Abstract
Contractility studies were undertaken to determine the nature of the receptors mediating responses to tachykinins in uteri of oestrogen-treated mice.
In the presence of thiorphan, (3 μM), captopril (10 μM) and bestatin (10 μM), substance P (SP), neurokinin A (NKA) and neurokinin B (NKB) produced concentration-related contractions of uterine preparations. The order of potency was SP ⩾NKA>NKB.
Neither atropine (0.1 μM) nor l-NOLA (100 μM), nor indomethacin (10 μM) alone or in combination with either ranitidine (10 μM) or mepyramine (10 μM), affected responses to SP. These findings indicate that SP actions are not mediated or modulated through the release of acetylcholine, nitric oxide, prostanoids or histamine.
In the presence of peptidase inhibitors, the tachykinin NK1 receptor-selective agonist [Sar9Met(O2)11]SP, produced a concentration-dependent contractile effect. The tachykinin NK2 and NK3 receptor-selective agonists [Lys5MeLeu9Nle10]NKA(4-10) and [MePhe7]NKB were relatively inactive. The potencies of SP analogues in which Glu replaced Gln5 and/or Gln6 were similar to that of SP.
The tachykinin NK1 receptor-selective antagonist, SR140333 (10 nM), alone or combined with the tachykinin NK2 receptor-selective antagonist, SR48968 (10 nM), shifted log concentration curves to SP, NKA and NKB to the right. SR140333 (10 nM) reduced the effect of [Sar9Met(O2)11]SP. SR48968 did not affect responses to SP or [Sar9Met(O2)11]SP, but reduced the effect of higher concentrations of NKA and shifted the log concentration-response curve to NKB to the right. The tachykinin NK3 receptor-selective antagonist, SR 142801 (0.3 μM), had little effect on responses to SP and NKB.
We conclude that the tachykinin NK1 receptor mediates contractile effects of SP, NKA and NKB and [Sar9Met(O2)11]SP in myometrium from the oestrogen-primed mouse. The tachykinin NK2 receptor may also participate in the responses to NKA and NKB.
Keywords: mouse myometrium, NK1 and NK2 receptors, neurokinin A, neurokinin B, oestrogen, substance P, tachykinins, uterine contractions, SR140333, SR48968
Introduction
Tachykinin-immunoreactive sensory neurones supply the female genital tract in a number of species including the human (Samuelson et al., 1985; Heinrich et al., 1986; Franco-Cereceda et al., 1987), rat (Huang et al., 1984; Springall et al., 1984), guinea-pig (Huang et al., 1984; Alm & Lundberg, 1988), and mouse (Huang et al., 1984). The tachykinins expressed in these nerves include SP and NKA, which can interact with three receptor types, designated NK1, NK2 and NK3. While SP, NKA and NKB are tachykinin NK1, NK2 and NK3 receptor-preferring, respectively; high concentrations of each can activate all three receptor subtypes (Maggi, 1995). Although NKB is not generally believed to be present in peripheral sensory nerves it has been reported to be expressed in the human placenta (Page et al., 2000) and the gene that encodes it, pre-protachykinin-B, is present in the rat uterus (Pinto et al., 2001). Tachykinins have also been reported to be present in a number of other cell types present in the genito-urinary tract (Patak et al., 2000a).
While it is now well established that sensory neurotransmitters, including the tachykinins, can be released from the peripheral terminals of capsaicin-sensitive sensory neurones to produce a number of local effects (Lembeck & Holzer, 1979; Maggi & Meli, 1988), the possible effects of release from these nerves or other sources on uterine functions are still unclear. Investigations of the actions of tachykinins on uterine function so far have indicated that SP may participate in stress-induced abortion in the mouse and possibly the human (Arck et al., 1995; Markert et al., 1997; Marx et al., 1999; Joachim et al., 2001). This effect has been attributed to a local action of SP via tachykinin NK1 receptors to release TNF-α.
There have been several investigations of the effects of tachykinins on myometrial contractility in the last decade; the large majority of these have been on the rat uterus (Barr et al., 1991; Fisher et al., 1993; Fisher & Pennefather, 1997; 1998; 1999; Magraner et al., 1997; 1998; Moodley et al., 1999; Shintani et al., 2000; Candenas et al., 2001). These studies have established that in that species, although NK1 and NK3 receptors are expressed in the uterus, the contractile effects of the mammalian tachykinins in tissues from non-pregnant animals are mediated predominantly by NK2 receptors. In this respect the rat is similar to the human (Patak et al., 2000a,b,c). In the present study we describe the effects of tachykinins on the smooth muscle of the mouse uterus since this species potentially affords the possibility of using genetically modified animals such as gene knockouts for studying dysfunctions of the uterus. Moreover in the mouse as in the human it has been proposed, as stated above, that tachykinins may participate in stress-induced abortion (Arck et al., 1995; Markert et al., 1997; Marx et al., 1999; Joachim et al., 2001).
Preliminary communication of some of the results of this study has been presented previously (Fleming et al., 1998).
Methods
Prior ethical approval for this study was obtained from the Monash University Standing Committee on Ethics in Animal Experimentation.
Animals and treatments
Female, virgin Balb C mice were housed in the Departmental Animal house at 22°C where they were exposed to a photo-period of 12 h light and 12 h dark and were allowed access to food and water ad libitum. Twenty-four hours prior to experimentation mice were administered a single injection of oestradiol cypionate (200 μg kg−1) in peanut oil (0.1 ml kg−1 s.c.).
Animals weighed 20–25 g at the time of experimentation. They were killed by firstly anaesthetizing with CO2 followed by decapitation. Vaginal smears were taken to confirm the presence of cornified epithelial cells indicating an oestrous-like state. These were stained with Giemsa stain which was made by dissolving Giemsa in citric acid/phosphate buffer (pH 4.0). This buffer contained 0.1 M of citric acid, 0.2 M disodium hydrogen peroxide phosphate in 25% methanol. Slides were dried and left overnight in the stain before rinsing with methanol.
Preparations
Both uterine horns were removed, cleared of surrounding fat and connective tissue and placed in a petri dish containing a modified Krebs-Henseleit solution of the following composition (mM: NaCl 118.0; KCl 4.7; MgSO4.7H2O 1.1; KH2PO4 1.18; NaHCO3 25.0; glucose 11.66; CaCl2.2H2O 1.9). Each horn was opened along the mesometrial border and transected medially allowing for four preparations per animal. These were tied to tissue holders so as to record contractile force produced by the longitudinally-oriented smooth muscle fibres. Preparations were placed into siliconized, 5 ml isolated organ baths containing the modified Krebs-Henseleit solution warmed to 37°C and continuously bubbled with 95% O2, in 5% CO2, pH=7.4. Each preparation was attached to a Grass FT03 force transducer that was connected to a MACLAB data acquisition system.
Agonist log concentration-response-curves
Each tissue was allowed to equilibrate for 60 min under 1 g force and washed every 20 min before drugs were added. To prevent peptide inactivation, which is prominent in the uterus (Fisher & Pennefather, 1997; Magraner et al., 1998) the peptidase inhibitors thiorphan (3 μM), captopril (10 μM) and bestatin (10 μM) were present throughout the experiments, being replaced each time the bath was washed out. Agonist log concentration-response curves, usually using a progression ratio of half a log unit over 5.5 orders of magnitude, were constructed on each tissue using the discrete method. In a subset of experiments with SP the progression ratio was 1 log unit. Each agonist concentration remained in contact with the tissue for 5 min, the tissue was then washed with 2–3 times the bath volume and a higher concentration of agonist was added 15 min later. Only one concentration-response curve was constructed on each preparation. At the conclusion of each experiment each tissue was exposed for 5 min to a single concentration (0.1 μM) of methacholine. Following this the tissue was exposed to a modified Krebs solution (KPSS) in which 40 mM KCl replaced 40 mM NaCl; the responses to KPSS were measured over the 5 min exposure period. Each preparation was weighed at the end of each experiment; the mean tissue weights of preparations used in this study were 29.1±0.42 mg (n=440 preparations from 110 mice).
Antagonist studies
The effects of the antagonists on responses to each agonist were determined by comparing the control agonist log concentration response curve constructed on one preparation from each animal with curves obtained on the other preparations from the same animal in the presence of the antagonist alone or in combination. Each antagonist was added at the beginning of the equilibration period and replaced each time the bath was washed out. In the series of experiments with the NK1 and NK2 antagonists, SR140333 and SR48968 respectively, using mammalian tachykinins, the ethanol vehicle used for solution of the antagonists was present in a concentration of 0.001% in all four preparations.
Data analysis
Responses to all agonists were measured as area under the force-time curve (gs), for the 5 min period that the agonist remained in contact with the tissue. Responses were also expressed as a percentage of the corresponding response to 40 mM KPSS. Results are expressed as mean±s.e.mean; n values refer to the numbers of mice used.
To determine agonist potencies in the absence and/or the presence of antagonists mean log concentration-response curves were constructed using non-linear regression analysis in GRAPHPAD PRISM 3 to determine pD2 values. Emax%KPSS was defined as the maximum response to an agonist, expressed as a percentage of the response to KPSS. If pairs of mean regression lines over the linear range of the log concentration-response curves were parallel, a potency ratio with 95% confidence limits was obtained using the analysis described in Documenta Geigy (1970) as described previously (Fisher & Pennefather, 1997). Shifts were considered significant when the 95% confidence limits did not include one. pKb estimates (pKb=log (concentration ratio-1) −log (antagonist concentration) were calculated when shifts in the positions of log concentration-response curves were parallel, with no evidence for significant depression of Emax. Other statistical procedures used included one- and two-way analyses of variance followed by Student Newman Keuls' pairwise test for multiple comparisons and Student's unpaired ‘t' tests to compare the means of two groups using SIGMASTAT or INSTAT (Version 2). Statistical significance was accepted when P<0.05.
Drugs and solutions
The drugs used were: acetyl-β-methylcholine chloride (methacholine chloride), atropine sulphate, bestatin (Sigma); captopril (D-3-mercapto-2-methyl propanoyl-L-proline) (Sigma); histamine (2-[-4-imidazolyl]ethylamine dihydrochloride (Sigma); indomethacin (Sigma); [Lys5MeLeu9Nle10]NKA(4-10) (RBI); mepyramine maleate (Sigma); neurokinin A (Auspep); neurokinin B (Auspep); [N-MePhe7]NKB (Auspep); N(ω)-nitro-L-arginine (L-NOLA, Sigma) oestradiol-17 β cypionate (Sigma); ranitidine(Sigma); [Sar9Met(O2)11]SP (Auspep); SR140333 (1-{2-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl) piperidin-3-yl]ethyl}-4phenyl-1-azonia-bicyclo[2.2.2]octane, SR48968 ((S)-N-methyl-N[4-acetylamino-4-phenylpiperidino)-2-((3,4-dichlorophenyl)butyl]benzamide) and SR142801 ((S)-(N)-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl)propyl)-4-phenylpiperidin-4-yl)-N-methylacetamide) (all generous gifts from Dr X. Emonds-Alt, Sanofi Recherche, France); substance P (SP, Auspep); [Glu5]SP, [Glu5,6]SP, [Glu6]SP (all gifts from Auspep); DL-Thiorphan (Sigma). Atropine, captopril, histamine, mepyramine, ranitidine and thiorphan were dissolved in distilled water. Neurokinin B (NKB) and [MePhe7]NKB were dissolved in 0.1 M ammonia. Indomethacin was dissolved in a 0.1 M and L-NOLA in 1 M sodium carbonate and were further diluted in distilled water. SR140333, SR48968 and SR142801 were dissolved in absolute ethanol and further diluted in distilled water. All remaining compounds were dissolved in dilute hydrochloric acid (0.01 M). Stock solutions of bestatin (10 mM), captopril (10 mM), histamine (1 mM), indomethacin (10 mM), mepyramine (10 mM), methacholine (10 mM), ranitidine (10 mM), SR140333 (1 mM), SR48968 (1 mM) and SR142801 (1 mM) were stored in the refrigerator. Standard solutions (1 mM) of all peptides and thiorphan were aliquoted into Eppendorf tubes and stored at −20°C. Oestradiol cypionate was prepared by first dissolving it in 0.1 ml of ethanol and then diluted in peanut oil (Crisco) up to 10 ml. It was protected from light by foil wrapping and storage in the dark.
Results
Vaginal smears
Histological examination of vaginal smears from oestrogen-treated mice used in this investigation confirmed cornification of the vaginal epithelium.
Agonist studies
All preparations initially exhibited spontaneous activity. Figure 1 shows a typical log concentration response curve to SP, together with responses to methacholine (0.1 μM) and KPSS. No significant differences in the mean responses to KPSS were observed in experiments in which the potencies of the agonists were compared (Table 1; one-way ANOVA, P>0.05). The mean responses to methacholine were also similar in these experiments in which the potencies of agonists were compared (one-way ANOVA, P>0.05). The overall mean response to methacholine (0.1 μM) from the agonist experiments shown in Table 1 was 52.4±4.38% (n=6 groups of 6–10 preparations) of the response to KPSS.
Figure 1.
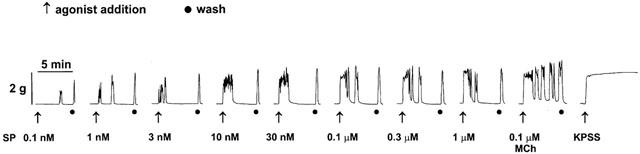
Trace showing contractile responses of a myometrial preparation obtained from an oestrogen-primed mouse (200 μg kg−1 oestradiol cypionate s.c.) to increasing concentrations of SP in the presence of peptidase inhibitors thiorphan (3 μM), captopril (10 μM) and bestatin (10 μM). After construction of the concentration-response curve, the tissue was exposed to methacholine (MCh, 0.1 μM), washed, and exposed to a high potassium bathing solution (KPSS).
Table 1.
Effects of peptides, methacholine (0.1 μM) and KPSS on myometrium from oestrogen-primed mice
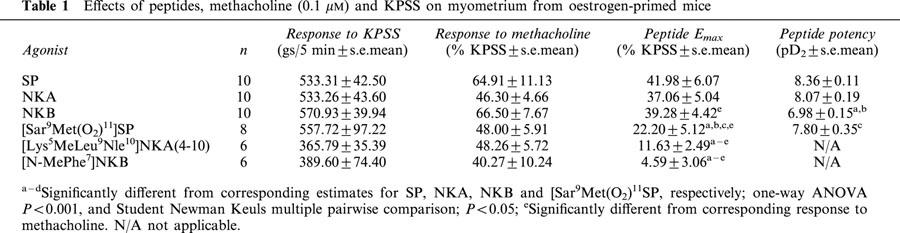
The mean maximal responses to the mammalian tachykinins were similar to or significantly lower than the mean responses to methacholine (0.1 μM) (Table 1). Figure 2a shows log concentration-response curves to SP, NKA and NKB. All three agonists produced maximal responses that did not differ significantly from one another (Table 1, one-way ANOVA, P>0.05) whether expressed as gs over the 5 min contact period (data not shown), or as a percentage of the KPSS response (Emax%KPSS).
Figure 2.
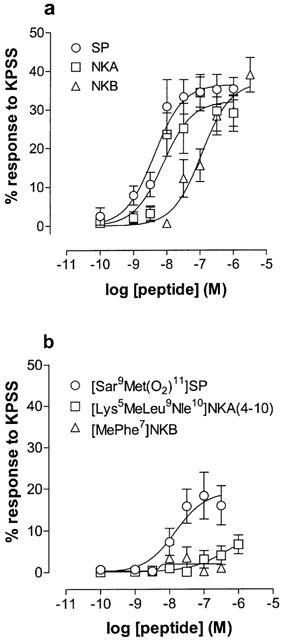
Log concentration-response curves to tachykinin peptides on oestrogen-primed mouse uterus. Data points are mean responses±s.e.mean and are expressed as a percentage of the response to KPSS. (a) Mean responses to SP (n=10), NKA (n=10) and NKB (n=10). The curve for SP lies to the left of that for NKA and that for NKB lies to the right of both SP and NKA. (b) Mean responses to [Sar9Met(O2)11]SP (tachykinin NK1 receptor-selective, n=8), [Lys5MeLeu9Nle10]NKA(4-10) (tachykinin NK2 receptor-selective, n=6), and [MePhe7]NKB (tachykinin NK3 receptor-selective, n=6). Only [Sar9Met(O2)11]SP was an effective agonist over the concentration range applied.
The relative order of agonist potency was SP⩾NKA>NKB (Figure 2a, Table 1).
Responses to tachykinin receptor-preferring agonists
The effects of [Sar9Met(O2)11]SP (tachykinin NK1 receptor-preferring), [Lys5MeLeu9Nle10]NKA(4-10) (tachykinin NK2 receptor-preferring) and [N-MePhe7]NKB (tachykinin NK3 receptor-preferring) were investigated. Figure 2b shows log concentration-response curves to these peptides. Only [Sar9Met(O2)11]SP acted as an agonist in these experiments, its maximal effect was lower than that of SP but its potency was similar to that of SP (Figure 2b, Table 1).
Responses to Glu5 analogues of SP
In a separate series of experiments (n=6) the potencies of three analogues of SP in which Gln5 and/or Gln6 were replaced with Glu were compared with SP. In this series of experiments the mean responses to KPSS and methacholine were similar in each of the four treatment groups (one-way ANOVA, P>0.05). All three analogues acted as full agonists, thus the mean Emax%KPSS did not differ significantly from those for SP (one-way-ANOVA, P>0.05) in this series of experiments, and the pD2 values were similar to those for SP.
Effects of atropine, L-NOLA, and indomethacin alone and in the presence of ranitidine or mepyramine on the response to SP
Two series of experiments were conducted to determine whether the effects of SP were mediated or modified through the release/formation of acetylcholine, nitric oxide, histamine or prostanoids. In the first series the effects of atropine (0.1 μM) or L-NOLA (100 μM) on log concentration-response curves to SP were determined (n=4). Neither shifted the SP curves (two-way ANOVA, P>0.05).
In the second series of experiments (n=11) the effects of indomethacin (10 μM), alone or in combination with mepyramine (0.1 μM) or ranitidine (10 μM) on responses to SP were determined. None of the inhibitor combinations led to significant shifts in the log concentration-response curves to SP. Nor did they alter the mean responses to KPSS or methacholine (two-way ANOVAs, P>0.05).
Tachykinin receptor antagonist studies
Effects of SR140333 and SR48968
Neither antagonist significantly affected the responses to KPSS or methacholine in these experiments using SP, NKA, NKB or [Sar9Met(O2)11]SP, (one-way ANOVAs P>0.05). Figure 3a shows the effects of SR140333 and SR48968, alone and in combination, on responses to SP. The tachykinin NK1 receptor-preferring antagonist, SR140333 (10 nM), significantly shifted the log concentration-response curve to the right (potency ratio 108.3; 95% confidence limits 34.6, 1049.3; d.f.=64). This yielded an approximate pKb value of 10 for SR140333 versus SP. There was no significant effect on the Emax value (one-way ANOVA, P>0.05).
Figure 3.
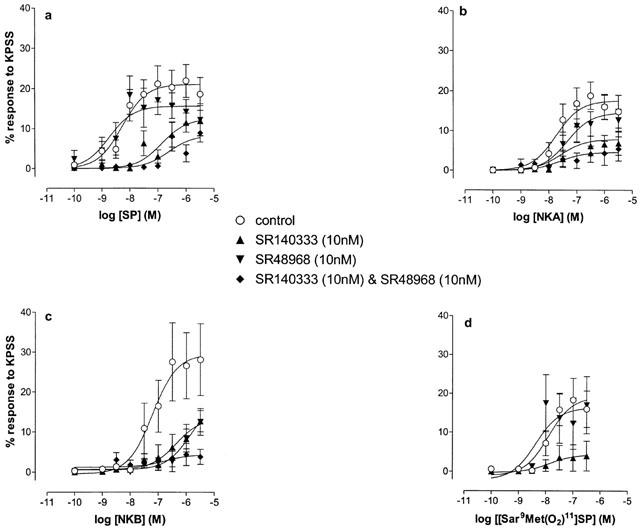
Log concentration-response curves to SP (a; n=10), NKA (b; n=9), NKB (c; n=9) and [Sar9Met(O2)11]SP (d; n=6) on oestrogen-primed mouse uterus in the absence and presence of SR140333 (tachykinin NK1 receptor-selective antagonist, 10 nM), SR48968 (tachykinin NK2 receptor-selective antagonist, 10 nM) and both SR140333 and SR48968 (each 10 nM). Data points are mean responses±s.e.mean and are expressed as a percentage of the response to KPSS. SR140333, alone and in combination with SR48968, significantly reduced responses to the peptides (ANOVA, P<0.05). SR48968 caused a marked rightward shift in the position of the log concentration-response curve to NKB, a lesser effect on the curve to NKA (ANOVA, P<0.05), but was without significant effect on responses to SP and [Sar9Met(O2)11]SP (ANOVA, P>0.05).
SR140333 (10 nM) and SR48968 (10 nM) in combination produced a significant depression in the Emax value for SP (one-way ANOVA, P<0.05; Student Newman Keuls P<0.05), as well as a marked rightward shift in the position of the log concentration-response curve (Figure 3a).
The tachykinin NK2 receptor-preferring antagonist, SR48968 (10 nM) given alone, was without significant effect on the log concentration-response curve to SP (Figure 3a).
Figure 3b shows that SR140333 (10 nM) produced significant rightward shifts in the position of the log concentration-response curve to NKA (3.7 fold, 95% CL=1.4, 14.7; d.f.=68). SR48968 (10 nM) also caused a rightward shift (4.7 fold, 95% CL=1.7, 14.8; d.f.=87). In combination the two antagonists produced a non-parallel shift in the log concentration-response curve to NKA (Figure 3b).
Both SR140333 (10 nM) and SR48968 (10 nM), produced significant rightward shifts in the log concentration-response curves to NKB (Figure 3c). The rightward shifts were 63.6 fold (95% CL=7.9, 3663.0; d.f.=47) and 53.6 fold (95% CL=14.1, 513.1; d.f.=59) respectively. In combination, the antagonists produced a very marked shift of the log concentration-response curves to NKB, including a depression of maximal response (Figure 3c). Responses to KPSS and methacholine were unaffected by the combination of these antagonists (one-way ANOVAs, P>0.05).
SR140333 (10 nM) produced a significant attenuation of the log concentration-response curve to [Sar9Met(O2)11]SP, but SR48968 (10 nM) was without effect (Figure 3d).
Effects of SR142801
SR142801 (0.3 μM) was without effect on responses to KPSS and methacholine in these experiments (one-way ANOVA, P>0.05). The effects of this antagonist on responses to SP and NKB are shown in Figure 4. Mean pD2 and Emax %KPSS estimates for SP and NKB were not significantly altered by the presence of the antagonist.
Figure 4.
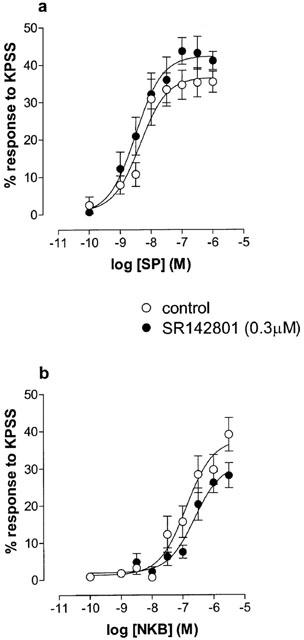
Log concentration-responses curves to SP (a; n=10) and NKB (b; n=10) on oestrogen-primed mouse uterus in the absence and presence of SR142801 (tachykinin NK3 receptor-selective antagonist, 0.3 μM). Data points are mean responses±s.e.mean and are expressed as a percentage of the response to KPSS. SR142801 did not antagonize the responses to either peptide (ANOVA, P>0.05).
Discussion
Although a number of studies have reported enhancement of rat myometrial activity by tachykinin peptides (Barr et al., 1991; Fisher et al., 1993; Pennefather et al., 1993; Magraner et al., 1997; 1998; Fisher & Pennefather, 1997; 1998; 1999; Shintani et al., 2000; Candenas et al., 2001) there have been few previous studies of tachykinin actions on mouse myometrium. We have herein demonstrated that the mammalian tachykinin peptides, SP, NKA and NKB cause contractions of longitudinal myometrium from the oestrogen-primed mouse. While the three mammalian tachykinin peptides produced similar maximal effects, the order of agonist potency differed from that previously reported for the rat uterus, and more recently the human uterus.
In the oestrogen-primed rat uterus the order of potency of the mammalian tachykinin peptides is NKA>SP⩾NKB (Pennefather et al., 1993; Fisher & Pennefather, 1997; Magraner et al., 1998). We have recently reported a similar order of agonist potency in myometrium from non-pregnant women as well as from women undergoing elective Caesarean section near term (Patak et al., 2000b,c). However in this study with the oestrogen-primed mouse, the order of potency was SP⩾NKA>NKB. This indicates that the tachykinin receptor mediating contraction of the oestrogen-primed mouse uterus differs from that of the oestrogen-primed rat uterus which has now been confirmed to be the NK2 receptor (Magraner et al., 1998; Fisher & Pennefather, 1998; 1999). The experiments reported herein suggest that, in contrast to the rat and the human, tachykinin-induced uterine contractions in the oestrogen-primed mouse are mediated primarily by the NK1 receptor, while the NK2 receptor plays a less important role.
Given that the mammalian tachykinins, SP, NKA and NKB in high concentrations are not totally selective for tachykinin NK1, NK2 and NK3 receptors, respectively, we undertook additional experiments with tachykinin receptor-selective agonists. The results of these experiments lend additional strong support to the notion of the involvement of the NK1 receptor in mouse uterine contraction. In mouse myometrium, the tachykinin NK1 receptor-selective agonist, [Sar9Met(O2)11]SP (Drapeau et al., 1987) was approximately equipotent with SP in causing uterine contraction, although its maximal effect was less than that of SP. In contrast, neither the tachykinin NK2 receptor-selective agonist [Lys5MeLeu9Nle10]NKA(4-10) (Chassaing et al., 1991) nor the tachykinin NK3 receptor-selective agonist, [N-MePhe7]NKB (Drapeau et al., 1987), were effective in producing myometrial contraction. This finding again differs from previous studies in the rat (Fisher et al., 1993; Fisher & Pennefather, 1997; Moodley et al., 1999) and the human (Patak et al., 2000b,c) in which, of these peptides, only [Lys5MeLeu9Nle10]NKA(4-10) had efficacy and potency approaching or exceeding that of NKA, consistent with activation of tachykinin NK2 rather than NK1 receptors.
Additional support for the involvement of tachykinin NK1 receptors in the mouse uterus comes from our findings that the three SP analogues, in which Gln at positions 5 and/or 6 were replaced with Glu were effective and full agonists on the mouse myometrium.
Strong evidence for the involvement of the tachykinin NK1 receptor in mediating responses to SP, NKA and NKB comes from studies using the selective tachykinin receptor antagonists, SR140333, SR48968, and SR142801. The tachykinin NK1-selective antagonist, SR140333 (Emonds-Alt et al., 1993) in a concentration of 10 nM, effectively antagonized the actions of SP, NKA and NKB. The rightward shift in the response to SP was approximately 100 fold, indicating a pA2 value for SR140333 versus SP of approximately 10, in keeping with the estimates obtained in a variety of smooth muscle preparations by Emonds-Alt et al. (1993). It should be noted that Emonds-Alt et al. (1993) despite quoting pA2 values, noted that the antagonism produced by this antagonist was apparently non-competitive. Some depression of responses to higher concentrations of agonists by SR140333 was also evident in the present study although only in the presence of SR48968 was this clearly significant statistically. The rightward shifts produced by SR140333 in the log concentration-response curves to NKA, NKB as well as those of SP and [Sar9Met(O2)11] SP indicate the involvement of a tachykinin NK1 receptor in responses to all four peptides. It should be noted that this antagonist is also a potent antagonist of septide in some tissues (Oury-Donat et al., 1994). It is not clear whether the ‘septide' variant of the tachykinin NK1 receptor, for which NKA and NKB have some affinity (Torrens et al., 1997; Wijkhuisen et al., 1999) is present in mouse tissues. The effects of NKB, and to a lesser extent NKA, but not those of SP or [Sar9Met(O2)11]SP, were, however, susceptible to antagonism by the potent non-peptide tachykinin NK2 receptor-selective antagonist, SR48968 (Advenier et al., 1992; Emonds-Alt et al., 1992). This indicates that the NKA and NKB may exert some of their effects on the oestrogen-primed mouse uterus through activation of a tachykinin NK2 receptor.
In contrast, the non-peptide tachykinin NK3 receptor antagonist, SR142801 (Emonds-Alt et al., 1995) was ineffective in blocking the effects of SP and NKB, indicating the non-involvement of NK3 receptors in eliciting uterine contraction in the oestrogen-primed mouse. However, it should be noted that the affinity of this antagonist for the tachykinin NK3 receptors is species dependent (Patacchini et al., 1995; Beaujouan et al., 1997). The affinity of this antagonist for the mouse tachykinin NK3 receptor has not been established, however there are reports describing its efficacy as an antagonist of NKB in that species (Inoue et al., 1996). It is of interest that Barr et al. (1991) reported the presence of a tachykinin NK3 receptor in rat uterus, however later investigations suggested that this receptor is down-regulated by oestrogen (Pinto et al., 1997). Whether this is also the case for the mouse uterus has yet to be determined.
In the series of experiments in which the effects of SP in the presence of atropine, L-NOLA and indomethacin, alone or in the presence of the H1 histamine receptor antagonist, mepyramine or the H2 receptor antagonist, ranitidine, were examined, no changes in the response to SP were observed. These experiments suggest (a) that in the oestrogen-primed mouse uterus, neither acetylcholine, acting at muscarinic receptors, nor histamine acting at either H1 or H2 histamine receptors mediate or modulate the uterotonic effects of SP, and further (b) that neither nitric oxide nor prostanoids mediate or modulate the effects of SP.
In conclusion, the results of the present experiments using mammalian tachykinins, tachykinin receptor-selective agonists, and non-peptide tachykinin receptor antagonists, indicate the importance of the tachykinin NK1 receptor in mediating the uterotonic effects of tachykinins on the oestrogen-primed mouse uterus. The tachykinin NK2 receptor may contribute to the effects of higher concentrations of the mammalian tachykinins NKA and NKB. The effects of SP are probably directly mediated by tachykinin NK1 receptors located on uterine smooth muscle. The present study constitutes the first evidence for a species difference in the nature of the predominant tachykinin receptor mediating myometrial contraction, and indicates that the non-pregnant mouse may not be an appropriate model of tachykinin action on human myometrium.
Acknowledgments
This work was supported in part by a grant from NH&MRC to J.N. Pennefather. SR140333, SR48968 and SR142801 were generous gifts from Dr Xavier Emonds-Alt.
Abbreviations
- KPSS
High potassium-containing modified physiological saline solution
- L-NOLA
N(ω)-nitro-L-arginine, NKA, neurokinin A
- NKB
neurokinin B
- SP
substance P
- SR140333
(1-{2-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl) piperidin-3-yl]ethyl}-4phenyl-1-azonia-bicyclo[2.2.2]octane
- SR48968
((S)-N-methyl-N[4-acetylamino-4-phenylpiperidino)-2-((3,4-dichlorophenyl)butyl]benzamide)
- SR142801
((S)-(N)-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl)propyl)-4-phenylpiperidin-4-yl)-N-methylacetamide)
References
- ADVENIER C., ROUISSI N., NGUYEN Q.T., EMONDS-ALT X., BRELIERE J.C., NELIAT G., NALINE E., REGOLI D. Neurokinin A (NK2) receptor revisited with SR48968, a potent non-peptide antagonist. Biochem. Biophys. Res. Commun. 1992;184:1418–1424. doi: 10.1016/s0006-291x(05)80041-5. [DOI] [PubMed] [Google Scholar]
- ALM P., LUNDBERG L.M. Co-existence and origin of peptidergic and adrenergic nerves in the guinea pig uterus. Retrograde tracing and immunocytochemistry, effects of chemical sympathectomy, capsaicin treatment and pregnancy. Cell Tissue Res. 1988;254:517–530. doi: 10.1007/BF00226501. [DOI] [PubMed] [Google Scholar]
- ARCK P.C., MERALI F.S., STANISZ A.M., STEAD R.H., CHAOUAT G., MANUEL J., CLARK D.A. Stress-induced murine abortion associated with substance P-dependent alteration in cytokines in maternal uterine decidua. Biol. Reprod. 1995;53:814–819. doi: 10.1095/biolreprod53.4.814. [DOI] [PubMed] [Google Scholar]
- BARR A.J., WATSON S.P., BERNAL A.L., NIMMO A.J. The presence of NK3 tachykinin receptors on rat uterus. Eur. J. Pharmacol. 1991;203:287–290. doi: 10.1016/0014-2999(91)90726-7. [DOI] [PubMed] [Google Scholar]
- BEAUJOUAN J.C., SAFFROY M., TORRENS Y., GLOWINSKI J. Potency and selectivity of the tachykinin NK3 receptor antagonist SR 142801. Eur. J. Pharmacol. 1997;319:307–316. doi: 10.1016/s0014-2999(96)00848-5. [DOI] [PubMed] [Google Scholar]
- CANDENAS M.L., MAGRANER J., ARMESTO C.P., ANSELMI E., NIETO P.M., MARTIN J.D., ADVENIER C., PINTO F.M. Changes in the expression of tachykinin receptors in the rat uterus during the course of pregnancy. Biol. Reprod. 2001;65:538–543. doi: 10.1095/biolreprod65.2.538. [DOI] [PubMed] [Google Scholar]
- CHASSAING G., LAVIELLE S., LOEUILLET D., ROBILLIARD P., CARRUETTE A., GARRET C., BEAUJOUAN J.C., SAFFROY M., PETITET F., TORRENS Y., GLOWINSKI J. Selective agonists of NK-2 binding sites highly active on rat portal vein (NK-3 bioassay) Neuropeptides. 1991;19:91–95. doi: 10.1016/0143-4179(91)90137-8. [DOI] [PubMed] [Google Scholar]
- DRAPEAU G., D'ORLEANS-JUSTE P., DION S., RHALEB N.E., ROUISSI N.E., REGOLI D. Selective agonists for substance P and neurokinin receptors. Neuropeptides. 1987;10:43–54. doi: 10.1016/0143-4179(87)90088-6. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., BICHON D., DUCOUX J.P., HEAULME M., MILOUX B., PONCELET M., PROIETTO V., VAN BROECK D., VILAIN P., NELIAT G., SOUBRIE P., LE FUR, G., BRELIERE J. SR 142801, the first potent non-peptide antagonist of the tachykinin NK3 receptor. Life Sci. 1995;56:L27–L32. doi: 10.1016/0024-3205(94)00413-m. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., DOUTREMEPUICH J.D., HEAULME M., NELIAT G., SANTUCCI V., STEINBERG R., VILAIN P., BICHON D., DUCOUX J.P., PROIETTO V., VAN BROECK D., SOUBRIE P., LE-FUR G., BRELIERE J.C. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., VILAIN P., GOULAOUIC P., PROIETTO V., VAN BROECK D., ADVENIER C., NALINE E., NELIAT G., LE FUR G., BRELIERE J.C. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sci. 1992;50:L101–L106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N. Potencies of agonists acting at tachykinin receptors in the oestrogen-primed rat uterus: effects of peptidase inhibitors. Eur. J. Pharmacol. 1997;335:221–226. doi: 10.1016/s0014-2999(97)01229-6. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N. Structure-activity studies of analogues of neurokinin A mediating contraction of rat uterus. Neuropeptides. 1998;32:405–410. doi: 10.1016/s0143-4179(98)90063-4. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N. Tachykinin receptors mediating contractions of oestrogen-primed rat uterus: classification using non-peptide antagonists. Clin. Exp. Pharmacol. Physiol. 1999;26:729–735. doi: 10.1046/j.1440-1681.1999.03119.x. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N., HALL S. Tachykinin receptors in the rat isolated uterus. Regul. Pept. 1993;46:396–398. doi: 10.1016/0167-0115(93)90098-s. [DOI] [PubMed] [Google Scholar]
- FLEMING A.J., PENNEFATHER J.N., STORY M.E. Effects of tachykinins on contractile activity of the oestrogen-primed mouse uterus. Proc. ASCEPT. 1998;5:176. [Google Scholar]
- FRANCO-CERECEDA A., HENKE H., LUNDBERG J.M., PETERMANN J.B., HOKFELT T., FISCHER J.A. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987;8:399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- HEINRICH D., REINECKE M., FORSSMANN W.G. Peptidergic innervation of the human and guinea pig uterus. Arch. Gynecol. 1986;237:213–219. doi: 10.1007/BF02133783. [DOI] [PubMed] [Google Scholar]
- HUANG W.M., GU J., BLANK M.A., ALLEN J.M., BLOOM S.R., POLAK J.M. Peptide-immunoreactive nerves in the mammalian female genital tract. Histochem. J. 1984;16:1297–1310. doi: 10.1007/BF01003727. [DOI] [PubMed] [Google Scholar]
- INOUE H., NAGATA N., KOSHIHARA Y. Involvement of tachykinin receptors in oedema formation and plasma extravasation induced by substance P, neurokinin A, and neurokinin B in mouse ear. Inflamm. Res. 1996;45:316–323. doi: 10.1007/BF02252943. [DOI] [PubMed] [Google Scholar]
- JOACHIM R.A., HILDEBRANDT M., ODER J., KLAPP B.F., ARCK P.C. Murine stress-triggered abortion is mediated by increase of CD8+ TNF- alpha+ decidual cells via substance P. Am. J. Reprod. Immunol. 2001;45:303–309. doi: 10.1111/j.8755-8920.2001.450506.x. [DOI] [PubMed] [Google Scholar]
- LEMBECK F., HOLZER P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch. Pharmacol. 1979;310:175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., MELI A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen. Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- MAGRANER J., MORCILLO E., AUSINA P., PINTO F.M., MARTIN J.D., MOREAU J., ANSELMI E., BARRACHINA M.D., CORTIJO J., ADVENIER C., CANDENAS M.L. Effects of Mn2+ on the responses induced by different spasmogens in the oestrogen-primed rat uterus. Eur. J. Pharmacol. 1997;326:211–222. doi: 10.1016/s0014-2999(97)85416-7. [DOI] [PubMed] [Google Scholar]
- MAGRANER J., PINTO F.M., ANSELMI E., HERNANDEZ M., PEREZ-AFONSO R., MARTIN J.D., ADVENIER C., CANDENAS M.L. Characterization of tachykinin receptors in the uterus of the oestrogen-primed rat. Br. J. Pharmacol. 1998;123:259–268. doi: 10.1038/sj.bjp.0701613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKERT U.R., ARCK P.C., MCBEY B.A., MANUEL J., CROY B.A., MARSHALL J.S., CHAOUAT G., CLARK D.A. Stress triggered abortions are associated with alterations of granulated cells into the decidua. Am. J. Reprod. Immunol. 1997;37:94–100. doi: 10.1111/j.1600-0897.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- MARX L., ARCK P., KIESLICH C., MITTERLECHNER S., KAPP M., DIETL J. Decidual mast cells might be involved in the onset of human first-trimester abortion. Am. J. Reprod. Immunol. 1999;41:34–40. doi: 10.1111/j.1600-0897.1999.tb00073.x. [DOI] [PubMed] [Google Scholar]
- MOODLEY N., LAU W.A., PENNEFATHER J.N., STORY M.E., FISHER L. NK2 receptors mediate tachykinin-induced contractions of rat uterus during the oestrous cycle. Eur. J. Pharmacol. 1999;376:53–60. doi: 10.1016/s0014-2999(99)00359-3. [DOI] [PubMed] [Google Scholar]
- OURY-DONAT F., LEFEVRE I.A., THURNEYSSEN O., GAUTHIER T., BORDEY A., FELTZ P., EMONDS-ALT X., LE FUR G., SOUBRIE P. SR 140333, a novel, selective, and potent nonpeptide antagonist of the NK1 tachykinin receptor: characterization on the U373MG cell line. J. Neurochem. 1994;62:1399–1407. doi: 10.1046/j.1471-4159.1994.62041399.x. [DOI] [PubMed] [Google Scholar]
- PAGE N.M., WOODS R.J., GARDINER S.M., LOMTHAISONG K., GLADWELL R.T., BUTLIN D.J., MANYONDA I.T., LOWRY P.J. Excessive placental secretion of neurokinin B during the third trimester causes pre-eclampsia. Nature. 2000;405:797–800. doi: 10.1038/35015579. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., BARTHO L., HOLZER P., MAGGI C.A. Activity of SR 142801 at peripheral tachykinin receptors. Eur. J. Pharmacol. 1995;278:17–25. doi: 10.1016/0014-2999(95)00090-8. [DOI] [PubMed] [Google Scholar]
- PATAK E.N., PENNEFATHER J.N., STORY M.E. Effects of tachykinins on uterine smooth muscle. Clin. Exp. Pharmacol. Physiol. 2000a;27:922–927. doi: 10.1046/j.1440-1681.2000.03362.x. [DOI] [PubMed] [Google Scholar]
- PATAK E.N., ZICCONE S., LILLEY A., PENNEFATHER J.N., STORY M.E. Tachykinins acting at NK2 receptors cause contraction of human uterine tissue from non-pregnant women. Proc. ASCEPT. 2000c;7:65. [Google Scholar]
- PATAK E.N., ZICCONE S., STORY M.E., FLEMING A.J., LILLEY A., PENNEFATHER J.N. Activation of neurokinin NK(2) receptors by tachykinin peptides causes contraction of uterus in pregnant women near term. Mol. Hum. Reprod. 2000b;6:549–554. doi: 10.1093/molehr/6.6.549. [DOI] [PubMed] [Google Scholar]
- PENNEFATHER J.N., ZENG X.P., GOULD D., HALL S., BURCHER E. Mammalian tachykinins stimulate rat uterus by activating NK-2 receptors. Peptides. 1993;14:169–174. doi: 10.1016/0196-9781(93)90025-c. [DOI] [PubMed] [Google Scholar]
- PINTO F.M., CINTADO C.G., DEVILLIER P., CANDENAS M.L. Expression of preprotachykinin-B, the gene that encodes neurokinin B, in the rat uterus. Eur. J. Pharmacol. 2001;425:R1–R2. doi: 10.1016/s0014-2999(01)01186-4. [DOI] [PubMed] [Google Scholar]
- PINTO F.M., MAGRANER J., AUSINA P., ANSELMI E., MARTIN J.D., CANDENAS M.L. Regulation by oestrogens of tachykinin NK3 receptor expression in the rat uterus. Eur. J. Pharmacol. 1997;324:125–127. doi: 10.1016/s0014-2999(97)00150-7. [DOI] [PubMed] [Google Scholar]
- SAMUELSON U.E., DALSGAARD C.J., LUNDBERG J.M., HOKFELT T. Calcitonin gene-related peptide inhibits spontaneous contractions in human uterus and fallopian tube. Neurosci. Lett. 1985;62:225–230. doi: 10.1016/0304-3940(85)90359-3. [DOI] [PubMed] [Google Scholar]
- SHINTANI Y., NISHIMURA J., NIIRO N., HIRANO K., NAKANO H., KANAIDE H. Mechanisms underlying the neurokinin A-induced contraction of the pregnant rat myometrium. Br. J. Pharmacol. (2000;130:1165–1173. doi: 10.1038/sj.bjp.0703410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRINGALL D.R., HACKER G.W., GRIMELIUS L., POLAK J.M. The potential of the immunogold-silver staining method for paraffin sections. Histochemistry. 1984;81:603–608. doi: 10.1007/BF00489542. [DOI] [PubMed] [Google Scholar]
- TORRENS Y., SAFFROY M., GLOWINSKI J., BEAUJOUAN J.C. Substance P(6-11) and natural tachykinins interact with septide-sensitive tachykinin receptors coupled to a phospholipase C in the rat urinary bladder. Neuropeptides. 1997;31:243–251. doi: 10.1016/s0143-4179(97)90055-x. [DOI] [PubMed] [Google Scholar]
- WIJKHUISEN A., SAGOT M.A., FROBERT Y., CREMINON C., GRASSI J., BOQUET D., COURAUD J.Y. Identification in the NK1 tachykinin receptor of a domain involved in recognition of neurokinin A and septide but not of substance P. FEBS Lett. 1999;447:155–159. doi: 10.1016/s0014-5793(99)00298-7. [DOI] [PubMed] [Google Scholar]


