Abstract
This study was designed to investigate the effect of 5-hydroxytryptamine (5-HT) and to characterize the 5-HT receptors involved in 5-HT responses in the pig intravesical ureter.
5-HT (0.01–10 μM) concentration-dependently increased the tone of intravesical ureteral strips, whereas the increases in phasic contractions were concentration-independent. The 5-HT2 receptor agonist α-methyl 5-HT, mimicked the effect on tone whereas weak or no response was obtained with 5-CT, 8-OH-DPAT, m-chlorophenylbiguanide and RS 67333, 5-HT1, 5-HT1A, 5-HT3 and 5-HT4 receptor agonists, respectively. 5-HT did not induce relaxation of U46619-contracted ureteral preparations. Pargyline (100 μM), a monoaminooxidase A/B activity inhibitor, produced leftward displacements of the concentration-response curves for 5-HT.
5-HT-induced tone was reduced by the 5-HT2 and 5-HT2A receptor antagonists ritanserine (0.1 μM) and spiperone (0.2 μM), respectively. However, 5-HT contraction was not antagonized by cyanopindolol (2 μM), SDZ–SER 082 (1 μM), Y-25130 (1 μM) and GR 113808 (0.1 μM), which are respectively, 5-HT1A/1B, 5-HT2B/2C, 5-HT3, and 5-HT4 selective receptor antagonists.
Removal of the urothelium did not modify 5-HT-induced contractions. Blockade of neuronal voltage-activated sodium channels, α-adrenergic receptors and adrenergic neurotransmission with tetrodotoxin (1 μM), phentolamine (0.3 μM) and guanethidine (10 μM), respectively, reduced the contractions to 5-HT. However, physostigmine (1 μM), atropine (0.1 μM) and suramin (30 μM), inhibitors of cholinesterase activity, muscarinic- and purinergic P2-receptors, respectively, failed to modify the contractions to 5-HT.
These results suggest that 5-HT increases the tone of the pig intravesical ureter through 5-HT2A receptors located at the smooth muscle. Part of the 5-HT contraction is indirectly mediated via noradrenaline release from sympathetic nerves.
Keywords: 5-hydroxytryptamine, smooth muscle contraction, 5-HT agonists and antagonists, 5-HT2A receptors, pig intravesical ureter
Introduction
5-Hydroxytryptamine (5-HT) produces a wide variety of mechanical effects such as contraction, relaxation or both responses in the mammalian urinary tract by either a direct action on the smooth muscle cells or by indirect effects on the autonomic intramural neurons. Thus, 5-HT induces contraction of the detrusor muscle through a mechanism which involves ketanserine-sensitive muscular 5-HT2 receptors in man (Klarskov & Hørby-Petersen, 1986) and dog (Cohen, 1990) and neural 5-HT3 receptors in the rabbit (Chen, 1990) and guinea-pig (Messori et al., 1995), by enhancing cholinergic transmission. Moreover, an inhibitory action of 5-HT in monkey urinary bladder, through muscular 5-HT4 receptors, has been described (Waikar et al., 1994).
5-HT receptors are classified into four major subtypes, namely 5-HT1 (1A, 1B, 1D, 1e, 1f) (negatively coupled toadenylate cyclase), 5-HT2 (2A, 2B, 2C) (positively coupled to phospholipase C), 5-HT3 (ligand-gated ion channel) and 5-HT4 (positively coupled to adenylate cyclase). In addition, molecular cloning studies have identified three other less well-defined subtypes (5-HT5, 5-HT6, 5-HT7), which can be discriminated by their relative agonist potency. Thus, lysergic acid (LSD) shows relatively high affinity, in comparison to 5-HT and 5-carboxamidotryptamine (5-CT), at the 5-HT5 subtype (LSD>5-CT>5-HT). At 5-HT6 receptors, 5-CT is less potent than 5-HT and 5-methoxytryptamine (5-MeOT) (5-MeOT>5-HT>5-CT). Finally, 5-CT shows higher affinity than 5-HT, LSD and 5-MeOT at 5-HT7 (5-CT>5-HT>5-MeOT>LSD) (Boess & Martin, 1994; Hoyer et al., 1994).
5-HT has been localized in the urothelium of the mammalian urinary tract (Fetissof et al., 1983) and its role on ureteral activity is not yet well understood. Either in vitro (Gidener et al., 1995, 1999) or in vivo (Hauser et al., 2002) pharmacological studies have shown that 5-HT evokes ureteral contractions. However, variations have been evidentiated with regard to the subtype of 5-HT receptor involved in these responses on the basis of the pharmacogical model (in vitro or in vivo) and the species used. The present study was undertaken, therefore, to analyse the effect(s) of 5-HT and to characterize further the 5-HT receptors mediating these responses of the isolated pig intravesical ureter.
Methods
Adult pigs of either sex with no lesions in their urinary tract were selected from the local slaughterhouse. Urinary bladders with attached ureters were removed immediately after the animals were killed, and kept in chilled (4°C) physiological saline solution (PSS). The adjacent connective and fatty tissues were removed with care and longitudinal preparations (4–6 mm long and 2–3 mm wide) of the intravesical ureter were isolated from the bladder by dissection, as previously described (Hernández et al., 1992). The ureteral strips were suspended horizontally with one end connected to an isometric transducer (Grass FT 03C) and the other one to a micrometer screw which regulates the tension applied to the preparations, in 5 ml organ baths. The signal was continuously recorded on a polygraph (Graphtec Multicorder MC 6621, Hugo Sachs Elektronik, Germany). Passive tension of 2 g was applied to the preparations and they were allowed to equilibrate for 60 min.
Experimental procedure
At the beginning of each experiment, the contractile capacity of the samples was tested by exposing the ureteral strips to 124 mM potassium enriched PSS (KPSS). Increases in basal tone (g) were examined by single application of increasing concentrations of 5-HT and tryptamine analogues with a washout period between two consecutive concentrations.
Due to the development of a strong tachyphylaxis of the tissue to the agonists, two consecutive concentration-response curves could not be constructed in the same preparation. Thus, the antagonist study was performed in adjacent preparations of the same animal run in parallel, one of them used as control and the other one treated with the 5-HT receptor antagonist under study for 30 min before 5-HT was added. Treatment with pargyline, an irreversible MAO A/B activity inhibitor, was during a period of 1 h and then this drug was removed from the organ bath. Incubation with guanethidine to block the adrenergic neurotransmission was during a period of 1 h washing every 20 min and this drug was present throughout the experiment.
To investigate whether 5-HT or the 5-HT1 and 5-HT4 receptor agonist 5-CT and RS 67333, respectively, induced relaxation, the strips were incubated for 30 min with the 5-HT2 receptor antagonist ritanserine (0.1 μM), to block the 5-HT-induced contraction. The preparations were contracted with the thromboxane analogue, U46619 (0.1 μM), and when the contraction was stable increasing concentrations of the 5-HT receptor agonists were added.
Drugs and solutions
The following drugs were used: atropine (Sigma, U.S.A.), m-chlorophenylbiguanide (Tocris, U.K.), 5-CT (5-carboxamidotryptamine, Tocris), cyanopindolol (Tocris), GR 113808 (1-methyl-1H-indole-3-carboxylic acid, [1-2[(methylsulfonyl)amino]ethyl]-4-piperidinyl]methyl ester, Tocris), guanethidine (Sigma), 8-OH-DPAT (8-hydroxy-2-dipropylaminotetralin hydrobromide, Tocris), 5-HT (5-hydroxytryptamine, Sigma), α-methyl-5-HT (α-methyl-5-hydroxytryptamine, Tocris), pargyline (Sigma), phentolamine (Sigma), physostigmine (Sigma), ritanserine (Sigma), RS 67333 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-butyl-4-piperidinyl]-1-propanone hydrochloride, Tocris), SDZ SER 082 ((+)-cis-4,5,7a,8,9,10, 11,11a-octahydro-7H-10-methylindolo[1,7-bc][2,6]-naphthyridine fumarate, Tocris), spiperone (Tocris), suramin (Sigma), TTX (tetrodotoxin, Sigma), U46619 (9,11-dideoxy-11(,9α-epoxy-methanoprostaglandin F2α, Sigma), VIP (vasoactive intestinal peptide, Sigma), Y-25130 (N-(1-azabicyclo[2,2,2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxamide hydrochloride, Tocris).
Physostigmine, spiperone and U46619 were dissolved in 96% ethanol, while GR 113808 and cyanopindolol were dissolved in dimethyl sulphoxide and further diluted in distilled water. The other drugs were dissolved in distilled water. The solvents had no effect on the preparation.
The composition of PSS was (mM): NaCl 119, KCl 4.6, MgCl2 1.2, NaHCO3 24.9, glucose 11, CaCl2 1.5, KH2PO4 1.2, EDTA (ethylenediamine tetraacetic acid) 0.027. The solution was continuously gassed at 37°C with 95% O2 and 5% CO2, to maintain pH at 7.4. KPSS (124 mM) was PSS with KCl exchanged for NaCl on an equimolar basis. Stock solutions were prepared daily in distilled water.
Calculations and statistics
For each concentration-response curve, the concentration required to give half-maximal response (EC50) to 5-HT and related analogues was estimated by computerized non linear regression analysis (GraphPad InPlot 4, U.S.A.). The sensitivity of the drugs is expressed in terms of pD2, where pD2 is defined as the negative logarithm of EC50 (pD2=−logEC50 [M]). Each parameter was determined from ureters of at least 4–6 different animals. Results are showed as percentage of maximal reponse induced by KPSS. Statistical significance of differences was calculated by Student's t-test, for unpaired observations for individual concentrations and variance analysis (ANOVA) for multiple comparisons, followed by an a posteriori Bonferroni test. Differences were considered significant with a probability level of P<0.05.
Results
The pig intravesical ureteral strips were allowed to equilibrate to a passive tension of 1.7±0.4 g (n=93). Under these conditions the exposition of samples to 124 mM KPSS evoked a contraction of 2.3±0.4 g (n=93).
Contraction induced by 5-HT and tryptamine analogues in the pig intravesical ureter
5-HT (0.01–10 μM) induced increases in the two contractile components (phasic activity and basal tone) of pig intravesical ureter. In all strips, concentrations up to 0.3 μM 5-HT essentially evoked the elevation of intravesical ureteral basal tone (Figures 1, 2, Table 1). 5-HT also caused the generation or the increase of phasic contractions in quiescent segments or in those which showed spontaneous activity, respectively, but this effect was manifested as concentration independent and often masked by the superimposed increased tone.
Figure 1.
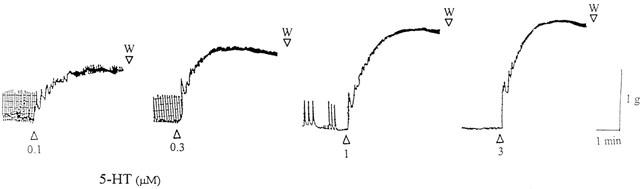
Isometric force recordings showing the contractions to 5-hydroxytryptamine (5-HT) (0.1–3 μM) in isolated pig intravesical ureter. Vertical bar shows tension in g and horizontal bar time in min. Numbers indicate micromolar concentration in the organ bath. W: wash out.
Figure 2.
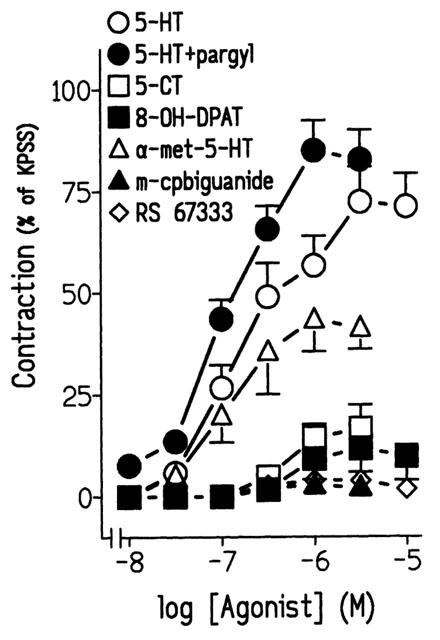
Log concentration-response contraction curves to 5-hydroxytryptamine (5-HT), 5-HT plus pargyline (100 μM), 5-carboxamidotryptamine (5-CT), 8-hydroxy-DPAT (8-OH-DPAT), α-methyl-5-hydroxytryptamine (α-methyl-5-HT), m-chlorophenylbiguanide (m-cpbiguanide) and 1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-butyl-4-piperidinyl]-1-propanone hydrochloride (RS 67333) in pig intravesical ureter. Contractions are expressed as a percentage of the 124 mM KPSS-induced contraction. Results represent means and vertical line s.e. of the mean of 6–10 preparations.
Table 1.
Contractions induced by 5-HT and tryptamine analogues in isolated pig intravesical ureter
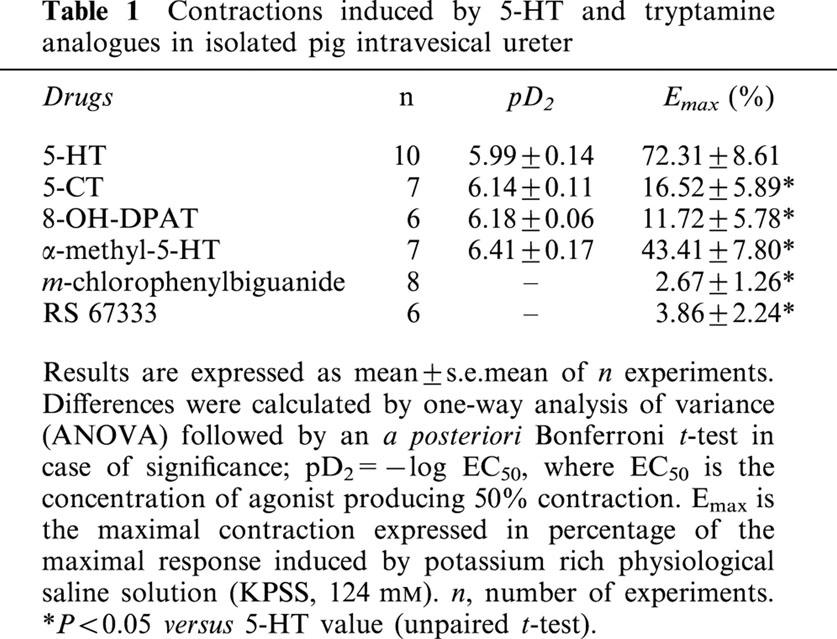
α-methyl-5-HT, a selective 5-HT2 receptor agonist, induced a significant increase of the ureteral tone. 5-CT, 8-OH-DPAT, m-chlorophenylbiguanide and RS 67333, which are respectively, 5-HT1, 5-HT1A, 5-HT3 and 5-HT4 receptor agonists, caused either no or only small increases in ureteral tone (Figure 2, Table 1).
Treatment with pargyline (100 μM), a monoaminooxidase (MAO) A/B activity inhibitor, produced a leftward displacement yielding a monophasic sigmoidal concentration-contraction curve to 5-HT. Furthermore, the pD2 values and maximal contraction to 5-HT were 6.10±0.07 and 7.01±0.09 (P<0.05, unpaired t-test, n=10) and 74.33±8.36% and 84.96±7.43% (P>0.05, unpaired t-test, n=10) (Figure 2) in the absence and presence of pargyline, respectively. Pargyline enhanced 5-HT-induced tone with no effect on the ureteral phasic activity. Due to the marked effect of the MAO blocker, the subsequent experiments, were carried out after the preparations had been treated with pargyline.
Effects of ritanserine, spiperone, SDZ-SER 082, cyanopindolol, Y-25130 and GR 113808 on5-HT-induced tone
Pretreatment of ureteral strips with ritanserine (0.1 μM) and spiperone (0.2 μM), 5-HT2 and 5-HT2A receptor antagonists, respectively, reduced the contractions to 5-HT (Figure 3a,b, Table 2) . Such contractions, however, were resistant to incubation with SDZ SER 082 (1 μM), cyanopindolol (2 μM), Y-25130 (1 μM) and GR 113808 (0.1 μM), which are respectively, 5-HT2B/2C, 5-HT1A/1B, 5-HT3, and 5-HT4 selective receptor antagonists (Figures 3c; 4a,b,c, Table 2). Combined treatments of Y-25130 plus ritanserine and GR 113808 plus ritanserine evoked a reduction of the 5-HT-induced contraction similar to that induced by ritanserine alone (Table 2).
Figure 3.
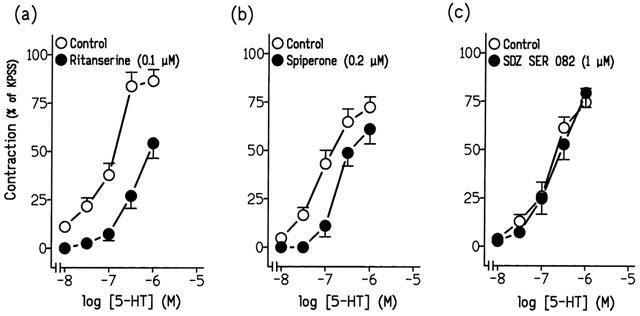
Log concentration-response contraction curves to 5-hydroxytryptamine (5-HT) in pig intravesical ureteral strips in control conditions or in the presence of (a) 0.1 μM ritanserine, (b) 0.2 μM spiperone or (c) 1 μM SDZ SER 082. The contractions are expressed as a percentage of the 124 mM KPSS-induced contraction. Results represent means and vertical line s.e. of the mean of 6–8 preparations.
Table 2.
Effect of ritanserine, spiperone, SDZ SER 082, cyanopindolol, Y-25130, GR 113808 and Y-25130 (1 μM) or GR 113808 (0.1 μM) plus ritanserine (0.1 μM) on 5-HT-induced tone of isolated pig intravesical ureter
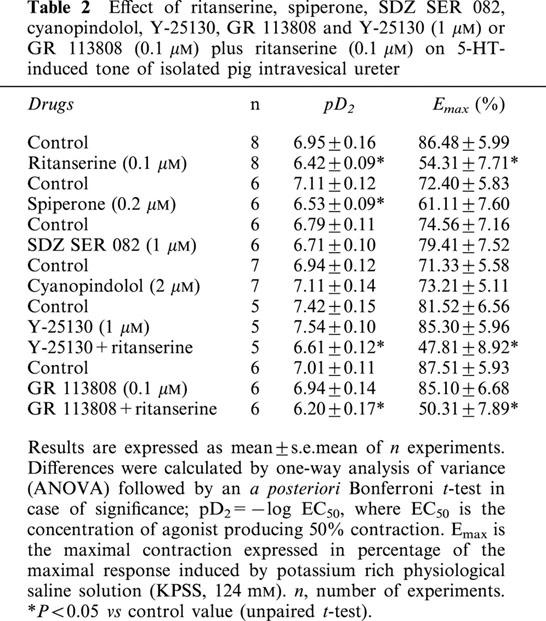
Figure 4.
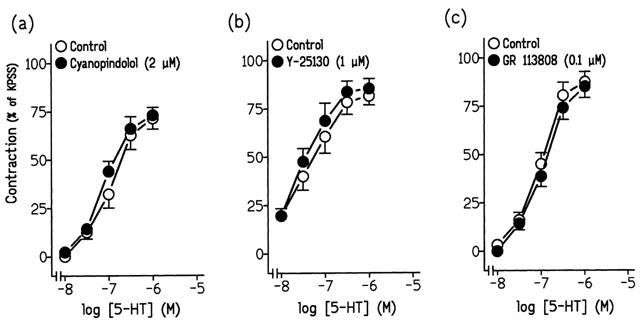
Log concentration-response contraction curves to 5-hydroxytryptamine (5-HT) in pig intravesical ureteral strips in control conditions or in the presence of (a) 2 μM cyanopindolol, (b) 1 μM Y-25130 or (c) 0.1 μM GR 113808. The contractions are expressed as a percentage of the 124 mM KPSS-induced contraction. Results represent means and vertical line s.e. of the mean of 5–7 preparations.
Effects of urothelium denudation, tetrodotoxin, guanethidine, phentolamine, atropine, physostigmine and suramin on 5-HT-induced tone
Exposition of urothelium-denuded strips to 124 mM KPSS induced a contraction of 2.2±0.6 g (n=6). Urothelium removal failed to modify the contractions to 5-HT. Thus, the pD2 values and maximal response to 5-HT were 7.22±0.07 and 7.19±0.09 (P>0.05, unpaired t-test, n=6) and 63.95±6.27% and 68.90±6.31% (P>0.05, unpaired t-test, n=6) (Figure 5a) in the presence and absence of urothelium, respectively.
Figure 5.
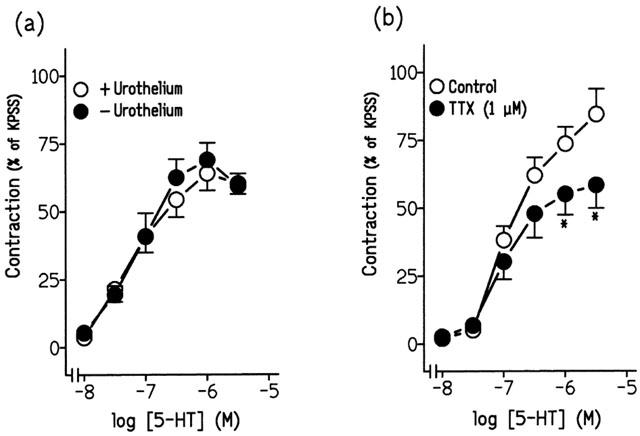
(a) Log concentration-response contraction curves to 5-hydroxytryptamine (5-HT) in pig intravesical ureteral strips in the presence and in the absence of urothelium. (b) Log concentration-response contraction curves to 5-HT in control conditions or in the presence of 1 μM tetrodotoxin (TTX). The contractions are expressed as a percentage of the 124 mM KPSS-induced contraction. Results represent means and vertical line s.e. of the mean of six preparations. *P<0.05 vs control value (unpaired t-test).
Incubation with TTX (1 μM) (Figure 5b, Table 3), guanethidine (10 μM) and phentolamine (0.3 μM), inhibitors of neuronal voltage-activated sodium channels, adrenergic neurotransmission and α-adrenergic receptors, respectively, reduced the contractions to 5-HT (Figure 6a,b, Table 3). However, physostigmine (1 μM), atropine (0.1 μM) and suramin (30 μM), inhibitors of cholinesterase activity, muscarinic- and purinergic P2-receptors, respectively, failed to modify these contractions (Table 3).
Table 3.
Effect of tetrodotoxin (TTX), guanethidine, phentolamine, atropine, physostigmine and suramin on 5-HT-induced tone of isolated pig intravesical ureter
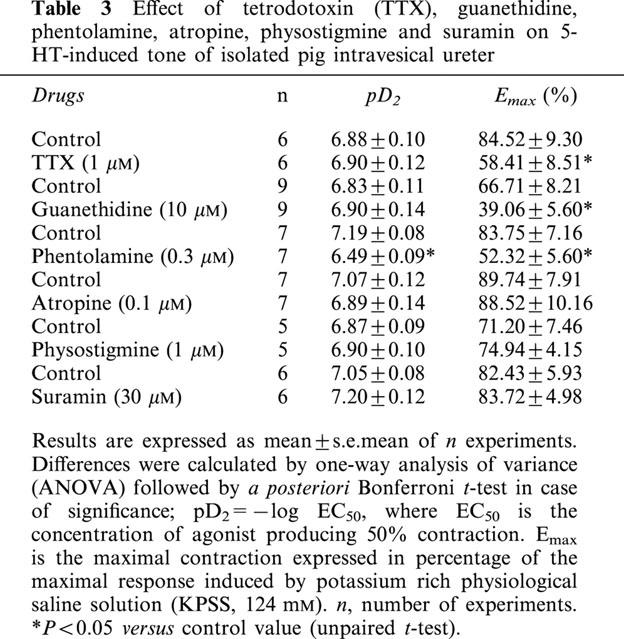
Figure 6.
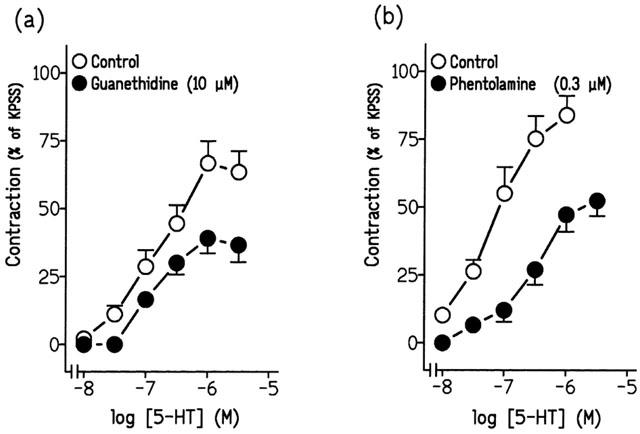
Log concentration-response contraction curves to 5-hydroxytryptamine (5-HT) in pig intravesical ureteral strips in control conditions or in the presence of (a) 10 μM guanethidine or (b) 0.3 μM phentolamine. The contractions are expressed as a percentage of the 124 mM KPSS-induced contraction. Results represent means and vertical line s.e. of the mean of 7–9 preparations.
Effect of 5-HT, 5-CT and RS 67333 on U46619-induced tone in porcine intravesical ureter
In ritanserine (0.1 μM)-treated ureteral samples, the thromboxane analogue, U46619 (0.1 μM) evoked a sustained contraction of 1.5±0.3 g (n=16). On the U46619-induced tone, 5-HT, 5-CT and RS 67333, did not induce any relaxation of the porcine intravesical ureteral strips. VIP (0.1 μM) relaxed the same preparations by 81.6±5.1% (n=16).
Discussion
The present study was designed in order to show the effect of 5-HT and to characterize the 5-HT receptor(s) involved in the activity of the isolated pig intravesical ureter. For this aim we used selective agonists and antagonists to determine the 5-HT receptor subtypes that mediate these effects. 5-HT and α-methyl-5-HT, a selective 5HT2 receptor agonist, concentration-dependently contracted the pig intravesical ureter, whereas 5-CT, 8-OH-DPAT, m-chlorophenylbiguanide and RS 67333, 5-HT1, 5-HT1A, 5-HT3 and 5-HT4 receptor agonists, respectively, failed to increase the ureteral tone. 5-HT-induced relaxation was not observed in our preparation. These results, together with the observations that ritanserine and spiperone antagonize 5-HT-induced contraction, suggest that 5-HT increases tone in the pig intravesical ureter by activation of 5-HT2A receptors. The lack of effect of the urothelium removal and the inhibitory effect caused by TTX, guanethidine and phentolamine on 5-HT-induced contractions suggests that part of this contractile effect is indirectly mediated through NA release from sympathetic nerves.
Recently, in vivo studies performed in the pig ureter showed that the intravenous administration of 5-HT and DOI, a 5-HT2A/2C receptor agonist, increases the frequency of ureteral contractions in a concentration-dependent manner (Hauser et al., 2002). In the present study 5-HT preferentially increased tone in the isolated intravesical ureter. Phasic contractions were induced or increased in the presence of 5-HT in the pig intravesical ureter, but the frequency of the contractions were not related to the concentration of 5-HT. Moreover, the phasic contractions were usually abolished by the marked 5-HT-evoked increases in ureteral tone. The different treatments used in our experimental protocol inhibited the 5-HT-evoked tone without affecting the frequency of ureteral phasic activity. The observed difference between the contraction produced by 5-HT in the in vivo (stimulation of peristaltic activity) and in vitro (elevation of basal tone) models could be explained on the basis of the ureteral segment used in the present study. Thus, isolated intravesical ureter is the ureteral component of the ureterovesical junction and it functions as a sphincter whose peristaltic contractions facilitate progression of the urine bolus during bladder filling and its closure avoids vesicoureteral reflux at micturition (Blok et al., 1985; Hernández et al., 1993; 1995; 1999; Prieto et al., 1997). At this level, 5-HT could be more related to the closure of the ureter once the urine bolus has been discharged into the urinary bladder, rather than to the stimulation of phasic contractions.
In some preparations as rat stomach, contractions evoked by tryptamine and analogues were potentiated by MAO inhibitors (Handschumacher & Vane, 1967). In the present study, pargyline (100 μM), an irreversible MAO A/B inhibitor, also caused a potent leftward displacement of the concentration-dependent contraction curve to 5-HT, suggesting the presence of a MAO barrier with high enzymatic activity which precludes the access of 5-HT to its receptor(s) in this preparation. Due to this reason the rest of our experimental protocol was performed in the presence of the MAO A/B activity inhibitor.
The possible involvement of 5-HT1 receptors in the ureteral contractions to 5-HT is still unclear. Thus, in the isolated human ureter (Gidener et al., 1999) 5-CT, a selective agonist of these receptors (Humphrey, 1984), evoked smooth muscle contraction. However, in our study, the slight contractile response induced by 5-CT and 8-OH-DPAT (Bjork et al., 1991), which show high and moderate affinity for 5-HT1A and 5-HT7, respectively, as well as the lack of inhibitory effect shown by cyanopindolol (Engel et al., 1986), a selective 5-HT1A/1B antagonist, indicate that these receptors do not seem to be involved in the 5-HT-induced tone.
5-HT2 receptors have previously been reported to mediate 5-HT contraction in human (Klarskov & Hørby-Petersen, 1986) and dog (Cohen, 1990) urinary bladder. In the pig intravesical ureter, α-methyl-5-HT, a selective 5-HT2 agonist (Humphrey, 1984) was the unique tryptamine analogue that increased ureteral basal tone. In addition, the antagonism afforded by ritanserine and spiperone (Hoyer et al., 1994) and the lack of effect of SDZ SER 082 (Nozulak et al., 1995), a selective 5HT2B/2C receptor antagonist with low affinity for 5-HT2A, on contractions induced by 5-HT in the ureteral strips, indicate that the effects of 5-HT are mediated through activation of a 5-HT2A receptor subtype. These results differ from those found in the human isolated upper ureter where the lack of contractile effect of DOI, a 5-HT2A receptor agonist, and the weak antagonism of ketanserine suggested that these receptors were not involved (Gidener et al., 1999). Our results, however, are consistent, in part, with those reported in the pig in vivo model, where the excitatory action of 5-HT and DOI on ureteral phasic contractions was reversed by the 5-HT2A/2C receptor antagonist ketanserine indicating, thus, the activation of these receptors (Hauser et al., 2002). In the pig isolated intravesical ureter, the involvement of a sole 5-HT2A subtype was supported by the reduction caused by spiperone, a selective 5-HT2A antagonist, and by the fact that incubation with SDZ SER 082 (Nozulak et al., 1995), an antagonist that shows high and low affinity for 5-HT2B/2C and 5HT2A, respectively, failed to modify the 5-HT-induced contractions.
5-HT3 receptors have been described in the urinary bladder of the rabbit (Chen, 1990), where they induce indirect contractile effects which are due to neurally released ACh and ATP. In the pig intravesical ureter, m-chlorophenylbiguanide (Kilpatrick et al., 1990), a selective 5-HT3 receptor agonist, failed to increase the ureteral tone. Moreover, Y-25130, a potent selective 5-HT3 receptor antagonist (Fukuda et al., 1991), did not modify the contractions induced by 5-HT and combined treatment of Y-25130 plus ritanserine did not cause greater antagonism than that caused by ritanserine alone. These results exclude the idea that 5-HT3 receptors are involved in the 5-HT-induced ureteral tone.
5-HT4 receptors have been described in the urinary bladder, where they locate prejunctionally causing ACh and ATP release, as reported in the guinea-pig (Messori et al., 1995) and man (Tonini et al., 1994). In the pig intravesical ureter, RS 67333 (Eglen et al., 1995), a selective 5-HT4 receptor agonist, did not increase the ureteral basal tone. Moreover, GR 113808, a selective 5-HT4 receptor antagonist (Gale et al., 1994), failed to reduce 5-HT contractions. The antagonism exerted by this blocker plus ritanserine was indistinguishable from that afforded by ritanserine alone. Therefore it is unlikely that 5-HT4 receptors are involved in the contraction to 5-HT in the pig intravesical ureter.
In our study, mechanical denudation of the urothelium failed to modify the tone induced by 5-HT, indicating that these responses are mediated via a receptor located at the ureteral smooth muscle. These results agree with previous studies showing 5-HT evokes contraction of the isolated human ureter (Gidener et al., 1995; 1999) by activation of smooth muscle 5-HT receptors. However, the inhibition caused by TTX, guanethidine and phentolamine on 5-HT-induced tone suggests that part of this excitatory action is evoked indirectly via NA release from noradrenergic terminals.
It has previously been reported that 5-HT may differentially affect ACh or ATP release from guinea-pig urinary bladder by activating at least three different excitatory receptors, presumably located at different sites including the neuronal cell body (5-HT3 receptors) and axon (5-HT2A and 5-HT4 receptors) (Messori et al., 1995). In the present study, the involvement of a neuronal mechanism in 5-HT contraction of the pig intravesical ureter was supported by the action of TTX which attenuated the contraction induced by high concentrations (1 and 3 μM) of 5-HT. However, the lack of inhibitory effect exhibited by physostigmine, atropine and suramin, cholinesterase, muscarinic- and purinergic P2-receptor inhibitors, respectively, on 5-HT-induced contractions excludes that 5-HT contraction involves ACh and ATP release in the pig intravesical ureter. These results agree with those reported in the human ureter ruling out a facilitatory role for 5-HT in ureteral cholinergic transmission (Gidener et al., 1995).
5-HT is known to modulate peripheral sympathetic neurotransmission primarily by inhibition of NA release through prejunctional 5-HT1-like receptors now identified as a mixture of 5-HT1B, 5-HT1D and 5-HT7 (Saxena et al., 1998), but also by enhancing the adrenergic neurotransmitter release through facilitatory presynaptic 5-HT1A receptors (Cohen et al., 1999). In our study, guanethidine and phentolamine, adrenergic neurotransmission and α-adrenergic receptors blockers, respectively, markedly reduced the 5-HT-induced contractions, thus suggesting that part of the contraction induced by 5-HT is indirectly mediated by NA release from adrenergic nerves acting on α-adrenoceptors. NA stimulates both phasic and tonic contractions of the pig intravesical ureter through α1 and α2 adrenoceptors (Hernández et al., 1992) and therefore, NA released in response to 5-HT could contribute to the contractile effect of 5-HT at this level of the urinary tract.
The lack of effect shown by the selective 5-HT1, 5-HT3 and 5-HT4 receptor antagonists on 5-HT-induced contractions, seems to discard the involvement of these receptor subtypes as putative prejunctional excitatory 5-HT receptors causing NA release in our preparation. Moreover, indirect effects of high concentrations of 5-HT to enhance NA release have been also reported, effects thought to be mediated by 5-HT uptake into the adrenergic terminals and the subsequent displacement of NA from neuronal stores (Marín et al., 1981). This could explain the observed effects of 5-HT releasing NA in the pig intravesical ureter.
In addition to its contractile effect, a relaxation to 5-HT mediated by 5-HT4 receptors has been reported in monkey urinary bladder (Waikar et al., 1994). Due to the fact that 5-HT2A receptors mediate the contractions to 5-HT in the pig intravesical ureter, the blockade of these receptors is a requisite for the direct relaxant activity of 5-HT and some 5-HT receptor agonists can be manifested. Thus, on U46619-contracted intravesical ureteral samples pretreated with 0.1 μM ritanserine to block the 5-HT2A subtype, 5-HT, 5-CT and RS 67333 failed to induce smooth muscle relaxation suggesting that 5-HT only evokes contraction in the pig intravesical ureter.
In conclusion, the results of the present work suggest that 5-HT induces a contractile effect through activation of 5-HT2A receptors in the pig intravesical ureter. This effect involves both neuronal and non-neuronal mechanisms, the former being related to NA release from noradrenergic nerves that would enhance 5-HT excitatory action at smooth muscle. The 5-HT-induced tone at this level suggests that 5-HT would be involved in the closure of intravesical ureteral wall rather than in the stimulation of phasic peristaltic activity.
Acknowledgments
The authors thank Mr Manuel Perales and Mr Francisco Puente for their technical assistance. They also wish to thank GIPISA slaughterhouse (Pozuelo de Alarcón, Madrid) for kindly donating the ureters.
Abbreviations
- 5-CT
5-carboxamidotryptamine
- GR 113808
1-methyl-1H-indole-3-carboxylic acid, [1-2[(methylsulfonyl)amino]ethyl]-4-piperidinyl]methyl ester
- 8-OH-DPAT
8-hydroxy-2-dipropylaminotetralin hydrobromide
- α-methyl-5-HT
α-methyl-5-hydroxytryptamine
- RS 67333
1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-butyl-4-piperidinyl]-1-propanone hydrochloride
- SDZ SER 082
(+)-cis-4,5,7a,8,9,10,11,11a-Octahydro-7H-10-methylindolo[1,7-bc][2,6]-naphthyridine fumarate
- U46619
9,11-dideoxy-11α,9α-epoxy-methanoprostaglandin F2α;
- Y-25130
N-(1-azabicyclo[2,2,2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxamidehydrochloride
References
- BJORK L., CORNFIELD L.J., NELSON D.L., HILLVER S.E., ANDEN N.E., LEWANDER T., HACKSELL U. Pharmacology of the novel 5-hydroxytryptamine1A receptor antagonist (S)-5-fluoro-8-hydroxy-2-(dipropylamino)tetralin: inhibition of (R)-8-hydroxy-2-(dipropylamino)tetralin-induced effects. J. Pharmacol. Exp. Ther. 1991;258:58–65. [PubMed] [Google Scholar]
- BLOK C., VAN VENROOIJ G.E., MOKHLESS I., COOLSAET B.L. Dynamics of the ureterovesical junction: its fluid transport mechanism in the pig. J. Urol. 1985;134:175–178. doi: 10.1016/s0022-5347(17)47051-0. [DOI] [PubMed] [Google Scholar]
- BOESS F.G., MARTIN I.L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- CHEN H.I. Evidence for the presynaptic action of 5-hydroxytryptamine and the involvement of purinergic innervation in the rabbit lower urinary tract. Br. J. Pharmacol. 1990;101:212–216. doi: 10.1111/j.1476-5381.1990.tb12115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN M.L. Canine, but not rat bladder contracts to serotonin via activation of 5-HT2 receptors. J. Urol. 1990;143:1037–1040. doi: 10.1016/s0022-5347(17)40178-9. [DOI] [PubMed] [Google Scholar]
- COHEN M.L., SCHENCK K.W., HEMRICK-LUECKE S.H. 5-Hydroxytryptamine1A receptor activation enhances norepinephrine release from nerves in the rabbit saphenous vein. J. Pharmacol. Exp. Ther. 1999;290:1195–1201. [PubMed] [Google Scholar]
- EGLEN R.M., BONHAUS D.W., JOHNSON L.G., LEUNG E., CLARK R.D. Pharmacological characterization of two novel and potent 5-HT4 receptor agonists, RS 67333 and RS 67506 in vitro and in vivo. Br. J. Pharmacol. 1995;115:1387–1392. doi: 10.1111/j.1476-5381.1995.tb16628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGEL G., GOTHER M., HOYER D., SCHLICKER E., HILLEBRAND K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs' Arch. Pharmacol. 1986;332:1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- FETISSOF F., DUBOIS M.P., ARBEILLE-BRASSART B., LANSON Y., BOIVIN F., JOBARD P. Endocrine cells in the prostate gland, urothelium and Brenner tumors. Immunohistological and ultrastructural studies. Virchows Arch. B. Cell Pathol. Incl. Mol. Pathol. 1983;42:53–64. doi: 10.1007/BF02890370. [DOI] [PubMed] [Google Scholar]
- FUKUDA T., SETOGUCHI M., INABA K., SHOJI H., TAHARA T. The antiemetic profile of Y-25130, a new selective 5-HT3 receptor antagonist. Eur. J. Pharmacol. 1991;196:299–305. doi: 10.1016/0014-2999(91)90443-t. [DOI] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W., OXFORD A.W., BUNCE K.T., HUMPHREY P.P. GR 113808: a novel selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIDENER S., GUMUSTEKIN M., KIRKALI Z. Pharmacological analysis of 5-hydroxytryptamine effects on human isolated ureter. Pharmacol. Res. 1999;39:487–491. doi: 10.1006/phrs.1999.0469. [DOI] [PubMed] [Google Scholar]
- GIDENER S., KIRKALI Z., GUVEN H. Influence of serotonin on the human ureter: an in vitro pharmacological study. Urol. Int. 1995;55:202–204. doi: 10.1159/000282786. [DOI] [PubMed] [Google Scholar]
- HANDSCHUMACHER R.E., VANE J.R. The relationship between the penetration of tryptamine and 5-hydroxytryptamine into smooth muscle and the associated contractions. Br. J. Pharmacol. 1967;29:105–118. doi: 10.1111/j.1476-5381.1967.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSER D.S., MEVISSEN M., WEISS R., PORTIER C.J., SCHOLTYSIK G., STUDER U.E., DANUSER H. Effects of ketanserine and DOI on spontaneous and 5-HT-evoked peristalsis of the pig ureter in vivo. Br. J. Pharmacol. 2002;135:1026–1032. doi: 10.1038/sj.bjp.0704536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁNDEZ M., BARAHONA M.V., BUSTAMANTE S., GARCÍA-SACRISTÁN A., ORENSANZ L.M. A2B adenosine receptors mediate relaxation of the pig intravesical ureter: adenosine modulation of non adrenergic non cholinergic excitatory neurotransmission. Br. J. Pharmacol. 1999;126:969–978. doi: 10.1038/sj.bjp.0702386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁNDEZ M., PRIETO D., ORENSANZ L.M., BARAHONA M.V., GARCÍA-SACRISTÁN A., SIMONSEN U. Nitric oxide is involved in the non-adrenergic non-cholinergic inhibitory neurotransmission of the pig intravesical ureter. Neurosci. Lett. 1995;186:33–36. doi: 10.1016/0304-3940(94)11275-n. [DOI] [PubMed] [Google Scholar]
- HERNÁNDEZ M., PRIETO D., SIMONSEN U., RIVERA L., BARAHONA M.V., GARCÍA-SACRISTÁN A. Noradrenaline modulates smooth muscle activity of the isolated intravesical ureter of the pig through different types of adrenoceptors. Br. J. Pharmacol. 1992;107:924–931. doi: 10.1111/j.1476-5381.1992.tb13387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁNDEZ M., SIMONSEN U., PRIETO D., RIVERA L., GARCÍA P., ORDAZ E., GARCÍA-SACRISTÁN A. Different muscarinic receptor subtypes mediating the phasic activity and basal tone of pig isolated intravesical ureter. Br. J. Pharmacol. 1993;110:1413–1420. doi: 10.1111/j.1476-5381.1993.tb13978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HUMPHREY P.P. Peripheral 5-hydroxytryptamine receptors and their classification. Neuropharmacology. 1984;23:1503–1510. doi: 10.1016/0028-3908(84)90094-7. [DOI] [PubMed] [Google Scholar]
- KILPATRICK G.J., BUTLER A., BURRIDGE J., OXFORD A.W. 1-(m-chlorophenyl)-biguanide, a potent high affinity 5-HT3 receptor agonist. Eur. J. Pharmacol. 1990;182:193–197. doi: 10.1016/0014-2999(90)90513-6. [DOI] [PubMed] [Google Scholar]
- KLARSKOV P., HØRBY-PETERSEN J. Influence of serotonin on lower urinary tract smooth muscle in vitro. Br. J. Pharmacol. 1986;58:507–513. doi: 10.1111/j.1464-410x.1986.tb05456.x. [DOI] [PubMed] [Google Scholar]
- MARÍN J., SALAICES M., GOMEZ B., LLUCH S. Noradrenergic component in the vasoconstriction induced by 5-hydroxytryptamine in goat cerebral arteries. J. Pharm. Pharmacol. 1981;33:715–719. doi: 10.1111/j.2042-7158.1981.tb13911.x. [DOI] [PubMed] [Google Scholar]
- MESSORI E., RIZZI C.A., CANDURA S.M., LUCCHELLI A., BALESTRA B., TONINI M. 5-Hydroxytryptamine receptors that facilitate excitatory neuromuscular transmission in the guinea-pig isolated detrusor muscle. Br. J. Pharmacol. 1995;115:677–683. doi: 10.1111/j.1476-5381.1995.tb14986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOZULAK J., KALKMAN H.O., FLOERSHEIM P., HOYER D., SCHOEFFER P., BUERKI H.R. (+)-cis-4, 5, 7a, 8, 9, 10, 11, 11a-octahydro-7H-10-methylindolo[1, 7-bc][2, 6]-naphthyridine: a 5HT2C/2B receptor antagonist with low 5HT2A receptor affinity. J. Med. Chem. 1995;38:28–33. doi: 10.1021/jm00001a007. [DOI] [PubMed] [Google Scholar]
- PRIETO D., HERNÁNDEZ M., RIVERA L., GARCÍA-SACRISTÁN A., SIMONSEN U. Distribution and functional effects of neuropeptide Y on equine ureteral smooth muscle and resistance arteries. Regul. Pept. 1997;69:155–165. doi: 10.1016/s0167-0115(97)00003-7. [DOI] [PubMed] [Google Scholar]
- SAXENA P.R., DE VRIES P., VILLALÓN C.M. 5-HT1-like receptors: a time to bid goodbye. Trends Pharmacol. Sci. 1998;19:311–316. doi: 10.1016/s0165-6147(98)01228-0. [DOI] [PubMed] [Google Scholar]
- TONINI M., MESSORI E., FRANCESCHETTI G.P., RIZZI C.A., CASTOLDI A.F., COCCINI T., CANDURA S.M. Characterization of the 5-HT receptor potentiating neuromuscular cholinergic transmission in strips of human isolated detrusor muscle. Br. J. Pharmacol. 1994;113:1–2. doi: 10.1111/j.1476-5381.1994.tb16163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAIKAR M.V., FORD A.P.D.W., CLARKE D.E. Evidence for an inhibitory 5-HT4 receptor in urinary bladder of Rhesus and Cynomolgus monkeys. Br. J. Pharmacol. 1994;111:213–218. doi: 10.1111/j.1476-5381.1994.tb14046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


