Abstract
ATP-gated ion channels (P2X receptors) contain two hydrophobic segments that are presumed to span the plasma membrane (TM1 and TM2). Pairs of cysteines were introduced by mutagenesis into the rat P2X2 receptor, one in TM1 one in TM2, at positions where single substitutions have previously been shown to confer sensitivity to methanethiosulfonates. The receptors were expressed in HEK293 cells; interactions between the cysteines were sought by measuring the effects on ionic currents of dithiothreitol and methanethiosulfonates.
Nine pairs gave normally functioning channels: F44C/I328C, F44C/N333C, F44C/L338C, Q37C/I328C, Q37C/N333C, Q37C/T336C, Q37C/L338C, G30C/I328C, G30C/N333C. None formed functionally detectable disulfide bonds.
Currents at the F44C/L338C receptor had time course and ATP-sensitivity similar to those for the F44C mutation alone. Methyl-methanethiosulfonate bound to L338C but did not inhibit ionic current. Methyl-methanethiosulfonate inhibited currents at F44C, but not at F44C/L338C.
Ethylammonium-methylthiosulfonate inhibited currents at both F44C and L338C, but not at F44C/L338C. It reversed the rapid current deactivation at F44C/L338C.
The results suggest that a methanethiosulfonate binding to L338C prevents binding to F44C; this might indicate proximity of these two residues.
Keywords: P2X receptors, electrophysiology, cysteine mutagenesis, sulfhydryl group, methanethiosulfonates
Introduction
P2X receptors are multimeric proteins that function as ATP receptors and membrane ion channels. An ion permeation pathway opens upon activation by extracellular ATP at concentrations in the low micromolar range. The subunits are 379–595 amino acids in length, with intracellular N- and C-termini, two membrane-spanning domains (TM1 and TM2), and an ectodomain of about 280 amino acids (North & Barnard, 1997). Current evidence suggests that three or perhaps six subunits form a channel (Nicke et al., 1998). The P2X receptor subunits are unrelated in sequence to any other proteins or domains. Approaches to understanding the modus operandi of these channels have therefore depended on site-directed mutagenesis combined with expression in heterologous cells and measurements of ionic currents.
One of the most useful of such approaches has been cysteine mutagenesis. In the native receptor, the ectodomain of each protein has 10 cysteine residues, and their positions are completely conserved among all P2X receptor subunits. The normal function of the receptors, measured as the ability of ATP to open the ion channel, is unaffected by reducing agents such as dithiothreitol (DTT); this indicates that these cysteines are either not disulfided, or that they are disulfided but inaccessible to DTT (Rassendren et al., 1997; Clyne et al., 2002; Ennion & Evans, 2002). Substitution of these cysteines individually by alanine leads to the appearance of a free sulfhydryl group that can be labelled with biotinylated methanethiosulfonate (MTS) (Ennion & Evans, 2002) and systematic mutagenesis has been used to assign possible disulfide bonds in the native receptors (Clyne et al., 2002; Ennion & Evans, 2002).
The absence of any action of DTT or MTS derivatives on the native receptor has permitted approaches in which new cysteines have been introduced by mutagenesis; the function of the receptor has then been probed with sulfhydryl reactive MTS compounds or silver (Rassendren et al., 1997; Egan et al., 1998; Stoop et al., 1999; Jiang et al., 2000; 2001; Haines et al., 2001). Such experiments have identified positions in TM2 where, after introduction of cysteine, one or more MTS compounds reduce the currents elicited by ATP (Rassendren et al., 1997; Egan et al., 1998). From comparing the effects of MTS compounds of different size and charge, and by measuring the relative effects on inward and outward currents, it has been inferred that Thr336 lies in the outer vestibule of the channel but close to the conducting pathway. Ile328 and Asn333 may also be in the outer vestibule. However, currents through channels with substitutions L338C and D349C were inhibited by the permeant cation ethylammonium-methanethiosulfonate (MTSEA), but not by the less permeant cation trimethylethylammonium-methanethiosulfonate (MTSET) or impermeant anion sulfonatoethyl-methanethiosulfonate (MTSES); this suggests that these positions are still accessible to the aqueous environment, but further towards the cell interior. Because D349C was more accessible when the channel had been opened, it was suggested that it lies internal to a channel ‘gate'.
Positions in and around TM1 have also been identified at which the introduction of cysteine confers sensitivity to inhibition by MTS. A region some 15 amino acids towards the C-terminus from the end of TM1 (Ser65–Lys71) has been implicated in the binding of ATP. The attachment of negatively charged MTS compounds at I67C reduced the current evoked by ATP, but this was surmountable when the ATP concentration was increased. Within TM1 itself, G30C, Q37C and F44C substitutions result in channels blocked by the small neutral methyl-methanethiosulfonate (MTSM), but not by relatively larger, charged MTS compounds. V48C, positioned at the outer edge of TM1, shows block by all MTS compounds. Finally, in the double mutant V48C/I328C, ATP did not elicit any membrane current until the receptor has been treated with and reduced by DTT; this indicates that a disulfide bond formed between these two substituted cysteines prevents the protein movement necessary for channel opening. Other evidence also implicates the outer end of TM1 in channel gating (Jiang et al., 2001; Haines et al., 2001).
The demonstration of disulfide formation between cysteines at Val48 and Ile328 is an important finding not only because it indicates movements necessary for channel opening, but also because it places clear constraints on the proximity of these residues within the protein (Cα – Cα<8.6 Å calculated from bond lengths). The pattern of MTSM-accessible cysteines substituted into TM1 was suggestive of a helical conformation (Gly30, Gln37, Phe44, Val48: see Jiang et al., 2001), and we considered that others parts of such a helix might also lie in proximity to residues in TM2. Therefore, the main purpose of the present experiments was to identify further potential disulfide formation by introducing cysteines in pairs, one into each TM domain.
Methods
Site-directed mutagenesis of rat P2X2 receptor
All the single cysteine mutant rat P2X2 receptors were made in our previous studies (Rassendren et al., 1997; Jiang et al., 2001). The second cysteine mutation was introduced into the receptor already containing one single cysteine mutation by subcloning, and all the cDNA constructs were confirmed by sequencing.
Electrophysiological recording
Human embryonic kidney (HEK) 293 cells were used to express both wild-type and mutant rat P2X2 receptors by transient transfection of cDNA using Lipofectamine 2000 according to the manufacturer's instructions (Gibco). The transfected cells were re-plated on 35 mm coverslips and incubated at 37°C for 5–12 h prior to recording. Whole cell recordings were performed at room temperature using a HEKA EPC9 patch-clamp amplifier as described previously (Jiang et al., 2000). The membrane potential was held at −60 mV. The extracellular solution contained (mM): NaCl 147, KCl 2, MgCl2 1, CaCl2 2, HEPES 10, and glucose 13, and the intracellular solution comprised (mM): NaF 147, HEPES 10 and EGTA 10. All solutions were maintained at pH 7.3 and 300–315 mosmol l−1.
Chemicals used were purchased from Sigma, unless otherwise specified. MTS compounds were obtained from Toronto Research Chemicals (Ontario, Canada); MTSM is CH3-S(O2)-S-CH3, MTSEA is CH3-S(O2)-S-CH2-CH2-NH+, MTSES is CH3-S(O2)-S-CH2-CH2-SO3−, MTSET is CH3-S(O2)-S-CH2-CH2-CH2-N+(CH3)3). MTSM was made as 1 M stock in Me2SO and kept in frozen aliquots; other MTS compounds were made daily as 0.1 M stock in the extracellular solution and kept on ice. All MTS compounds were diluted to 1 mM in the extracellular solution immediately (⩽2 min) prior to application. Agonist and MTS compound applications were carried out using a RSC 200 system (Biologic Science Instruments, Grenoble, France).
ATP concentration-current curves were fitted using Kaleidagraph (Synergy Software, Reading, PA, U.S.A.) with I/Imax=([A]n/([A]n+EC50n)), where I is the current activated by a given agonist concentration ([A]) and expressed as % of the maximal currents (Imax). The data in the figures are shown as mean±s.e.mean, and tests for statistical significance were done using Student's t-test or ANOVA followed by Tukey post-hoc test.
Immunocytochemistry
All the P2X2 receptor cDNAs used in this study encoded an EYMPME epitope tag at the C terminus (Rassendren et al., 1997). This was detected by immunocytochemistry using a mouse monoclonal antibody (Babco, Richmond, CA, U.S.A.) at 1 : 1000 dilution. The secondary antibody (1 : 200) was tetramethyl rhodamine-isothiocyanate conjugated to anti-mouse IgG.
Results
Effects of pairs of cysteines in the transmembrane domain
We studied the properties of thirteen paired substitutions (F44C with I328C, N333C, T336C, or L338C; Q37C with I328C, N333C, T336C or L338C; G30C with I328C, N333C, T336C, I351C or L352C). In all except four pairs, ATP (30 μM) evoked substantial inward currents at −60 mV (0.5–5 nA) that were reproducible with repeated applications. For F44C/T336C, currents were <100 pA and immunocytochemistry indicated very poor membrane expression; for G30C/L352C, there were not detectable current, although immunocytochemistry indicated strong membrane expression. In the case of G30C/T336C and G30C/I351C, the current amplitude declined strongly with repeated applications of ATP. Currents were smaller (0.5–1 nA) in constructs containing Q37C than in the other mutants, in agreement with the smaller currents observed for Q37C alone (Jiang et al., 2001).
DTT (10 mM, 4–20 min) did not change the currents evoked by ATP (30 μM for 2 s every 2 min) in any of the 13 double mutants now tested. Thus, it appears that none of the pairs of cysteines that we have now tested are able to form detectable disulfides.
Scanning double cysteine mutants with MTSM
We chose MTSM for initial scanning studies because the side chain that it would attach is small and relatively non-polar(-S-CH3). This has the advantage of more accessibility to surfaces of the protein that might face lipid as well as water. It has the disadvantage that it may be less likely than a larger or charged MTS compound to alter the properties of the P2X receptor in the event that it does thiolate a free cysteine. Figure 1 summarizes the effect of MTSM on the nine double cysteine mutants that expressed well. In all except two cases (F44C/L338C and Q37C/L338C) the inhibition by MTSM of currents through the double-mutant channel was as might be expected from the inhibition observed for either one or other of the mutations expressed alone. For F44C/L338C, the double mutant showed almost no inhibition by MTSM (Figure 1) even though F44C alone was inhibited by about 70%; we therefore chose this pair for further study with a range of methanethiosulfonates.
Figure 1.
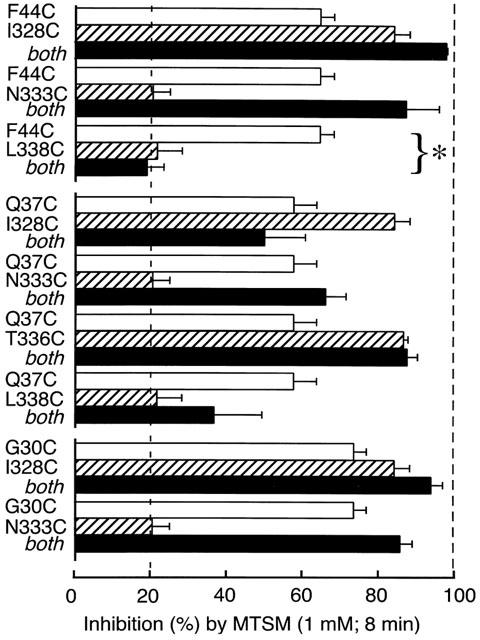
Summary of the effect of MTSM on single and double cysteine-substituted receptors. ATP (30 μM, 2 s) was used to elicit currents. Open bars indicate inhibition observed with single substitutions in TM1 (values from Jiang et al., 2001). Hatched bars indicate inhibition observed with single substitution in TM2. Solid bars indicate inhibition observed in double cysteine substitution. MTSM did not inhibit the double substitution F44C/L338C although it strongly inhibited the single substitution F44C (P<0.01). Bars show mean±s.e.mean (n=3–6 cells).
Effects of methanethiosulfonates on F44C, L338 and F44C/L338C
F44C alone
We have previously reported that substituting cysteine for phenylalanine at position 44 in TM1 leads to (a) a left-shift in the ATP concentration-response curve, (b) enhanced desensitization of the current, and (c) a slower decline of the current when ATP is washed out (Jiang et al., 2001). We also found that F44C is substantially inhibited by MTSM, though much less by MTSET or MTSES (Jiang et al., 2001). In the present experiments we again observed the changes associated with the mutation F44C, and showed that MTSEA also inhibited the currents evoked by ATP.
L338 alone
We found that application of MTSM (1 mM) did not inhibit currents in P2X2 [L338C]. This result was surprising, because currents in these channels are almost completely inhibited by MTSEA, though not by MTSES and MTSET (Rassendren et al., 1997). We wondered whether MTSM was attaching to the cysteine but not inhibiting the current. We found that after exposure to MTSM the blocking effect of MTSEA was completely lost for L338C, indicating that MTSM binds at these cysteines but the -S-CH3 side-chain is not effective to inhibit the current (Figure 2). MTSES and MTSET did not prevent subsequent inhibition by MTSEA, indicating that these compounds did not inhibit the current because they were not able to access the sulfhydryl group at L338C (Figure 2).
Figure 2.
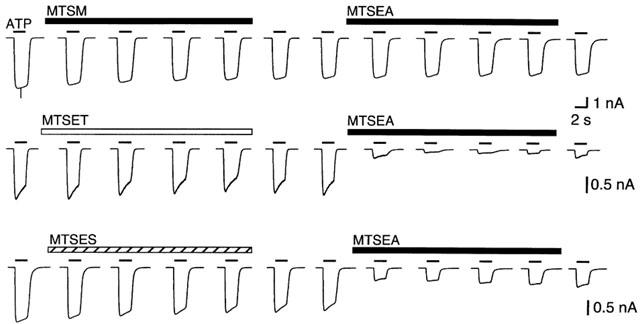
MTSM binds at L338C but does not inhibit currents. MTSM applied to the L338C receptor does not inhibit the current evoked by ATP (30 μM, 2 s, bars above traces), but it prevents the effect of a subsequent application of MTSEA, MTSET and MTSES do not inhibit the current themselves, and also do not block the inhibition by subsequent MTSEA. All MTS compounds applied at 1 mM for 8 min, as indicated by bars. Similar results were observed for at least three cells in each case.
F44C/L338C together
The concentration-response curve for the doubly-mutated channel (P2X2[F44C/L338C]) was similar to left-shifted curve of the P2X2[F44C] mutation (Figure 3). The desensitizing kinetics of the current also resembled those of the P2X2[F44C] (Figure 4). MTSM had little or no effect on the currents at the doubly-mutated channel (Figure 4A). We know that cysteines introduced at Phe44 and Leu338 are accessible from the aqueous phase, because MTSEA produced a significant inhibition in each of the single substitutions (F44C: 79.2±11.6%, n=3; L338C: 76.9±3.4%, n=4; see Rassendren et al., 1997; Jiang et al., 2001). However, MTSEA also had only a small effect on the peak amplitude of the current evoked by ATP (30 μM) in the case of the double mutant (inhibition was 26.2±4.3%, n=4) (Figure 4B).
Figure 3.
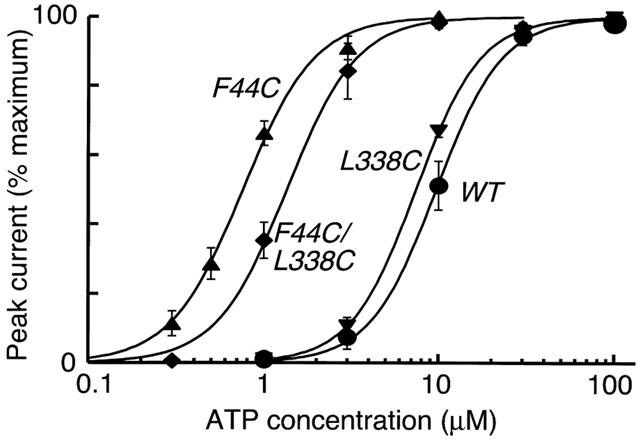
ATP sensitivity of F44C and F44C/L338C. Cysteine substitution for Phe44 causes a 10 fold increase in sensitivity to ATP (EC50 0.71±0.1 μM, n=5), whereas substitution of Leu338 has no effect (EC50 7.1±0.5 μM, n=4). The double substitution also has increased sensitivity (EC50 1.3±0.2 μM, n=3). Wild-type EC50 is 9.7±1.3 μM (n=3). F44C and F44C/L338C EC50 s are different; P<0.05 unpaired t-test.
Figure 4.
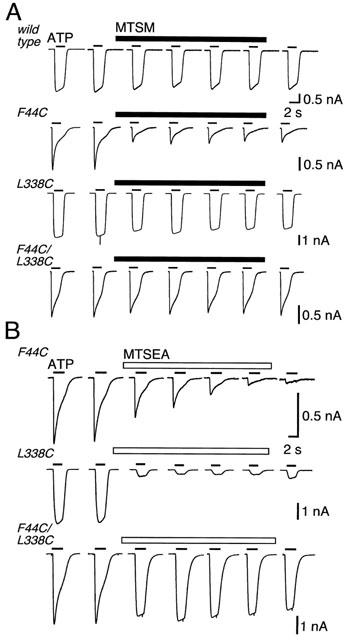
Methanethiosulfonates inhibit at F44C, L338C but not at F44C/L338C. Currents were evoked by application of ATP (30 μM, 2 s, bars above traces). (A) MTSM (1 mM, 8 min) does not inhibit currents at wild-type receptors or L338C receptors but it does inhibit currents at F44C. There is no inhibition of current at the double mutant F44C/L338C. (B) MTSEA (1 mM, 8 min) inhibits current at F44C and L338C receptors, but not at the double mutant F44C/L338C.
This could suggest that MTSM and MTSEA do not access either cysteine in the double-mutant channel. This seems unlikely; although MTSEA did not reduce the peak current elicited by 30 μM ATP, it still clearly altered the kinetics of the current (Figure 4B). Moreover, this action of MTSEA was prevented by prior exposure to MTSM; this indicates that MTSM can also attach at L338C in the double mutant. We interpret this to indicate that the access pathway to F44C is available when the side-chain at 338 is -CH2-CH-(CH3)2(leucine) or simply -CH2-SH (cysteine), but that it is blocked when the side chain is modified to -CH2-S-S-CH3 (with MTSM) or -CH2-S-S-CH2-CH2-NH4+ (with MTSEA).
We attempted to probe further the interaction between these two positions by studying the effect of MTSEA on the ATP dose-response curve. In some circumstances, this approach can provide information regarding the mechanism of inhibition by the methanethiosulfonates (Jiang et al., 2000; Colquhoun, 1998). We measured the currents elicited by different ATP concentrations before and 2 min after addition of MTSEA; there was no significant difference in the EC50 values for ATP.
Discussion
There are two main results from the present work. The first is that the pairs of cysteines introduced into TM1 and TM2 are insufficiently close to form disulfide bonds. One could argue that a disulfide bond is formed but this results in no detectable difference to the function of the channel; however, this seems to be unlikely because application of MTSM to the double-substituted channels had the effect that might be expected from its binding to one or other of the cysteines when they were studied in the singly-substituted channels (Figure 1; with the exception of F44C/L338C, see below). Therefore, we can conclude that V48C/N333C, V48C/T336C (Jiang et al., 2001), F44C/I328C, F44C/N333C, Q37C/I328C, Q37C/N333C, Q37C/T336C, Q37C/L338C, G30C/I328C, G30C/N333C, G30C/T336C, G30C/I351C and G30C/L352C seem not to form disulfides. We have examined only a limited number of pairs, and it might be considered that an extension of the approach would have resulted in the discovery of further pairs that can disulfide bridge.
The second main result is the interaction between Phe44 and Leu338 that is evident from the double cysteine substitution. It will be helpful to recapitulate the properties of the channels with single substitutions. We found that the introduction of cysteine alone into TM1 (F44C) alters the properties of the channel (it becomes more sensitive to ATP and it exhibits a rapidly desensitizing state; see Figures 3 and 4). The currents evoked by 30 μM ATP were strongly inhibited by MTSM and MTSEA (Figure 4); we have found previously that MTSES and MTSET cause only little inhibition at F44C (Jiang et al., 2001). It is known that MTSEA (but not MTSET or MTSES) can readily permeate the open channel, so these results might suggest that F44C is exposed to the aqueous environment of the ion channel. Introduction of cysteine alone into TM2 (L338C) gives rise to a channel that is not different from wild-type in terms of ATP sensitivity or kinetics of the current (Figures 2 and 3). The currents are strongly inhibited by MTSEA (Rassendren et al., 1997; MTSM can bind at this position but it does not cause inhibition; MTSET and MTSES do not bind and therefore do not inhibit (Figure 2). This finding is therefore consistent with earlier conclusions that L338 lies deeper within the ion channel than the cluster I328, N333, T336, for which current is inhibited by MTSET and MTSES, as well as MTSEA (Rassendren et al., 1997).
We found further that the double-substitution F44C/L338C produced a channel with the properties of F44C (desensitizing current, increased sensitivity to ATP). If we regard the properties of the F44C channel as the baseline phenotype, then the subsequent replacement of the -CH2-CH-(CH3)2 side chain by -CH2-SH at position 338 had no detectable effects on the channel function. However, in this double mutant, the presence of the cysteine at position Leu338 essentially abrogated the ability of MTS compounds to inhibit current by binding to F44C (Figures 1 and 4). This presumably occurs as a result of MTSM or MTSEA binding to the cysteine at position 338; MTSEA did act to alter the time course of the current (Figure 4) and prior exposure to MTSM prevented this effect (data not shown), indicating that MTSM could also still bind at L338C. Taken together, these results suggest that attachment of MTSM or MTSEA at L338C prevents any attachment at F44C. We consider less likely, but we cannot exclude, the possibility that the introduction of cysteine at L338C alters the orientation of the side-chain at F44C in such a way that thiolation by MTS compounds no longer has a detectable functional effect. Similarly, it is possible that the properties of the channel are also altered in unknown ways by the substitutions of polar cysteine residues in place of the hydrophobic phenylalanine and leucine.
Secondary structure prediction algorithms indicate that TM1 ends close to Val48, and TM2 begins around Asn333. In this case, the two residues would both be four or five residues toward the cell interior from the extracellular ends of the transmembrane domains (Figure 5). Inhibition by the water soluble MTSEA indicates that the side chains at Phe44 and Leu338 are exposed to the aqueous environment, but they do not show that they lie on the ion permeation pathway. The lack of inhibition by the larger MTSET and MTSES would be consistent with the residues being positioned on a conducting pathway that excludes these ions. It is also possible that they are within a water-filled pocket elsewhere in the protein, and that MTS side-chains at L338C stericly inhibit access to F44C (and Q37C). Finally, it should be borne in mind that none of the results presented here address the question of whether the interactions observed occur between residues in the same subunit, or between F44C in the TM1 of one subunit, and L338C in the TM2 of a different subunit of the homomeric channel. Notwithstanding these caveats, the present finding contributes further to our evolving view of the structure and function of the P2X receptor proteins.
Figure 5.
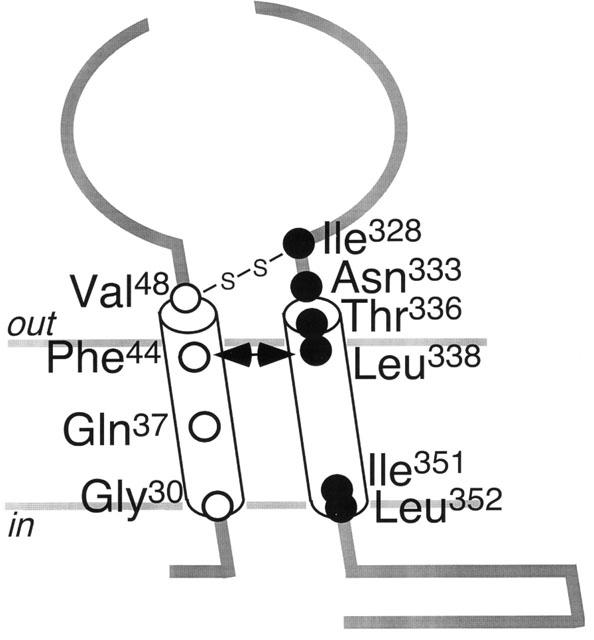
Schematic indication of the relative positions of residues substituted by cysteine. The connection between Val48 and Ile328 indicates a potential disulfide bond. The double-headed arrow indicates the interaction between Phe44 and Leu338.
Acknowledgments
We are grateful to François Rassendren for advice, and to Daniele Estoppey and Gareth Evans for help with cell culture. This work was supported by grants from the Wellcome Trust.
Abbreviations
- DTT
dithiothreitol
- EC50
concentration giving half maximal response
- HEK
human embryonic kidney
- MTS
methanethiosulfonate
- MTSEA
ethylammonium-methanethiosulfonate
- MTSES
sulfonatoethyl-methanethiosulfonate
- MTSET
trimethylethylammonium-methanethiosulfonate
- MTSM
methyl-methanethiosulfonate
- TM1
first transmembrane segment
- TM2
second transmembrane segment
References
- CLYNE J.D., WANG L.-F., HUME R.I. Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J. Neurosci. 2002;22:3873–3880. doi: 10.1523/JNEUROSCI.22-10-03873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLQUHOUN D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGAN T.M., HAINES W.R., VOIGHT M.M. A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J. Neurosci. 1998;18:2350–2359. doi: 10.1523/JNEUROSCI.18-07-02350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNION S.J., EVANS R.J. Conserved cysteine residues in the extracellular loop of the human P2X1 receptor form disulfide bonds and are involved in receptor trafficking to the cell surface. Mol. Pharmacol. 2002;61:303–311. doi: 10.1124/mol.61.2.303. [DOI] [PubMed] [Google Scholar]
- HAINES W.R., VOIGT M.M., MIGITA K., TORRES G.E., EGAN T.M. On the contribution of the first transmembrane domain to whole-cell current through an ATP-gated ionotropic P2X receptor. J. Neurosci. 2001;21:5885–5892. doi: 10.1523/JNEUROSCI.21-16-05885.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG L.-H., RASSENDREN F., SPELTA V., SURPRENANT A., NORTH R.A. Amino acid residues involved in gating identified in the first membrane-spanning domain of the rat P2X2 receptor. J. Biol. Chem. 2001;276:14902–14908. doi: 10.1074/jbc.M011327200. [DOI] [PubMed] [Google Scholar]
- JIANG L.-H., RASSENDREN F., SURPRENANT A., NORTH R.A. Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J. Biol. Chem. 2000;275:34190–34196. doi: 10.1074/jbc.M005481200. [DOI] [PubMed] [Google Scholar]
- NICKE A., BAUMERT H.G., RETTINGER J., EICHELE A., LAMBRECHT G., MUTSCHLER E., SCHMALZING G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A., BARNARD E.A. Nucleotide receptors. Curr. Opin. Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- RASSENDREN F., BUELL G., NEWBOLT A., NORTH R.A., SURPRENANT A. Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO J. 1997;16:3446–3454. doi: 10.1093/emboj/16.12.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOOP R., THOMAS S., RASSENDREN F., KAWASHIMA E., BUELL G., SURPRENANT A., NORTH R.A. Contribution of individual subunits to the multimeric P2X2 receptor: estimates based on methanethiosulfonate block at T336C. Mol. Pharmacol. 1999;56:973–981. doi: 10.1124/mol.56.5.973. [DOI] [PubMed] [Google Scholar]


