Abstract
Accumulation of inositol (poly)phosphates (InsPx) has been studied in rat hepatocytes labelled with [3H]inositol. Stimulation with ADP resulted in a significant increase in total [3H]InsPx, whereas 2-MeSADP had only a small effect and ADPβS was ineffective. UTP and ITP also stimulated substantial increases in [3H]InsPx.
The dose–response curve to ADP was largely unaltered by the presence of the P2Y1 antagonist, adenosine-3′-phosphate-5′-phosphate (A3P5P). Similarly, inclusion of MRS 2179, a more selective P2Y1 antagonist, had no effect on the dose–response curve to ADP.
The inclusion of hexokinase in the assay reduced, but did not abolish, the response to ADP.
HPLC analysis revealed that ADP in the medium was rapidly converted to AMP and ATP. The inclusion of hexokinase removed ATP, but exacerbated the decline in ADP concentration, leading to increased levels of AMP. 2-MeSADP was stable in the medium and ATP was largely unaffected.
The addition of the adenylate kinase inhibitor, diadenosine pentaphosphate (Ap5A) significantly reduced the ADP response. HPLC analysis conducted in parallel demonstrated that this treatment inhibited conversion of ADP to ATP and AMP.
Inclusion of the P1 antagonist CGS 15943 had no effect on the dose–response curve to ADP.
These observations indicate that hepatocytes respond to ADP with an increase in inositol (poly)phosphates following conversion to ATP. P2Y1 activation in hepatocytes does not appear to be coupled to inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) production.
Keywords: Rat hepatocytes, P2Y receptors, inositol (poly)phosphates, HPLC, nucleotide interconversion, ADP, 2-methylthioADP, adenylate kinase
Introduction
P2Y receptors comprise a family of G-protein-coupled receptors responding to the native nucleotides ADP, ATP, UDP and UTP (Burnstock, 1996). In common with many other cell types rat hepatocytes co-express multiple P2Y subtypes, although these have not been conclusively identified (Dixon et al., 1990; 2000; Keppens, 1993). Reverse transcriptase-polymerase chain reaction (RT–PCR) has demonstrated the presence in primary rat hepatocytes of mRNA transcripts encoding all four of the cloned rat P2Y subtypes investigated (P2Y1, P2Y2, P2Y4, P2Y6; Dixon et al., 2000). However, P2Y6 receptors appear not to be expressed, as UDP (the most potent agonist at this receptor subtype; Nicholas et al., 1996) had no effect on intracellular free Ca2+ concentration ([Ca2+]i) in rat hepatocytes (Dixon et al., 2000). Similarities in the responses to ATP and UTP have indicated that these nucleotides act at a common receptor (Dixon et al., 2000; Keppens et al., 1992). On the basis of sensitivity of the responses to suramin, we have argued for expression of P2Y2 and not P2Y4 receptors (Dixon et al., 2000); rat P2Y2 receptors are inhibited by suramin (Chen et al., 1996) whereas rat P2Y4 receptors are unaffected (Bogdanov et al., 1998). Of the more recently identified members of the P2Y family (P2Y11-13), the rat homologue of P2Y12 has been cloned but its presence not determined in hepatocytes. Rat P2Y11 and P2Y13 receptor clones have not yet been reported.
In single perfused rat hepatocytes, the transients in [Ca2+]i generated in response to ADP, 2-methylthioADP (2-MeSADP) and 2-methylthioATP (2-MeSATP) are indistinguishable, indicating a common receptor (Dixon, 2000; Dixon et al., 1995). The recently cloned canine P2Y11 receptor is activated by these agonists (Qi et al., 2001; Torres et al., 2002; Zambon et al., 2001). Studies of P2Y11 receptors show a species difference; the human homologue is activated most effectively by triphosphates (Communi et al., 1997) whereas the canine receptor responds to nucleotide diphosphates (Qi et al., 2001; Torres et al., 2002; Zambon et al., 2001). As the rat P2Y11 receptor has yet to be cloned its agonist profile is not known. It must, however, be considered as a possible mediator of the effects of 2-MeSADP, 2-MeSATP and ADP. Functional data, however, suggest that these responses are mediated by P2Y1 receptors (Dixon et al., 1995; 2000; Dixon, 2000). Thus the [Ca2+]i responses of single cells to both 2-MeSADP and 2-MeSATP were blocked by the P2Y1 antagonists, PPADS (Dixon et al., 2000) and adenosine-3′-phosphate-5′-phosphate (A3P5P; Dixon, 2000). Surprisingly, the [Ca2+]i response to 2-MeSATP in rat hepatocytes occurred in the absence of detectable increases in inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) (Keppens & De Wulf, 1991). This was in contrast to ADP which produced a substantial Ins(1,4,5)P3 response (Keppens & De Wulf, 1995). These observations are difficult to reconcile: while ADP, 2-MeSADP and 2-MeSATP are believed to act on a common P2Y1 receptor, only ADP stimulates PLC. However, in the earlier Ins(1,4,5)P3 experiments, consideration was not given to the possible effects of agonist interconversion in the recorded responses.
Here we have investigated the accumulation of total [3H]-inositol (poly)phosphates ([3H]-InsPx) in response to the P2Y1 agonists, ADP, 2-MeSADP and ADPβS, and have studied the effects of the P2Y1 antagonist, A3P5P (Boyer et al., 1996) and its highly selective derivative, MRS 2179 (Boyer et al., 1998). The effects of ATP, UTP and ITP are also reported. In parallel experiments HPLC analysis revealed that the response to ADP was likely to be secondary to its conversion to AMP and ATP through the action of an ecto-adenylate kinase. Consistent with this, inclusion of the adenylate kinase inhibitor, Ap5A (Lienhard & Secemski, 1973) greatly reduced the effect of ADP.
Methods
Cell preparation and culture
Hepatocytes were isolated from fed, male Wistar-strain rats (150–250 g) by collagenase perfusion as described previously (Dixon et al., 1995). Briefly, the rat was killed by cervical dislocation in accordance with institutional guidelines, and the hepatic portal vein cannulated. An initial Ca2+-free perfusion was followed by perfusion with collagenase (0.04% w v−1) and Ca2+ (3.8 mM) for 15 min. The perfusion rate was 30 ml min−1 throughout. The cells were harvested and cultured for 24 h at a density of 1×105 cells well−1 in 24-well plates in William's medium E with 10% FCS, 50 iu ml−1 penicillin, 50 μg ml−1 streptomycin and 50 μg ml−1 gentamicin.
Total [3H]-inositol (poly)phosphates
Cells were labelled for 48 h at 37°C in 5% CO2 with myo-[2-3H]inositol (0.037 Mbq ml−1, 0.5 ml well−1) in Medium M199 with the same additions of antibiotics. Twenty min stimulations, in the presence of 10 mM LiCl, were made without change of medium. In experiments with the P2Y1 antagonists, A3P5P and MRS 2179 (100 μM), the P1 antagonist, CGS 15943 (1 μM), and the adenylate kinase inhibitor, Ap5A (300 μM), cells were pre-incubated for 10 min prior to agonist addition. Hexokinase was included at 3 units well−1 where indicated. Total [3H]-InsPx were extracted on small Dowex-1 (Cl−) columns.
HPLC analysis
In parallel experiments medium was collected from above cells in 24-well plates following the 20 min stimulation period under identical experimental conditions as the [3H]-InsPx assay. HPLC analysis of the nucleotide content was conducted to determine the extent of agonist interconversion. Nucleotides were identified by co-chromatography.
Statistical analysis
Data presented in figures are representative of three experiments (mean±s.e.mean) from different hepatocyte preparations and expressed as d.p.m. after subtraction of controls. Data pooled across experiments are presented in the text; statistical analysis was by two-way ANOVA with Bonferroni's post-test. Since the dose–response curves did not saturate, true EC50 values could not be reported. Instead, we report apparent EC50 values, calculated as the concentration of agonist which generates 50% of the response to the maximal concentration used.
Materials
Cell culture medium was from GIBCO BRL (Paisley, U.K.). Myo-[2-3H]inositol was purchased from Amersham Pharmacia Biotech (Little Chalfont, Bucks, U.K.). Collagenase was from Boehringer (Lewes, U.K.). Hexokinase, nucleotides, A3P5P, MRS 2179, Ap5A, CGS 15943 and all other chemicals were from Sigma-Aldrich (Poole, U.K.). 10 mM stock solutions of ADP, ADPβS and Ap5A were incubated for 1 h with 80 units ml−1 hexokinase and 20 mM glucose (ADP) and 20 units ml−1 grade VII apyrase (ADPβS and Ap5A) and HPLC analysis used to confirm purity. The apyrase-treated Ap5A stock was then heated to 100°C for 10 min to remove apyrase activity. Samples of ADP and ATP (300 μM) were incubated for 20 min at 37°C with 1 mM heat-treated Ap5A, and HPLC analysis used to ensure that the apyrase activity had been destroyed.
Results
Stimulation of [3H]-inositol-labelled hepatocytes with ADP or ATP led to a concentration-dependent accumulation of [3H]-InsPx. Figure 1 shows that at the same concentration the response to ADP was significantly smaller than the response to ATP (data pooled across three experiments, P<0.05 by two-way ANOVA). At the maximal concentration used (300 μM) the ADP response was 76.7±7.3% (n=3 separate experiments) of the ATP response (P<0.05, Bonferroni's post-test). Whilst potencies cannot be determined for responses that do not form a plateau, the apparent EC50 values, assuming that 300 μM agonist gave a maximal response, were ATP, 21 μM (log EC50=−4.7±0.1) and ADP, 63 μM (log EC50=−4.3±0.2). These apparent EC50 values for ATP and ADP were not significantly different. In contrast to the effect of ADP, 2-MeSADP yielded only a small [3H]-InsPx response as shown in Figure 1 (16.7±3.2% of the ATP response at 300 μM; n=3). ADPβS treated with apyrase to remove contaminating ADP and ATP, was ineffective (6.5±2.8% of the ATP response at 300 μM; n=3; Figure 1).
Figure 1.
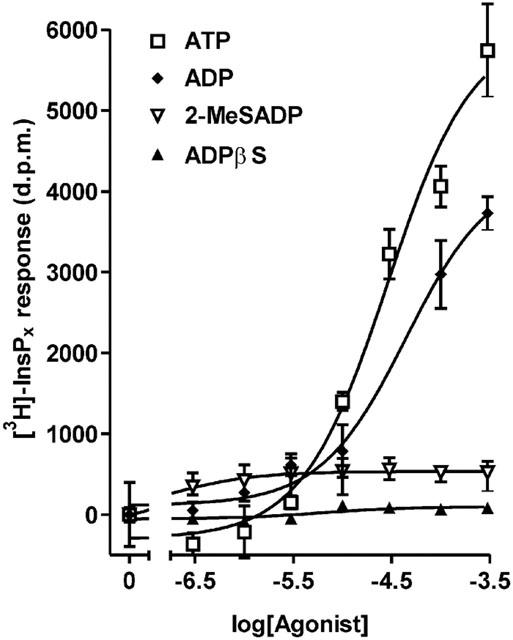
Effect of ADP, ATP, ADPβS and 2-MeSADP on inositol (poly)phosphate accumulation in rat hepatocytes. Total [3H]InsPx accumulation was measured in [3H]inositol-labelled cells in response to incubation for 20 min with increasing concentrations of agonist. Results, shown as d.p.m. after subtraction of unstimulated controls, are from a single representative experiment, with stimulations performed in triplicate. Data pooled from three separate experiments are reported in the text.
As shown in Figure 2, substantial increases in [3H]-InsPx were measured in response to UTP and ITP. The response to the maximal UTP concentration was indistinguishable from that to ATP, pooled across three separate experiments. However, the response to ITP was significantly different from ATP (P<0.001 by two-way ANOVA of pooled data); 300 μM ITP yielded 83.7±8.5% (n=3) of the response to 300 μM ATP. The apparent EC50 values for ATP and ITP were not significantly different; ATP, 15 μM (log EC50=−4.9±0.1) and ITP, 60 μM (log EC50=−4.3±0.2).
Figure 2.
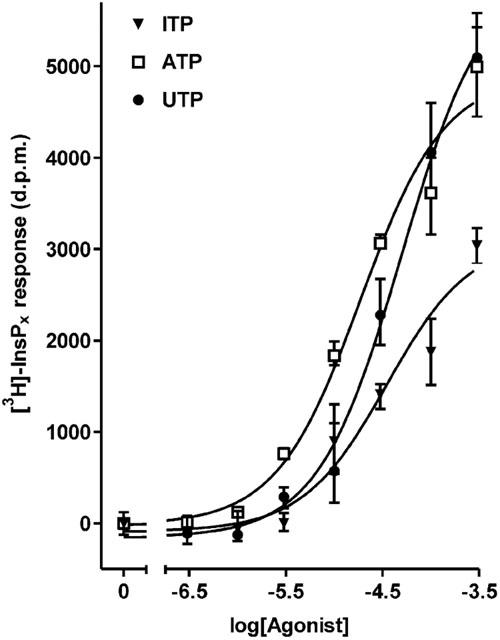
Effect of ATP, UTP and ITP on inositol (poly)phosphate accumulation in rat hepatocytes. Total [3H]InsPx accumulation was measured in [3H]inositol-labelled cells in response to incubation for 20 min with increasing concentrations of agonist. Results, shown as d.p.m. after subtraction of unstimulated controls, are from a single representative experiment, with stimulations performed in triplicate. Data pooled from three separate experiments are reported in the text.
To investigate whether the [3H]-InsPx effect of ADP was mediated by P2Y1 receptors, the antagonists A3P5P (Boyer et al., 1996) and MRS 2179 (Boyer et al., 1998) were used. The inclusion of 100 μM A3P5P led to a slight reduction in the effect of ADP at concentrations above 10 μM, as illustrated in Figure 3a. At the maximal concentration of ADP the response in the presence of A3P5P was reduced by 17.3±2.5% (P<0.05, data pooled across three experiments). As shown in Figure 3b, the inclusion of MRS 2179 had no effect on the concentration-response curve to ADP (the response to 300 μM ADP was reduced by 4.8±7.3%, n=3).
Figure 3.
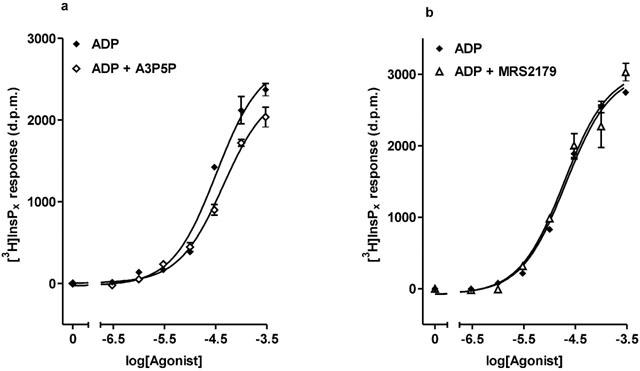
Effects of the P2Y1 antagonists, A3P5P and MRS 2179 on the inositol (poly)phosphate response to ADP. Dose–response curves to increasing concentrations of ADP were constructed in the presence and absence of 100 μM A3P5P (a) or 100 μM MRS 2179 (b). Results, shown as d.p.m. after subtraction of unstimulated controls, are from a single representative experiment, with stimulations performed in triplicate. Data pooled from three separate experiments are reported in the text.
To explore the possibility that the ADP effect was due to conversion to ATP in the extracellular compartment, experiments with hexokinase were conducted. ADP stock was treated with hexokinase to eliminate contaminating ATP, and an additional 3 units of enzyme were included in each well to prevent conversion of ADP to ATP during the stimulation period. The effect was to significantly reduce the effectiveness of ADP (Figure 4); over three experiments the response to 100 μM ADP was reduced by 55.7±9.8% (P<0.001, Bonferroni's post-test). This result indicated that interconversion of ADP to ATP was contributing to the observed ADP response. HPLC analysis was performed to further elucidate the changes occurring in the nucleotide composition of the medium. UTP, ITP and 2-MeSADP were all found to be stable for the 20 min stimulation period. The relative amounts of nucleotides recovered after incubating cells for 20 min with 300 μM ATP are shown in Table 1. The ATP stock was determined to be essentially free of contaminants. The stock ADP solution used was found to be contaminated with 3% ATP and 11% AMP (no adenosine was detected). As shown in Table 1 ADP was actively converted to AMP and ATP during the assay period. The inclusion of hexokinase in the incubation served to exacerbate the decline in ADP concentration, with a coincident decrease in ATP levels as expected. The overall effect was to channel ADP into AMP (Table 1).
Figure 4.
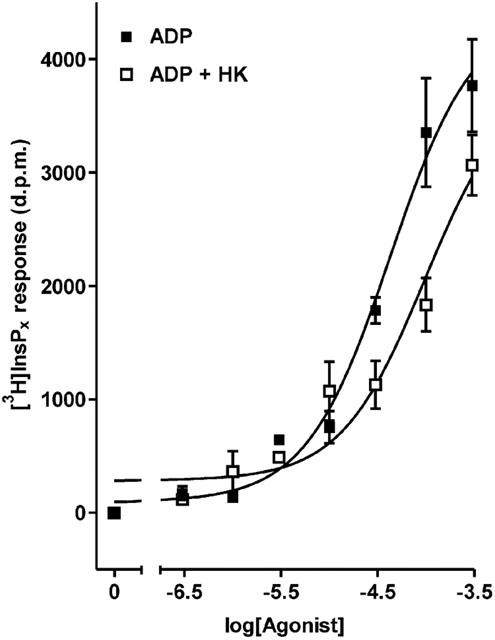
Effect of hexokinase-treatment on the inositol (poly)phosphate response to ADP. Dose–response curves to increasing concentrations of untreated, and hexokinase-treated ADP. Hexokinase was also included in the assay (3 units well−1) to counteract conversion of ADP to ATP during the 20 min stimulation period. Results, shown as d.p.m. after subtraction of unstimulated controls, are from a single representative experiment, with stimulations performed in triplicate. Data pooled from three separate experiments are reported in the text.
Table 1.
Conversion of ADP and ATP by cultured hepatocytes
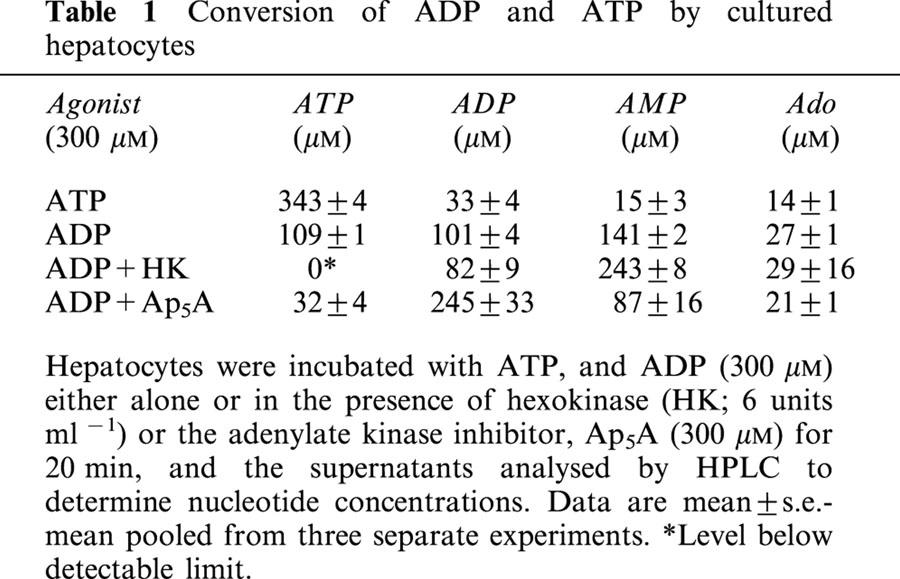
The enzyme adenylate kinase catalyses the reaction 2.ADP↔AMP+ATP and could account for the observed interconversions. We therefore conducted experiments with the adenylate kinase inhibitor, Ap5A (Lienhard & Secemski, 1973) to examine the contribution of this enzyme to ADP-stimulated [3H]-InsPx accumulation. As shown in Table 1, incubating cells with 300 μM ADP in the presence of a maximally effective concentration of Ap5A (300 μM), largely inhibited the interconversion of ADP. Although some ATP and AMP were still present, increasing the Ap5A concentration to 1 mM did not further protect ADP concentrations. ADP-stimulated accumulation of [3H]-InsPx was significantly reduced by inclusion of 300 μM Ap5A as shown in Figure 5. At concentrations of ADP below 30 μM, Ap5A effectively blocked the accumulation of [3H]-InsPx. At higher concentrations the response was reduced (at 100 μM ADP by 69.5±4.9% and 300 μM by 32.8±9.2%; n=3).
Figure 5.
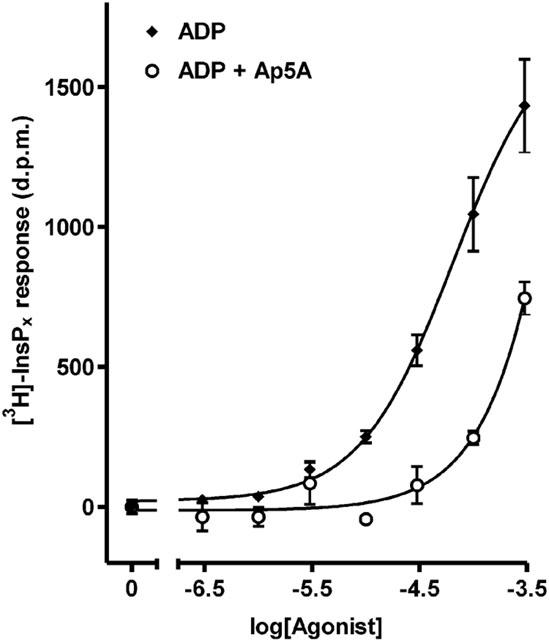
Effect of the adenylate kinase inhibitor, Ap5A on the inositol (poly)phosphate response to ADP. Dose–response curves to increasing concentrations of ADP were constructed in the presence and absence of 300 μM Ap5A. Results, shown as d.p.m. after subtraction of unstimulated controls, are from a single representative experiment, with stimulations performed in triplicate. Data pooled from three separate experiments are reported in the text.
The adenylate kinase-catalysed breakdown of ADP produces AMP in addition to ATP (Table 1). We therefore investigated the contribution of AMP to the ADP response through use of a P1 antagonist. The inclusion of the non-selective P1 antagonist, CGS 15943 (1 μM; Alexander et al., 1996) in the assay led to a small, although not significant, increase in the response at 300 μM ADP (111.1±15%, P>0.05, n=3; data not shown). The apparent EC50 of ADP was not affected by the presence of CGS 15943 (logEC50 for ADP, −4.5±0.3; logEC50 for ADP+1 μM CGS 15943, 4.4±0.2).
Discussion
Rat hepatocytes are known to express multiple P2Y receptors mediating the effects of extracellular nucleotides (Dixon et al., 1990; 2000; Keppens, 1993). In a previous report we concluded that rat hepatocytes express both P2Y1 and P2Y2 receptors, and that these receptors mediate the effects of ADP and ATP/UTP respectively (Dixon et al., 2000). In the absence of selective antagonists, the conclusion that functional P2Y2 receptors are expressed was based upon similarities in [Ca2+]i transients induced by ATP and UTP (Dixon et al., 2000; Keppens et al., 1992), and on the sensitivity of the responses to suramin (Dixon et al., 2000). The similarity in the inositol phosphate responses reported here to UTP and ATP is consistent with the expression of P2Y2 receptors, at which ATP and UTP are equipotent. However, rat P2Y4 receptors are also activated equally by ATP and UTP (Bogdanov et al., 1998; Webb et al., 1998), unlike the human homologue which displays a marked preference for UTP (Communi et al., 1995; Nguyen et al., 1995). The reduced response to ITP reported here compared with ATP and UTP argues against the sole involvement of P2Y4 receptors, as all three of these nucleotides are full and equipotent agonists at this receptor (Bogdanov et al., 1998). The results presented here then are consistent with the earlier conclusion that P2Y2 receptors mediate the effects of UTP and ATP in rat hepatocytes, although a contribution to the ATP/UTP response by the P2Y4 receptor cannot be completely discounted. The definitive test of these conclusions awaits the advent of selective ligands/antagonists for P2Y2 and P2Y4 receptors.
2-MeSADP stimulates a [Ca2+]i response in rat hepatocytes which is blocked by the P2Y1 antagonist, A3P5P (Dixon, 2000). However, as demonstrated here this nucleotide evokes only a small rise in InsPx. Similarly, ADPβS, a full agonist at the human P2Y1 receptor (Leon et al., 1997), failed to evoke a [3H]-InsPx response but does elicit a rise in [Ca2+]i in rat hepatocytes (Keppens et al., 1993). 2-MeSATP also stimulated [Ca2+]i transients which are inhibited by A3P5P (Dixon, 2000), yet this nucleotide failed to increase Ins(1,4,5)P3 levels (Keppens & De Wulf, 1995). The rat hepatocyte P2Y1 receptor then appears not to be coupled to increases in Ins(1,4,5)P3. In contrast, the cloned rat P2Y1 receptor has been shown to couple to inositol phosphate accumulation when transfected into 1321N1 human astrocytoma cells and C6 rat glioma cells (Schachter et al., 1997). The reason for this disparity is not clear; it may reflect differences in cloned and native systems, in terms of receptor number or signal transduction systems. Inositol phosphate-independent increases in [Ca2+]i have also been noted in rat brain capillary endothelial cells in response to ADP and 2-MeSATP (Frelin et al., 1993; Vigne et al., 1994; Albert et al., 1997).
Previous work has indicated that ADP acts at the same receptor as 2-MeSADP on rat hepatocytes (Dixon, 2000). Therefore the observation that ADP stimulation led to [3H]-InsPx accumulation, whilst 2-MeSADP evoked only a small response was surprising. The P2Y1 receptor antagonist, A3P5P had little effect on the dose-response curve to ADP. The reported pKB for this antagonist at the human P2Y1 receptor is ∼6 (Boyer et al., 1996). Therefore the minimal shift in the ADP curve seen here in the presence of 100 μM A3P5P is inconsistent with the major part of the [3H]InsPx response being mediated by P2Y1 receptors. Furthermore the highly selective P2Y1 receptor antagonist, MRS 2179 (pKB ∼7; Boyer et al., 1998) had no effect on the ADP-mediated [3H]-InsPx accumulation. It appears from these results that the response to ADP is not mediated by P2Y1 receptors.
HPLC analysis revealed that ADP in the medium was actively converted to AMP and ATP so that similar amounts of all three nucleotides were recovered following the 20 min stimulation period. Adenylate kinase catalyzes the reaction 2.ADP↔ATP+AMP and could therefore account for the observed nucleotide conversions. This enzyme is inhibited by Ap5A (Lienhard & Secemski, 1973) and consistent with its presence on rat hepatocytes, Ap5A protected ADP from conversion to AMP and ATP. (Following 20 min stimulation with 300 μM ADP ∼65% was preserved, compared with 27% in the absence of Ap5A.) The accumulation of [3H]-InsPx measured in response to ADP was therefore likely to be dependent upon its conversion to AMP and ATP. Inclusion of the non-selective P1 antagonist, CGS 15943 had no effect on ADP-stimulated [3H]-InsPx accumulation. This indicates that AMP formed through the action of adenylate kinase does not contribute to the ADP response. However, consistent with the proposal that the ADP response is dependent on the action of adenylate kinase, there was a coincident decrease in [3H]-InsPx accumulation in response to ADP when Ap5A was included in the assay. At less than 30 μM ADP, the response was effectively blocked by Ap5A. The residual response to ADP at concentrations over 30 μM in the presence of Ap5A can be accounted for by the small amounts of ATP formed (∼30 μM ATP when 300 μM ADP applied). It is clear then from these data that most, if not all, of the ADP response is secondary to its conversion to ATP by an ecto-adenylate kinase and action at a P2Y2 receptor. Although traditionally regarded as an intracellular enzyme, ecto-adenylate kinase activities have been reported on human umbilical vein endothelial cells (Yegutkin et al., 2001) and human airway surfaces (Donaldson et al., 2002). The presence of adenylate kinase will play an important role in determining the nucleotide composition of the extracellular milieu, and will therefore act as a regulator of hepatocyte function.
Another enzyme capable of extracellular conversion of ADP to ATP is ecto-nucleoside diphosphokinase (Lazarowski et al., 1997). However, if this enzyme were responsible for ATP formation in the present study, AMP would not be generated as reported. In addition the [3H]-InsPx response to ADP and the formation of ATP would not be inhibited by Ap5A. We conclude, therefore, that diphosphokinase activity is not responsible for the hepatocyte response to ADP.
Hexokinase treatment is used to convert ATP formed during the incubation back into ADP. The reduced effect of ADP seen in the presence of hexokinase therefore confirms the significance of formation of ATP in mediating this response. However, removing ATP with hexokinase did not have as great an effect as may be expected. It is possible that the levels of nucleotides monitored in the bulk phase by HPLC may not mirror those at the cell surface, where both ecto-enzyme and P2Y receptors are located. However, it also remains a possibility that ADP acts in part at a hitherto uncloned receptor which is not stimulated by ADPβS or 2MeSADP.
In conclusion, we have provided evidence that the ADP-stimulated inositol (poly)phosphate formation in rat hepatocytes is secondary to its conversion to ATP by adenylate kinase. It is striking that while ADP was very rapidly metabolized by cultured hepatocytes, ATP was relatively stable. This indicates that these cells have little ecto-nucleotidase activity, but a very active ecto-adenylate kinase. The consequence will be to limit stimulation of P2Y1 receptors, whilst enhancing P2Y2 activation, in response to released native nucleotides. AMP, generated along with ATP by adenylate kinase, will act at P1 receptors on hepatocytes which are coupled to increases in cAMP (Okajima et al., 1987), further potentiating the response to ATP; it has previously been shown that elevated cAMP levels enhance the hepatocyte [Ca2+]i response to ATP (Green et al., 1994).
Acknowledgments
We are grateful for funding from The Wellcome Trust.
Abbreviations
- ADPβS
adenosine 5′-O-(2-thiodiphosphate)
- Ap5A
diadenosine pentaphosphate
- A3P5P
adenosine-3′-phosphate-5′-phosphate
- [Ca2+]i
intracellular free Ca2+ concentration
- HPLC
high pressure liquid chromatography
- InsPx
inositol (poly)phosphates
- Ins(1,4,5)P3
inositol 1,4,5-trisphosphate
- 2-MeSADP
2-methylthioADP
- 2-MeSATP
2-methylthioATP
- PLC
phospholipase C
- WME
William's medium E
References
- ALBERT J.L., BOYLE J.P., ROBERTS J.A., CHALLISS J., GUBBY S.E., BOARDER M.R. Regulation of brain capillary endothelial cells by P2Y receptors coupled to Ca2+, phospholipase C and mitogen-activated protein kinase. Br. J. Pharmacol. 1997;122:935–941. doi: 10.1038/sj.bjp.0701453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., COOPER J., SHINE J., HILL S.J. Characterization of the human brain putative A2B adenosine receptor expressed in Chinese hamster ovary (CHO.A2B4) cells. Br. J. Pharmacol. 1996;119:1286–1290. doi: 10.1111/j.1476-5381.1996.tb16035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGDANOV Y.D., WILDMAN S.S., CLEMENTS M.P., KING B.F., BURNSTOCK G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br. J. Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER J.L., MOHANRAM A., CAMAIONI E., JACOBSON K.A., HARDEN T.K. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′ -bisphosphate. Br. J. Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER J.L., ROMERO-AVILA T., SCHACHTER J.B., HARDEN T.K. Identification of competitive antagonists of the P2Y1 receptor. Mol. Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- BURNSTOCK G.P2 purinoceptors: historical perspective and classification P2 purinoceptors: localization, function and transduction mechanisms 1996Chichester: John Wiley & Sons Ltd; 1–29.Ciba Foundation Symposium 198 [DOI] [PubMed] [Google Scholar]
- CHEN Z.-P., KRULL N., XU S., LEVY A., LIGHTMAN S.L. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology. 1996;137:1833–1840. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., GOVAERTS C., PARMENTIER M., BOEYNAEMS J.-M. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J. Biol. Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., PIROTTON S., PARMENTIER M., BOEYNAEMS J.M. Cloning and functional expression of a human uridine nucleotide receptor. J. Biol. Chem. 1995;270:30849–30852. doi: 10.1074/jbc.270.52.30849. [DOI] [PubMed] [Google Scholar]
- DIXON C.J. Evidence that 2-methylthioATP and 2-methylthioADP are both agonists at the rat hepatocyte P2Y1 receptor. Br. J. Pharmacol. 2000;130:664–668. doi: 10.1038/sj.bjp.0703350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., COBBOLD P.H., GREEN A.K. Actions of ADP, but not ATP, on cytosolic free Ca2+ in single rat hepatocytes mimicked by 2-methylthioATP. Br. J. Pharmacol. 1995;116:1979–1984. doi: 10.1111/j.1476-5381.1995.tb16401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., WOODS N.M., CUTHBERTSON K.S.R., COBBOLD P.H. Evidence for two calcium-mobilizing purinoceptors on rat hepatocytes. Biochem. J. 1990;269:499–502. doi: 10.1042/bj2690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., WOODS N.M., WEBB T.E., GREEN A.K. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. Br. J. Pharmacol. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALDSON S.H., PICHER M., BOUCHER R.C. Secreted and cell-associated adenylate kinase and nucleotide diphosphokinase contribute to extracellular nucleotide metabolism on human airway surfaces. Am. J. Respir. Cell Mol. Biol. 2002;26:209–215. doi: 10.1165/ajrcmb.26.2.4650. [DOI] [PubMed] [Google Scholar]
- FRELIN C., BREITTMAYER J.P., VIGNE P. ADP induces inositol-phosphate-independent intracellular Ca2+ mobilization in brain capillary endothelial cells. Br. J. Pharmacol. 1993;268:8787–8792. [PubMed] [Google Scholar]
- GREEN A.K., COBBOLD P.H., DIXON C.J. Elevated intracellular cyclic AMP exerts different modulatory effects on cytosolic free Ca2+ oscillations induced by ADP and ATP in single hepatocytes. Biochem. J. 1994;302:949–955. doi: 10.1042/bj3020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPPENS S. The complex interaction of ATP and UTP with isolated hepatocytes. How many receptors. Gen. Pharmacol. 1993;24:283–289. doi: 10.1016/0306-3623(93)90304-g. [DOI] [PubMed] [Google Scholar]
- KEPPENS S., DE WULF H. Characterization of the biological effects of 2-methylthio-ATP on rat hepatocytes: clear-cut differences with ATP. Br. J. Pharmacol. 1991;104:301–304. doi: 10.1111/j.1476-5381.1991.tb12426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPPENS S., DE WULF H. Some P2 purinergic agonists increase cytosolic calcium but not inositol 1,4,5-trisphosphate in isolated rat hepatocytes. Biochem. Biophys. Acta. 1995;1269:316–322. doi: 10.1016/0167-4889(95)00132-7. [DOI] [PubMed] [Google Scholar]
- KEPPENS S., VANDEKERCKHOVE A., DE WULF H. Extracellular ATP and UTP exert similar effects on rat isolated hepatocytes. Br. J. Pharmacol. 1992;105:475–479. doi: 10.1111/j.1476-5381.1992.tb14278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPPENS S., VANDEKERCKHOVE A., DE WULF H. Characterization of the effects of adenosine 5′-[β-thio]-diphosphate in rat liver. Br. J. Pharmacol. 1993;108:663–668. doi: 10.1111/j.1476-5381.1993.tb12858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., HOMOLYA L., BOUCHER R.C., HARDEN T.K. Identification of an ecto-nucleoside diphosphokinase and its contribution to interconversion of P2 receptor agonists. J. Biol. Chem. 1997;272:20402–20407. doi: 10.1074/jbc.272.33.20402. [DOI] [PubMed] [Google Scholar]
- LEON C., HECHLER B., VIAL C., LERAY C., CAZENAVE J.-P., GACHET C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- LIENHARD G.E., SECEMSKI I.I. P1,P5-Di(adenosine-5′)pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J. Biol. Chem. 1973;248:1121–1123. [PubMed] [Google Scholar]
- NGUYEN T., ERB L., WEISMAN G.A., MARCHESE A., HENG H.H.Q., GARRAD R.C., GEORGE S.R., TURNER J.T., O'DOWD B.F. Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J. Biol. Chem. 1995;270:30845–30848. doi: 10.1074/jbc.270.52.30845. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A., WATT W.C., LAZAROWSKI E.R., LI Q., HARDEN T.K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific rceptor. Mol. Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- OKAJIMA F., TOKUMITSU Y., KONDO Y., UI M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J. Biol. Chem. 1987;262:13483–13490. [PubMed] [Google Scholar]
- QI A.D., ZAMBON A.C., INSEL P.A., NICHOLAS R.A. An arginine/glutamine difference at the juxtaposition of transmembrane domain 6 and the third extracellular loop contributes to the markedly different nucleotide selectivities of human and canine P2Y11 receptors. Mol. Pharmacol. 2001;60:1375–1382. doi: 10.1124/mol.60.6.1375. [DOI] [PubMed] [Google Scholar]
- TORRES B., ZAMBON A.C., INSEL P.A. P2Y11 receptors activate adenylyl cyclase and contribute to nucleotide-promoted cAMP formation in MDCK-D-1 cells – a mechanism for nucleotide-mediated autocrine-paracrine regulation. J. Biol. Chem. 2002;277:7761–7765. doi: 10.1074/jbc.M110352200. [DOI] [PubMed] [Google Scholar]
- SCHACHTER J.B., BOYER J.L., LI Q., NICHOLAS R.A., HARDEN T.K. Fidelity in functional coupling of the rat P2Y1 receptor to phospholipase C. Br. J. Pharmacol. 1997;122:1021–1024. doi: 10.1038/sj.bjp.0701479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIGNE P., PACAUD P., LOIRAND G., BREITTMAYER J.P., FRELIN C. PPADS inhibits P2Y1 purinoceptors in rat brain capillary endothelial cells and in rat ileal myocytes by an indirect mechanism. Biochem. Biophys. Res. Comm. 1994;244:332–335. doi: 10.1006/bbrc.1998.8262. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., HENDERSON D.J., ROBERTS J.A., BARNARD E.A. Molecular cloning and characterization of the rat P2Y4 receptor. J. Neurochem. 1998;71:1348–1357. doi: 10.1046/j.1471-4159.1998.71041348.x. [DOI] [PubMed] [Google Scholar]
- YEGUTKIN G.G., HENTTINEN T., JALKANEN S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- ZAMBON A.C., BRUNTON L.L., BARRETT K.E., HUGHES R.J., TORRES B., INSEL P.A. Cloning, expression, signaling mechanisms, and membrane targeting of P2Y11 receptors in Madin Darby canine kidney cells. Mol. Pharmacol. 2001;60:26–35. doi: 10.1124/mol.60.1.26. [DOI] [PubMed] [Google Scholar]


