Abstract
The presence of β3-adrenoceptors and the low affinity state of the β1-adrenoceptor (formerly ‘putative β4-adrenoceptor') was investigated in ring preparations of rat isolated aorta preconstricted with phenylephrine or prostaglandin F2α (PGF2α). Relaxant responses to isoprenaline, selective β3-adrenoceptor agonists (BRL 37344, SR 58611A, CL 316243) and non-conventional partial agonists (CGP 12177A, cyanopindolol, pindolol) were obtained.
In phenylephrine-constricted, but not PGF2α-constricted rings, relaxations to isoprenaline showed a propranolol-resistant component.
In phenylephrine-constricted rings, relaxations to BRL 37344 (pEC50, 4.64) and SR 58611A (pEC50, 4.94) were not antagonized by the selective β3-adrenoceptor antagonist SR 59230A (⩽1 μM). CL 316243 (⩽100 μM) failed to produce relaxation. In PGF2α-constricted rings only SR 58611A produced relaxation, which was not affected by SR 59230A (⩽3 μM).
Non-conventional partial agonists produced relaxation in phenylephrine-constricted but not PGF2α-constricted rings. The relaxation to CGP 12177A was unaffected by SR 59230A (⩽1 μM) or by CGP 20712A (10 μM), reported to block the low affinity state of the β1-adrenoceptor.
β-adrenoceptor antagonists also produced relaxation in phenylephrine-constricted rings with an order of potency of (pEC50 values): bupranolol (5.5)≈38;SR 59230A (5.47)≈38;cyanopindolol (5.47)>pindolol (5.30)>alprenolol (5.10)>propranolol (4.83)>ICI 118551 (4.60)>CGP 12177A (4.38)≈38;CGP 20712A (4.35). Bupranolol (100 μM), alprenolol (30 μM), propranolol (100 μM) and SR 59230A (10 μM) produced no relaxation in PGF2α-constricted rings.
These results provide no evidence for the presence of functional β3-adrenoceptors or the low affinity state of the β1-adrenoceptor in rat aorta.
Keywords: β-adrenoceptors, β3-adrenoceptors, putative β4-adrenoceptors, low affinity state of β1-adrenoceptors, rat aorta, β3-adrenoceptor agonists, non-conventional partial agonists, β-adrenoceptor antagonists
Introduction
The term ‘atypical' has been used extensively to describe β-adrenoceptors not corresponding pharmacologically to the β1/-β2-classification. It is now known that ‘atypical' β-adrenoceptors correspond to β3-adrenoceptors and to the low affinity state of β1-adrenoceptors (formerly ‘putative β4-adrenoceptors'). The pharmacological properties of these receptors are now well established (Arch & Kaumann, 1993; Kaumann, 1997). β3-adrenoceptors are pharmacologically characterized by (i) low affinity of classical β-adrenoceptor antagonists, such as propranolol, (ii) activation by selective β3-adrenoceptor agonists, such as BRL 37344 (Arch et al., 1984), SR 58611A (Bianchetti & Manara, 1990) and CL 316243 (Bloom et al., 1992), (iii) activation by non-conventional partial agonists (potent β1/β2-adrenoceptor antagonists with β3-adrenoceptor agonist activity at higher concentrations (Kaumann, 1989)) such as CGP 12177A (Mohell & Dicker, 1989), cyanopindolol (Engel et al., 1981) and pindolol (Walter et al., 1984), (iv) blockade by selective β3-adrenoceptor antagonists such as SR 59230A (Manara et al., 1996). A fourth subtype of β-adrenoceptor, the ‘putative β4-adrenoceptor' was also postulated to exist in the heart, sharing properties (i) and (iii) above, but not (ii) and (iv) (Kaumann, 1989; Malinowska & Schlicker, 1996; Kaumann & Molenaar, 1996; Galitzky et al., 1997). However, the pharmacology of the ‘putative β4-adrenoceptor' is not seen in β1-adrenoceptor knockout mice (Konkar et al., 2000; Kaumann et al., 2001) and it is now understood that this receptor corresponds to a low-affinity state of the β1-adrenoceptor (Kompa & Summers, 1999; Konkar et al., 2000; Kaumann et al., 2001).
Vascular β-adrenoceptors were originally classified as β2- (Lands et al., 1967) although β1-adrenoceptors also mediate vasorelaxation (O'donnell & Wanstall, 1985). A number of recent studies have also suggested the presence of atypical β-adrenoceptors, i.e. β3-adrenoceptors or ‘putative β4-adrenoceptors' in blood vessels. For example, β3-adrenoceptor agonists have been shown to have peripheral vasodilator effects in dogs (Tavernier et al., 1992; Shen et al., 1994; 1996), rats (Shen et al., 1996) and in mice (Rohrer et al., 1999). β3-adrenoceptor agonists have also been shown to have vasorelaxant properties in vitro (Oriowo, 1994; 1995; Sooch & Marshall, 1997; Tamaoki et al., 1998; MacDonald et al., 1999; Shafiei & Mahmoudian, 1999; Trochu et al., 1999; Brawley et al., 2000). The effects of the β3-adrenoceptor agonists, however, may not all be mediated via β3-adrenoceptors. For example, Oriowo (1995) found that the relaxations to BRL 37344 and CGP 12177A in rat aorta and carotid artery were not desensitized by BRL 37344 or antagonized by cyanopindolol, a β3-adrenoceptor antagonist in the gastrointestinal tract (Blue et al., 1989; McLaughlin & MacDonald, 1990; 1991), in contrast to the well-characterized β3-adrenoceptor-mediated relaxations of colon and fundus. Shafiei & Mahmoudian (1999) found that relaxations to cyanopindolol in rat aorta were not blocked by SR 59230A and suggested that the effects of non-conventional partial agonists in rat aorta may be mediated via the ‘putative β4-adrenoceptor'. Brawley et al. (2000) also found that relaxations to BRL 37344 and CGP 12177A were not blocked by the selective β3-adrenoceptor antagonist SR 59230A and similarly suggested the presence of the ‘putative β4-adrenoceptor'. However the latter authors conceded that not all of their data supported this conclusion since, firstly, the putative β4-adrenoceptor is not activated by selective β3-agonists (Kaumann, 1997) and secondly, the order of potency obtained for cyanopindolol and CGP 12177A (cyanopindolol>CGP 12177A) was the wrong way round for the cardiac ‘putative β4-adrenoceptor' (Kaumann & Molenaar, 1996; Malinowska & Schlicker, 1996). In contrast to these studies, Trochu et al. (1999) reported that relaxations to SR 58611A in rat aorta were mediated via β3-adrenoceptors since they were antagonized by SR 59230A. Thus there is conflicting evidence for the presence of β3-adrenoceptors and/or the low affinity state of the β1-adrenoceptor (‘putative β4-adrenoceptor') in rat aorta.
The aim of the present study was to investigate further the nature of the relaxations mediated by β3-adrenoceptor agonists and by non-conventional partial agonists in rat isolated aorta. An important aspect of the study was the choice of preconstrictor. The most commonly employed preconstrictors for investigation of β-adrenoceptor-mediated relaxation are α1-adrenoceptor agonists. The possibility that relaxant effects of β3-adrenoceptor agonists or non-conventional partial agonists were due to non-specific blockade of α1-adrenoceptors or interference with the α1-adrenoceptor signalling pathway was investigated by additionally employing a preconstrictor with a different mechanism, prostaglandin F2α (PGF2α). Preliminary accounts of these results have been presented to the British Pharmacological Society (Brahmadevara et al., 2001; 2002).
Methods
Male Wistar rats (200–250 g) were stunned and killed by cervical dislocation followed by exsanguination. The thoracic aorta was isolated, removed carefully to prevent endothelium damage and cleared of fat and connective tissue. In some experiments, distal colon was also removed.
Thoracic aorta
Aortae were cut into 3 mm ring segments and mounted on stainless steel wires in 20 ml organ baths containing Krebs' medium with the following composition in (mM): NaCl 19, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, D-glucose 11.1. The Krebs' medium also contained ethylene diamine tetra-acetic acid (30 μM) and ascorbic acid (30 μM). The medium was maintained at 37°C and gassed continuously with 95% O2 and 5% CO2. Each tissue was placed under 1.5 g resting tension and equilibrated for 60 min prior to the execution of experimental protocols. During this period tissues were washed with Krebs every 15 min and tension was readjusted to 1.5 g. Isometric muscle tension was recorded with Grass transducers and displayed on a Goerz Servogor 400 oscillograph.
Phenylephrine and PGF2α produced concentration-dependent contractions of aortic rings (pEC50s: 6.90±0.03, n=8; 5.45±0.01, n=7, respectively) from which sub-maximal concentration of phenylephrine (0.6 μM, approximately EC80) and PGF2α (3 μM, approximately EC50) were chosen for subsequent experiments. After the equilibration period, artery rings were constricted with phenylephrine (10 μM) twice or PGF2α (10 μM) followed by washout for 30 min. Functional endothelium was checked by the presence of at least 80% relaxation in response to acetylcholine (1 μM) after pre-constricting the tissues with a sub-maximal concentration of phenylephrine (0.6 μM) or a sub-maximal concentration of PGF2α (3 μM). In some experiments endothelium was denuded by gentle abrasion of the intimal surface of the aortic rings with the tip of a stainless steel wire. Endothelium removal was confirmed by the absence of relaxation to acetylcholine (1 μM). After washout, some tissues were incubated with the appropriate antagonist for 30 min with control tissues receiving vehicle treatment. The rings were then contracted again with phenylephrine (0.6 μM) or PGF2α (3 μM) and cumulative concentration-response curves to relaxants were obtained following stabilization of constrictor-induced tone. In experiments with PGF2α, tissues were incubated with prazosin (0.75 μM) to block α1-adrenoceptors when isoprenaline was used as a relaxant.
Distal colon
Longitudinal segments (c. 2.5 cm) of distal colon were mounted in organ baths containing Krebs' medium (composition and conditions as described above), with care taken not to occlude the lumen. Each tissue was placed under 1 g resting tension and equilibrated for 40 min prior to contraction with 50 mM KCl for 15 min followed by a 30 min wash. Tissues were again contracted with a sub-maximal concentration of KCl (30 mM) and washed for 30 min. Tissues were then incubated with the appropriate antagonist for 30 min with control tissues receiving vehicle treatment. The tissues were then contracted again with KCl (30 mM) and cumulative concentration-response curves to relaxants were obtained, after contraction was stabilized.
Drugs
The following were dissolved in distilled water: L-phenylephrine hydrochloride, acetylcholine chloride, (−)-isoprenaline bitartrate, (±)-propranolol hydrochloride, alprenolol hydrochloride, CL 316243 (disodium (R,R)-5-[2-[[2-3-Chlorophenyl) - 2 - hydroxyethyl] - amino]propyl] -1,3 - benzodioxole-2,2-dicarboxylate) (all from Sigma), CGP 12177A hydrochloride, ((±)-4-(3-tbutylamino-2-hydroxypropoxy)-benzimidazol-2-one hydrochloride) (gift from Novartis Pharma), BRL 37344 ((R*, R*)-(±)-4-[2-[(3-chlorophenyl)-2-hydroxyethyl) amino] propyl] phenoxyacetic acid) (Tocris Cookson), (±)-cyanopindolol hemifumarate (Tocris Cookson), CGP 20712A (2-hydroxy-5 (2-((2-hydroxy-3-4 ((1-methyl-4-trifluoromethyl) 1H-imidazole-2-yl)-phenoxy) propyl) amino) ethoxy)-benzamide monomethane sulphonate) (gift from Novartis Pharma), (−)-bupranolol hydrochloride (gift from Schwartz Pharma), SR 58611A (RS-N-(7-carbethoxymethoxyl 1,2,3,4-tetrahydronophth-2-yl)-2 hydroxy 2-(3-chlorophenyl)-ethanamine) (gift from Sanofi, Italy), ICI 118551 ((±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3- [(1 - methylethyl)amino]-2-butanol) (Tocris Cookson). Pindolol (Sigma) was dissolved in 0.1 M HCl. PGF2α, (Biomol) was dissolved in 100% ethanol. SR 59230A (3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-2S-2-propanol oxalate) (Sigma) was dissolved in 40% dimethyl sulphoxide (DMSO). The final concentration of DMSO in the organ baths was 0.06%. At this concentration the solvent alone had no effect on the tissues.
Calculations and statistical analysis
Responses to relaxants were calculated as percentage of inhibition of the constrictor-induced contraction and expressed as mean±s.e.mean. Mean concentration response curves to relaxants were analysed by fitting to a four parameter logistic equation (given below) using non-linear regression (Graph Pad Prism),
where X is the logarithm of molar concentration of the relaxant, Y is the response and P is the Hill slope. Rmax and pEC50 values were obtained where Rmax is the maximum percentage of relaxation obtained and EC50 is the concentration (M) of relaxant that produces 50% of its maximum response. Concentration ratios (r) were determined from EC50 values and antagonist affinities were expressed as pKB values using the following equation (Schild, 1949):
Statistical analyses were performed using Student's t-test to compare two groups and one-way analysis of variance followed by the Tukey multiple comparison post test for comparison of three or more groups. P<0.05 was considered to be significant.
Results
Isoprenaline in phenylephrine-constricted rings
Isoprenaline produced a concentration-dependent, full relaxation of phenylephrine-constricted rings (Figure 1). The β1/β2-adrenoceptor antagonist propranolol (30 nM, 0.3 μM and 1 μM) caused rightward shifts of the isoprenaline concentration-response curve with no reduction in the maximum response (Figure 1a, Table 1) and no significant difference in Hill slopes (P>0.05). A higher concentration of propranolol (3 μM) produced a small reduction in the maximum relaxation (Rmax, %: control, 102±0.9; propranolol, 3 μM, 90±4, P<0.05, n=8) without producing any further shift (Figure 1a, Table 1). The corresponding Schild plot, using concentrations of propranolol up to 1 μM, gave a slope of 0.42 (95% CL: 0.24 to 0.60), significantly different from unity (n=24, P<0.05) (Figure 1b). Calculation of pKB values for each individual propranolol concentration showed that the higher concentrations (0.3, 1 and 3 μM) caused reduced rightward shifts compared to the lower concentration (30 nM) indicating a propranolol resistant component (Table 1).
Figure 1.
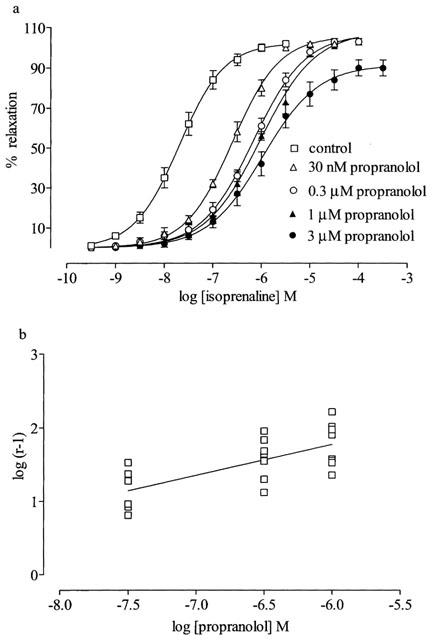
(a) Effect of propranolol on relaxations induced by (−)-isoprenaline in rat thoracic aorta preconstricted with phenylephrine (0.6 μM). Results are expressed as percentage reduction of tone induced by phenylephrine. Values are mean±s.e.mean of eight observations for each curve. (b) Schild plot for propranolol against isoprenaline with slope of 0.42 (95% C.L. 0.24 to 0.60), significantly different from unity (P<0.05, n=24).
Table 1.
pEC50s for isoprenaline in the presence of propranolol and calculated pKBs

Selective β3-agonists in phenylephrine-constricted rings
BRL 37344 and SR 58611A produced concentration-dependent, full relaxation of phenylephrine-constricted rings (Table 2, Figure 2a). CL 316243 (30 nM to 100 μM) had no relaxant effect (n=14, Figure 2a). Even at a lower constrictor concentration of phenylephrine of 0.1 μM, approximately the EC50, CL 316243 (⩽100 μM) had no relaxant effect (n=7). The relaxations to BRL 37344 and SR 58611A took up to 8 and 6 min respectively to reach a plateau. Relaxations to these compounds were unaffected by propranolol (0.3 or 1 μM) or SR 59230A (⩽1 μM) (Table 2). Preliminary experiments with concentrations of SR 59230A higher than 1 μM indicated that these concentrations reduced the level of pre-constriction (see below). Bupranolol, which also blocks β3-adrenoceptors at μM concentrations, could not be tested as it also depressed the phenylephrine constriction at these concentrations.
Table 2.
Relaxant effects of β3-adrenoceptor agonists and non-conventional partial agonists in rat aortic rings preconstricted with phenylephrine

Figure 2.
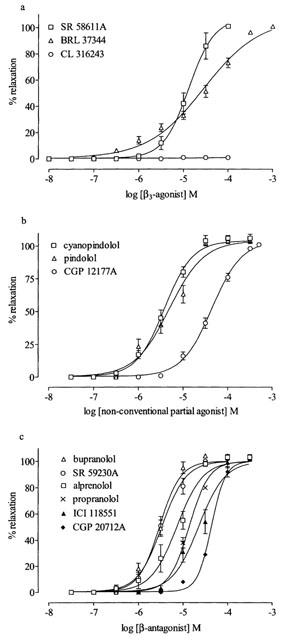
Relaxant effect of (a) β3-adrenoceptor agonists, (b) non-conventional partial agonists and (c) β-adrenoceptor antagonists in rat thoracic aortae preconstricted with phenylephrine (0.6 μM). Results are expressed as percentage reduction of tone induced by phenylephrine. Values are mean±s.e.mean of 3–14 observations.
Non-conventional partial agonists inphenylephrine-constricted rings
The non-conventional partial agonists, CGP 12177A, cyanopindolol and pindolol also produced concentration-dependent full relaxation of phenylephrine-constricted rings (Table 2, Figure 2b). Relaxations to CGP 12177A or to pindolol were unaffected by propranolol (1 μM). Relaxations to CGP 12177A were unaffected by SR 59230A (⩽1 μM) or CPG 20712A (⩽10 μM) (Table 2).
Effect of endothelium removal on relaxation to SR 58611A and CGP 12177A in phenylephrine-constricted rings
Removal of endothelium had little or no effect on relaxation to SR 58611A or CGP 12177A (Table 3). The effectiveness of endothelium removal was confirmed by complete abolition of the relaxation to 1 μM acetylcholine (>80% relaxation in endothelium intact preparations).
Table 3.
Effect of endothelium removal on relaxation to SR 58611A and CGP 12177A in rat aortic rings constricted with phenylephrine

Effect of β-adrenoceptor antagonists on phenylephrine-constricted rings
Since preliminary experiments with SR 59230A and bupranolol indicated that at μM concentrations a reduction in the size of the phenylephrine constriction was produced (see above), the relaxant effects of SR 59230A, bupranolol and other β-adrenoceptor antagonists on phenylephrine-constricted rings were investigated. SR 59230A, bupranolol, alprenolol, propranolol, CGP 20712A and ICI 118551 all produced concentration-dependent, full relaxation of phenylephrine-constricted rings (Table 4, Figure 2c). The effect of bupranolol was unaltered by propranolol (1 μM) (Table 4).
Table 4.
Relaxant effects of β-adrenoceptor antagonists in rat aortic rings preconstricted with phenylephrine

Effect of isoprenaline in PGF2α-constricted rings
Isoprenaline produced a concentration-dependent relaxation of PGF2α-constricted rings (pEC50, 7.42±0.04, Rmax, %, 76±4, n=6). In the presence of propranolol (1 μM), isoprenaline produced no relaxation at concentrations ⩽30 μM (n=6).
Effect of selective β3-agonists on PGF2α-constricted aorta
CL 316243 (30 nM to 100 μM, n=7) and BRL 37344 (30 nM to 30 μM, n=6) had no relaxant effect in PGF2α-constricted aortic rings. SR 58611A did produce concentration-dependent relaxation in PGF2α-constricted aortae, which was unaffected by propranolol (1 μM), bupranolol (10 μM and 30 μM), alprenolol (30 μM) or SR 59230A (⩽3 μM) (Table 5).
Table 5.
Relaxant effect of SR 58611A in rat aortic rings constricted with PGF2α

Non-conventional partial agonists in PGF2α-constricted aorta
CGP 12177A (⩽200 μM, n=8) and cyanopindolol (10 μM, n=6) failed to produce relaxation in PGF2α constricted aortic rings.
β-adrenoceptor antagonists in PGF2α-constricted aorta
Bupranolol (100 μM, n=6), alprenolol (30 μM, n=6), propranolol (100 μM, n=5) or SR 59230A (10 μM, n=7) did not produce any relaxation in PGF2α-constricted aortic rings.
β3-adrenoceptor agonists and antagonists in rat colon
In order to confirm potency and selectivity of β3-adrenoceptor agonists and antagonists, experiments were carried out in distal colon, a tissue known to contain β3-adrenoceptors (McLaughlin & MacDonald, 1990). Selective β3-adrenoceptor agonists BRL 37344 and CL 316243 produced concentration-dependent relaxation of KCl (30 mM)-contracted distal colon at low concentrations (pEC50 values: BRL 37344, 8.11±0.02, n=6; CL 316243, 8.40±0.02, n=5) (Figure 3). Relaxations took up to 25 min and 20 min to reach a plateau to BRL 37344 and CL 316243 respectively. Pre-incubation with SR 59230A (1 μM) produced 93 and 99 fold shifts of the BRL 37344 and CL 316243 concentration-response curves respectively without reducing the maximum response (Figure 3) giving pKB values of 7.96±0.11 (n=6) and 7.97±0.78 (n=5) respectively.
Figure 3.
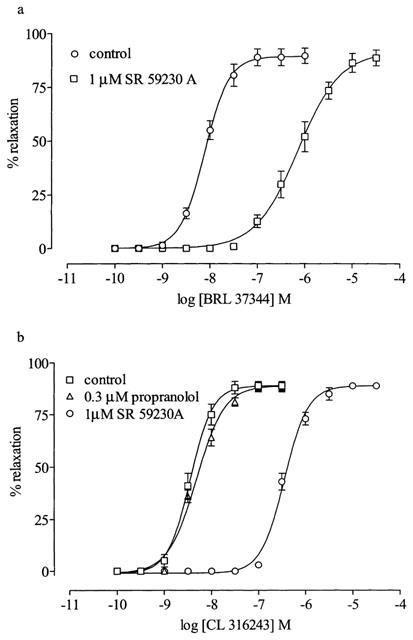
(a) Effect of SR 59230A (1 μM) on relaxation to BRL 37344 in rat distal colon preconstricted with KCl (30 mM). Values are mean±s.e.mean of six observations. (b) Effect of SR 59230A (1 μM) and propranolol (0.3 μM) on relaxation to CL 316243 in rat distal colon preconstricted with KCl (30 mM). Values are mean±s.e.mean of five observations.
Discussion
A characteristic pharmacological property of both β3-adrenoceptors and the low affinity state of β1-adrenoceptors is resistance to blockade by propranolol (Arch & Kaumann, 1993; Kaumann, 1997). In the present study, relaxations to isoprenaline in phenylephrine-constricted arteries were inhibited by propranolol in a non-competitive manner, with a propranolol-resistant component of the response, confirming previous observations in blood vessels (Oriowo, 1994; 1995; Sooch & Marshall, 1997; Brawley et al., 2000). The shift produced by 30 nM propranolol (pKB, 8.64) is consistent with an action of isoprenaline at β1-/β2-subtypes (pA2 8.2 to 8.8, Wilson et al., 1984). Higher concentrations of propranolol produced smaller than expected shifts, indicating the appearance of a propranolol-resistant component at higher concentrations of isoprenaline. This propranolol-resistant component to isoprenaline has been taken as evidence of vascular ‘atypical' β-adrenoceptors (Oriowo, 1994; 1995; Sooch & Marshall, 1997; Shafiei & Mahmoudian, 1999; Brawley et al., 2000). However, in the present study no evidence for a propranolol-resistant component was found in the PGF2α-constricted rings. It appears therefore that the apparent propranolol-resistance in phenylephrine-constricted rings is constrictor-dependent. The reason for an apparent propranolol-resistant component in phenylephrine-constricted, but not PGF2α-constricted preparations, is not clear, but as most of the other studies using blood vessels in vitro employ either noradrenaline or phenylephrine as the constrictor then further studies with alternative constrictors are required.
A second characteristic property of β3-adrenoceptors, but not the low affinity state of β1-adrenoceptors, is activation by selective β3-adrenoceptor agonists (Kaumann, 1997). In the present study relaxations in phenylephrine-constricted rings to the selective β3-adrenoceptor agonists BRL 37344 and SR 58611A were obtained, although rings failed to relax to CL 316243. Relaxation to selective β3-adrenoceptor agonists has been taken as evidence of the presence of β3-adrenoceptors in blood vessels (Sooch & Marshall, 1997; Tamaoki et al., 1998; MacDonald et al., 1999; Trochu et al., 1999). However, in contrast to the colon where β3-adrenoceptors are known to be present (McLaughlin & MacDonald, 1990), the relaxations to BRL 37344 occurred only with low potency (pEC50s: aorta, 4.64; colon, 8.11). The low potency of β3-adrenoceptor agonists in rat aorta in this study is in agreement with that found in other studies in the same organ (Oriowo, 1995; Sooch & Marshall, 1997; Trochu et al., 1999; Brawley et al., 2000). The relaxations were also different in nature from those seen in the colon, where the relaxations are slow. In addition, the selective β3-adrenoceptor antagonist SR 59230A failed to antagonize BRL 37344 or SR 58611A-induced relaxation in phenylephrine-constricted aorta at a concentration (1 μM) that produced more than 90 fold shifts of BRL 37344 and CL 316243 in the colon. The pKB values obtained in colon (7.96 and 7.97 against BRL 37344 and CL 316243 respectively) are in agreement with the reported pA2 for SR 59230A against BRL 37344 at β3-adrenoceptors in rabbit jejunum (pA2, 7.58, MacDonald & Watt, 1999). The lack of effect of SR 59230A on SR 58611A-induced relaxation is in contrast to the results of Trochu et al. (1999) who showed that SR 59230A (10 μM) abolished SR 58611A-induced relaxation and reported that a lower concentration (1 μM) reduced the relaxation. In the present study, we were unable to use concentrations of SR 59230A higher than 1 μM since they reduced the size of the phenylephrine constriction (see later). For the same reason, we were unable to use bupranolol as a β3-adrenoceptor antagonist since it reduced the size of the phenylephrine constriction at concentrations reported to block β3-adrenoceptors (Kaumann & Molenaar, 1996). Thus the low potency of BRL 37344 and SR 58611A and the lack of antagonism of their relaxant effects by SR 59230A support the conclusion that the relaxations induced by BRL 37344 and SR 58611A in phenylephrine-constricted aortic rings do not appear to be mediated by β3-adrenoceptors. The absence of β3-adrenoceptors is supported by the lack of any relaxant effect in phenylephrine-constricted rings of CL 316243, whose potency and β3-selectivity (Bloom et al., 1992; Kaumann & Molenaar, 1996) was confirmed in the colon in the present study (pEC50, 8.4). Further evidence for the lack of β3-adrenoceptors was provided by the experiments in PGF2α-constricted rings. In these rings the selective β3-adrenoceptor agonists BRL 37344 and CL 316243 has no relaxant effects although isoprenaline produced relaxation with only a small difference in potency from phenylephrine-constricted rings. SR 58611A did produce relaxation in PGF2α-constricted rings, which was not prevented by the selective β3-adrenoceptor antagonist, SR 59230A, or by μM concentrations of bupranolol or alprenolol, both reported to block β3-adrenoceptors at these concentrations (Kaumann & Molenaar, 1996; Blue et al., 1990).
A characteristic property of both β3-adrenoceptors and the low affinity state of β1-adrenoceptors is activation by non-conventional partial agonists. The non-conventional partial agonists, CGP 12177A, cyanopindolol and pindolol also relaxed phenylephrine-constricted rings. Relaxation to CGP 12177A in blood vessels has been quoted as evidence for the presence of β3-adrenoceptors or ‘atypical' β-adrenoceptors (Oriowo, 1994; 1995; Trochu et al., 1999; Brawley et al., 2000). The relaxations to CGP 12177A were unaffected by the selective β3-adrenoceptor agonist SR 59230A, in agreement with a previous study (Brawley et al., 2000) and in agreement with the present results with β3-adrenoceptor agonists (see above). Relaxations to CGP 12177A were not blocked with CGP 20712A (3 and 10 μM), which is reported to block the low affinity state of the β1-adrenoceptor at high concentrations (Kaumann & Molenaar, 1996; Galitzky et al., 1997; Malinowska & Schlicker, 1997). This is in disagreement with our previous study in noradrenaline-constricted rat aorta where CGP 20712A antagonized the effects of CGP 12177A (Brawley et al., 2000). The reasons for the different results are not clear but as the antagonism reported was clearly not simply competitive, then some other factors may have contributed to the results. The functional absence of the low affinity state of the β1-adrenoceptor was confirmed in this study by a complete lack of relaxant effect of CGP 12177A and cyanopindolol in PGF2α-constricted rings.
It was reported previously that removal of endothelium attenuates the relaxant response of the β3-adrenoceptor agonist SR 58611A, leading to the conclusion that β3-adrenoceptors are located on endothelial cells (Trochu et al., 1999). In the present study, endothelium removal had little or no effect on the relaxations obtained to SR 58611A and CGP 12177A in phenylephrine-constricted rings. Thus the ‘non-specific' relaxant effect of SR 58611A and CGP 12177A in phenylephrine-constricted rings is not endothelium-dependent.
Since preliminary experiments had shown that the β-adrenoceptor antagonists SR 59230A and bupranolol at μM concentrations could depress phenylephrine constrictions, we considered whether the relaxant effect of the non-conventional partial agonists was shared by other β-adrenoceptor antagonists. All of the β-adrenoceptor antagonists tested produced relaxation of phenylephrine-constricted rings (order of potency: bupranolol≅SR 59230A≅cyanopindolol>pindolol>alprenolol>propranolol>ICI 118551>CGP 12177A≅CGP 20712A) (Figure 2, Tables 2 and 4). In contrast, in PGF2α-constricted rings no relaxation was seen with the β-adrenoceptor antagonists tested (bupranolol, alprenolol, propranolol, SR 59230A). Thus all of the β-adrenoceptor antagonists tested, including the non-conventional partial agonists, produce relaxation in phenylephrine-constricted but not PGF2α-constricted rings, supporting the conclusion that the relaxant effects of the non-conventional partial agonists is not related to their agonist activity at β3-adrenoceptors or at the low affinity state of the β1-adrenoceptor. The fact that the relaxant effects occur in phenylephrine-constricted but not PGF2α-constricted arteries suggests some interference with the α1-adrenoceptor signalling pathway, possibly α1-adrenoceptor blocking activity. These results suggest that phenylephrine is not a suitable constrictor for investigation of β3- or low affinity state of β1-adrenoceptor-mediated vasorelaxation.
In conclusion, although some β3-adrenoceptor agonists and the non-conventional partial agonists produce relaxation in phenylephrine-constricted rat aorta, the relaxation is unrelated to stimulation of β3-adrenoceptors or to the low affinity state of the β1-adrenoceptor. The selective β3-adrenoceptor agonists BRL 37344 and SR 58611A appear to mediate effects in rat aorta other than those previously reported at β3-adrenoceptors. CL 316243 lacks these effects and thus may be a suitable agonist for investigation of β3-adrenoceptors in blood vessels. β-adrenoceptor antagonists, including the non-conventional partial agonists, relax phenylephrine-constricted but not PGF2α-constricted arteries, suggesting interference with the α1-adrenoceptor signalling pathway. These results provide no evidence for the presence of functional β3-adrenoceptors or the low affinity state of the β1-adrenoceptor in rat isolated aorta and it is suggested that phenylephrine is an unsuitable constrictor for investigation of these receptors in blood vessels.
Acknowledgments
We are grateful to Novartis Pharma A.G. (CGP 20712A, CGP 12177A), Sanofi Reserche (SR 58611A) and Schwarz Pharma A.G. (bupranolol) for gifts of drugs.
Abbreviations
- DMSO
dimethyl sulphoxide
- pEC50
negative logarithm of the concentration (M) of relaxant that produces 50% of its maximum response
- PG
prostaglandin
- Rmax
%, maximum % relaxation
References
- ARCH J.R.S., AINSWORTH A.T., CAWTHORNE M.A., PIERCEY V., SENITT M.V., THODY V.E., WILSON S. Atypical β-adrenoceptor brown adipocytes as target for anti-obesity drugs. Nature. 1984;309:163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- ARCH J.R.S., KAUMANN A.J. β3- and atypical β-adrenoceptors. Med. Res. Rev. 1993;13:663–729. doi: 10.1002/med.2610130604. [DOI] [PubMed] [Google Scholar]
- BIANCHETTI A., MANARA L. In vitro inhibition of intestinal motility by phenylethanolaminotetralines: evidence of atypical β-adrenoceptors in rat colon. Br. J. Pharmacol. 1990;100:831–839. doi: 10.1111/j.1476-5381.1990.tb14100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOOM J.D., DUTIA M.D., JOHNSON B.D., WISSNER A., BURNS M.G., LARGIS E.E., DOLAN J.A., CLAUS T.H. Disodium (R,R) - 5- [2 -[[2- (3-Chlorophenyl)-2-hydroxymethyl]-amino]propyl]-1,3-benzodioxole-2,2-dicarboxylate (CL 316,243). A potent β-adrenergic agonist virtually specific for β3-adrenoceptors. A promising antidiabetic and antiobesity agent. J. Med. Chem. 1992;35:3081–3084. doi: 10.1021/jm00094a025. [DOI] [PubMed] [Google Scholar]
- BLUE D.R., BOND R.A., ADHAM N., DELMENDO R., MICHEL A., EGLEN R.M., WHITING R.L., CLARKE D.E. Interaction of dihydroalprenolol and cyanopindolol with atypical β-adrenoceptors in guinea-pig ileum. Br. J. Pharmacol. 1989;96:242P. [PubMed] [Google Scholar]
- BLUE D.R., BOND R.A., ADHAM N., DELMENDO R., MICHEL A., EGLEN R.M., WHITING R.L., CLARKE D.E. Antagonist characterisation of atypical beta adrenoceptors in guinea pig ileum: blockade by alprenolol and dihydroalprenolol. J. Pharmacol. Exp. Ther. 1990;252:1034–1042. [PubMed] [Google Scholar]
- BRAHMADEVARA N., SHAW A.M., MACDONALD A. Pharmacological differences between atypical β-adrenoceptors in rat aorta and β3-adrenoceptors in rat colon. Br. J. Pharmacol. 2001;134:110P. [Google Scholar]
- BRAHMADEVARA N., SHAW A.M., MACDONALD A. Lack of functional β3- or putative β4-adrenoceptors in rat isolated aorta. Br. J. Pharmacol. 2002;135:203P. [Google Scholar]
- BRAWLEY L., SHAW A.M., MACDONALD A. β1-, β2- and atypical β-adrenoceptor-mediated relaxation in rat isolated aorta. Br. J. Pharmacol. 2000;129:637–644. doi: 10.1038/sj.bjp.0703091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGEL G., HOYER D., BERTHOLD R., WAGNER H. (±)[125Iodo]cyanopindolol, a new ligand for β-adrenoceptors: identification and quantitation of subclasses of β-adrenoceptors in guinea pig. Naunyn-Schmiedeberg's Arch. Pharmacol. 1981;317:277–285. doi: 10.1007/BF00501307. [DOI] [PubMed] [Google Scholar]
- GALITZKY J., LANGIN D., VERWAERDE P., MONTASTRUC J.-L., LAFONTAN M., BERLAN M. Lipolytic effects of conventional β3-adrenoceptor agonists and of CGP 12177 in rat and human fat cells: preliminary pharmacological evidence for a putative β4-adrenoceptor. Br. J. Pharmacol. 1997;122:1244–1250. doi: 10.1038/sj.bjp.0701523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J. Is there a third heart β-adrenoceptor. Trends Pharmacol. Sci. 1989;10:316–320. doi: 10.1016/0165-6147(89)90065-5. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Four β-adrenoceptor subtypes in the mammalian heart. Trends Pharmacol. Sci. 1997;18:70–76. doi: 10.1016/s0165-6147(96)01033-4. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., ENGELHARDT S., HEIN L., MOLENAAR P., LOHSE M. Abolition of (-) CGP 12177-evoked cardiostimulation in double β1/β2-adrenoceptor knockout mice. Obligatory role of β1-adrenoceptors for putative β4-adrenoceptor pharmacology. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:87–93. doi: 10.1007/s002100000336. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Differences between the third cardiac β-adrenoceptor and the colonic β3-adrenoceptor in the rat. Br. J. Pharmacol. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMPA A.R., SUMMERS R.J. Desensitization and resensitization of β1- and putative β4-adrenoceptor mediated response occur in parallel in a rat model of cardiac failure. Br. J. Pharmacol. 1999;128:1399–1406. doi: 10.1038/sj.bjp.0702920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONKAR A.A., ZHAI Z., GRANNEMAN J.G. β1-adrenergic receptors mediate β3-adrenergic-independent effects of CGP 12177 in brown adipose tissue. Mol. Pharmacol. 2000;57:252–258. [PubMed] [Google Scholar]
- LANDS A.M., ARNOLD A., MCAULIFF J.P., LUDUENA F.P., BROWN T.G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967;214:597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- MACDONALD A., MCLEAN M., MACAULAY L., SHAW A.M. Effects of propranolol and L-NAME on β-adrenoceptor-mediated relaxation in rat carotid artery. J. Autonom. Pharmacol. 1999;19:145–149. doi: 10.1046/j.1365-2680.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- MACDONALD A., WATT K. Characterisation of the atypical β-adrenoceptor in rabbit isolated jejunum using BRL 37344, cyanopindolol and SR 59230A. J. Autonom. Pharmacol. 1999;19:91–95. doi: 10.1046/j.1365-2680.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., SCHLICKER E. Mediation of the positive chronotropic effect of CGP 12177 and cyanopindolol in the pithed rat by atypical β-adrenoceptors, different from β3-adrenoceptors. Br. J. Pharmacol. 1996;117:943–949. doi: 10.1111/j.1476-5381.1996.tb15285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINOWSKA B., SCHLICKER E. Further evidence for differences between cardiac atypical β-adrenoceptors and brown adipose tissue β3-adrenoceptors in the pithed rat. Br. J. Pharmacol. 1997;122:1307–1314. doi: 10.1038/sj.bjp.0701516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANARA L., BADONE D., BARONI M., BOCCARDI G., CECCHI R., CROCI T., GIUDICE A., GUZZI U., LANDI M., LE FUR G. Functional identification of rat atypical β-adrenoceptors by the first β3-selective antagonists, aryloxypropanolaminotetralines. Br. J. Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAUGHLIN D.P., MACDONALD A. Evidence for the existence of ‘atypical' β-adrenoceptors (β3-adrenoceptors) mediating relaxation in the rat distal colon in vitro. Br. J. Pharmacol. 1990;101:569–574. doi: 10.1111/j.1476-5381.1990.tb14122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAUGHLIN D.P., MACDONALD A. Characterization of catecholamine-mediated relaxations in rat gastric fundus – evidence for an atypical β-adrenoceptor. Br. J. Pharmacol. 1991;103:1351–1356. doi: 10.1111/j.1476-5381.1991.tb09792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHELL N., DICKER A. The β-adrenergic radioligand [3H] CGP-12177, generally classified as an antagonist, is a thermogenic agonist in brown adipose tissue. Biochem. J. 1989;261:401–405. doi: 10.1042/bj2610401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'DONNELL S.R., WANSTALL J.C. Responses to the β2-selective agonist procaterol of vascular and atrial preparations with different functional β-adrenoceptor populations. Br. J. Pharmacol. 1985;84:227–235. [PMC free article] [PubMed] [Google Scholar]
- ORIOWO M.A. Atypical beta-adrenoceptors in the rat isolated common carotid artery. Br. J. Pharmacol. 1994;113:699–702. doi: 10.1111/j.1476-5381.1994.tb17049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORIOWO M.A. Different atypical β-adrenoceptors mediate isoprenaline-induced vascular relaxation in vascular and non-vascular smooth muscles. Life Sci. 1995;56:PL269–PL275. doi: 10.1016/0024-3205(95)00076-3. [DOI] [PubMed] [Google Scholar]
- ROHRER D.K., CHRUSCINSKI A., SCHAUBLE E.H., BERNSTEIN D., KOBILKA B.K. Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J. Biol. Chem. 1999;274:16701–16708. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- SCHILD H.O. PAx and competitive drug antagonism. Br. J. Pharmacol. Chemother. 1949;4:227–280. doi: 10.1111/j.1476-5381.1949.tb00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAFIEI M., MAHMOUDIAN M. Atypical β-adrenoceptors of rat thoracic aorta. Gen. Pharmacol. 1999;32:557–562. doi: 10.1016/s0306-3623(98)00283-3. [DOI] [PubMed] [Google Scholar]
- SHEN Y.T., CERVONI P., CLAUS T., VATNER S.F. Differences in Beta-3 adrenergic receptor cardiovascular regulation in conscious primates, rats and dogs. J. Pharmacol. Exp. Ther. 1996;278:1435–1443. [PubMed] [Google Scholar]
- SHEN Y.T., ZHANG H., VATNER S.F. Peripheral vascular effects of Beta-3 adrenergic receptor stimulation in conscious dogs. J. Pharmacol. Exp. Ther. 1994;268:466–473. [PubMed] [Google Scholar]
- SOOCH S., MARSHALL I. Atypical β-adrenoceptors in the rat vasculature. Ann. N.Y. Acad. Sci. 1997;812:211–212. doi: 10.1111/j.1749-6632.1997.tb48178.x. [DOI] [PubMed] [Google Scholar]
- TAMAOKI J., TAGAYA E., ISONO K., NAGAI A. Atypical adrenoceptor-mediated relaxation of canine pulmonary artery through a cAMP-dependent pathway. Biochem. Biophys. Res. Comm. 1998;248:722–727. doi: 10.1006/bbrc.1998.9047. [DOI] [PubMed] [Google Scholar]
- TAVERNIER G., GALITZKY J., BOUSQUET-MELOU A., MONTASTRUC J.L., BRELAN M. The positive chronotropic effect induced by BRL 37344 and CGP 12177, two beta-3 adrenergic agonists, does not involve cardiac beta adrenoceptors but baroreflux mechanisms. J. Pharmacol. Exp. Ther. 1992;263:1083–1090. [PubMed] [Google Scholar]
- TROCHU J.N., LEBLAIS V., RAUTUREAU Y., BEVERELLI F., LE MAREC H., BERDEAUX A., GAUTHIER C. Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br. J. Pharmacol. 1999;128:69–76. doi: 10.1038/sj.bjp.0702797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTER M., LEMOINE H., KAUMANN A.J. Stimulant and blocking effects of optical isomers of pindolol on the sinoatrial node and trachea of guinea pig. Role of β-adrenoceptor subtypes in the dissociation between blockade and stimulation. Naunyn Schmiedeberg's Arch. Pharmacol. 1984;327:159–175. doi: 10.1007/BF00500912. [DOI] [PubMed] [Google Scholar]
- WILSON C., WILSON S., PIERCY V., SENNIT M.V., ARCH J.R.S. The rat lipolytic β-adrenoceptor: studies using novel β-adrenoceptor agonists. Eur. J. Pharmacol. 1984;100:309–319. doi: 10.1016/0014-2999(84)90007-4. [DOI] [PubMed] [Google Scholar]


