Abstract
We determined the effects of nociceptin/orphanin FQ and the NOP receptor ligands acetyl-Arg-Tyr-Tyr-Arg-Ile-Lys-NH2 (Ac-RYYRIK-NH2) and naloxone benzoylhydrazone on transmitter release in vitro.
The electrically evoked tritium overflow from guinea-pig and mouse striatal slices and guinea-pig retinal discs preincubated with [3H]-dopamine was inhibited by nociceptin/orphanin FQ (pEC50 7.9, 7.6 and 8.6; Emax 30, 50 and 55%). Ac-RYYRIK-NH2 0.032 μM and naloxone benzoylhydrazone 5 μM antagonized the effect of nociceptin/orphanin FQ in striatal slices of the guinea-pig (apparent pA2 9.1 and 6.8) and the mouse (apparent pA2 9.2 and 7.5) and strongly attenuated the effect of nociceptin/orphanin FQ 0.1 μM in guinea-pig retinal discs. Ac-RYYRIK-NH2 0.032 μM did not affect the evoked overflow by itself whereas naloxone benzoylhydrazone 5 μM inhibited it in each tissue.
The electrically evoked tritium overflow from mouse brain cortex slices preincubated with [3H]-noradrenaline was inhibited by nociceptin/orphanin FQ (pEC50 7.9, Emax 85%), Ac-RYYRIK-NH2 (pEC50 8.3, Emax 47%) but not affected by naloxone benzoylhydrazone 5 μM. Ac-RYYRIK-NH2 and naloxone benzoylhydrazone showed apparent pA2 values of 8.6 and 6.9.
In conclusion, the inhibitory effect of nociceptin/orphanin FQ on dopamine release in the striatum and retina and on noradrenaline release in the cerebral cortex is mediated via NOP receptors. Ac-RYYRIK-NH2 behaves as an extremely potent NOP receptor antagonist in the striatum and retina and as a partial agonist in the cortex.
Keywords: NOP receptor, nociceptin, orphanin FQ, dopamine release, noradrenaline release, striatum, guinea-pig retina, mouse cerebral cortex, Ac-RYYRIK-NH2, naloxone benzoylhydrazone
Introduction
A fourth type of opioid receptor, which has a homology in the amino acid sequence to the three classical opioid receptors of about 50%, was first identified in 1994 (for review, see Mogil & Pasternak, 2001). This receptor, termed NOP according to the nomenclature of IUPHAR (International Union of Pharmacology; Cox et al., 2000), is activated by a heptadecapeptide, termed nociceptin/orphanin FQ(N/OFQ) (for review, see Mogil & Pasternak, 2001). The NOP and the classical opioid receptors resemble each other with respect to the transduction mechanisms, i.e. inhibition of adenylyl cyclase (Hawes et al., 2000) and of voltage-dependent Ca2+ channels and activation of voltage-dependent K+ channels (Moran et al., 2000). However, the NOP receptor markedly differs from the classical opioid receptors in its pharmacological properties (e.g. lack of antagonistic effect of the opioid receptor blocking agent naloxone) (Calo et al., 2000) and its distribution in the brain (Mollereau & Mouledous, 2000). The most striking difference, however, occurs with respect to the influence on nociception. Thus, N/OFQ elicits hyperalgesia rather than analgesia when administered at a supraspinal site (for review, see Mogil & Pasternak, 2001). The action of N/OFQ is, however, not restricted to nociception since the heptadecapeptide also affects feeding (Polidori et al., 2000), learning and memory (Noda et al., 2000) and anxiety (Jenck et al., 1997) as well as numerous vegetative functions (Mogil & Pasternak, 2001).
One aspect in which the classical opioid and the NOP receptors resemble each other is that they occur presynaptically on a variety of peripheral and central neurones where they cause inhibition of the release of the respective neurotransmitter (Moran et al., 2000; Schlicker & Morari, 2000). N/OFQ e.g. affects the release of dopamine and this has been shown by direct measurement of transmitter release or electrophysiological techniques for the (i) nigrostriatal (Konya et al., 1998; Schlicker et al., 1998a; Shieh & Pan, 2001), (ii) mesolimbic (Murphy et al., 1996; Murphy & Maidment, 1999; Lutfy et al., 2001; Shieh & Pan, 2001; Zheng et al., 2002), (iii) tuberoinfundibular (Wagner et al., 1998; Shieh & Pan, 2001) and (iv) incertohypothalamic system (Shieh & Pan, 2001). The involvement of NOP receptors in the effect of N/OFQ has so far been proven only in the studies by Shieh & Pan (2001; use of an antisense oligodeoxynucleotide) and by Zheng et al. (2002; use of the weakly potent NOP receptor antagonist [Phe1Ψ(CH2-NH)Gly2]-N/OFQ(1-13)NH2). The need for such experiments is highlighted by a study on conscious rats in which intrastriatal administration of N/OFQ increased dopamine release in a manner sensitive to naloxone thereby arguing against the involvement of NOP receptors (Konya et al., 1998).
We therefore examined in two in vitro models of the striatum, i.e. superfused guinea-pig and mouse striatal slices, in which N/OFQ inhibits dopamine release in the presence of naloxone (Schlicker et al., 1998a), whether this effect is counteracted by NOP receptor antagonists. We used the selective and highly potent NOP receptor antagonist acetyl-Arg-Tyr-Tyr-Arg-Ile-Lys-NH2 (Ac-RYYRIK-NH2) (Dooley et al., 1997; Berger et al., 1999) and a non-selective and less potent NOP receptor antagonist, naloxone benzoylhydrazone, used by our own group (Schlicker et al., 1998b; Malinowska et al., 2000a) and by other investigators (Bucher, 1998; Siniscalchi et al., 1999; Ho et al., 2000; Bigoni et al., 2002). Furthermore, we studied whether N/OFQ affects dopamine release also in guinea-pig retinal discs since the influence of N/OFQ on the retinal dopamine system has so far not been examined. Finally, we compared the potencies of Ac-RYYRIK-NH2 and naloxone benzoylhydrazone at the NOP receptor causing inhibition of noradrenaline release in the mouse brain cortex, i.e. at an NOP receptor previously identified and characterized by our group (Schlicker et al., 1998b; Bauer et al., 1999; Werthwein et al., 1999).
Methods
Superfusion studies
Male Dunkin-Hartley guinea-pigs and male NMRI mice were decapitated and the brains and/or eyes were removed from the skull. The retina was carefully detached from other layers of the guinea-pig eye using a spatula and discs (diameter 3 mm) were punched out. Furthermore, 0.3 mm thick slices were prepared from the guinea-pig and mouse striatum (diameter 2 mm) and the mouse cerebral cortex (diameter 3 mm). Retinal discs and striatal slices were incubated for 30 min with physiological salt solution (PSS) of 37°C containing [3H]-dopamine 100 nM; cortex slices were incubated for 60 min with PSS of 37°C containing [3H]-noradrenaline 25 nM. After incubation, discs or slices were superfused with PSS of 37°C for 110 min at a flow rate of 1 ml min−1. The superfusate was collected in 5 min samples. The PSS was composed as follows (mM): NaCl 118, KCl 4.8, NaHCO3 25, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.3, glucose 11.1, ascorbic acid 0.06, Na2EDTA 0.03; it was aerated with 95% O2 and 5% CO2.
Tritium overflow was evoked by two 2 min periods of electrical field stimulation 40 (S1) and 90 min (S2) after onset of superfusion. The stimulation parameters were 1 Hz 200 mA, 2 ms for discs or slices preincubated with [3H]-dopamine and 0.3 Hz, 50 mA, 2 ms for slices precincubated with [3H]-noradrenaline. Usually, N/OFQ or tetrodotoxin was added to (or Ca2+ ions were omitted from) the PSS from 62 min of superfusion onward whereas other drugs were present in the PSS throughout superfusion. At the end of superfusion, the radioactivity of the discs and slices and of the superfusate samples was determined by liquid scintillation counting.
Tritium overflow was calculated as the fraction of the tritium content of the slices at the beginning of the respective collection period (fractional rate of tritium efflux). Basal tritium efflux was quantified by calculating the ratio of the fractional rate in the 5-min period immediately before S2 (i.e. from 85–90 min; t2) over that in the collection period from 55–60 min (t1). Stimulation-evoked tritium overflow was calculated by subtraction of the basal from the total tritium efflux during stimulation and the subsequent 13 min and was expressed as per cent of tritium present in the slice at the onset of stimulation (basal tritium efflux was assumed to decline linearly from the 5 min collection period before to that 15 to 20 min after onset of stimulation). To quantify the effect of N/OFQ on the stimulated tritium overflow, the ratio of the overflow evoked by S2 over that evoked by S1 was determined. To determine the effects of antagonists on the evoked overflow, the S1 values obtained in the presence and absence of the respective antagonist were compared. To quantify agonist potencies, pEC50 values (negative logarithms of the concentration causing the half-maximal effect) were determined. Apparent pA2 values for antagonists were calculated according to formula 4 of Furchgott (1972).
Statistical analysis
Results are given as means±s.e.mean of n experiments. Student's t-test was used for comparison of mean values; if more than one experimental series was compared to the same control, the Bonferroni correction was applied.
Drugs used
[Ring-2,5,6-3H]-dopamine (specific activity (spec. act.) 53.6–60.0 Ci mmol−1), (R)-(−)-[ring-2,5,6-3H]-noradrenaline (spec. act. 56.3 Ci mmol−1; NEN, Zaventem, Belgium); acetyl-Arg-Tyr-Tyr-Arg-Ile-Lys-NH2 (Ac-RYYRIK-NH2), nociceptin/orphanin FQ (N/OFQ; Bachem, Heidelberg, Germany); desipramine hydrochloride (Ciba-Geigy, Wehr, Germany); naloxone hydrochloride, naloxone benzoylhydrazone, (−)-sulpiride (Sigma, München, Germany); nomifensine (Hoechst, Frankfurt); rauwolscine hydrochloride, tetrodotoxin (Roth, Karlsruhe, Germany). Stock solutions of the drugs were prepared with water, citrate buffer (0.1 mM, pH 4.8; tetrodotoxin) or ethanol (naloxone benzoylhydrazone) and diluted with PSS to the concentration required. The solvents did not affect basal and evoked tritium overflow by themselves.
Results
Superfusion experiments on striatal slices and retinal discs
Guinea-pig and mouse striatal slices and guinea-pig retinal discs were preincubated with [3H]-dopamine and superfused with medium routinely containing nomifensine 10 μM, (−)-sulpiride 3.2 μM and naloxone 10 μM. Basal tritium overflow was not affected or slightly altered (<22%) by addition of tetrodotoxin, N/OFQ, Ac-RYYRIK-NH2 and naloxone benzoylhydrazone and by omission of Ca2+ ions (not shown). Under control conditions (i.e. in the absence of tetrodotoxin, N/OFQ, Ac-RYYRIK-NH2 and naloxone benzoylhydrazone and in the presence of Ca2+ ions), the electrically (1 Hz) evoked tritium overflow (S2/S1) amounted to 0.79±0.03 in 20 guinea-pig striatal slices, to 0.76±0.03 in 11 mouse striatal slices and to 0.87±0.07 in 12 guinea-pig retinal discs. The amount of tritium evoked by S1 under various experimental conditions is given in Table 1.
Table 1.
Effect of Ac-RYYRIK-NH2 and naloxone benzoylhydrazone on the electrically evoked tritium overflow from superfused brain slices or retinal discs preincubated with [3H]-dopamine or [3H]-noradrenaline

Addition of tetrodotoxin 1 μM or omission of Ca2+ ions before and during S2 inhibited the evoked tritium overflow (S2/S1) by at least 90% in each of the three tissues (n=4–5; results not shown). N/OFQ (added to the medium before and during S2) concentration-dependently inhibited the evoked tritium overflow in each of the three tissues (Figure 1). The maximum inhibitory effect amounted to about 30, 50 and 55% for guinea-pig and mouse striatal slices and guinea-pig retinal discs, respectively; for pEC50 values, see Table 2. Ac-RYYRIK-NH2 0.032 μM (present in the medium throughout superfusion) shifted to the right the concentration–response curves of N/OFQ in guinea-pig (Figure 2A) and mouse striatal slices (Figure 2C), yielding apparent pA2 values of about 9 (Table 2). The effect of N/OFQ was also antagonized by naloxone benzoylhydrazone 5 μM in either tissue (Figure 2B,D); the apparent pA2 values were about 7 (Table 2). The inhibitory effect of N/OFQ 0.1 μM on the electrically evoked tritium overflow in guinea-pig retinal discs was markedly attenuated (P<0.015) by Ac-RYYRIK-NH2 0.032 μM and abolished by naloxone benzoylhydrazone 5 μM (Figure 3). Ac-RYYRIK-NH2 0.032 μM by itself did not affect the evoked tritium overflow (S1) whereas naloxone benzoylhydrazone 5 μM inhibited it by about 30% in guinea-pig and mouse striatal slices and guinea-pig retinal discs (Table 1).
Figure 1.
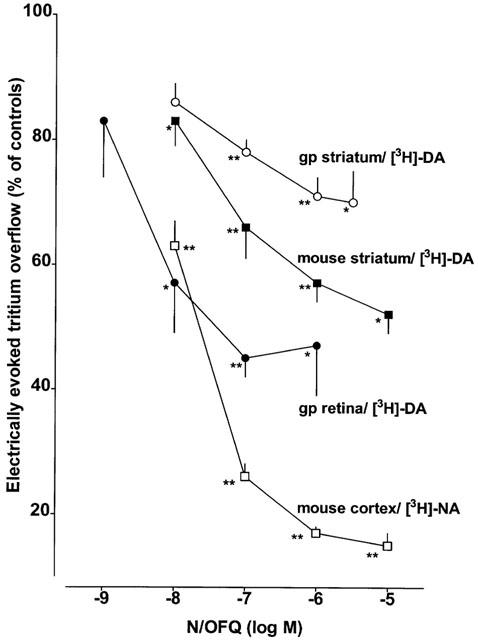
Effect of nociceptin/orphanin FQ (N/OFQ) on the electrically evoked tritium overflow from superfused brain slices and retinal discs. Guinea-pig (gp) and mouse striatal slices and guinea-pig retinal discs preincubated with [3H]-dopamine ([3H]-DA) were superfused with medium containing nomifensine 10 μM, (−)-sulpiride 3.2 μM and naloxone 10 μM; tritium overflow was evoked at 1 Hz. Mouse cortex slices preincubated with [3H]-noradrenaline ([3H]-NA) were superfused with medium containing desipramine 1 μM, rauwolscine 1 μM and naloxone 10 μM; tritium overflow was evoked at 0.3 Hz. N/OFQ was added to the medium from 62 min of superfusion onward. Tritium overflow was evoked twice, after 40 (S1) and 90 min (S2) of superfusion, and the ratio of the overflow evoked by S2 over that evoked by S1 was determined. Tritium overflow is given as per cent of the S2/S1 value in the corresponding controls (not shown). Means±s.e.mean of 5–19 experiments. *P<0.05, **P<0.001.
Table 2.
Potencies of N/OFQ, Ac-RYYRIK-NH2 and naloxone benzoylhydrazone at the NOP receptors examined in the present studya
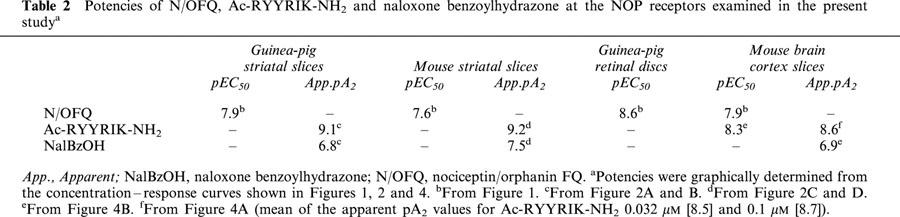
Figure 2.
Interaction of Ac-RYYRIK-NH2 (A, C) and naloxone benzoylhydrazone (NalBzOH) (B, D) with the effect of nociceptin/orphanin FQ (N/OFQ) on the electrically (1 Hz) evoked tritium overflow from superfused guinea-pig (gp) (A, B) and mouse (C, D) striatal slices preincubated with [3H]-dopamine ([3H]-DA). The medium contained nomifensine 10 μM, (−)-sulpiride 3.2 μM, naloxone 10 μM and when necessary Ac-RYYRIK-NH2 or NalBzOH throughout superfusion and N/OFQ from 62 min of superfusion onward. Tritium overflow was evoked twice, after 40 (S1) and 90 min (S2) of superfusion, and the ratio of the overflow evoked by S2 over that evoked by S1 was determined. Tritium overflow is given as per cent of the S2/S1 value in the corresponding controls (not shown). Means±s.e.mean of 4–19 experiments (guinea-pig striatal slices) and 4–15 experiments (mouse striatal slices). *P<0.05, **P<0.001.
Figure 3.

Interaction of Ac-RYYRIK-NH2 and naloxone benzoylhydrazone (NalBzOH) with the effect of nociceptin/orphanin FQ (N/OFQ) on the electrically (1 Hz) evoked tritium overflow from superfused guinea-pig (gp) retinal discs preincubated with [3H]-dopamine ([3H]-DA). The medium contained nomifensine 10 μM, (−)-sulpiride 3.2 μM, naloxone 10 μM and when necessary Ac-RYYRIK-NH2 or NalBzOH throughout superfusion and N/OFQ from 62 min of superfusion onward. Tritium overflow was evoked twice, after 40 (S1) and 90 min (S2) of superfusion, and the ratio of the overflow evoked by S2 over that evoked by S1 was determined. Tritium overflow is given as per cent of the S2/S1 value in the corresponding controls. Means±s.e.mean of 7–10 experiments. *P<0.05, **P<0.001.
Superfusion experiments on cerebral cortex slices
Mouse cortex slices were preincubated with [3H]-noradrenaline and superfused with medium routinely containing desipramine 1 μM, rauwolscine 1 μM and naloxone 10 μM. Basal tritium overflow was not affected by N/OFQ, Ac-RYYRIK-NH2 and naloxone benzoylhydrazone (not shown). Under control conditions (i.e. in the absence of N/OFQ, Ac-RYYRIK-NH2 and naloxone benzoylhydrazone), the electrically (0.3 Hz) evoked tritium overflow (S2/S1) amounted to 1.15±0.05 in six mouse cortex slices. The amount of tritium evoked by S1 under various experimental conditions is given in Table 1.
The effects of N/OFQ, Ac-RYYRIK-NH2 and naloxone benzoylhydrazone on the evoked tritium overflow were studied in two experimental series. In the first one (Figure 4A), N/OFQ was added to the medium before and during S2 whereas Ac-RYYRIK-NH2 was present throughout superfusion. N/OFQ inhibited the evoked tritium overflow (S2/S1) concentration-dependently; the maximum extent of inhibition amounted to 85% (Figures 1 and 4A). The concentration–response curve of N/OFQ was shifted to the right by Ac-RYYRIK-NH2 0.032 and 0.1 μM (apparent pA2 values of about 8.5; Table 2). Ac-RYYRIK-NH2 by itself inhibited the electrically evoked tritium overflow (S1) (Table 1).
Figure 4.
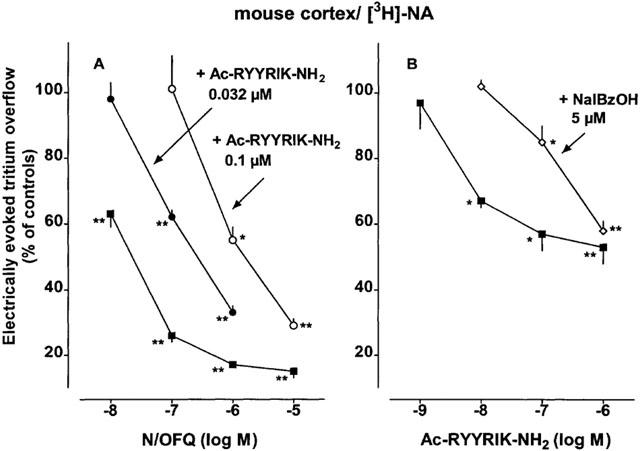
Interaction of Ac-RYYRIK-NH2 with nociceptin/orphanin FQ (N/OFQ) (A) and of naloxone benzoylhydrazone (NalBzOH) with Ac-RYYRIK-NH2 (B) in superfused mouse brain cortex slices preincubated with [3H]-noradrenaline ([3H]-NA). The medium contained desipramine 1 μM, rauwolscine 1 μM and naloxone 10 μM. In panel (A), Ac-RYYRIK-NH2 was present in the medium throughout superfusion whereas N/OFQ was added from 62 min onward. In panel (B), NalBzOH was present in the medium throughout superfusion whereas Ac-RYYRIK-NH2 was added from 62 min onward. Two periods of electrical stimulation (0.3 Hz) were administered, after 40 (S1) and 90 min (S2) of superfusion, and the ratio of the overflow evoked by S2 over that evoked by S1 was determined. Tritium overflow is given as per cent of the S2/S1 value in the corresponding controls (not shown). Means±s.e.mean of 4–6 experiments. *P<0.05, **P<0.001.
In the second series of experiments (Figure 4B), Ac-RYYRIK-NH2 was added to the medium before and during S2 only (like N/OFQ in the first series) whereas naloxone benzoylhydrazone was present in the medium throughout superfusion. Ac-RYYRIK-NH2 inhibited the electrically evoked tritium overflow (S2/S1) in a concentration-dependent manner. The pEC50 value resembles that of N/OFQ (Table 2) whereas the maximum extent of inhibition (by 47%) is markedly lower (compar e Figure 4B with 4A), yielding an intrinsic activity relative to N/OFQ of 0.55. The concentration-response curve of Ac-RYYRIK-NH2 was shifted to the right by naloxone benzoylhydrazone 5 μM (for apparent pA2 value, see Table 2). Naloxone benzoylhydrazone 5 μM by itself did not affect the electrically evoked tritium overflow (S1) (Table 1).
Discussion
The present study shows that N/OFQ inhibits monoamine release in various tissues via NOP receptors. Monoamine release was studied in superfused brain slices or retinal discs and the fact that the electrically evoked tritium overflow in tissues preincubated with [3H]-dopamine and [3H]-noradrenaline is tetrodotoxin-sensitive and Ca2+-dependent suggests that we measure quasi-physiological dopamine and noradrenaline release, respectively (Schlicker et al., 1992; present study). Besides naloxone (used to block the classical opioid receptors; see Introduction and below), an inhibitor of the respective uptake mechanism (nomifensine and desipramine) and an antagonist of the respective autoreceptor ((−)-sulpiride and rauwolscine) were added to the medium in order to increase the amount of the evoked tritium overflow. Another reason to block the autoreceptors was our previous finding that rauwolscine increased the extent of NOP receptor-mediated inhibition of noradrenaline release in the mouse brain cortex (Werthwein et al., 1999). The auxiliary drugs do not possess an affinity for NOP receptors since [3H]-N/OFQ binding to mouse brain cortex membranes was not affected (pKi<4.5) by naloxone, desipramine, rauwolscine (Werthwein et al., 1999), nomifensine and (−)-sulpiride (unpublished results).
We found that, besides the nigrostriatal, mesolimbic, tuberoinfundibular and incertohypothalamic system (for references, see Introduction), dopaminergic cells in the retina (i.e. amacrine cells; Oh et al., 1999) represent the fifth dopaminergic system affected by N/OFQ. Previous radioligand studies have revealed that NOP receptors occur at high densities in the retina of the rat (85% of the Bmax in the rat striatum; Makman & Dvorkin, 1997) and the rabbit (25% of the Bmax in the rabbit brain; Neal et al., 1997). Moreover, N/OFQ has been shown to inhibit the light-induced acetylcholine release in the rabbit retina (Neal et al., 1997). Since only few retinal discs (when compared to the number of striatal slices) could be prepared from the same guinea-pig, only one concentration of Ac-RYYRIK-NH2 and naloxone benzoylhydrazone was used for the interaction experiments with N/OFQ.
An inhibitory effect of N/OFQ on dopamine release has been shown previously in the striatum of the rat (Shieh & Pan, 2001) and the mouse (Schlicker et al., 1998a) and we show here that such an effect also occurs in the striatum of the guinea-pig. The maximum extent of inhibition in the guinea-pig striatum is lower than that obtained in the mouse striatum. This species difference is reminiscent of the NOP receptor involved in inhibition of cortical noradrenaline release; in this case, again the extent of inhibition in the guinea-pig was lower than that in the mouse (Schlicker et al., 1998b). In order to prove that the effect of N/OFQ in the striatum of either species is mediated via NOP receptors we used antagonists. By contrast, Shieh & Pan (2001) had used an antisense oligodeoxynucleotide to prove the involvement of NOP receptors in the N/OFQ-induced inhibition of striatal dopamine release in the rat.
For the sake of comparison, the inhibitory effect of N/OFQ on noradrenaline release in mouse cortex slices was studied as well; the maximum extent of N/OFQ-induced inhibition markedly exceeded that obtained for the other three models. The antagonism of Ac-RYYRIK-NH2 against N/OFQ confirms that the peptide acts via NOP receptors; the same conclusion had been reached in our previous studies in which the less potent and/or less selective antagonists (Phe1Ψ(CH2-NH)Gly2]-N/OFQ(1-13)NH2, naloxone benzoylhydrazone (Schlicker et al., 1998b) and Mr 2266 were used (Bauer et al., 1999).
The apparent pA2 values for Ac-RYYRIK-NH2 obtained in three functional models of the present study (range 8.6–9.2) at least equal the pA2 values reported in the literature for the standard NOP receptor antagonist CompB (Kawamoto et al., 1999) or its racemate, ranging from 7.8 to 8.7 (Bigoni et al., 2000; Chiou & Fan, 2002; Olianas & Onali, 2002; Rominger et al., 2002). On the other hand, Ac-RYYRIK-NH2 (although not affecting dopamine release in our three models) inhibited noradrenaline release in the mouse cortex by itself. The concentration–response curve of Ac-RYYRIK-NH2 was shifted to the right by naloxone benzoylhydrazone and the apparent pA2 value (6.9) was very similar to that obtained in our previous study against N/OFQ (6.6; Schlicker et al., 1998b). Furthermore, the potency of Ac-RYYRIK-NH2 as an antagonist (apparent pA2 8.6) closely resembles its potency as an agonist (pEC50 8.3), a typical feature of partial agonists. The fact that Ac-RYYRIK-NH2 is a partial agonist at the NOP receptor in the mouse cortex is not surprising since partial agonistic effects of this compound have been reported for a variety of NOP receptor models (Berger et al., 2000; Ho et al., 2000; Malinowska et al., 2000b; Mason et al., 2001; Olianas & Onali, 2002).
Interesting enough, naloxone benzoylhydrazone 5 μM inhibited dopamine release in each of the three models but failed to affect noradrenaline release in the mouse brain cortex at this concentration (present study) and even at 30 μM (Schlicker et al., 1998b). Therefore, the explanation that this compound, which has previously been shown to behave as a partial agonist at recombinant human NOP receptors (Bigoni et al., 2002), is a partial agonist also at the NOP receptors causing inhibition of striatal and retinal dopamine release is not plausible. Although no further attempts were made to clarify the unexpected findings with naloxone benzoylhydrazone, the possibility has to be considered that this drug inhibited dopamine release due to an agonistic effect on classical opioid receptors. This view is supported by recent results by Bigoni et al. (2002), who showed that naloxone benzoylhydrazone 3 μM inhibited the electrically induced twitch response of the isolated guinea-pig ileum and that naloxone 30 μM was necessary to abolish this effect.
In conclusion, our study shows (i) that N/OFQ inhibits dopamine release in the retina, (ii) that this effect and its inhibitory effect on monoamine release in another three models involves NOP receptors and (iii) that Ac-RYYRIK-NH2 is a very potent antagonist at these receptors although it proves to be a partial agonist at the NOP receptor involved in inhibition of noradrenaline release in the mouse brain cortex.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Graduiertenkolleg 246) and by the Land Nordrhein-Westfalen (BONFOR programme). We wish to thank Mrs D. Petri for her skilled technical assistance and Ciba-Geigy (Novartis) and Hoechst (Aventis) for gifts of drugs.
Abbreviations
- Ac-RYYRIK-NH2
acetyl-Arg-Tyr-Tyr-Arg-Ile-Lys-NH2
- [3H]-DA
[3H]-dopamine
- [3H]-NA
[3H]-noradrenaline
- NalBzOH
naloxone benzoylhydrazone
- N/OFQ
nociceptin/orphanin FQ
- PSS
physiological salt solution
References
- BAUER U., NAKAZI M., KATHMANN M., GÖTHERT M., SCHLICKER E. The stereoselective κ opioid receptor antagonist Mr 2266 does not exhibit stereoselectivity as an antagonist at the orphan opioid (ORL1) receptor. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:17–20. doi: 10.1007/pl00005317. [DOI] [PubMed] [Google Scholar]
- BERGER H., ALBRECHT E., WALLUKAT G., BIENERT M. Antagonism by acetyl-RYYRIK-NH2 of G protein activation in rat brain preparations and of chronotropic effect on rat cardiomyocytes evoked by nociceptin/orphanin FQ. Br. J. Pharmacol. 1999;126:555–558. doi: 10.1038/sj.bjp.0702353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGER H., CALO G., ALBRECHT E., GUERRINI R., BIENERT M. [Nphe1]NC(1-13)NH2 selectively antagonizes nociceptin/orphanin FQ-stimulated G-protein activation in rat brain. J. Pharmacol. Exp. Ther. 2000;294:428–433. [PubMed] [Google Scholar]
- BIGONI R., CALO G., RIZZI A., GUERRINI R., DE RISI C., HASHIMOTO Y., HASHIBA E., LAMBERT D., REGOLI D. In vitro characterization of J-113397, a non-peptide nociceptin/orphanin FQ receptor antagonist. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;361:565–568. doi: 10.1007/s002100000220. [DOI] [PubMed] [Google Scholar]
- BIGONI R., CALO G., RIZZI A., OKAWA H., REGOLI D., SMART D., LAMBERT D.G. Effects of naloxone benzoylhydrazone on native and recombinant nociceptin/orphanin FQ receptors. Can. J. Physiol. Pharmacol. 2002;80:407–412. doi: 10.1139/y02-040. [DOI] [PubMed] [Google Scholar]
- BUCHER B. ORL1 receptor-mediated inhibition by nociceptin of noradrenaline release from perivascular sympathetic nerve endings of the rat tail artery. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:682–685. doi: 10.1007/pl00005312. [DOI] [PubMed] [Google Scholar]
- CALO G., GUERRINI R., RIZZI A., SALVADORI S., REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic agent. Br. J. Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIOU L.C., FAN S.H. CompB (J-113397), selectively and competitively antagonizes nociceptin activation of inwardly rectifying K+ channels in rat periaqueductal gray slices. Neuropharmacology. 2002;42:987–992. doi: 10.1016/s0028-3908(02)00051-5. [DOI] [PubMed] [Google Scholar]
- COX B.M., CHAVKIN C., CHRISTIE M.J., CIVELLI O., EVANS C., HAMON M.D., HOELLT V., KIEFFER B., KITCHEN I., MCKNIGHT A.T., MEUNIER J.C., PORTOGHESE P.S.Opioid receptors The IUPHAR compendium of receptor characterization and classification 2000London: IUPHAR Media; 321–333.ed. Girdlestone, D. pp [Google Scholar]
- DOOLEY C.T., SPAETH C.G., BERZETEI-GURSKE I.P., CRAYMER K., ADAPA I.D., BRANDT S.R., HOUGHTEN R.A., TOLL L. Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J. Pharmacol. Exp. Ther. 1997;283:735–741. [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Handbook of experimental pharmacology, vol. XXXIII 1972Berlin: Springer; 283–335.ed. Blaschko, H. & Muscholl, E. pp [Google Scholar]
- HAWES B.E., GRAZIANO M.P., LAMBERT D.G. Cellular actions of nociceptin: transduction mechanisms. Peptides. 2000;21:961–967. doi: 10.1016/s0196-9781(00)00232-1. [DOI] [PubMed] [Google Scholar]
- HO M., CORBETT A.D., MCKNIGHT A.T. Characterization of the ORL1 receptor on adrenergic nerves in the rat anococcygeus muscle. Br. J. Pharmacol. 2000;131:349–355. doi: 10.1038/sj.bjp.0703583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENCK F., MOREAU J.L., MARTIN J.R., KILPATRICK G.J., REINSCHEID R.K., MONSMA F.J., NOTHACKER H.P., CIVELLI O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAMOTO H., OZAKI S., ITOH Y., MIYAJI M., ARAI H., NAKASHIMA H., KATO T., OHTA H., IWASAWA Y. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidinyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one (J-113397) J. Med. Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- KONYA H., MASUDA H., ITOH K., NAGAI K., KAKISHITA E., MATSUOKA A. Modification of dopamine release by nociceptin in conscious rat striatum. Brain Res. 1998;788:341–344. doi: 10.1016/s0006-8993(98)00075-4. [DOI] [PubMed] [Google Scholar]
- LUTFY K., DO T., MAIDMENT N.T. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berlin) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKMAN M.H., DVORKIN B. Presence of nociceptin (orphanin FQ) receptors in rat retina: Comparison with receptors in striatum. Eur. J. Pharmacol. 1997;338:171–176. doi: 10.1016/s0014-2999(97)81945-0. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., KOZŁOWSKA H., BERGER H., SCHLICKER E. Acetyl-RYYRIK-NH2 is a highly efficacious OP4 receptor agonist in the cardiovascular system of anesthetized rats. Peptides. 2000b;21:1875–1880. doi: 10.1016/s0196-9781(00)00330-2. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., KOZŁOWSKA H., KONECZNY B., SCHLICKER E. Nociceptin inhibits the neurogenic vasopressor response in the pithed rat via prejunctional ORL1 receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000a;361:80–84. doi: 10.1007/s002109900160. [DOI] [PubMed] [Google Scholar]
- MASON S.L., HO M., NICHOLSON J., MCKNIGHT A.T. In vitro characterization of Ac-RYYRWK-NH2, Ac-RYYRIK-NH2 and [Phe1Ψ(CH2-NH)Gly2]nociceptin(1-13)NH2 at rat native and recombinant ORL1 receptors. Neuropeptides. 2001;35:244–256. doi: 10.1054/npep.2001.0882. [DOI] [PubMed] [Google Scholar]
- MOGIL J.S., PASTERNAK G.W. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- MOLLEREAU C., MOULEDOUS L. Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides. 2000;21:907–917. doi: 10.1016/s0196-9781(00)00227-8. [DOI] [PubMed] [Google Scholar]
- MORAN T.D., ABDULLA F.A., SMITH P.A. Cellular neurophysiological actions of nociceptin/orphanin FQ. Peptides. 2000;21:969–976. doi: 10.1016/s0196-9781(00)00235-7. [DOI] [PubMed] [Google Scholar]
- MURPHY N.P., LY H.T., MAIDMENT N.T. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- MURPHY N.P., MAIDMENT N.T. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J. Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- NEAL M.J., CUNNINGHAM J.R., PATERSON S.J., MCKNIGHT A.T. Inhibition by nociceptin of the light-evoked release of ACh from retinal cholinergic neurones. Br. J. Pharmacol. 1997;120:1399–1400. doi: 10.1038/sj.bjp.0701135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NODA Y., MAMIYA T., MANABE T., NISHI M., TAKESHIMA H., NABESHIMA T. Role of nociceptin systems in learning and memory. Peptides. 2000;21:1063–1069. doi: 10.1016/s0196-9781(00)00244-8. [DOI] [PubMed] [Google Scholar]
- OH S., KIM I., LEE E., KIM K., KIM H., CHUN M. Immunocytological localization of dopamine in the guinea-pig retina. Cell Tiss. Res. 1999;298:561–565. doi: 10.1007/s004419900122. [DOI] [PubMed] [Google Scholar]
- OLIANAS M.C., ONALI P. Pharmacological properties of nociceptin/orphanin FQ-induced stimulation and inhibition of cyclic AMP formation in distinct layers of rat olfactory bulb. Br. J. Pharmacol. 2002;135:233–238. doi: 10.1038/sj.bjp.0704477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLIDORI C., DE CARO G., MASSI M. The hyperphagic effect of nociceptin/orphanin FQ in rats. Peptides. 2000;21:1051–1062. doi: 10.1016/s0196-9781(00)00243-6. [DOI] [PubMed] [Google Scholar]
- ROMINGER A., FÖRSTER S., ZENTNER J., DOOLEY D.J., MCKNIGHT A.T., FEUERSTEIN T.J., JACKISCH R., VLASKOVSKA M. Comparison of the ORL1 receptor-mediated inhibition of noradrenaline release in human and rat neocortical slices. Br. J. Pharmacol. 2002;135:800–806. doi: 10.1038/sj.bjp.0704523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLICKER E., BEHLING A., LÜMMEN G., MALINOWSKA B., GÖTHERT M. Mutual interaction of histamine H3-receptors and α2-adrenoceptors on noradrenergic terminals in mouse and rat brain cortex. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;345:639–646. doi: 10.1007/BF00164577. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., MORARI M. Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides. 2000;21:1023–1029. doi: 10.1016/s0196-9781(00)00233-3. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., WERTHWEIN S., KATHMANN M. Orphan opioid receptor-mediated inhibition of noradrenaline, dopamine and serotonin release in the mouse, rat and guinea-pig brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998a;357 Suppl.:R90. doi: 10.1007/pl00005162. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., WERTHWEIN S., KATHMANN M., BAUER U. Nociceptin inhibits noradrenaline release in the mouse brain cortex via presynaptic ORL1 receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998b;358:418–422. doi: 10.1007/pl00005273. [DOI] [PubMed] [Google Scholar]
- SHIEH K.R., PAN J.T. Effects of orphanin FQ on central dopaminergic neuronal activities and prolactin secretion. Am. J. Physiol. Regulatory Integrative Comp. Physiol. 2001;280:R705–R712. doi: 10.1152/ajpregu.2001.280.3.R705. [DOI] [PubMed] [Google Scholar]
- SINISCALCHI A., RODI D., BEANI L., BIANCHI C. Inhibitory effect of nociceptin on [3H]-5-HT release from rat cerebral cortex slices. Br. J. Pharmacol. 1999;128:119–123. doi: 10.1038/sj.bjp.0702793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER E.J., RONNEKLEIV O.K., GRANDY D.K., KELLY M.J. The peptide orphanin FQ inhibits beta-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82. doi: 10.1159/000054301. [DOI] [PubMed] [Google Scholar]
- WERTHWEIN S., BAUER U., NAKAZI M., KATHMANN M., SCHLICKER E. Further characterization of the ORL1 receptor-mediated inhibition of noradrenaline release in the mouse brain in vitro. Br. J. Pharmacol. 1999;127:300–308. doi: 10.1038/sj.bjp.0702534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG F., GRANDY D.K., JOHNSON S.W. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br. J. Pharmacol. 2002;136:1065–1071. doi: 10.1038/sj.bjp.0704806. [DOI] [PMC free article] [PubMed] [Google Scholar]


