al. (1993) and Takemura et al. (1999) have shown 5-HT1 receptors mediating relaxation in guinea-pig proximal stomach in vitro. 5-HT7 receptors are found to mediate relaxation in both distal (Prins et al., 2001a) and proximal (Janssen et al., 2002) parts of the canine stomach. Data from in vivo studies have also indicated that 5-HT1 receptors mediate relaxation, since sumatriptan (a 5-HT1 receptor agonist) relaxed the proximal stomach of cat (Coulie et al., 1999) and of man (Tack et al., 2000). To date it is not known what mechanism underlies this effect linked to 5-HT1 receptors. Takemura et al. (1999) demonstrated that the 5-HT-induced contraction of guinea-pig fundus strips was antagonized by ketanserin, suggesting 5-HT2A receptor involvement. 5-HT2A receptors were also found to mediate contraction in the canine proximal stomach (Janssen et al., 2002). However, rat gastric fundus contraction is mediated by 5-HT2B receptors (Baxter et al., 1994). 5-HT3 receptors mediate stomach contraction in the guinea-pig, in vitro (Buchheit & Buhl, 1994). In conscious dogs, m-chlorophenylbiguanide (a selective 5-HT3 receptor agonist) stimulates antral motility (Nagakura et al., 1997). 5-HT4 receptor agonists potentiate electrically evoked contractions in longitudinal muscle of dog gastric corpus (Prins et al., 2001b) and circular muscle of the guinea-pig gastric fundus and corpus (Hegde & Eglen, 1996).
The digestive system of man and pig show physiological and anatomical similarity (Miller & Ullrey, 1987). Therefore we found it of interest to investigate the 5-HT receptor pharmacology of the porcine proximal stomach. With respect to 5-HT receptor pharmacology, data from studies with porcine or human stomach are scarce. Although 5-HT contracts the pig proximal stomach in vitro (Lefebvre & Vandekerckhove, 1998), it is yet unknown which 5-HT receptor mediates this contraction. We have set out to characterize the receptors involved in 5-HT-induced contraction of pig proximal stomach longitudinal muscle.
Methods
Tissue preparation
Piglets of either sex, weighing between 12 and 20 kg, were sacrificed by decerebration and successive exsanguination through the carotid artery. The entire stomach was dissected and placed in Krebs-Henseleit solution (composition in mM: glucose 10.1, CaCl2 2.51, NaHCO3 25, MgSO4 1.18, KH2PO4 1.18, KCl 4.69 and NaCl 118). The stomach was opened by cutting along the lesser curvature and the contents were rinsed out. A piece of proximal stomach, clearly showing longitudinal muscle bundles along the greater curvature, was carefully cleared of mucosa, submucosa and omentum. Longitudinal muscle strips (maximum 16 per piglet) of approximately 1.5 cm length and 2–3 mm width were prepared and mounted onto tissue holders. These were placed in an organ bath set-up containing Krebs–Henseleit solution (20 ml) at 37°C, continuously gassed with 95% O2 and 5% CO2. The mechanical activity of the preparations was recorded via isotonic transducers (2 g load) (Harvard apparatus) on a chart recorder (Model BD 112; Kipp & Sons). All strips were studied on the day of preparation.
Experimental protocols
All experiments were conducted in the presence of indomethacin (1 μM) to avoid spontaneous contractions due to prostaglandin synthesis. Still, after indomethacin addition 24% (from 27 out of 111 pigs) of all strips showed a spontaneous rise in tone during the stabilization period; these strips were not used.
After a 30 min stabilization period antagonist or solvent was added to the organ bath and left to incubate for 30 min, after which a cumulative concentration-response curve to an agonist was established with half log-units ascending concentration increments from 1 nM onwards. The time interval between two consecutive concentrations was approximately 10 min. Then, compounds were washed out by replacing the organ bath solution twice. Maximal relaxation to nitroglycerin (10 μM) was achieved after which maximal contraction to KCl (0.16 M) was obtained. All agonist-induced responses were expressed as percentage of the contraction to KCl (0.16 M) after nitroglycerin relaxation (10 μM). Only one agonist was studied per muscle strip.
Data analysis
Monophasic curves were used for an iterative fitting procedure using the Hill equation to obtain estimates for mid-point location (pEC50), upper asymptote (α), and Hill slope (nH). Curves displaying a biphasic curve shape were fitted by iterative fitting procedure to a double Hill equation:
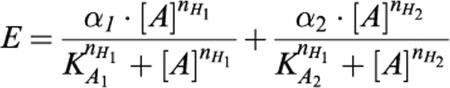 |
In this equation, E represents the response elicited by the agonist, [A] represents concentration of agonist, α indicates the maximum effect asymptote, KA the dissociation constant and nH the Hill slope. The indices 1 and 2 refer to the first and to the second phase response, respectively. It was not feasible to selectively eliminate the high-affinity contraction by pharmacological antagonism, therefore, we did not know whether the low-affinity contraction is added to the high-affinity contraction or not, as a result it was not feasible to interpret the curve parameters for the low-affinity contraction. We thus only used this biphasic curve fitting procedure to obtain agonist curve parameter estimates of the high affinity phase.
Concentration–dependent antagonism of 5-HT by methysergide and of the α-methyl-5-HT curve by ketanserin was quantified by an iterative fitting procedure using a derivative of the Schild equation (as presented by Black et al., 1985).
Statistical evaluation
To test the influence of a treatment on the curve parameters of an agonist concentration-response curve, one-way ANOVA was performed. A level of P<0.05 was considered to indicate significance. To test the criteria for Schild-analysis (curves must have equal slopes and upper asymptotes), one-way ANOVA was performed, followed by a post-hoc Bonferroni's test for multiple comparisons. All data are expressed as the mean±s.e.means, where n represents the number of piglets used in one experimental protocol.
Drugs
The following drugs were used (abbreviations and respective suppliers in parentheses): 5-methoxytryptamine (5-MeOT), 2-methyl-5-HT (2-Me-5-HT), granisetron HCl, mesulergine HCl, ritanserin, ketanserin tartrate, 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine HCl (NAN-190), N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea (SB204741), [1-[2-(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl1-methyl-1H-indole-3-carboxylate (GR113808; Janssen Research Foundation, Belgium), atropine sulphate (Janssen Chimica, Belgium), 5-hydroxytryptamine creatinine sulphate (5-HT), tetrodotoxin (TTX; Serva, Germany), α-methyl-5-HT (α-Me-5-HT), fluoxetine HCl, ω-conotoxin GVIA (ω-conotoxin), 5-carboxamidotryptamine (5-CT; Tocris Cookson, U.K.), potassium chloride (KCl; Sigma, Belgium), methysergide maleate (RBI, U.S.A.), 2-methyl-4-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid [4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]amide HCl (GR127935; kindly donated by Glaxo Group Research, Ware, U.K.), cocaine HCl, glycerol trinitrate 1% (nitroglycerin; Merck, Germany) and pargyline HCl (Abbott, U.S.A.). All compounds were dissolved in distilled water, except for ketanserin, pargyline and 5-HT. Ketanserin was dissolved in distilled water acidified with tartaric acid in the stock solution; pargyline was dissolved in distilled water with 10% cyclodextrine in the stock solution. 5-HT was prepared with ascorbic acid (44 μg ml−1) in the stock solution. The solvents had no effect on the baseline muscle strip length or the curves to agonists. All stock solutions were freshly prepared on the day of the experiment and dilutions were prepared using distilled water.
Results
Although 24% of all muscle strips showed spontaneous tone rise (see Methods), muscle strips did not display phasic activity. 5-HT and the tryptamine analogues induced slowly equilibrating contractions that were maintained. In this manner, it was feasible to establish cumulative concentration–contraction curves to these agonists. There was substantial variation in the response induced by the agonists tested, which was further enhanced by the interaction of the agonists with more than one receptor.
Inhibition of re-uptake-1 by cocaine (30 μM), of selective 5-HT re-uptake by fluoxetine (0.3 μM) and of monoamine oxidase by pargyline (0.1 mM) did not change the curve parameters of the 5-HT curve (n=5); tetrodotoxin (TTX; 0.3 μM), ω-conotoxin (0.3 μM) and atropine (1 μM) did not affect the concentration–contraction curve to 5-HT (n=6, results not shown).
Influence of tryptamine analogues and 5-HT receptor antagonists on the response to 5-HT
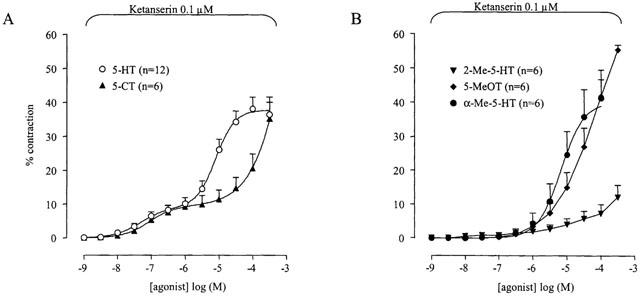
All agonists tested induced contraction of the muscle strips (Figure 1); pEC50, α and nH obtained from the monophasic curve fitting procedure are given in Table 1. The following rank order of agonist potency was found: 5-HT>5-CT>α-Me-5-HT>2-Me-5-HT>5-MeOT. A representative tracing showing the concentration-response curve to 5-HT can be seen in Figure 2A. A decline in contractile response was seen from 10 μM onwards. This was regularly seen and we believe that this is due to desensitisation. Only the concentrations up to the maximum contraction were taken into account calculating the mean results.
Figure 1.
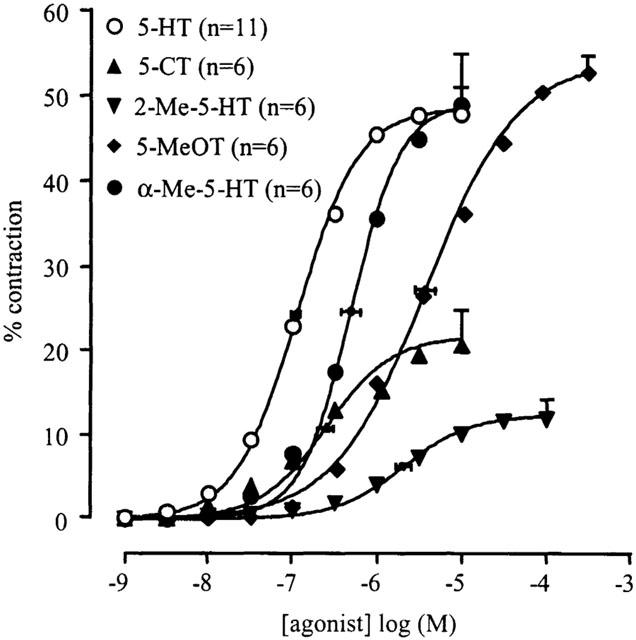
Concentration–contraction curves to 5-HT, 5-CT, 2-Me-5-HT, 5-MeOT and α-Me-5-HT of porcine proximal stomach longitudinal muscle preparations. Contractions were expressed as percentage of muscle strip contraction to KCl (0.16 M). The curves shown superimposed on the vertically averaged data points represent simulations using the Hill equation and the estimates for α (with vertical error bars) and pEC50 (with horizontal error bars) that were obtained from the iterative fitting procedure.
Table 1.
Curve parameters for the concentration–response curves to 5-HT and tryptamine analogues
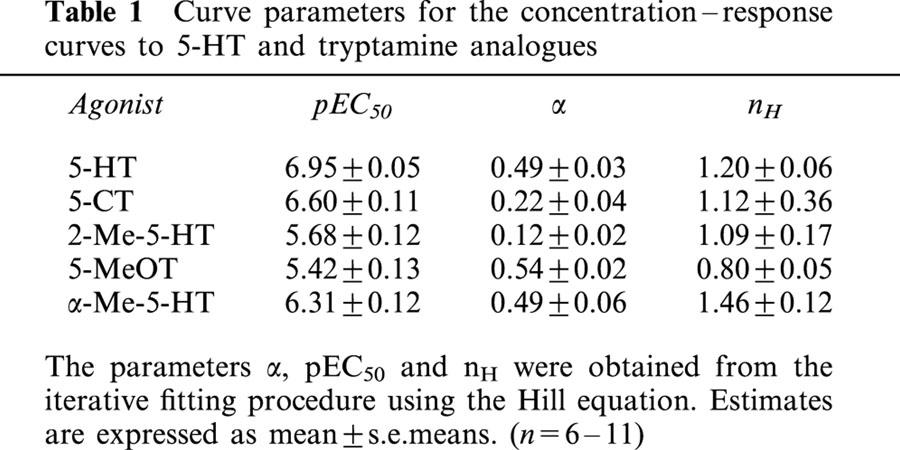
Figure 2.
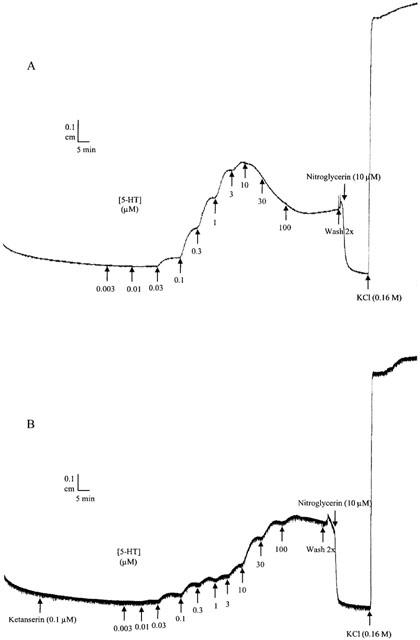
Representative recorder tracings showing the response to 5-HT in pig proximal stomach muscle strips. 5-HT was administered with half log unit concentration increments as indicated by the arrows. The upper tracing (A) represents a concentration–response curve in control condition, while the lower tracing (B) is in the presence of ketanserin (0.1 μM).
Neither the selective 5-HT3 receptor antagonist granisetron (Sanger & Nelson, 1989; 0.3 μM) nor the selective 5-HT4 receptor antagonist GR113808 (Gale et al., 1994; 0.3 μM) altered the concentration-response curve to 5-HT (n=6–8; Table 2).
Table 2.
Curve parameters for the concentration–response curves to 5-HT in control conditions and in the presence of the antagonists indicated
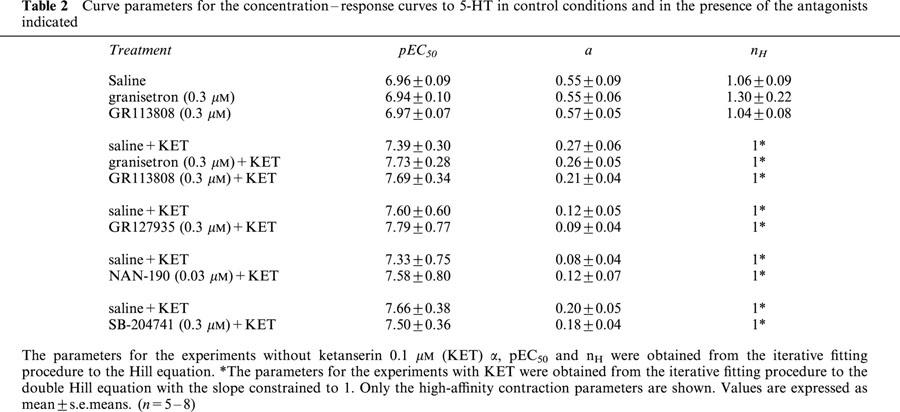
Methysergide (5-HT1, 5-HT2, 5-HT5, 5-HT6 and 5-HT7 receptor antagonist; Gommeren et al., 1998; Figure 3A), mesulergine (5-HT2 and 5-HT7 receptor antagonist; Hoyer et al., 1994; Figure 3B) and ketanserin (5-HT2A receptor antagonist; Hoyer et al., 1994; Figure 3C) induced a concentration-dependent rightward shift of the concentration-response curve to 5-HT without significantly altering the maximum effect. Methysergide competitively antagonized the 5-HT-induced concentration–response curves. A pKB value of 8.60±0.12 was estimated. In the presence of increasing concentrations of ketanserin and mesulergine, the concentration–response curve to 5-HT became progressively biphasic, indicative of multiple receptors mediating the response to 5-HT. Ketanserin antagonized the low-affinity phase of the response to 5-HT while it did not modify the high-affinity phase. Similar results were obtained with higher concentrations of ketanserin (up to 1 μM; Figure 3D). With 1 μM of ketanserin, a significant depression of the maximal effect to 5-HT was observed. A representative tracing of the response to 5-HT in the presence of ketanserin (0.1 μM) can be seen in Figure 2B.
Figure 3.

Influence of increasing concentrations of methysergide (MET; A), mesulergine (MES; B) and ketanserin (KET; C and D) on the 5-HT-induced contractions of porcine proximal stomach longitudinal muscle strips. The vertically averaged experimental data points were expressed as mean percentage±s.e.means of the contraction to KCl (0.16 M) and connected by a line.
Ketanserin (10–100 nM) produced a parallel rightward displacement of the concentration-contraction curve to α-Me-5-HT (Figure 4), yielding a Schild slope of 1.16±0.14, this was not significantly different from unity. After constraining the Schild slope to unity, a pKB estimate of 8.23±0.09 was obtained.
Figure 4.

Antagonism by ketanserin of the α-Me-5-HT-induced contraction of porcine proximal stomach longitudinal muscle preparations. The curves shown superimposed on the vertically averaged data points represent simulations using the Hill equation and the estimates for α (with vertical error bars) and pEC50 (with horizontal error bars) that were obtained from the iterative fitting procedure.
Contractions to 5-HT and tryptamine analogues in the presence of ketanserin
While antagonizing the low-affinity contraction to 5-HT, ketanserin (7 nM–1 μM) did not affect the high-affinity contraction to 5-HT. To investigate which receptors are involved in the high-affinity contraction to 5-HT, it was necessary to antagonize the 5-HT receptor population mediating the low-affinity contractions to 5-HT. In order to do this, the following experiments were performed in the presence of ketanserin (0.1 μM).
Neither TTX (0.3 μM) nor atropine (1 μM) affected the concentration-response curve to 5-HT in the presence of ketanserin (n=6; results not shown).
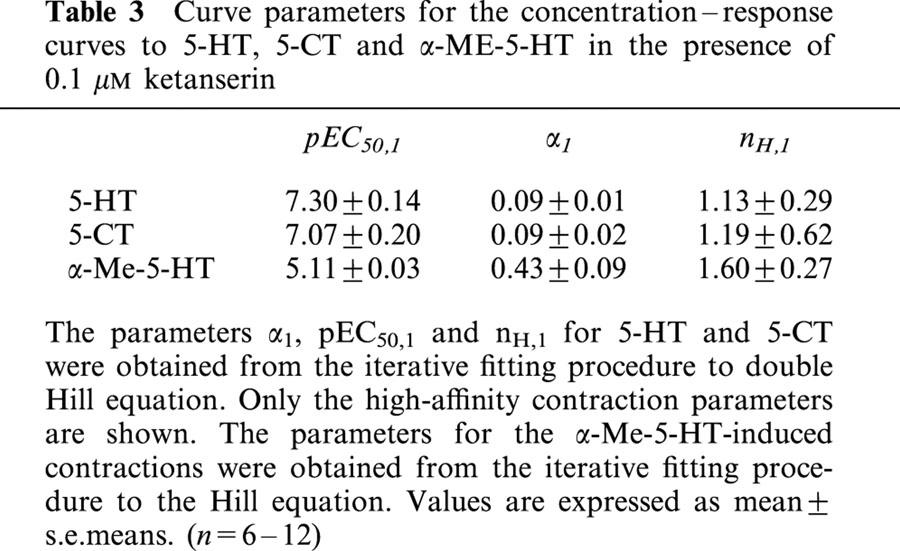
5-HT and tryptamine analogues all induced contraction (Figure 5). 5-CT and 5-HT induced a biphasic concentration–response curve revealing a high- and a low-affinity phase. The curves were fitted to the double Hill equation to obtain curve parameters for the high-affinity contraction to 5-HT and 5-CT (Table 3). The concentration-response curve to α-Me-5-HT was monophasic and was fitted to the Hill equation for monophasic curves, yielding values for α and nH that were not statistically different from those obtained in the absence of ketanserin. However the pEC50 value was significantly lower compared to the pEC50 in the absence of ketanserin (Table 1 vs Table 3). The concentration–response curve to 5-MeOT did not reach a maximum (results not shown), and could therefore not be fitted; also in the presence of lower concentrations of ketanserin (10–30 nM), the maximal effect was not reached by 5-MeOT at 300 μM (results not shown). The 2-Me-5-HT-induced contraction curve appeared neither monophasic nor biphasic, therefore no curve fit was performed.
Figure 5.
Concentration-contraction curves to 5-HT, 5-CT (A) and 2-Me-5-HT, 5-MeOT and α-Me-5-HT (B) of porcine proximal stomach longitudinal muscle preparations in the presence of ketanserin (0.1 μM). The curves shown superimposed on the vertically averaged data points±s.e.means for 5-HT and 5-CT represent simulations using the double Hill equation. The curve shown superimposed on the vertically averaged data points±s.e.means for α-Me-5-HT represent simulation using the Hill equation. The curves to 2-Me-5-HT and 5-MeOT were not fitted. The vertically averaged experimental data points±s.e.means of these agonists were connected by a line.
Table 3.
Curve parameters for the concentration–response curves to 5-HT, 5-CT and α-ME-5-HT in the presence of 0.1 μM ketanserin
Sumatriptan (1 nM–0.1 mM), a 5-HT1B, 5-HT1D and 5-HT1F receptor agonist (Hoyer et al., 1994) was not able to significantly contract the muscle strips (results not shown; n=5).
Influence of 5-HT receptor antagonists on the 5-HT induced contractions in the presence of ketanserin
Mesulergine (1 μM), GR127935 (5-HT1B, 5-HT1D receptor antagonist; Terron, 1996; 0.1 μM), granisetron (0.3 μM), GR113808 (0.3 μM), NAN-190 (5-HT1A receptor antagonist; Cao & Rodgers, 1997; 0.03 μM) and SB-204741 (5-HT2B receptor antagonist; Forbes et al., 1995; 0.3 μM) did not alter the high-affinity phase of the concentration–response curve to 5-HT (n=5–7; Table 2). Ritanserin (5-HT2A, 5-HT2B, 5-HT2C and 5-HT7 receptor antagonist; Gommeren et al., 1998; 0.3 μM) did not alter the Hill slope or pEC50 of the high-affinity phase of the 5-HT curve but depressed the maximal effect (n=7; Figure 6A). Methysergide (1 μM) affected the high-affinity contraction to 5-HT with a depression of the maximum effect (n=7; Figure 6B).
Figure 6.
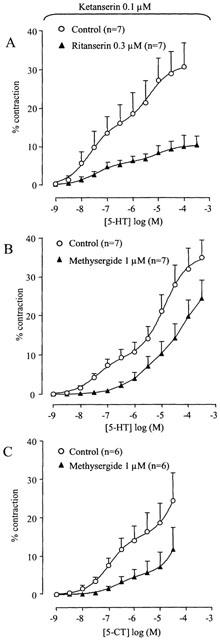
Effect of ritanserin (A) on the 5-HT-induced concentration–contraction curve and of methysergide (1 μM) on the 5-HT- (B) and 5-CT- (C) induced concentration–contraction curve of porcine proximal stomach longitudinal muscle strips. The experiments were performed in the presence of ketanserin (0.1 μM). The curves shown superimposed on the vertically averaged data points±s.e.means represent simulations using the double Hill equation. The 5-HT-induced concentration–contraction curve in the presence of methysergide was not fitted, the vertically averaged experimental data points±s.e.means were connected by a line.
Mesulergine (1 μM), GR127935 (0.1 μM) and NAN-190 (0.03 μM) did not alter the concentration-response curve to 5-CT. Methysergide (1 μM) affected the high-affinity contraction to 5-CT with a depression of the maximum effect (n=6; Figure 6C).
Discussion
The data presented in this study suggest that at least two receptor populations mediate the contraction to 5-HT of pig proximal stomach longitudinal muscle. One receptor population has the characteristics of the 5-HT2A receptor, whilst it was not possible to identify the other receptor population with the pharmacological tools that were used.
The experiments with pargyline, fluoxetine and cocaine imply that breakdown or re-uptake of 5-HT does not impact the interaction of 5-HT with its receptors. Furthermore, the inability of TTX to affect the 5-HT-induced contractions, either in the absence or presence of ketanserin, indicates that the receptors involved are most likely located on the smooth muscle.
Involvement of a low and high affinity receptor
The rank order of agonist potency of the tryptamine analogues can be used to roughly identify the 5-HT receptor subtype involved in a particular response (Baxter et al., 1994; Leff & Martin, 1988). Our rank order of agonist potency is difficult to interpret, perhaps as a result of the heterogeneous nature of the 5-HT receptor population. In this manner, multiple rank orders of agonist potencies overlap. Nevertheless, of the tryptamine analogues used, α-Me-5-HT and 5-CT were clearly more potent than 2-Me-5-HT and 5-MeOT, suggesting the possible involvement of 5-HT2 receptors, and 5-HT1 and 5-HT7 receptors respectively. 2-Me-5-HT and 5-MeOT were markedly less potent agonists, thus implying that 5-HT3 and 5-HT4 receptors are not involved or of minor importance. In the presence of ketanserin, the curves to 5-HT and 5-CT, but not those to the other tryptamine analogues, became biphasic. These findings are consistent with a heterogeneous receptor population consisting of at least two different receptor subtypes. One is stimulated by 5-HT, α-Me-5-HT, 5-CT, 2-Me-5-HT and 5-MeOT and ketanserin-sensitive, hence is most likely a 5-HT2 receptor. The other ketanserin-insensitive receptor displays a relatively high affinity (both using 5-HT or 5-CT) but a relatively low efficacy in comparison to the ketanserin-sensitive receptor. To distinguish between the two receptors, the ketanserin-sensitive receptor was thus called the low-affinity receptor.
The involvement of 5-HT3 and 5-HT4 receptors is unlikely. Indeed, the preferential 5-HT3 receptor agonist 2-Me-5-HT induces a low potency, low efficacy response and the selective 5-HT3 receptor antagonist granisetron (Sanger & Nelson, 1989) did not affect the curve to 5-HT in the absence and the presence of ketanserin. The putative 5-HT4 receptor agonist 5-MeOT was clearly less potent than 5-HT, α-Me-5-HT and 5-CT. In the presence of ketanserin, no biphasic response to 5-MeOT was induced indicating that 5-MeOT has little affinity for the high-affinity contraction receptors. Furthermore, GR113808 did not alter the 5-HT-induced concentration-response curve either in the absence or presence of ketanserin. This emphasizes that neither 5-HT3 nor 5-HT4 receptors are involved in either phase of the 5-HT-response.
Characterization of the low-affinity receptor
The competitive antagonism produced by ketanserin (10–100 nM) on the α-Me-5-HT-induced contraction pointed strongly to 5-HT2A receptor involvement. The pKB estimate for ketanserin (8.23±0.09) against α-Me-5-HT agrees well with literature affinities at 5-HT2A receptors (dog colon: pKB of 8.4±0.1; Prins et al., 1997), rat caudal artery: pA2 of 8.4±0.1; (Blackburn et al., 1988)). The results with ketanserin also show that α-Me-5-HT has little or no affinity for the high-affinity receptor.
This confirms the presence of contractile 5-HT2A receptors in pig proximal stomach LM strips and explains the effect of ketanserin on the curve to 5-HT. Ketanserin, as well as mesulergine, antagonized the 5-HT-induced curve, revealing a biphasic nature of the 5-HT-induced contraction curve. Methysergide on the other hand competitively antagonizes the low-affinity phase. The pKB estimate for methysergide is well in line with literature affinities for the 5-HT2 receptors (pKi of 8.6 (Hoyer & Schoeffter, 1991)).
Characterization of the high-affinity receptor
To study the high-affinity receptor, experiments were performed in the presence of ketanserin (0.1 μM). Only 5-CT and 5-HT display affinity for the high-affinity receptor, suggesting the apparent involvement of 5-HT1 or 5-HT7 receptors. Although ritanserin depressed the curve to 5-HT (Figure 6A) it is very unlikely that 5-HT2 receptors were involved, since (1) 5-HT2A receptors were already blocked by ketanserin; (2) the selective 5-HT2B receptor antagonist SB-204741 was ineffective; (3) ketanserin, at high concentrations also blocks 5-HT2C receptors, the non-effect of ketanserin and mesulergine rules out the involvement of 5-HT2C receptors. 5-HT7 receptors are most likely not involved for two reasons. First, 5-HT7 receptors located on smooth muscle are expected to mediate relaxation, in view of their positive coupling to adenylate cyclase (Jasper et al., 1997). Second, mesulergine, a potent 5-HT7 receptor antagonist (Terron & Falcon-Neri, 1999), did not affect the high-affinity response to both 5-HT or 5-CT. Thus, this would point to the involvement of 5-HT receptors belonging to the 5-HT1 receptor class. This seems to be corroborated by the influence of methysergide (prevents interactions with 5-HT1, 5-HT2, 5-HT7 receptors) on the response to 5-HT and 5-CT in the presence of ketanserin. However, GR127935 and NAN-190 did not affect the high-affinity contraction to 5-HT. Furthermore, it is unlikely that 5-HT1E and 5-HT1F receptors are involved, since 5-CT (weak 5-HT1E receptor agonist; Hoyer et al., 1994) is relatively potent and sumatriptan (efficacious 5-HT1F receptor agonist, Hoyer et al., 1994) is ineffective. These results can correlate with three possibilities: (1) the receptor involved in the high-affinity contraction to 5-HT can not be characterized within the actually described subtypes of 5-HT receptors; (2) the used tools are not correctly labelled for the pig 5-HT receptor sequence; or (3) the receptor involved is not a 5-HT receptor.
In conclusion, at least two different receptors mediate the contraction observed in porcine proximal stomach LM strips upon 5-HT administration. A 5-HT2A receptor mediates the low-affinity, high-efficacy contraction, becoming the second phase of contractions in the presence of a 5-HT2A receptor antagonist. A high-affinity receptor population mediates a less efficacious contraction that becomes the first phase of contractions in the presence of a 5-HT2A receptor antagonist. This high-affinity receptor to 5-HT is methysergide-, ritanserin- and 5-CT-sensitive. This receptor could not be characterized between the actually known classes of 5-HT receptors with the pharmacological tools that were used.
Acknowledgments
The authors wish to thank Luc Hoskens and Marcel Sysmans for their technical assistance.
Abbreviations
- 2-Me-5-HT
2-methyl-5-HT
- 5-CT
5-carboxamidotryptamine
- 5-MeOT
5-methoxytryptamine
- α-Me-5-HT
α-methyl-5-HT
- GR113808
[1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl 1-methyl-1H-indole-3-carboxylate
- GR127935
2-methyl-4-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid [4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]amide HCl
- KCl
potassium chloride
- NAN-190
1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine HCl
- SB204741
N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea
- TTX
tetrodotoxin
- ω-conotoxin
ω-conotoxin GVIA
References
- BAXTER G.S., MURPHY O.E., BLACKBURN T.P. Further characterization of 5-hydroxytryptamine receptors (putative 5-HT2B) in rat stomach fundus longitudinal muscle. Br. J. Pharmacol. 1994;112:323–331. doi: 10.1111/j.1476-5381.1994.tb13072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK J.W., LEFF P., SHANKLEY N.P. Further analysis of anomalous pKB values for histamine H2-receptor antagonists on the mouse isolated stomach assay. Br. J. Pharmacol. 1985;86:581–587. doi: 10.1111/j.1476-5381.1985.tb08934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKBURN T.P., THORNBER C.W., PEARCE R.J., COX B. In vitro studies with ICI 169,369, a chemically novel 5-HT antagonist. Eur. J. Pharmacol. 1988;150:247–256. doi: 10.1016/0014-2999(88)90005-2. [DOI] [PubMed] [Google Scholar]
- BUCHHEIT K.H., BUHL T. Stimulant effects of 5-hydroxytryptamine on guinea pig stomach preparations in vitro. Eur. J. Pharmacol. 1994;262:91–97. doi: 10.1016/0014-2999(94)90031-0. [DOI] [PubMed] [Google Scholar]
- CAO B.J., RODGERS R.J. Influence of 5-HT1A receptor antagonism on plus-maze behaviour in mice. II. WAY 100635, SDZ 216-525 and NAN-190. Pharm. Biochem. Behav. 1997;58:593–603. doi: 10.1016/s0091-3057(97)00279-7. [DOI] [PubMed] [Google Scholar]
- COULIE B., TACK J., SIFRIM D., ANDRIOLI A., JANSSENS J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am. J. Physiol. 1999;276:G373–G377. doi: 10.1152/ajpgi.1999.276.2.G373. [DOI] [PubMed] [Google Scholar]
- FORBES I.T., JONES G.E., MURPHY O.E., HOLLAND V., BAXTER G.S. N-(1-Methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea: A novel, High-affinity 5-HT2B Receptor Antagonist. J. Med. Chem. 1995;38:855–857. doi: 10.1021/jm00006a001. [DOI] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W., OXFORD A.W., BUNCE K.T., HUMPHREY P.P. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMMEREN W., RENDERS J., VAN GOMPEL P., LESAGE A., LEYSEN J.E., JURZAK M. Extensive pharmacological study of the G-protein coupled fraction of human 5-HT receptors using agonist radioligand binding. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:8–42. [Google Scholar]
- HEGDE S.S., EGLEN R.M. Peripheral 5-HT4 receptors. FASEB J. 1996;10:1398–1407. doi: 10.1096/fasebj.10.12.8903510. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HOYER D., SCHOEFFTER P. 5-HT receptors: subtypes and second messengers. J. Recept. Res. 1991;11:197–214. doi: 10.3109/10799899109066399. [DOI] [PubMed] [Google Scholar]
- JANSSEN P., PRINS N.H., MEULEMANS A.L., LEFEBVRE R.A. Pharmacological characterization of the 5-HT receptors mediating contraction and relaxation of canine isolated proximal stomach smooth muscle. Br. J. Pharmacol. 2002;136:321–329. doi: 10.1038/sj.bjp.0704716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JASPER J.R., KOSAKA A., TO Z.P., CHANG D.J., EGLEN R.M. Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7b) Br. J. Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJIMA S., ISHIZAKI R., SHIMO Y. Investigation into the 5-hydroxytryptamine-induced relaxation of the circular smooth muscle of guinea-pig stomach fundus. Eur. J. Pharmacol. 1992;224:45–49. doi: 10.1016/0014-2999(92)94816-e. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A., VANDEKERCKHOVE K. Effect of nitroglycerin and long-term electrical stimulation on nitrergic relaxation in the pig gastric fundus. Br. J. Pharmacol. 1998;123:143–149. doi: 10.1038/sj.bjp.0701582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFF P., MARTIN G.R. The classification of 5-Hydroxytryptamine Receptors. Med. Res. Rev. 1988;8:187–202. doi: 10.1002/med.2610080203. [DOI] [PubMed] [Google Scholar]
- MEULEMANS A.L., HELSEN L.F., SCHUURKES J.A. The role of nitric oxide (NO) in 5-HT-induced relaxations of the guinea-pig stomach. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:424–430. doi: 10.1007/BF00171343. [DOI] [PubMed] [Google Scholar]
- MILLER E.R., ULLREY D.E. The pig as a model for human nutrition. Annu. Rev. Nutr. 1987;7:361–382. doi: 10.1146/annurev.nu.07.070187.002045. [DOI] [PubMed] [Google Scholar]
- NAGAKURA Y., ITO H., KAMATO T., NISHIDA A., MIYATA K. Effect of a selective 5-HT3 receptor agonist on gastric motility in fasted and fed dogs. Eur. J. Pharmacol. 1997;327:189–193. doi: 10.1016/s0014-2999(97)89660-4. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., AKKERMANS L.M., LEFEBVRE R.A., SCHUURKES J.A. Characterization of the receptors involved in the 5-HT-induced excitation of canine antral longitudinal muscle. Br. J. Pharmacol. 2001a;134:1351–1359. doi: 10.1038/sj.bjp.0704376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., VAN DER GRIJN A., LEFEBVRE R.A., AKKERMANS L.M.A., SCHUURKES J.A.J. 5-HT4 receptors mediating enhancement of contractility in canine stomach; an in vitro and in vivo study. Br. J. Pharmacol. 2001b;132:1941–1947. doi: 10.1038/sj.bjp.0703985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., SCHUURKES J.A. Characterization of the contraction to 5-HT in the canine colon longitudinal muscle. Br. J. Pharmacol. 1997;120:714–720. doi: 10.1038/sj.bjp.0700954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER G.J., NELSON D.R. Selective and functional 5-hydroxytryptamine 3 receptor antagonism by BRL 43694 (granisetron) Eur. J. Pharmacol. 1989;159:113–124. doi: 10.1016/0014-2999(89)90695-x. [DOI] [PubMed] [Google Scholar]
- TACK J., COULIE B., WILMER A., ANDRIOLI A., JANSSENS J. Influence of sumatriptan on gastric fundus tone and on the perception of gastric distension in man. Gut. 2000;46:468–473. doi: 10.1136/gut.46.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMURA K., TAKADA K., MAMEYA S., KAIBARA M., TANIYAMA K. Regional and functional differences of 5-hydroxytryptamine-receptor subtypes in guinea pig stomach. Jap. J. Pharm. 1999;79:41–49. doi: 10.1254/jjp.79.41. [DOI] [PubMed] [Google Scholar]
- TERRON J.A. GR127935 is a potent antagonist of the 5-HT1-like receptor mediating contraction in the canine coronary artery. Eur. J. Pharmacol. 1996;300:109–112. doi: 10.1016/0014-2999(96)00041-6. [DOI] [PubMed] [Google Scholar]
- TERRON J.A., FALCON-NERI A. Pharmacological evidence for the 5-HT7 receptor mediating smooth muscle relaxation in canine cerebral arteries. Br. J. Pharmacol. 1999;127:609–616. doi: 10.1038/sj.bjp.0702580. [DOI] [PMC free article] [PubMed] [Google Scholar]


