Abstract
The function and autoradiographic binding expression of kinin B1 receptors were evaluated in the lungs of Streptozotocin (STZ)-diabetic rats.
The intrapleural injection (i.pl.) of des-Arg9-bradykinin (des-Arg9-BK) (50 and 100 nmol per site), a selective B1 receptor agonist, increased time-dependently the mononuclear and neutrophil cells influx in the pleural cavity of rats treated with STZ (65 mg kg−1, i.p., 4 days earlier). This effect was significantly less in control rats.
The influx of mononuclear and polymorphonuclear neutrophil cells induced by des-Arg9-BK was significantly inhibited by two B1 receptor antagonists (des-Arg10-Hoe140 or R-715, 100 nmol per site, 5 min earlier), but not by two B2 receptor antagonists (Hoe140, 10 nmol or NPC 18884, 100 nmol per site, 5 min earlier). However, Hoe140 prevented the higher basal leukocyte influx seen in STZ-diabetic rats.
Leukocyte infiltration induced by des-Arg9-BK in STZ-diabetic rats was significantly reduced after treatment with insulin (2 U per day, s.c. over 4 days) or with an anti-PMN antibody (0.1 ml of a 1 : 20 dilution, i.pl. 5 min earlier).
Specific B1 receptor binding sites were seen in lung sections from both control and STZ-diabetic rats, yet the density of labelling was much greater in diabetic rats and particularly after intrapleural injection of des-Arg9-BK. Treatment with insulin or with the anti-PMN antibody markedly reduced B1 receptor binding sites occurring after the injection of des-Arg9-BK in STZ-diabetic rats.
Data suggest that the B1 receptor is up-regulated in the lungs of STZ-diabetic rats, and its activation increases leukocyte infiltration into the pleural cavity. The overexpression of B1 receptors seems to depend on neutrophils influx and appears to be associated with hyperglycaemia.
Keywords: Bradykinin, des-Arg9-BK, B1 receptor, Hoe140, pleurisy, inflammation, leukocyte, diabetes
Introduction
Kinins exert their biological effects through the activation of two transmembrane G-protein-coupled receptors, denoted as B1 and B2 (Regoli & Barabé, 1980). Whereas the B2 receptor is better activated by the whole kinin sequence such as bradykinin (BK), the B1 receptor is preferentially activated by the kininase I metabolite des-Arg9-BK, which is elevated at sites of inflammation (Raymond et al., 1995; Décarie et al., 1996). Although the B1 receptor is constitutively present and functional in the canine cardiovascular system (Nakhostine et al., 1993; Lamontagne et al., 1996) and in peripheral tissues of the mouse (vas deferens, stomach, lung) (Mass et al., 1995; Nsa Allogho et al., 1995; 1998; Vianna & Calixto., 1998), this receptor is generally underexpressed in normal tissues and healthy animals, but can be up-regulated in inflammatory conditions. Induction and increased expression of the B1 receptor occur following tissue injury, treatment with bacterial endotoxins, cytokines or growth factors (Marceau, 1995; Marceau et al., 1998; Mclean et al., 2000). Evidence in human lung fibroblasts (IMR-90) suggests an up-regulation of B1 receptor by its own agonist involving the activation of protein kinase C, the transcriptional nuclear factor NF-κB, pertussis and cholera toxin-insensitive pathways (Schanstra et al., 1998). Most components of the kallikrein-kinin system were detected in immune cells, supporting an important role for kinins in inflammation and other pathological processes. Kinin precursors (kininogens) and kinin-generating enzymes (kallikreins) were found in neutrophils and macrophages of several species (Böckmann & Paegelow, 2000), and the expression of kinin B1 receptor was demonstrated in mouse neutrophils (Araujo et al., 2001).
Insulin-dependent diabetes mellitus is an autoimmune disease characterized by specific destruction of the insulin producing β cells of Langerhans islets, with infiltration of leukocytes in the islets, a process termed insulitis. The infiltration of leukocytes in the islets in Streptozotocin (STZ)-diabetic mice was inhibited by a selective B1 receptor antagonist (Navarro et al., 2001), suggesting the participation of B1 receptor in this model. Recent evidence suggests that B1 receptor is induced in the spinal cord (Cloutier & Couture, 2000), rat paw (Campos et al., 2001), isolated renal glomeruli (Mage et al., 2002), and retinas (Abdouh et al., 2001) of STZ-diabetic rats.
The aim of this study was to investigate the infiltration of leukocytes in response to B1 receptor activation in the pleural cavity of STZ-treated rats, and to correlate leukocyte trafficking with the presence of specific B1 receptor binding sites by autoradiography in the lung of control and STZ-diabetic rats. Treatments with insulin and an anti-PMN antibody were given to address the influence of hyperglycaemia and neutrophils in the cellular response and expression of B1 receptor binding sites in this inflammatory process.
Methods
Animals
Male Wistar rats (weighting 200–250 g, n=264) from Charles River (St-Constant, Québec, Canada), and housed two per cage were used in this study. The animals were maintained in an environment of controlled temperature (20°C), humidity (53%) under a 12 h light-dark cycle. Food and water were provided ad libitum. All animal procedures used were in strict accordance with the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals.
Intrapleural leukocyte infiltration
Leukocyte infiltration was induced by intrapleural (i.pl.) injection of 100 μl of sterile saline, containing des-Arg9-BK (10 to 100 nmol per site, n=6–10 for each dose) into the right pleural space through the chest skin of the rat under short halothane anaesthesia. An equal volume of sterile saline was injected i.pl. to control rats (n=6).
After i.pl. injection of des-Arg9-BK, the animals were sacrificed at different periods of time (1 to 48 h, n=6–10 for each end point) with an overdose of halothane. The thoracic cavity was opened and washed with 2 ml of PBS (pH 7.4 and containing 20 IU ml−1 heparin). The animals were pre-treated with a single dose of STZ (65 mg kg−1, i.p. diluted in sterile saline) or with the vehicle 4 days before des-Arg9-BK-induced pleurisy. Glucose concentration was measured in a blood sample obtained from tail punction, with a glucose oxidase-impregnated test strip and a reflectance meter (Accu-Check III, Boehringer Mannheim, Germany). Animals had a blood glucose concentration higher than 20 mM 4 days after treatment with STZ.
Leukocyte counts
Total leukocyte counts were measured in Neubauer chambers by means of an optical microscope, after diluting the pleural fluid in Turk solution (1 : 20). Differential cell counts were performed after cytocentrifugation (Cytospin 3) and staining with Hema 3 Stain Set, and the analysis was carried out under immersion objective. Around 100 cells were counted, the results being expressed as the number of each cell population in 1 ml.
Experimental protocols for drug treatments
The protocols for drug administration were as follows: des-Arg10-Hoe140 (10, 50 and 100 nmol per site, B1 antagonist, n=6 per dose), R-715 (100 nmol per site, B1 antagonist, n=6), Hoe140 (10 nmol per site, B2 antagonist, n=6), Hoe140 plus R-715 (10 and 100 nmol per site, respectively n=6) or NPC 18884 (100 nmol per site, B2 antagonist, n=6) were administered i.pl. 5 min before des-Arg9-BK-induced leukocyte infiltration. R-715 and Hoe140 were also injected alone in separate rats (n=5–6). The insulin implant which releases 2 U 24 h−1 was inserted under the back skin with a 12 G hypodermic needle (trocar/stylet) immediately after STZ injection under short halothane anaesthesia. The anti-PMN antibody (0.1 ml of a 1 : 20 dilution) was injected either in the pleural cavity 5 min or via the intraperitoneal route 30 min prior to carrageenan (1% i.pl. 4 h beforehand)-induced leukocyte migration into the pleural cavity of control rats in order to determine the best treatment schedule (n=5 in each group). STZ-diabetic rats received des-Arg9-BK (100 nmol i.pl. 4 h beforehand) either in the absence (n=10) or presence (n=10) of the anti-PMN antibody (0.1 ml of a 1 : 20 dilution injected i.pl. 5 min earlier) which was the most effective treatment with carrageenan.
Tissue preparation for autoradiography
Leukocyte infiltration was measured in all rats used for autoradiographic studies. Immediately after the withdrawal of leukocytes from the pleural cavity, the two lungs were frozen in 2-methyl butane cooled at −45 to −70°C with liquid nitrogen, mounted together in a gelatine bloc and serially cut into 40 μm thick coronal sections with a cryostat fixed at temperature varied between −11 to −13°C. The sections were alternatively thaw-mounted on 0.2% gelatine/0.033% chromium potassium sulphate coated slides. Each slide contains three sections of the left and right lungs from the same animal. Three slides were taken for the total binding and two slides (adjacent sections) for the non-specific binding and this was replicated four times for each animal. A total of 80 slides (480 lung slices from both sides) were obtained from four rats/group and kept at −80°C until use.
Peptide iodination
Iodination of HPP-desArg10-Hoe140 was made with the chloramine T method according to Hunter & Greenwood (1962). Briefly, 5 μg of peptide were incubated in 0.05 M PBS for 30 s in the presence of 0.5 mCi (18.5 Mbq) of Na 125I and 220 nmol of chloramine T in a total volume of 85 μL. The monoiodinated peptide was then immediately purified by high pressure liquid chromatography (HPLC) on a C4 Vydac column (0.4×250 mm) (The Separations group, Hesperia, CA, U.S.A.) with 0.1% trifluoroacetic acid and acetonitrile as mobile phases. The specific activity of the iodination peptide was calculated to be approximately 2000 c.p.m. fmol−1 that approximately yields 1212 Ci mmol−1.
Ligand binding to B1 receptor
B1 receptor binding densities were measured by quantitative autoradiography, using [125I]-HPPdesArg10-Hoe140 as radioligand (Cloutier et al., 2002). Sections were quickly warmed to room temperature, dipped for 30 s in 25 mM PIPES buffer (pH 7.40, 4°C), followed by 90 min of incubation at room temperature in 25 mM PIPES buffer, pH 7.40 containing (mM): 1,10-phenanthroline 1, dithiothreitol 1, bacitracin 0.014%, captopril 0.1, bovine serum albumine 0.2% (protease free), magnesium chloride 7.5 and [125I]-HPP-desArg10-Hoe140 150 pM, with 150 pM of unlabelled HPP-Hoe140, an antagonist of B2 receptor to prevent binding at B2 receptor. To determine the non-specific binding, 1 μM of unlabelled peptide HPP-desArg10-Hoe140 was included to the solution. After incubation, sections were rinsed in buffer (4°C) three times for 4 min each, and dipped for 15 s into distilled water (4°C) to remove the excess of salt and air-dried under cool air. The washed sections were exposed to [3H]-Hyperfilm at room temperature in the presence of [125I]-microscales for 3 days. The films were developed in D-19 (Kodak developer) and fixed in Kodak Ektaflo. Densitometric labellings were carried out with an image analysis system (MCIDTM, Imaging Research, ON, Canada). Standard curves from autoradiographic [125I]-microscales were used to convert density binding into fmol mg−1 of tissue. Specific binding was determined by subtracting non-specific labelling from total binding taken from adjacent sections.
Drugs
The following drugs were used: Streptozotocin (Zanosar) (Pharmacia & Upjohn, ON, Canada), heparin, Piperazine-N,N′-bis[2-ethanesulphonic-acid] (PIPES), 1,10-phenanthroline, dithiothreitol, bacitracin, captopril, carrageenan, PBS and bovine serum albumin (protease free) were all purchased from Sigma-Aldrich Canada Ltd. Des-Arg9-BK (B1 agonist) and des-Arg10-Hoe140 (B1 antagonist, Wirth et al., 1991) were from Bachem Bioscience Inc., PA, U.S.A. The B1 antagonist R-715 (AcLys[D-βNal7, Ile8]des-Arg9-BK) (Gobeil et al., 1996) and the B1 receptor radioligand HPP-desArg10-Hoe140 (3–4 hydroxyphenyl-propionyl-desArg9-D-Arg[Hyp3,Thi5,D-Tic7,Oic8]-BK) were synthesized in the laboratory of D. Regoli (Department of Pharmacology, Université de Sherbrooke, Sherbrooke, Canada). The B2 antagonist Hoe140 (D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]-BK) (Hock et al., 1991) was obtained from Aventis Pharma Deutschland GmbH. The nonpeptide B2 receptor antagonist NPC 18884 (Chakravarty et al., 1996; Saleh et al., 1998) was synthesized at and obtained from Scios Nova (Sunnyvale, CA, U.S.A.). The bovine insulin implant was purchased from LinShin Canada Inc. (Scarborough, ON, Canada). The adsorbed rabbit antiserum (Cat number AI-AD51140) directed against the rat polymorphonuclear (PMN) neutrophils was purchased from Accurate Chemical & Scientific Corp., NY, U.S.A. The Hema 3 Stain Set was purchased from Biochemical Sciences Inc. Stock solutions for all peptides were prepared in sterile saline. All drugs were maintained at −18°C, and diluted to the desired concentration just before use. Autoradiographic [125I]-microscales (20 μm) and [3H]-Hyperfilm (single coated, 24×30 cm) were purchased from Amersham Pharmacia Biotech, Canada.
Statistical analysis
Results are given as means±s.e.mean. The significance of differences between means was evaluated by Student's t-test for unpaired samples. For multiple comparisons to the same control group, significance was assessed by a one-way analysis of variance (ANOVA) followed by the test of Dunnett. P values less than 0.05 were considered to be statistically significant.
Results
Leukocyte infiltration induced by des-Arg9-BK in control and STZ-diabetic rats
The i.pl. injection of the selective B1 receptor agonist des-Arg9-BK (100 nmol per site) increased time-dependently leukocyte infiltration into the pleural cavity of STZ-pretreated rats (Figure 1). The polymorphonuclear neutrophil cell migration occurred at 4 h after des-Arg9-BK injection (P<0.001) and returned to normal values at 8–12 h. The neutrophil influx corresponds to about 20% of the total cell migration in response to des-Arg9-BK. The mononuclear cells (mainly macrophages and some lymphocytes) increased significantly 1 h after B1 agonist injection, peaked at 8 h and remained elevated for up to 24 h (P<0.001). Mononuclear cells migration was nearly back to control values 48 h after des-Arg9-BK injection. Whereas des-Arg9-BK (50 and 100 nmol per site) caused increases in neutrophil cell migration (P<0.001) at 4 h post-injection, the dose of 10 nmol was inactive when compared to saline values. On the other hand, the increase of mononuclear cells was maximal at 50 nmol and found significant at 10 nmol (Figure 2). Neutrophil and mononuclear cells migration induced by des-Arg9-BK (100 nmol per site) was also significantly increased in control rats, yet the effect was significantly smaller (P<0.01) than that induced by the B1 agonist in STZ-diabetic rats (Figure 3). Basal values of leukocytes measured in the thoracic cavity of animals not challenged with saline (resident cells) were 2.05±0.30×106 (mononuclear cells) and 0.0 (neutrophils) in control rats in comparison with 2.62±0.40×106 and 0.25±0.10×106 for mononuclear cells and neutrophils, respectively in STZ-treated rats. These values were not significantly different from those measured after i.pl. injection of saline (Figure 3). Thus basal values (saline) for mononuclear cells were similar in control and STZ-diabetic rats, but they were significantly higher for neutrophils in STZ-diabetic rats (P<0.01) (Figure 3).
Figure 1.
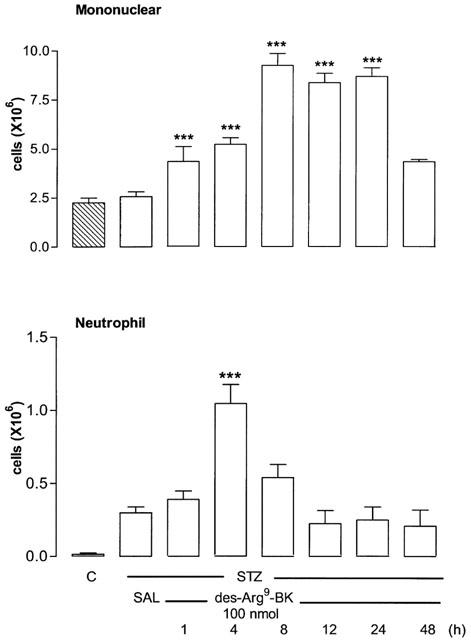
Time course profile of leukocyte migration (mononuclear and neutrophil cells) after intrapleural (i.pl.) injection of saline (SAL) or 100 nmol per site of des-Arg9-BK in streptozotocin (STZ)-treated rats. Control rats (C) received sterile saline instead of STZ. Shown are effects of the B1 agonist at different times post-injection. Data represent the means±s.e.mean of 6–10 rats for each column. Statistical comparison to STZ receiving saline was calculated with a one-way ANOVA with a post-hoc Dunnett test and is indicated by ***P<0.001.
Figure 2.
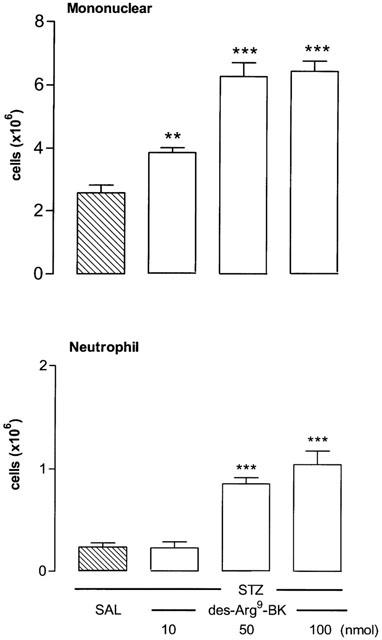
Dose-related increases of leukocyte migration (mononuclear and neutrophil cells) 4 h after the intrapleural (i.pl.) injection of des-Arg9-BK (10–100 nmol per site) in rats made diabetic with streptozotocin (STZ). Data represent the means±s.e.mean of 6–10 rats for each dose and saline (SAL). Statistical comparison to STZ receiving saline was calculated with a one-way ANOVA and a post-hoc Dunnett test and is indicated by **P<0.01; ***P<0.001.
Figure 3.
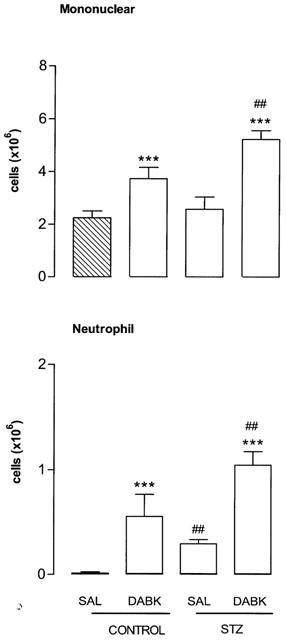
Comparison of the increases of leukocyte migration (mononuclear and neutrophil cells) 4 h after the intrapleural (i.pl.) injection of saline (SAL) or 100 nmol per site of des-Arg9-BK (DABK) in control rats and in rats made diabetic with streptozotocin (STZ). Data represent the means±s.e.mean of 6–10 rats in each group. Statistical comparison to the corresponding saline group (*) or between control and STZ-treated rats (#) was calculated with a Student's t-test for unpaired samples and is indicated by ##P<0.01, ***P<0.001.
The i.pl. injection of des-Arg9-BK increased fluid leakage into the pleural cavity of control rats (from 0 at 1 h to 100 μl±10 μl at 4 h, n=6; P<0.05) and STZ-diabetic rats (from 0 at 1 h to 200±20 μl at 4 h and 100±20 μl at 8 h, n=6; P<0.01). Whereas saline injection did not produce fluid leakage in control rats (n=6), saline injection caused an increase of 100±10 μl at 4 and 8 h post-injection in STZ-diabetic rats (n=6; P<0.05).
Effect of B1 and B2 antagonists on des-Arg9 BK-induced leukocyte migration
Leukocyte (mononuclear and neutrophil cells) migration induced by des-Arg9-BK (100 nmol per site) was significantly reduced by des-Arg10-Hoe140 (50 and/or 100 nmol per site, 5 min earlier) (Figure 4). R-715 (100 nmol) was more effective than des-Arg10-Hoe140 as it inhibited des-Arg9-BK-induced neutrophil infiltration to basal values. R-715 had no direct effect on leukocyte infiltration when compared to basal values in STZ-diabetic rats (Figure 4).
Figure 4.
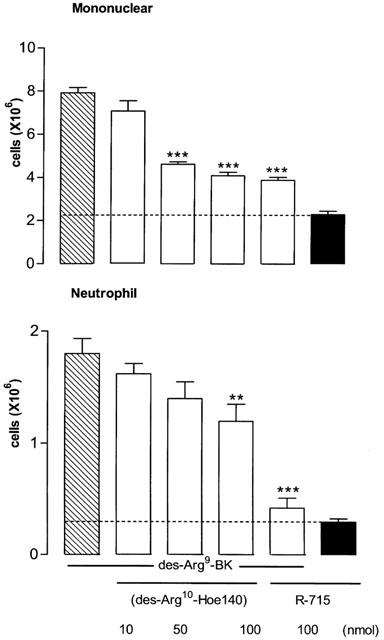
Increases of leukocyte migration (mononuclear and neutrophil cells) 4 h after the intrapleural (i.pl.) injection of 100 nmol per site of des-Arg9-bradykinin in rats made diabetic with streptozotocin (STZ). Shown are effects of the B1 agonist in the absence (hatched columns) and presence (open columns) of one of the two selective B1 receptor antagonists: des-Arg10-Hoe140 (10–100 nmol per site) and R715 (100 nmol per site). The direct effect of R-715 is represented by a black column. Data represent the means±s.e.mean of 6–10 rats in each group. Dotted lines represent basal values measured with saline. Statistical comparison to the agonist without antagonist was calculated with a one-way ANOVA and a post-hoc Dunnett test and is indicated by **P<0.01; ***P<0.001.
Two B2 receptor antagonists were also tested against the effects induced by des-Arg9-BK (100 nmol per site). While NPC18884 (100 nmol per site) did not modify the leukocyte response to the B1 agonist, Hoe140 (10 nmol per site) increased significantly both mononuclear and neutrophil cells infiltration induced by des-Arg9-BK (Figure 5). The enhancing effect of Hoe140 on mononuclear cells infiltration was not prevented by the co-injection of R-715 (100 nmol) while that of neutrophils was only slightly reduced. The baseline values measured with i.pl. injection of saline for both mononuclear and neutrophil cells measured in STZ-diabetic rats were significantly reduced by 10 nmol Hoe140 (P<0.001) (Figure 5).
Figure 5.
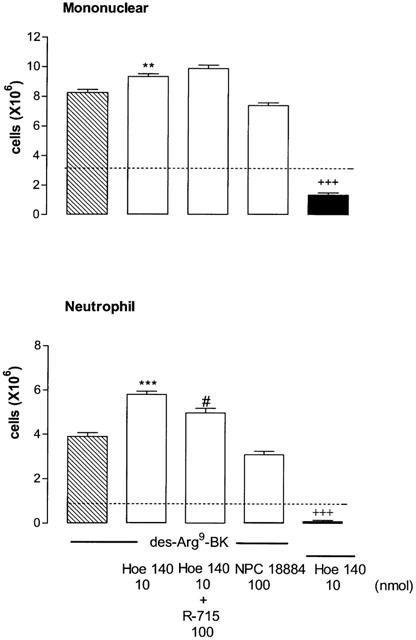
Increases of leukocyte migration (mononuclear and neutrophil cells) 4 h after the intrapleural (i.pl.) injection of 100 nmol per site of des-Arg9-BK in rats made diabetic with streptozotocin (STZ). Shown are effects of the B1 agonist in the absence (hatched columns) and presence (open columns) of one of the two selective B2 receptor antagonists: Hoe140 (10 nmol per site either alone or with R-715, 100 nmol per site) and NPC 18884 (100 nmol per site). The direct effect of Hoe140 is represented by a black column. Data represent the means±s.e.mean of 6–10 rats in each group. Dotted lines represent basal values measured with saline. Statistical comparison to saline (+), des-Arg9-BK (*) or to the agonist with Hoe140 (#) was calculated with a Student's t-test for unpaired samples and is indicated by #P<0.05; **P<0.01; +++***P<0.001.
Effect of insulin on des-Arg9-BK-induced leukocyte migration
Systemic treatment of diabetic rats for 4 days with insulin (2 U per day) prevented the hyperglycaemia caused by STZ (Table 1) and reduced significantly, although not completely, both mononuclear and neutrophil cells infiltration induced by des-Arg9-BK (100 nmol) in STZ-treated rats (Figure 6).
Table 1.
Glucose values (mmol l−1) in blood samples of streptozotocin (STZ)-diabetic rats without and with insulin (2 U per day) over 4 days

Figure 6.

Increases of leukocyte migration (mononuclear and neutrophil cells) 4 h after the intrapleural (i.pl.) injection of 100 nmol per site of des-Arg9-BK in rats made diabetic with streptozotocin (STZ). Shown are effects of the B1 agonist in the absence (hatched columns) and presence of insulin treatment (2 U per day ×4 days) (open columns) or anti-PMN (0.1 ml of a 1 : 20 dilution, i.pl. 5 min earlier) (black columns). Data represent the means±s.e.mean of 6–10 rats in each group. Dotted lines represent basal values measured with saline. Statistical comparison to des-Arg9-BK alone was calculated with a Student's t-test for unpaired samples and is indicated by ***P<0.001.
Effect of anti-PMN antibody on des-Arg9-BK-induced leukocyte migration
Pretreatment with an anti-PMN antibody (0.1 ml of a 1 : 20 dilution, i.pl. 5 min earlier) reduced significantly both mononuclear and neutrophil cells migration induced by des-Arg9-BK (100 nmol) in STZ-diabetic rats (Figure 6). The i.pl. treatment with the anti-PMN antibody was chosen because it was found more effective than the i.p. treatment to reduce neutrophils infiltration induced by carrageenan (1% i.pl.) in control rats. On the other hand, the injection of the anti-PMN antibody either i.p. or i.pl. increased significantly mononuclear cells infiltration caused by carrageenan (Figure 7).
Figure 7.
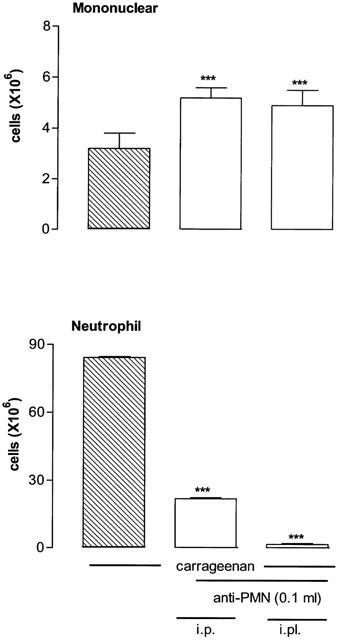
Increases of leukocyte migration (mononuclear and neutrophil cells) 4 h after the intrapleural (i.pl.) injection of 1% carrageenan in control rats. Shown are effects of carrageenan in the absence (hatched columns) and presence of anti-PMN (open columns) which was injected either i.p. (30 min earlier) or i.pl. (5 min earlier) with 0.1 ml of a 1 : 20 dilution. Data represent the means±s.e.mean of six rats in each group. Statistical comparison to carrageenan alone was calculated with a Student's t-test for unpaired samples and is indicated by ***P<0.001.
Specific kinin B1 receptor binding sites in the rat lungs
Figure 8 shows autoradiograms which permit to visualize the density of total and non-specific B1 receptor binding sites with [125I]-HPP-desArg10-Hoe140 on 40 μm thick sections of lungs from both control and STZ-diabetic rats receiving either saline (vehicle) or 100 nmol des-Arg9-BK. Specific B1 receptor binding sites were significantly increased after activation of the B1 receptor with des-Arg9-BK when compared with STZ-diabetic rats receiving saline (P<0.001) (Figure 9). Pretreatment with either insulin (2 U per day) or anti-PMN (0.1 ml of a 1 : 20 dilution, i.pl.) reduced significantly the density of specific B1 receptor binding sites in the lung of STZ-diabetic rats after challenge with 100 nmol des-Arg9-BK (Figures 8 and 9). In the lung of control rats, des-Arg9-BK (100 nmol) increased to a smaller extent specific B1 receptor binding sites (0.17±0.05 fmol mg−1 tissue, n=4) when compared with control receiving saline (0.12±0.09 fmol mg−1 tissue, n=4; P<0.01) (Figure 8).
Figure 8.
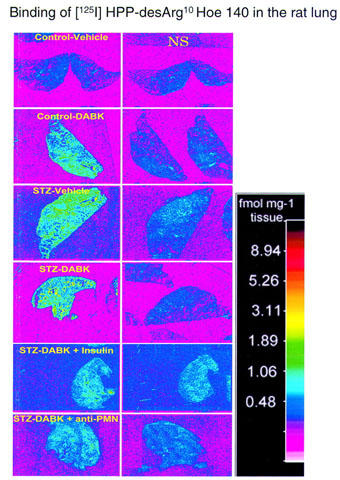
Autoradiograms of [125I]-HPP-desArg10-Hoe140 binding sites in lung sections taken from control rats or STZ-diabetic rats injected i.pl. 4 h earlier with vehicle or des-Arg9-BK (DABK) in the absence or presence of treatment with insulin (2 U per day ×4 days) or anti-PMN (0.1 ml of a 1 : 20 dilution, i.pl., 5 min earlier). Non-specific binding (NS) in the presence of 1 μM unlabelled radioligand is shown on the right panels. Note the high level of specific binding in lung sections of STZ-diabetic rats injected with des-Arg9-BK and its marked reduction under insulin or anti-PMN treatment.
Figure 9.
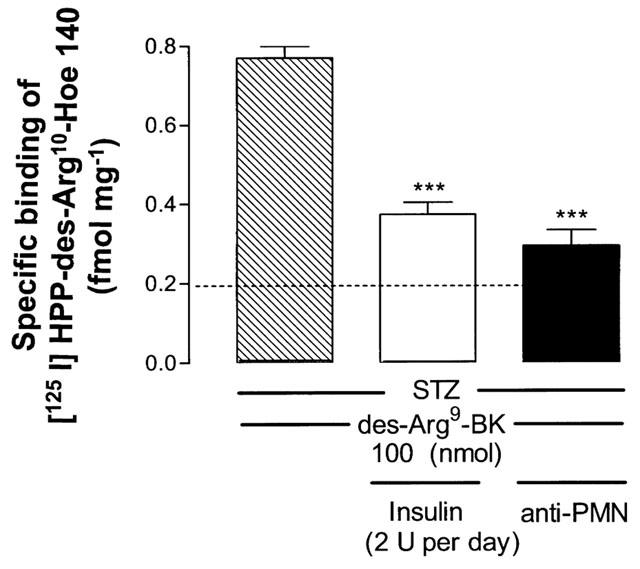
Quantification of specific [125I]-HPP-desArg10-Hoe140 binding sites in lung sections taken from STZ-diabetic rats injected 4 h earlier with des-Arg9-BK (100 nmol per site). Shown are the effects of insulin (2 U per day × 4 days) or anti-PMN (0.1 ml of a 1 : 20 dilution, i.pl., 5 min earlier) on des-Arg9-BK-induced increase of specific B1 receptor binding sites. Data represent the means ±s.e.mean of 120 lung slices per rat in each group of four animals. Statistical comparison to des-Arg9-BK without treatment was assessed by a one-way analysis of variance followed by a Dunnett test (***P<0.001).
Discussion
The i.pl. injection of the selective B1 agonist des-Arg9-BK promotes in a time- and dose-dependent manner the infiltration of leukocytes in the pleural cavity of the STZ-diabetic rat. This response is characterized by an influx of mononuclear and neutrophil cells that peaked at 8 and 4 h, respectively. Because the des-Arg9-BK-induced leukocyte migration into the pleural cavity of STZ-diabetic rats was reduced by i.pl. injection of selective B1 receptor antagonists, des-Arg10-Hoe140 and R-715, but not by the B2 receptor antagonists, Hoe140 and NPC 18884, it is suggested that the cellular response is mediated by B1 receptors. The B1 receptor antagonist R-715 was more effective than des-Arg10-Hoe 140 in inhibiting leukocyte infiltration which is consistent with its greater potency in vitro and resistance to degradation by peptidases (Regoli et al., 1998). The B2 receptor antagonist Hoe140 markedly enhanced des-Arg9-BK-induced neutrophil and mononuclear infiltration which confirms one of our earlier studies (Couture et al., 2001). This phenomenon did not occur with the selective non-peptide B2 receptor antagonist NPC 18884 at a dose that consistently antagonized BK-mediated effect in vivo (Saleh et al., 1998). Also, the potentiating effect of Hoe140 was still present under simultaneous blockade of B1 receptors with R-715, suggesting that it is mediated by other mechanisms not related to B2 and B1 receptor activation. Moreover, the enhancing effect of Hoe140 cannot be attributed to a residual agonist activity because the latter compound significantly reduced the basal values of leukocyte infiltration. The latter finding also suggests that B2 receptors (but not B1 receptors as R-715 had no effect per itself), which are likely activated by endogenous BK, are involved in the basal neutrophil response measured in STZ-diabetic rats. This could be pathologically relevant as higher circulating levels of high and low molecular weight kininogens and prekallikrein were found in STZ-diabetic rats, suggesting also that insulin could modulate BK release (Rothschild et al., 1999).
B1 receptor-mediated neutrophil recruitment was first demonstrated in dorsal air pouches of mice treated locally 4 h earlier with interleukinin-1β (Ahluwalia & Perretti, 1996). Leukocyte accumulation was partially inhibited by des-Arg9-Leu8-BK, but not by Hoe140. However, in normal mice, the intrapleural injection of B1 selective agonist without cytokine pre-treatment produced leukocyte accumulation, mainly neutrophils that peaked at 4 h, suggesting that the B1 receptor is constitutively expressed in this species (Vianna & Calixto., 1998). Surprisingly, the B1 receptor agonist also enhanced leukocyte infiltration in the pleural cavity of control rats, that may indicate that a basal expression of constitutive B1 receptors is present in rats as well. This hypothesis is strongly supported by the present autoradiographic study which reveals a significant increase in the number of B1 receptor binding sites in the lung of control rats which received an intrapleural injection of B1 agonist 4 h earlier. The neutralization of neutrophils with a specific anti-PMN antibody in STZ-treated rats led to a striking decrease of B1 receptor binding sites correlated with a diminished neutrophil infiltration into the pleural cavity of STZ-treated rats. Thus, it appears that most B1 receptors are located on neutrophils. Nevertheless, since the anti-PMN antibody failed to completely abolish the response of des-Arg9-BK on both neutrophil migration and receptor binding sites, and because the anti-PMN antibody abolished specifically the neutrophil infiltration induced by carrageenan, it is suggested that B1 receptors are also present on other elements such as endothelial cells, macrophages and primary sensory fibres as discussed earlier (Couture et al., 2001). For instance, the presence of B1 receptors was found on macrophages from several species (Böckmann & Paegelow, 2000) and on human lung fibroblasts (Schanstra et al., 1998; Koyama et al., 2000). Therefore, it is feasible that the release of cytokines from those cells could facilitate the further upregulation of B1 receptors on neutrophils and other target cells (Couture et al., 2001). The up-regulation of B1 receptors in STZ-diabetic rats was evidenced by a greater density of receptor binding sites in the absence and presence of B1 receptor agonist stimulation when compared with control rats.
Insulin treatment which normalized glycaemia blunted the leukocyte inflammatory response induced by des-Arg9-BK and caused a parallel reduction of B1 receptor binding sites in STZ-diabetic rats, supporting that B1 receptor induction is caused by the lack of insulin in these animals and the subsequent metabolic disturbances. The effect of insulin was more effective on neutrophil migration which was nearly reduced to control values. Thus hyperglycaemia is likely to contribute to the up-regulation of the B1 receptor in this model. Hyperglycaemia and the resulting oxidative stress can activate NF-κB (Yerneni et al., 1999), which is known to participate in the in vivo B1 receptor upregulation (Marceau et al., 1998; Campos et al., 1999; Cabrini et al., 2000). Therefore, both the overproduction of cytokines and hyperglycaemia could trigger the expression of B1 receptor through NF-κB-mediated mechanism confirming further this conclusion in diabetes. In the same STZ-animal model, des-Arg9-BK-induced mononuclear and neutrophil cells infiltration into the pleural cavity was significantly reduced by a systemic treatment with an NF-κB inhibitor (pyrrolidine-dithiocarbamate) (Couture et al., 2001).
Conclusion
Autoradiographic and in vivo studies suggest that the B1 receptor is upregulated in the lung of STZ-diabetic rats. Its activation leads to the migration of mononuclear and neutrophil cells into the pleural cavity. An over-expression of B1 receptor binding sites was found on neutrophils in STZ-diabetic rats. Experiments performed with insulin suggest that hyperglycaemia is a contributing factor involved in the up-regulation of neutrophil B1 receptors which is correlated with a greater neutrophil migration. Therefore, this study provides a useful model for investigating the role and expression of the B1 receptor in cellular inflammatory process.
Acknowledgments
This work was supported by a Grant-in-Aid from the Canadian Diabetes Association to R. Couture. Authors are thankful to Dr Denis deBlois (Department of Pharmacology, Université de Montréal) for the supply of Hoe140 and to Dr Gaétan Thibault (Clinical Research Institute of Montreal) for the iodination of the B1 radioligand. Brice Ongali holds a Studentship from the Republic of Gabon.
Abbreviations
- BK
bradykinin
- BSA
bovine serum albumin
- DTT
dithiothreitol
- i.pl.
intrapleural
- NF-κB
transcriptional nuclear factor kappa B
- PBS
phosphate buffered solution
- PIPES
piperazine-N,N′-bis[2-ethanesulphonic-acid]
- STZ
streptozotocin
References
- ABDOUH M., KHANJARI A., COUTURE R., REGOLI D., HASSÉSSIAN H.M.Vasodilator bradykinin receptor mechanisms in the retina of Wistar and streptozotocin treated diabetic rats International Symposium on Peptide Receptors, Montreal, Canada July 29–August 2 2001(Abstract P 70)
- AHLUWALIA A., PERRETTI M. Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1β in vivo in the mouse. J. Immunol. 1996;156:269–274. [PubMed] [Google Scholar]
- ARAUJO R.C., KETTRITZ R., FICHTNER I., PAIVA A.C.M., PESQUERO J.B., BADER M. Altered neutrophil homeostasis in kinin B1 receptor-deficient mice. Biol. Chem. 2001;382:91–95. doi: 10.1515/BC.2001.014. [DOI] [PubMed] [Google Scholar]
- BÖCKMANN S., PAEGELOW I. Kinins and kinin receptors: importance for the activation of leukocytes. J. Leukocyte Biol. 2000;68:587–592. [PubMed] [Google Scholar]
- CABRINI D.A., CAMPOS M.A., TRATSK K.S., MERINO V.F., SILVA J.A., JR, SOUZA G.E.P., AVELLAR M.C.W., PESQUERO J.B., CALIXTO J.B. Molecular and pharmacological evidence for modulation of kinin B1 receptor expression by endogenous glucocorticoids hormones in rats. Br. J. Pharmacol. 2000;132:567–577. doi: 10.1038/sj.bjp.0703846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., CABRINI D.A., CARDOZO A.H.M., RAE R.A., HUIDOBRO TORO J.P., CALIXTO J.B. Changes in paw oedema triggered via bradykinin B1 and B2 receptors in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2001;416:169–177. doi: 10.1016/s0014-2999(01)00883-4. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., CALIXTO J.B. In vivo B1 kinin-receptor upregulation. Evidence for involvement of protein kinases and nuclear factor-κB pathways. Br. J. Pharmacol. 1999;127:1851–1859. doi: 10.1038/sj.bjp.0702715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAKRAVARTY S., MAVUNKEL B.J., GOEHRING R.R., KYLE D.J. Novel bradykinin receptor antagonists from a structurally directed non-peptide combinatorial library. Immunopharmacology. 1996;33:61–67. doi: 10.1016/0162-3109(96)00057-4. [DOI] [PubMed] [Google Scholar]
- CLOUTIER F., COUTURE R. Pharmacological characterization of the cardiovascular responses elicited by kinin B1 and B2 receptor agonists in the spinal cord of streptozotocin-diabetic rats. Br. J. Pharmacol. 2000;130:375–385. doi: 10.1038/sj.bjp.0703319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOUTIER F., DE SOUSA BUCK H., ONGALI B., COUTURE R. Pharmacologic and autoradiographic evidence for an up-regulation of kinin B2 receptors in the spinal cord of spontaneously hypertensive rats. Br. J. Pharmacol. 2002;135:1641–1654. doi: 10.1038/sj.bjp.0704632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUTURE R., HARRISSON M., VIANNA R.M., CLOUTIER F. Kinin receptors in pain and inflammation. Eur. J. Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- DÉCARIE A., ADAM A., COUTURE R. Effects of captopril and icatibant on bradykinin (BK) and [des-Arg9]-BK in carrageenan-induced edema. Peptides. 1996;17:1009–1015. doi: 10.1016/0196-9781(96)00145-3. [DOI] [PubMed] [Google Scholar]
- GOBEIL F., NEUGEBAUER W., FILTEAU C., JUKIC D., NSA ALLOGHO S., PHENG L.H., NGUYEN-LE X.K., BLOUIN D., REGOLI D. Structure-activity studies of B1 receptor-related peptides. Antagonists. Hypertension. 1996;28:833–839. doi: 10.1161/01.hyp.28.5.833. [DOI] [PubMed] [Google Scholar]
- HUNTER W.M., GREENWOOD F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- HOCK F.J., WIRTH K., ALBUS U., LINZ W., GERHARDS H.J., WIEMER G., HENKE S., BREIPOHL G., KÖNIG W., KNOLLE J., SCHÖLKENS B.A. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br. J. Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOYAMA S., SATO E., NUMANAMI H., KUBO K., NAGAI S., IZUMI T. Bradykinin stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activity. Am. J. Respir. Cell Mol., Biol. 2000;22:75–84. doi: 10.1165/ajrcmb.22.1.3752. [DOI] [PubMed] [Google Scholar]
- LAMONTAGNE D., NAKHOSTINE N., COUTURE R., NADEAU R. Mechanisms of kinin B1-receptor-induced hypotension in the anesthetized dog. J. Cardiovasc. Pharmacol. 1996;28:645–650. doi: 10.1097/00005344-199611000-00006. [DOI] [PubMed] [Google Scholar]
- MAGE M., PÉCHER C., NEAU E., CELLIER E., DOS REISS M.L., SCHANSTRA J.P., COUTURE R., BASCANDS J.-L., GIROLAMI J.-P. Induction of B1 receptors in streptozotocin diabetic rats: possible involvement in the control of hyperglycemia-induced glomerular Erk 1 and 2 phosphorylation. Can. J. Physiol. Pharmacol. 2002;80:328–333. doi: 10.1139/y02-024. [DOI] [PubMed] [Google Scholar]
- MARCEAU F. Kinin B1 receptors: review. Immunopharmacology. 1995;30:1–26. doi: 10.1016/0162-3109(95)00011-h. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MASS J., RAE A.G., HUIDOBRO-TORO J., CALIXTO J.B. Characterization of kinin receptors modulating neurogenic contractions of the mouse isolated vas deferens. Br. J. Pharmacol. 1995;114:1471–1477. doi: 10.1111/j.1476-5381.1995.tb13372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLEAN P.G., AHLUWALIA A., PERRETTI M. Association between kinin B1 receptor expression and leukocyte trafficking across mouse mesenteric postcapillary venules. J. Exp. Med. 2000;192:367–380. doi: 10.1084/jem.192.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKHOSTINE N., RIBUOT C., LAMONTAGNE D., NADEAU R., COUTURE R. Mediation by B1 and B2 receptors of vasodepressor responses to intravenously administered kinins in anaesthetized dogs. Br. J. Pharmacol. 1993;110:71–76. doi: 10.1111/j.1476-5381.1993.tb13773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAVARRO M., FRONTERA M., ZUCCOLLO A., CATANZARO O.Nitric oxide (NO) changes accompanying the progression from early insulitis to diabetes International Symposium on Peptide Receptors, Montreal, Canada, July 29–August 2 2001(Abstract P 66)
- NSA ALLOGHO S., GOBEIL F., PHENG L.H., NGUYEN-LE X.K., NEUGEBAUER W., REGOLI D. Kinin B1 and B2 receptors in the mouse. Can. J. Physiol. Pharmacol. 1995;73:1759–1764. doi: 10.1139/y95-240. [DOI] [PubMed] [Google Scholar]
- NSA ALLOGHO S., GOBEIL F., PERRON S.I., HESS J.F., REGOLI D. Effects of kinins on isolated stomachs of control and transgenic knockout B2 receptor mice. Naunyn-Schm. Arch Pharmacol. 1998;357:191–196. doi: 10.1007/pl00005157. [DOI] [PubMed] [Google Scholar]
- RAYMOND P., DRAPEAU G., RAUT R., AUDET R., MARCEAU F., ONG H., ADAM A. Quantification of des-Arg9-bradykinin using a chemiluminescence enzyme immunoassay: application to its kinetic profile during plasma activation. J. Immunol. Methods. 1995;180:247–257. doi: 10.1016/0022-1759(94)00320-v. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- REGOLI D., NSA ALLOGHO S., RIZZI A., GOBEIL F., JR Bradykinin receptors and their antagonists. A review. Eur. J. Pharmacol. 1998;348:1–10. doi: 10.1016/s0014-2999(98)00165-4. [DOI] [PubMed] [Google Scholar]
- ROTHSCHILD A.M., MELO V.L., REIS M.L., FOSS M.C., GALLO L., JR Kininogen and prekallikrein increases in the blood of streptozotocin-diabetic rats are normalized by insulin in vivo and in vitro. Naunyn-Schm. Arch. Pharmacol. 1999;360:217–220. doi: 10.1007/s002109900068. [DOI] [PubMed] [Google Scholar]
- SALEH T.S.F., VIANNA R.M.J., CRECZYNSKI-PASA T.B., CHAKRAVARTY S., MAVUNKEL B.J., KYLE D.J., CALIXTO J.B. Oral anti-inflammatory action of NPC 18884, a novel bradykinin B2 receptor antagonist. Eur. J. Pharmacol. 1998;363:179–187. doi: 10.1016/s0014-2999(98)00778-x. [DOI] [PubMed] [Google Scholar]
- SCHANSTRA J.P., BATAILLÉ E., MARIN CASTAÑO M.E., BARASCUD Y., HIRTZ C., PESQUERO J.B., PECHER C., GAUTHIER F., GIROLAMI J.P., BASCANDS J.L. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-κB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J. Clin. Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIANNA R.M.J., CALIXTO. J.B. Characterization of the receptor and the mechanisms underlying the inflammatory response induced by des-Arg9-BK in mouse pleurisy. Br. J. Pharmacol. 1998;123:281–291. doi: 10.1038/sj.bjp.0701590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIRTH K., BREIPOHL G., STECHL J., KNOLLE J., HENKE S., SCHÖLKENS B.A. Des-Arg9-D-Arg[Hyp3, Thi5, D-Tic7, Oic8]-bradykinin (des-Arg10-[Hoe140]) is a potent bradykinin B1 receptor antagonist. Eur. J. Pharmacol. 1991;205:217–218. doi: 10.1016/0014-2999(91)90824-a. [DOI] [PubMed] [Google Scholar]
- YERNENI K.K.V., BAI W., KHAN B.V., MEDFORD R.M., NATARAJAN R. Hyperglycemia-induced activation of nuclear transcription factor kappa B in vascular smooth muscle cells. Diabetes. 1999;48:855–864. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]


