Abstract
This study characterizes the K+ channel(s) underlying charybdotoxin-sensitive hyperpolarization of porcine coronary artery endothelium.
Two forms of current-voltage (I/V) relationship were evident in whole-cell patch-clamp recordings of freshly-isolated endothelial cells. In both cell types, iberiotoxin (100 nM) inhibited a current active only at potentials over +50 mV. In the presence of iberiotoxin, charybdotoxin (100 nM) produced a large inhibition in 38% of cells and altered the form of the I/V relationship. In the remaining cells, charybdotoxin also inhibited a current but did not alter the form.
Single-channel, outside-out patch recordings revealed a 17.1±0.4 pS conductance. Pipette solutions containing 100, 250 and 500 nM free Ca2+ demonstrated that the open probability was increased by Ca2+. This channel was blocked by charybdotoxin but not by iberiotoxin or apamin.
Hyperpolarizations of intact endothelium elicited by substance P (100 nM; 26.1±0.7 mV) were reduced by apamin (100 nM; 17.0±1.8 mV) whereas those to 1-ethyl-2-benzimidazolinone (1-EBIO, 600 μM, 21.0±0.3 mV) were unaffected (21.7±0.8 mV). Substance P, bradykinin (100 nM) and 1-EBIO evoked charybdotoxin-sensitive, iberiotoxin-insensitive whole-cell perforated-patch currents.
A porcine homologue of the intermediate-conductance Ca2+-activated K+ channel (IK1) was identified in endothelial cells.
In conclusion, porcine coronary artery endothelial cells express an intermediate-conductance Ca2+-activated K+ channel and the IK1 gene product. This channel is opened by activation of the EDHF pathway and likely mediates the charybdotoxin-sensitive component of the EDHF response.
Keywords: Endothelium, EDHF, hyperpolarization, calcium-activated potassium channels, charybdotoxin, iberiotoxin, apamin, IK1 gene product
Introduction
The vascular endothelium controls vessel tone by releasing nitric oxide (Furchgott & Zawadzki, 1980) and prostacyclin (Moncada & Vane, 1979) as well as by a third pathway which involves hyperpolarization of the vascular smooth muscle (reviewed by Busse et al., 2002). This ‘endothelium-dependent hyperpolarizing factor (EDHF)' pathway is inhibited by a combination of the toxins apamin and charybdotoxin, but not apamin and iberiotoxin (Corriu et al., 1996; Zygmunt & Högestätt, 1996; Petersson et al., 1997; Chataigneau et al., 1998; Edwards et al., 1998; 2000). Given the specificities of these toxins (reviewed by Garcia et al., 1991; Castle, 1999), small- and intermediate-conductance Ca2+-activated K+ channels (SKCa and IKCa, respectively) but not large-conductance Ca2+-activated K+ channels (BKCa) are implicated in the EDHF pathway.
In the vasculature, SKCa and IKCa are expressed in endothelial cells (Sakai, 1990; Marchenko & Sage, 1996; Köhler et al., 2000; Burnham et al., 2002) but not in smooth muscle cells with the contractile phenotype while BKCa are mainly expressed in myocytes (Zygmunt et al., 1997; Neylon et al., 1999; Quignard et al., 2000). Furthermore, the combination of apamin and charybdotoxin blocks EDHF-mediated vasodilatation if selectively applied to the endothelium and inhibits the hyperpolarization of the endothelial cells produced by acetylcholine or bradykinin (Edwards et al., 1998; 2000; Doughty et al., 1999; Ohashi et al., 1999). Finally, the increase in endothelial intracellular calcium concentration, provoked by the agonist, is not inhibited by the two toxins (Ghisdal & Morel, 2001). Altogether, these experimental results suggest that the hyperpolarization of the endothelial cells is the critical initiating step in the EDHF-mediated responses (Quignard et al., 2000; Edwards et al., 2000; Busse et al., 2002) and that apamin plus charybdotoxin exert their effects at this site (Edwards et al., 1998).
In an earlier study, in porcine coronary artery endothelial cells, an apamin-sensitive K+ channel which is likely to be involved in the EDHF response was proposed to be an SKCa containing the SK3 subunit (Burnham et al., 2002). The purpose of the present study was, in the same cells, to characterize the K+ channels sensitive to charybdotoxin which are also involved in EDHF-mediated responses.
Methods
Tissue dissection
Pig hearts were taken from Large-White pigs (male or female, 25 kg) anaesthetized with a mixture of tiletamine plus zolazepam (i/m., each 10 mg kg−1) or obtained from the local abattoir and transported to the laboratory in ice-cold Krebs solution (mM, NaCl 118, KCl 3.4, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, glucose 11, gassed with 5% CO2 in O2). Left anterior descending coronary arteries were dissected free of surrounding tissue while maintained in ice-cold Krebs solution.
Micro-electrode studies
Segments of intact vessel were opened longitudinally, pinned to the Sylgard base of a heated 10 ml bath and superfused with Krebs solution (10 ml min−1, 37°C, gassed with 5% CO2 in O2) containing 300 μM NG-nitro-L-arginine and 10 μM indomethacin. Endothelial cells were impaled using micro-electrodes filled with 3 M KCl (resistance 40–80 MΩ). Successful impalements were signalled by a sudden change in membrane potential which remained stable for at least 2 min before the experiment was commenced. Substance P and 1-EBIO were each added as bolus injections directly into the bath in quantities calculated to give (transiently) the final concentrations indicated. Charybdotoxin and apamin were added to the reservoir of Krebs superfusing the bath. Only recordings from impalements that were maintained successfully for the entire protocol were analysed.
Recordings were obtained using a conventional high impedance amplifier (Intra 767, WPI Instruments) and digitized for analysis using a MacLab system (AD Instruments). 50 Hz interference at the amplifier output was selectively removed using an active processing circuit (Humbug, Digitimer).
Patch-clamp experiments
Endothelial cells were mechanically isolated and placed on cover-slips pretreated with Matrigel® basement membrane matrix. This isolation procedure did not allow to determine the side of the endothelial cells which was studied (luminal or abluminal). K+ currents were measured at room temperature (20–24°C) using whole-cell, outside-out or perforated-patch configurations of the patch-clamp technique. The external solution contained: (mM) NaCl 140, CaCl2 1.8, MgCl2 1, KCl 5.4, and Na-HEPES 10 (pH 7.4). Recording pipettes (3–8 MΩ resistance) were filled with a solution containing: (mM) K-aspartate 80, KCl 40, NaCl 20, MgCl2 1, Mg-ATP 3, EGTA 10, K-HEPES 5 (pH 7.4). EGTA was titrated with Ca2+ to provide solutions containing 100, 200 and 500 nM free Ca2+. For perforated-patch experiments, whole-cell access was achieved within 2–5 min by the inclusion of nystatin (50–100 μg ml−1) in the pipette solution.
Patch-clamp data were recorded at 5–10 kHz using an Axopatch 200B amplifier (Axon Instruments) with compensation for series resistance and cell capacitance. Data were filtered at 1 kHz, digitized using an Axon interface and data analysis was performed using pClamp-6 software (Axon Instruments). Variance analysis and histogram distribution were used to determine unitary conductance. Both methods were compared and provided similar results. Mean current (I) and variance (σ2) were calculated for the variance analysis. Least squared parabolic fits to the resulting values were used to derive estimates of single channels properties according to the equation σ2=Ii–(I2/N), where N is the number of channels in the patch and i the single channel current amplitude.
3′ and 5′ RACE
Samples of porcine coronary artery endothelium were obtained by scraping the luminal surface of an artery with a clean scalpel blade. cDNA for 3′ and 5′ RACE (Rapid Amplification of cDNA Ends) was produced using a GeneRacer kit (Qiagen). Amplification was performed at 56°C with 1.5 mM Mg2+ using the gene-specific, nested primers (forward) 5′-CTGAGAGGCAGGCTGTCAATGC and 5′-TCACGTTCCTGACCATTGGCTA and (reverse, in presence of 10% dimethylsulphoxide) 5′-CGAGTGTGCTTGTAGTACATCCA and 5′-GACTCCTTCATCTCTTTGGCATA. Products were cloned using TOPO-TA cloning kits (Invitrogen) and sequenced in triplicate using Big Dye Terminator kit (PE Applied Biosystems) by the central sequencing facility of the School of Biological Sciences, University of Manchester.
Materials
Apamin, synthetic charybdotoxin and iberiotoxin were obtained from Latoxan, France and Matrigel was obtained from Becton Dickinson (MA, U.S.A.). All other substances were supplied by Sigma, except 1-EBIO (1-ethyl-2-benzimidazolinone; Aldrich), and substance P (RBI).
Data analysis
All values are given as mean±standard error (s.e.mean). The number of tested cells or arteries from individual animals is given by n. Statistical analysis was carried out using the paired and unpaired Student's t-test and a value of P<0.05 was considered significant.
Results
Whole-cell currents and effects of toxins
Freshly-isolated endothelial cells were studied with the whole-cell configuration of the patch-clamp technique (500 nM free-Ca2+ pipette solution). The voltage steps were in increments of 10 mV (300–500 ms duration) between −150 and +150 mV from a holding potential of −60 mV. The cells displayed two forms of current-voltage (I/V) relationship (Figure 1). One group of cells (62%) exhibited ‘linear', voltage-independent whole-cell I/V relationships. The remaining cells exhibited ‘non-linear' I/V relationships at positive potentials, with a peak in outward current at +50 mV and another increase at potentials above +100 mV (Figure 1). The reversal potential of the whole-cell current was similar in cells exhibiting linear (−68.8±1.7 mV; n=16) and non-linear (−73.4±2.4 mV; n=10) I/V relationships and both were close to the calculated K+ equilibrium potential under these conditions (−79 mV for 120 mM K+ external and 5.4 mM K+ internal gradient). In the perforated patch configuration, after rupture of the cell membrane, dialysis of the cell with a concentration of 500 nM free calcium increased the net currents. Then, increasing the extracellular potassium concentration from 5 mM to 140 mM significantly increased the outward and inward potassium currents and shifted the reversal potential from −78 mV to 0 mV (data not shown).
Figure 1.
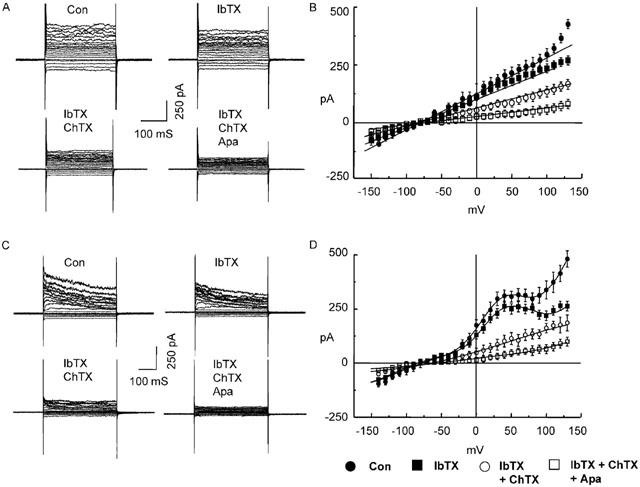
Effect of iberiotoxin, charybdotoxin and apamin on ‘linear' and ‘non-linear' whole-cell K+ currents in porcine coronary artery endothelial cells. Currents were elicited by 10 mV step pulses between −150 and +150 mV from a holding potential of −60 mV using 500 nM free Ca2+ pipette solution. Cells displayed ‘linear' (A and B) and ‘non-linear' (C and D) I/V relationships. Representative current traces (A and C) and the mean data (B, n=12 and D, n=9) obtained under control conditions (Con) and after the cumulative application of iberiotoxin (100 nM; IbTX), charybdotoxin (100 nM; ChTX) and apamin (100 nM; Apa).
The effects of cumulative addition to the bathing solution of the Ca2+-activated K+ channel blockers iberiotoxin, charybdotoxin and apamin (100 nM each) were determined on the whole-cell currents described above. Iberiotoxin significantly inhibited currents only at potentials more positive than +50 mV, in both cells with the linear and non-linear I/V relationships while the addition of charybdotoxin inhibited whole-cell currents at all tested potentials (Figure 1). Furthermore, charybdotoxin abolished the non-linear component of the I/V relationship observed between −20 and +80 mV in that group of cells. The addition of apamin produced a further decrease in whole-cell current in both types of cells (Figure 1).
As shown in Figure 1, the values of the reversal potentials of the currents (either the linear or the voltage-dependent current) is not affected by the presence of iberiotoxin or iberiotoxin+charybdotoxin. Furthermore, the subtracted charybdotoxin-sensitive current showed a similar reversal potential (data not shown).
Single-channel recordings
Single-channel recordings were obtained from freshly-isolated endothelial cells using the outside-out patch configuration with 250 nM free Ca2+ pipette solution under asymmetric, quasi-physiological K+ concentrations (120 mM K+ internal and 5.4 mM K+ external solutions). In addition to the two SKCa that we have previously described (Burnham et al., 2002), a third channel was detected with a unitary conductance of 17.1±0.4 pS (n=9) in the range −20 to +40 mV as determined by plotting unitary current amplitude against membrane potential (Figure 2). This 17.1 pS conductance showed weak voltage-sensitivity, with open probability decreasing at potentials above +50 mV (0.64±0.03 at +50 mV and 0.40±0.03 at +80 mV, n=5).
Figure 2.
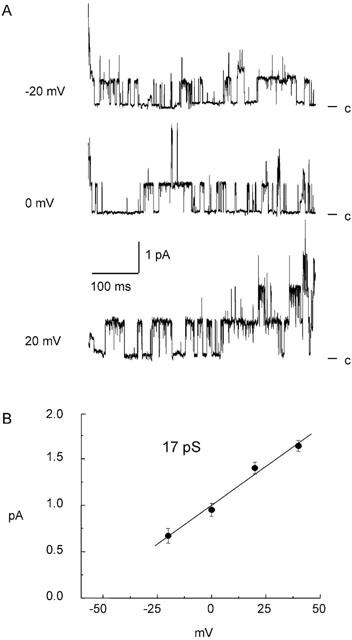
Single-channel recordings obtained from outside-out patches taken from porcine coronary artery endothelial cells. Activity was recorded at the indicated holding potentials under asymmetrical K+ gradients with Ca2+ fixed at 250 nM in the pipette solution. Representative traces are shown, with ‘c' indicating the closed stated (A). Mean unitary currents were plotted against holding potential and fitted with a linear function (B). The slope conductance obtained was 17.1±0.4 pS.
The gating effects of Ca2+ and toxins on the 17.1 pS conductance were determined in outside-out patches. With 100, 250 or 500 nM free Ca2+ in the pipette solution and at a holding potential of 0 mV, the open probability increased from 0.17±0.02 (n=8) to 0.38±0.04 (n=8) and to 0.65±0.03 (n=7), respectively (Figure 3). With 250 nM free Ca2+ in the pipette solution, addition of iberiotoxin (100 nM) to the bathing solution did not affect the channel open probability (0.47±0.11 and 0.44±0.08 in its absence and presence, respectively; n=7; Figure 3). However, supplementing the bathing solution with charybdotoxin (100 nM) decreased the channel open probability to 0.14±0.04 (P<0.05; n=7; Figure 3). The open probability of the channel was not significantly affected by apamin (100 nM) alone (0.42±0.03 and 0.44±0.02 in its absence and presence, respectively; n=6).
Figure 3.
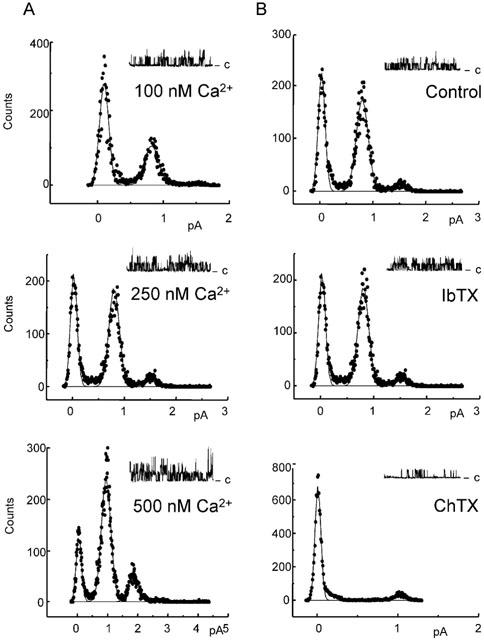
Effect of Ca2+ and toxins on 17.1 pS channel activity recorded from outside-out patches taken from porcine coronary artery endothelial cells. Distributions of unitary conductance amplitude recorded at 0 mV are shown, together with representative traces (closed state is indicated by ‘c'). A Gaussian fit was performed for every single channel experiment, and the amplitude of single channels was determined with the best fit. Then, the mean of the obtained amplitudes was calculated. The effect of Ca2+ was determined using pipette solutions with free Ca2+ fixed at 100, 250 and 500 nM (panel A). The sensitivity of the channel to the cumulative application of iberiotoxin (IbTX, 100 nM) and charybdotoxin (ChTX, 100 nM) was recorded using 250 nM free Ca2+ in the pipette solution (panel B).
In some experiments, either in the whole cell or the outside-out configuration the concentration of 1 μM free Ca2+ was studied. However, under these experimental conditions, within 2 min of the cell membrane rupture, Ca2+ elicited activation of other currents. In the whole cell configuration, these calcium-induced currents had a reversal potential which varied between −35 and −17 mV. In outside-out patches, noisy currents with no clear opened and closed states were activated by this elevated calcium concentration.
Substance P, bradykinin and 1-EBIO evoked currents
Microelectrode studies
In a previous study (Burnham et al., 2002), we tested for 1-EBIO-evoked activation of SKCa in porcine coronary artery endothelium as a charybdotoxin-resistant hyperpolarization. However, this activation would have been masked if the SKCa was partially charybdotoxin-sensitive. Consequently, we examined the apamin-sensitivity of 1-EBIO-evoked hyperpolarization in the present study. Under control conditions, intact porcine coronary artery endothelium impaled with 3 M KCl-filled microelectrodes displayed a membrane potential of −49.2±0.3 mV (n=4 for all; Figure 4). Under these conditions, substance P (100 nM) and the Ca2+-sensitive K+ channel opener 1-EBIO (600 μM) produced hyperpolarizations of 26.1±0.7 and 21.0±0.3 mV, respectively. With apamin (100 nM) present in the bathing solution, the response to substance P was reduced (17.0±1.8 mV) whereas the 1-EBIO response was unaffected (21.7±0.8). The combination of apamin and charybdotoxin (100 nM each) abolished both responses. The small magnitude of the levcromakalim-evoked control hyperpolarizations (∼10 mV, Figure 4) confirmed that the microelectrodes were impaled into endothelial cells, as myocytes produce much larger hyperpolarizations to this agent (∼30 mV; Edwards et al., 2001).
Figure 4.
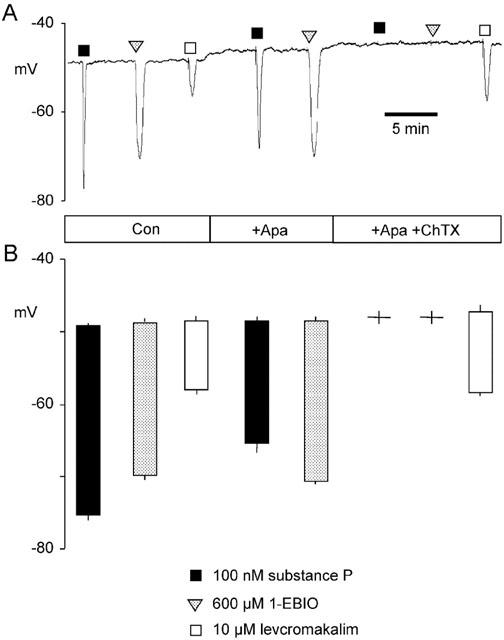
Effect of apamin and the combination of apamin plus charybdotoxin on substance P- and 1-EBIO-evoked hyperpolarizations of porcine coronary artery endothelium. Substance P and 1-EBIO were added as bolus injections, calculated to give transiently the required final concentrations (100 nM and 600 μM, respectively), to a recording chamber perfused with Kreb's solution containing apamin (Apa, 100 M) and charybdotoxin (ChTX, 100 nM) as indicated. Membrane potential responses were recorded using microelectrodes filled with 3 M KCl. A representative trace (A) and mean data derived from four such experiments (B: columns represent the mean±s.e.mean resting membrane potential immediately before exposure (top of column) and mean±s.e.mean peak hyperpolarization (bottom of column) during exposure to each agent) are shown. Levcromakalim (10 μM) was included as an internal control.
Perforated-patch recordings
Substance P (100 nM) did not affect K+ currents in endothelial cells dialysed with 10 mM EGTA (n=5, data not shown). Using the perforated-patch whole-cell configuration at a holding potential of −40 mV (500 nM free Ca2+ in the pipette solution), substance P produced a transient and rapid peak in the net outward current followed by a steady-state activation (Figure 5). This peak current (57.9±8.2 pA; n=5) was insensitive to iberiotoxin (100 nM) but inhibited by charybdotoxin (100 nM; 15.3±4.1 pA; n=5). In the steady-state phase, K+ currents were elicited by 10 mV voltage steps between −120 mV and +80 mV from a holding potential of −80 mV (Figure 5). Substance P increased the outward current at +50 mV by 152±8% and this was significantly reduced to 119±3% by the presence of charybdotoxin (100 nM; n=9).
Figure 5.
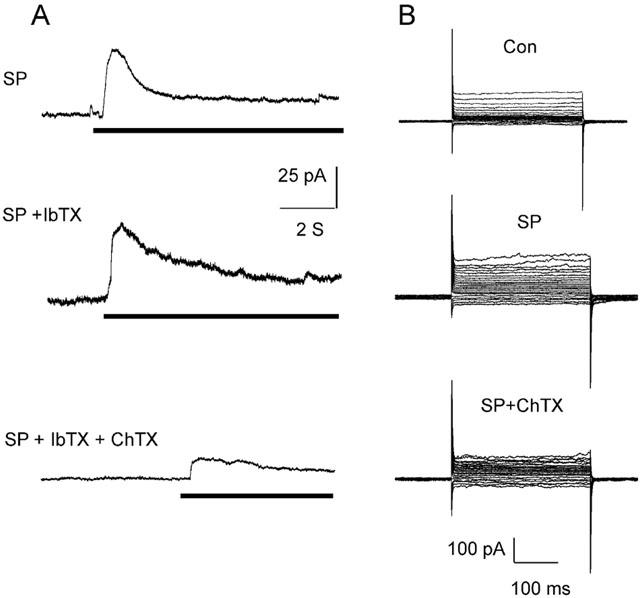
Characterization of substance P-evoked currents in porcine coronary artery endothelial cells determined using the perforated-patch configuration (holding potential of −40 mV using 500 nM free Ca2+ in the pipette solution). Panel A: Outward currents resulting from the application of substance P (SP, 100 nM, indicated by horizontal bar) to the bathing solution in the presence of iberiotoxin (IbTX, 100 nM) and charybdotoxin (ChTX, 100 nM) are shown. Panel B: During the steady-state phase of the SP-evoked currents, 10 mV voltage steps between −120 mV and +80 mV were applied from a holding potential of −80 mV. The effect of charybdotoxin is shown.
The effects of bradykinin were qualitatively similar to those of substance P. Voltage steps to +50 mV from a holding potential of −80 mV elicited outward currents that were potentiated by bradykinin. These currents (147.5±8.1% of control; n=7) were reduced by the addition of apamin (129.1±5.2%) and almost abolished by the further addition of charybdotoxin (108.3±3.4%; Figure 6). Similar protocols were used to assess the effects of 1-EBIO (300 μM). Application of 1-EBIO increased outward current by 164±8% of control (n=7; Figure 6) and this increase was unaffected by iberiotoxin (100 nM; 157±6%). Cumulative addition of apamin (100 nM) produced a small but statistically significant inhibition of this current (142±10%) while addition of charybdotoxin (100 nM) completely abolished the current (81±10%).
Figure 6.
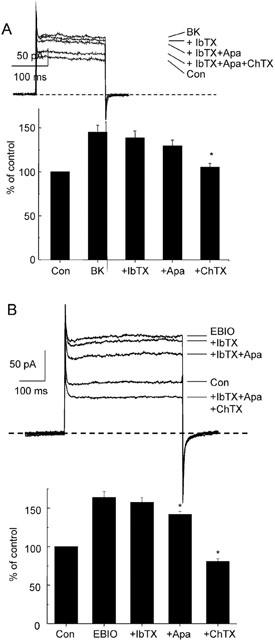
Characterization of bradykinin and 1-EBIO evoked currents in porcine coronary artery endothelial cells recorded using the perforated-patch configuration. K+ currents were elicited by voltage steps to +50 mV from a holding potential of −80 mV (500 nM free Ca2+ in the pipette solution) in control conditions (Con), after application of bradykinin (BK, 100 nM) and after application iberiotoxin (IbTX, 100 nM), apamin (Apa, 100 nM) and charybdotoxin (ChTX, 100 nM) Ms shown (panel A, representative traces and mean data are shown, n=7). Identical experiments were performed using 1-EBIO (300 μM) in place of BK (panel B, n=7).
The reversal potentials of the charybdotoxin-sensitive currents activated by 1-EBIO, substance P or bradykinin were included between −68 and −75 mV and therefore very similar to the equilibrium potential for K+ ions under these experimental conditions.
3′ and 5′ RACE
Gene-specific primers for 3′ and 5′ RACE were designed to homologous regions of rat and human IK1 sequences. Two RACE products were amplified from porcine coronary artery endothelium cDNA and constituted overlapping halves of the 1.97 kb full-length porcine homologue of IK1. This sequence was submitted to GenBank under accession number AY062036. The presence of an in-frame stop codon at position −84 in the 5′ untranslated region suggested that the initiator codon was valid although the GenBank EST database contained no porcine sequences for comparison. The deduced amino acid sequence shared 90% identity with human IK1 (Figure 7), displaying 41 amino acid substitutions and a single deletion (alanine-133). Residues surrounding the K+ selectivity motif (GYG) were highly conserved, with sequence divergence largely restricted to the S3–S4 region and the C terminus.
Figure 7.

Comparison of deduced amino acid sequences of porcine and human IK1. Porcine IK1 (submitted as GenBank AY062036) was sequenced from coronary artery endothelium cDNA using 3′ and 5′ RACE with gene-specific primers designed to homologous regions of human and rat IK1 sequences. Underlined residues constitute transmembrane spans (S1-S6) and the pore (P) region according to Vandorpe et al. (1998); boldfaced residues are conserved consensus calmodulin-binding domain (Conserved Domain Database, NCBI).
Discussion
This study demonstrates the presence of a charybdotoxin-sensitive, iberiotoxin-insensitive IKCa in freshly-isolated endothelial cells of the porcine coronary artery. This channel is activated by substance P, bradykinin and 1-EBIO and contributes to the endothelial cell hyperpolarization elicited by these compounds. Together with previously described SKCa (Burnham et al., 2002), these channels generate the endothelial hyperpolarization thought to initiate the EDHF pathway in porcine coronary arteries and likely represent the site of action for the characteristic blockade of this pathway by charybdotoxin and apamin.
Porcine coronary artery endothelial cells expressIKCa/IK1
Whole-cell currents were dominated by K+ conductances, with reversal potentials close to the calculated K+ equilibrium potential. In the presence of the BKCa-specific inhibitor iberiotoxin, charybdotoxin blocked a component of the current activated by 500 nM free Ca2+ pipette solution, indicating that native porcine coronary artery endothelial cells express a charybdotoxin-sensitive K+ channel. Single-channel recordings from outside-out patches confirmed the existence of a channel with 17.1 pS unitary conductance under quasi-physiological K+ gradients. This conductance was stimulated by internal Ca2+ and was blocked by external charybdotoxin but not iberiotoxin or apamin. In addition, we detected and sequenced the mRNA for an IKCa in samples taken from intact porcine coronary artery endothelium.
Few studies of Ca2+-activated K+ channels have been performed in native endothelial cells, although these channels are often reported in cultured endothelial cells. Sakai (1990) reported a 9pS Ca2+-activated K+ channel in native rabbit aortic endothelial cells. Sharma & Davis (1994) detected a Ca2+-activated, voltage-insensitive, inwardly-rectifying K+ conductance of 11–25 pS in porcine coronary artery endothelial cells that had been cultured only overnight. Although the toxin-sensitivity was not determined in these studies, these properties are representative of an SKCa or IKCa (typically 5–20 pS and 20–60 pS, respectively; Castle, 1999). Marchenko & Sage (1996) observed a charybdotoxin-sensitive Ca2+-activated K+ channel in rat aortic endothelium with a conductance of 6.7 pS under quasi-physiological and 18 pS in symmetric K+ solutions.
Several authors have reported a molecular identity for IKCa, at least in pancreas, foetal brain and T lymphocytes (Ishii et al., 1997; Joiner et al., 1997; Logsdon et al., 1997). Expression of the IK1 (also known as SK4; see Joiner et al., 1997) sequence in Xenopus oocytes and mammalian cells resulted in charybdotoxin-sensitive, iberiotoxin- and apamin-insensitive 12–42 pS Ca2+-activated K+ channels: the symmetry of the K+ gradient and inward-rectifier properties of the channel affecting conductance. To date, no other charybdotoxin-sensitive, iberiotoxin-insensitive Ca2+-activated K+ channels have been cloned and therefore the porcine homologue of IK1 sequenced in the present study likely represents the molecular identity of the 17.1 pS channel. The porcine IK1 is 90% identical at the amino acid level to the human form, homology comparable to that shown by mouse and human sequences (Vandorpe et al., 1998). Marked porcine sequence divergence, including a single amino acid deletion, occurs in the S3–S4 region (presumed second extracellular loop). This region also diverges in the mouse sequence, where it includes two amino acid deletions relative to the human form.
In the present study using freshly-isolated cells, BKCa currents were not observed in outside-out patches although a whole-cell current sensitive to iberiotoxin was detected, albeit at only very positive potentials above +50 mV. Of the few reported observations of BKCa currents in native endothelial cells, half-maximal activation has required high intracellular Ca2+ concentrations of 1–6 μM, possibly due to the absence in these cells of regulatory BKCa β subunits that enhance Ca2+-sensitivity (Rusko et al., 1992; Köhler et al., 2000; Papassotiriou et al., 2000). When we performed preliminary experiments with Ca2+ concentrations of 1 μM or higher, chloride and non-selective cation conductances were activated which shifted the whole-cell current reversal potential to more positive potential (>−35 mV). Even under these conditions, iberiotoxin only blocked an outward current at very positive potentials above +50 mV. Overall, the results of the present study suggest that BKCa is expressed in native porcine coronary artery endothelial cells but that for whatever reason, its activation at physiological potentials is not apparent.
In the present study, endothelial cells displayed two forms of whole-cell I/V relationship. The charybdotoxin-sensitive current component was responsible for the majority of the ‘non-linear' relationship observed in some cells, although a ‘linear' charybdotoxin-sensitive current was also present in the remaining cells. It is known that the rectification of charybdotoxin-sensitive IKCa can be altered by the symmetry of the K+ gradient (see Marchenko & Sage, 1996), although our observations were made under identical conditions. Köhler et al. (2000) found whole-cell currents in human mesenteric artery endothelial cells similar to the linear and non-linear currents observed in the present study. Their analysis correlated three forms of I/V relationship to the differential expression of hIK1 and hSlo (BKCa α subunit). Given that other studies have reported heterogeneous electrical properties in native endothelial cells (Jiang et al., 2001), this would appear to be the physiological state of the endothelium. The mechanisms which underlie the present observations are unknown although K+ channel properties are known to be affected by post-translational modification and auxillary subunits.
Agonist-evoked currents and implications for theEDHF pathway
The substance P- and bradykinin-evoked outward currents measured using the whole-cell perforated-patch technique were carried in part by a charybdotoxin-sensitive IKCa with little evidence for activation of an iberiotoxin-sensitive BKCa; data consistent with our conventional microelectrode recordings in intact endothelium (present study; Edwards et al., 2000; Burnham et al., 2002). These data from native endothelial cells are reproduced to some extent by observations from cultured ones, in that evidence for the activation of IKCa has been reported (Sharma & Davis, 1994). However, Frieden et al. (1999) reported that bradykinin activated an iberiotoxin-sensitive current via a cytochrome P450 pathway. Activation of BKCa by bradykinin was not observed in the present study. This might be explained by differences in experimental conditions, in that the present study used freshly-isolated endothelial cells while the previous study was performed in cultured endothelial cells. As endothelial cells in culture increase both the expression of BKCa α subunit mRNA and the appearance of BKCa currents, these conditions may encourage such observations (Kestler et al., 1998; Jow et al., 1999). Indeed, studies using prolonged incubations of intact arteries have demonstrated similar findings, in that bradykinin may activate an iberiotoxin-sensitive pathway distinct from the classic apamin and charybdotoxin-sensitive EDHF pathway (Edwards et al., 2001).
1-EBIO is an activator of SKCa and IKCa (Devor et al., 1996; Grunnet et al., 2001) thought to modulate the interaction between the channel and its intrinsically-bound calmodulin Ca2+ sensor (Pedarzani et al., 2001). BKCa, which is not gated by calmodulin, is insensitive to this compound and in the vasculature, K+ channels opened by 1-EBIO are limited to the endothelium (Edwards et al., 1999; Walker et al., 2001). In a previous study (Burnham et al., 2002), we showed that the hyperpolarization of porcine coronary artery endothelium evoked by 1-EBIO was entirely blocked by charybdotoxin alone, despite recombinant channel studies suggesting that SKCa should also be opened. In the present study, it was hypothesized that an SKCa could have been activated by 1-EBIO but, if it displayed some sensitivity to charybdotoxin, this effect would have been masked. Consequently, we tested whether application of apamin would reveal an activation of SKCa by 1-EBIO. Although the hyperpolarization to substance P recorded using conventional microelectrodes was reduced by apamin alone, the response to 1-EBIO was unaffected. Furthermore, our single-channel analysis of the apamin-sensitive SKCa and charybdotoxin-sensitive IKCa of porcine coronary artery endothelium indicated a lack of ‘cross-reactivity' to the other toxin (present study; Burnham et al., 2002). Although 1-EBIO-evoked whole-cell currents were, in addition to being charybdotoxin-sensitive, slightly inhibited by apamin, this may represent the inhibition of a basal SKCa current due to the presence of Ca2+ in the pipette solution.
In cultured porcine coronary artery endothelial cells, a recent study by Sollini et al. (2002) reported the presence of an apamin-insensitive Ca2+-activated K+ channel. This channel was activated by both substance P and bradykinin, similar to the channel described in the present study. However, despite the fact that this channel in cultured endothelial cells was inhibited by charybdotoxin and resistant to apamin (both hallmark pharmacological features of IKCa) and activated by 1-EBIO, Sollini et al. (2002) designated the channel as an SKCa (SKap-ins), largely on the basis of its relatively low conductance (10.7 pS in asymmetrical K+ conditions; 30.9 pS in symmetrical K+). Thus, SKap-ins shares all the characteristics of the IKCa reported here, except that its conductance is low for an IKCa (typically 20–60 pS). The present investigation has clearly shown that freshly-isolated porcine coronary artery endothelial cells expresses the porcine homologue of IK1. We thus favour the view that an IKCa is present on these endothelial cells and that this is the correct designation of the charybdotoxin-sensitive, apamin-insensitive channel involved in the EDHF pathway in the porcine coronary artery.
In summary, the present study has shown that native porcine coronary artery endothelial cells express an IKCa and the IK1 gene product. This channel is opened by vasodilators such as substance P and bradykinin, which activate the EDHF pathway. Inhibition of the EDHF pathway by charybdotoxin and apamin is due to their blockade of IKCa and SKCa located on the endothelial rather than on smooth muscle cells. The presence of IKCa and SKCa on endothelial cells is associated with expression of IK1 and SK (particularly SK3) gene products (present study, Köhler et al., 2001; Burnham et al., 2002) and these are likely to be the channels which generate the endothelial hyperpolarization that underlies the EDHF response.
Abbreviations
- BKCa
large conductance calcium-activated potassium channels
- EDHF
endothelium-derived hyperpolarizing factor
- IKCa
intermediate conductance calcium-activated potassium channels
- SKCa
small conductance calcium-activated potassium channels
- 1-EBIO
1-ethyl-2-benzimidazolinone
- RACE
rapid amplification of cDNA ends
References
- BURNHAM M.P., BYCHKOV R., FELETOU M., RICHARDS G.R., VANHOUTTE P.M., WESTON A.H., EDWARDS G. Characterization of an apamin-sensitive small conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br. J. Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concepts together. Trends Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- CASTLE N.A. Recent advances in the biology of small conductance calcium-activated potassium channels. Perspect. Drug. Discov. 1999;15/16:131–154. [Google Scholar]
- CHATAIGNEAU T., FÉLÉTOU M., DUHAULT J., VANHOUTTE P.M. Epoxyeicosatrienoic acids, potassium channel blockers and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br. J. Pharmacol. 1998;123:574–580. doi: 10.1038/sj.bjp.0701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRIU C., FÉLÉTOU M., CANET E., VANHOUTTE P.M. Inhibitors of the cytochrome P450-mono-oxygenase and endothelium-dependent hyperpolarizations in the guinea-pig isolated carotid artery. Br. J. Pharmacol. 1996;117:607–610. doi: 10.1111/j.1476-5381.1996.tb15233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVOR D.C., SINGH A.K., FRIZZELL R.A., BRIDGES R.J. Modulation of Cl− secretion by benzimidazolones. I. Direct activation of Ca(2+)-dependent K+ channel. Am. J. Physiol. 1996;271:L775–L784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- DOUGHTY J.M., PLANE F., LANGTON P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am. J. Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., FÉLÉTOU M., GARDENER M.J., GLEN C.D., RICHARDS G.R., VANHOUTTE P.M., & WESTON A.H. Further investigations into the endothelium-dependent hyperpolarizing effects of bradykinin and substance P in porcine coronary artery. Br. J. Pharmacol. 2001;133:1145–1153. doi: 10.1038/sj.bjp.0704157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., GARDENER M.J., FELETOU M., BRADY G., VANHOUTTE P.M., WESTON A.H. Further investigation of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br. J. Pharmacol. 1999;128:1064–1070. doi: 10.1038/sj.bjp.0702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., THOLLON C., GARDENER M.J., FÉLÉTOU M., VILAINE J.P., VANHOUTTE P.M., WESTON A.H. Role of gap junctions and EETs in endothelium-dependent hyperpolarization of porcine coronary artery. Br. J. Pharmacol. 2000;129:1145–1154. doi: 10.1038/sj.bjp.0703188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN M., SOLLINI M., BENY J.-L. Substance P and bradykinin activate different types of Kca currents to hyperpolarize cultured porcine coronary artery endothelial cells. J. Physiol. 1999;519:361–371. doi: 10.1111/j.1469-7793.1999.0361m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI JV. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GARCIA M.L., GALVEZ A., GARCIA-CALVO M., KING V.F., VASQUEZ J., KACZOROWSKI G.J. Use of toxins to study potassium channels. J. Bioenerg Biomembr. 1991;23:615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- GHISDAL P., MOREL N. Cellular target of voltage and calcium-dependent K+ channel blockers involved in EDHF-mediated responses in rat superior mesenteric artery. Br. J. Pharmacol. 2001;134:1021–1028. doi: 10.1038/sj.bjp.0704348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNNET M., JESPERSEN T., ANGELO K., FRØKJÆR-JENSEN C., KLAERKE D.A., OLESEN S.-P., JENSEN B.S. Pharmacological modulation of SK3 channels. Neuropharmacology. 2001;40:879–887. doi: 10.1016/s0028-3908(01)00028-4. [DOI] [PubMed] [Google Scholar]
- ISHII T.M., SILVIA C., HIRSCHBERG B., BOND C.T., ADELMAN J.P., MAYLIE J. A human intermediate-conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG Z.-G., SI J.-Q., LASAREV M.R., NUTTALL A.L. Two resting potential levels regulated by the inward-rectifier potassium channel in the guinea-pig spiral modiolar artery. J. Physiol. 2001;537:829–842. doi: 10.1111/j.1469-7793.2001.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOINER W.J., WANG L.-Y., TANG M.D., KACZMAREK L.K. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOW F., SULLIVAN K., SOKOL P., NUMANN R. Induction of Ca2+-activated K+ current and transient outward currents in human capillary endothelial cells. J. Membrane Biol. 1999;167:53–64. doi: 10.1007/s002329900471. [DOI] [PubMed] [Google Scholar]
- KESTLER H.A., JANKO S., HAUSSLER U., MUCHE R., HOMBACH V., HOHER M., WIECHA J. A remark on the high-conductance calcium-activated potassium channel in human endothelial cells. Res. Exp. Med. 1998;198:133–143. doi: 10.1007/s004330050097. [DOI] [PubMed] [Google Scholar]
- KÖHLER R., DEGENHARDT C., KÜHN M., RUNKEL N., PAUL M., HOYER J. Expression and function of endothelial Ca2+-activated K+ channels in human mesenteric artery–A single cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ. Res. 2000;87:496–503. doi: 10.1161/01.res.87.6.496. [DOI] [PubMed] [Google Scholar]
- KÖHLER R., BRAKEMEIER S., KÜHN M., BEHRENS C., REAL R., DEGENHARDT C., ORZECHOWSKI H-D., PRIES A.R., PAUL M., HOYER O. Impaired hyperpolarization in regenerated endothelium after balloon catheter injury. Circ. Res. 2001;89:174–179. doi: 10.1161/hh1401.093460. [DOI] [PubMed] [Google Scholar]
- LOGSDON N.J., KANG J., TOGO J.A., CHRISTIAN E.P., AIYAR J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J. Biol. Chem. 1997;272:32723–32726. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- MARCHENKO S.M., SAGE S.O. Calcium-activated potassium channels in the endothelium of intact rat aorta. J. Physiol. 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., VANE J.R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2 and prostacyclin. Pharmacol. Rev. 1979;30:293–331. [PubMed] [Google Scholar]
- NEYLON C.B., LANG R.J., FU Y., BOBIK A., REINHART P.H. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle. Relationship between KCa channel diversity and smooth muscle cell function. Circ. Res. 1999;85:1–11. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- OHASHI M., SATOH K., ITOH T. Acetylcholine-induced membrane potential changes in endothelial cells of rabbit aortic valve. Br. J. Pharmacol. 1999;126:19–26. doi: 10.1038/sj.bjp.0702262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPASSOTIRIOU J., KÖHLER R., PRENEN J., KRAUSE H., AKBAR M., EGGERMONT J., PAUL M., DISTLER A., NILIUS B., HOYER J. Endothelial K+ channel lacks the Ca2+ sensitivity-regulating β subunit. FASEB J. 2000;14:885–894. [PubMed] [Google Scholar]
- PEDARZANI P., MOSBACHER J., RIVARD A., CINGOLANI L. A., OLIVER D., STOCKER M., ADELMAN J.P., & FAKLER B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J. Biol. Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- PETERSSON J., ZYGMUNT P.M., HÖGESTÄTT E.D. Characterization of the potassium channels involved in EDHF-mediated relaxation in cerebral arteries. Br. J. Pharmacol. 1997;120:1344–1350. doi: 10.1038/sj.bjp.0701032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIGNARD J.-F., FELETOU M., EDWARDS G., DUHAULT J., WESTON A.H., VANHOUTTE P.M. Role of endothelial cells hyperpolarization in EDHF-mediated responses in the guinea-pig carotid artery. Br. J. Pharmacol. 2000;129:1103–1112. doi: 10.1038/sj.bjp.0703175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSKO J., TANZI F., VAN BREEMEN C., ADAMS D.J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J. Physiol. 1992;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAI T. Acetylcholine induces Ca-dependent K currents in rabbit endothelial cells. Japan J. Pharmacol. 1990;53:235–246. doi: 10.1254/jjp.53.235. [DOI] [PubMed] [Google Scholar]
- SHARMA N.R., DAVIS M.J. Mechanism of substance P-induced hyperpolarization of porcine coronary artery endothelial cells. Am. J. Physiol. 1994;266:H156–H164. doi: 10.1152/ajpheart.1994.266.1.H156. [DOI] [PubMed] [Google Scholar]
- SOLLINI M., FRIEDEN M., BÉNY J.-L. Charybdotoxin-sensitive small conductance KCa channel activated by bradykinin and substance P in endothelial cells. Br. J. Pharmacol. 2002;136:1201–1209. doi: 10.1038/sj.bjp.0704819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDORPE D.H., SHMUKLER B.E., JIANG L., LIM B., MAYLIE J., ADELMAN J.P., DE FRANCESCHI L., CAPPELLINI M.C., BRUGNARA C., ALPER S. cDNA Cloning and Functional Characterization of the Mouse Ca2+-gated K+ Channel, mIK1. J. Biol. Chem. 1998;273:21542–21553. doi: 10.1074/jbc.273.34.21542. [DOI] [PubMed] [Google Scholar]
- WALKER S.D., DORA K.A., INGS N.T., CRANE G.J., GARLAND C.J. Activation of endothelial cell IKCa with 1-ethyl-2-benzimidazolinone evokes smooth muscle hyperpolarization in rat isolated mesenteric artery. Br. J. Pharmacol. 2001;134:1548–1554. doi: 10.1038/sj.bjp.0704415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P., EDWARDS G., WESTON A.H., LARSSON B., HÖGESTATT E.D. Involvement of voltage-dependent potassium channels in the EDHF-mediated relaxation of the rat hepatic artery. Br. J. Pharmacol. 1997;121:141–149. doi: 10.1038/sj.bjp.0701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HÖGESTÄTT E.D. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br. J. Pharmacol. 1996;117:1600–1606. doi: 10.1111/j.1476-5381.1996.tb15327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


